Severe Acute Malnutrition: Recognition and Management of Marasmus and Kwashiorkor
Severe Acute Malnutrition: Recognition and Management of Marasmus and Kwashiorkor
Introduction
Severe acute malnutrition (SAM) is a life-threatening condition affecting millions of children worldwide and is characterized by inadequate weight relative to height. SAM is clinically categorized into 2 primary forms: marasmus, caused by an overall calorie deficiency, and kwashiorkor, primarily due to severe dietary protein deficiency despite adequate or near-adequate energy intake. A mixed form, marasmic-kwashiorkor, may also occur, combining features of both. The term "kwashiorkor" is derived from the Ga language spoken in Ghana and loosely translates to "the sickness a baby gets when the new baby comes," referring to early weaning to a low-protein, high-carbohydrate diet.[1]
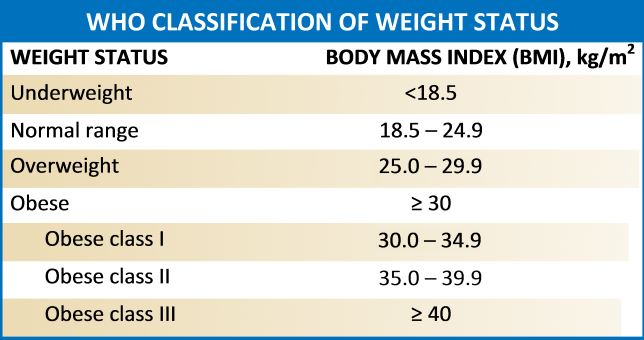
Anthropometry, the measurement of a child's physical size and dimensions, is the most commonly used tool to quantify pediatric undernutrition. Children with the marasmus form of SAM exhibit severe wasting, defined by a weight-for-height z-score that is greater than 3 standard deviations below the mean, based on age and gender, or, in children aged 6 to 59 months, a mid-upper arm circumference of 115 mm or less. The World Health Organization (WHO) and the Centers for Disease Control and Prevention growth charts are the most frequently used references for growth standards (see Image. World Health Organization Classification of Weight Status Table).[2] Children with severe wasting exhibit a marked loss of both fat and muscle mass. Those with kwashiorkor present with bilateral edema, often with minimal or no visible wasting.[2]
Children with both forms of SAM typically have concomitant micronutrient deficiencies, particularly of iron, zinc, vitamin A, and iodine, which are associated with faltering growth, impaired cognitive development, and compromised host defenses, resulting in an increased risk of severe infections and mortality.[3] Understanding these conditions' distinct presentations and underlying pathophysiology is critical for timely diagnosis and effective management. This article reviews the etiology, epidemiology, clinical features, diagnosis, and management of marasmus and kwashiorkor within the broader context of SAM, guided by WHO criteria and evidence-based clinical practice.
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
Undernutrition, including SAM, underweight, and chronic malnutrition, contributes to an estimated 35% to 45% of global child mortality and remains a leading cause of disease burden and death in children worldwide.[4] Malnutrition is a broad term referring to any nutrient imbalance, encompassing both undernutrition and overnutrition, such as obesity. Undernutrition refers explicitly to a deficiency in calories, protein, or micronutrients. In clinical and public health practice, the terms malnutrition and undernutrition are often used interchangeably in the context of pediatric populations.
The etiology of SAM is multifactorial, involving a combination of inadequate dietary intake (calories and/or protein), recurrent or chronic infections such as human immunodeficiency virus (HIV) and tuberculosis, suboptimal maternal and fetal nutrition, food insecurity, poverty, and environmental factors such as poor sanitation, famine, other natural disasters, and war. Underlying causes include both primary factors (insufficient nutritious food) and secondary factors (disease states that increase metabolic demands, impair absorption, or reduce intake).[2] Marasmus develops when there is a prolonged energy and protein deficiency, causing severe wasting without edema. The impact of marasmus is exacerbated by chronic or recurrent infections and by underlying social or economic deprivation.[3] In contrast, children with kwashiorkor present with bilateral pitting edema, which can falsely elevate weight and mid-upper arm circumference measurements. While protein deficiency was historically considered the primary cause of kwashiorkor, current evidence suggests that a combination of inadequate protein and micronutrient intake, oxidative stress, alterations in gut microbiota, and exposure to environmental toxins (eg, aflatoxins) also contributes to its development.
The underlying social root cause of SAM in children is poverty.[5][6] Poverty may stem from factors such as low socioeconomic status and limited parental education, as well as broader systemic issues, including war, natural disasters, and civil unrest. Poverty directly influences a household's ability to secure a reliable food source for children and supply their caloric and nutritional needs. Family displacement and adverse living conditions—such as inadequate shelter and lack of clean water—undermine the ability to care for children, increasing their risk of infections and diarrheal diseases.
The HIV/acquired immunodeficiency virus (AIDS) epidemic in Sub-Saharan Africa has significantly increased the caregiving burden on households, often limiting adults' ability to engage in agricultural work due to illness or the need to care for affected family members.[7] Maternal education is another key factor in the development of childhood malnutrition. Results from one study showed that Kenyan mothers with a primary school education had children with a 94% lower risk of chronic malnutrition (growth stunting) compared to mothers with no formal education.[8] Biological contributors to malnutrition include HIV/AIDS and other infectious diseases.[9] Children infected with HIV have worse nutritional outcomes compared to uninfected children.[10][11] Malaria is associated with stunting in children younger than 2, but rarely causes marasmus or kwashiorkor.[12]
Epidemiology
Approximately 19 to 20 million children worldwide experience SAM, as estimated by global epidemiological analyses and reviews of prevalence data in low- and middle-income countries. SAM causes 300,000 deaths per year and contributes to half of the deaths reported in young children.[6] This condition is associated with higher rates of pediatric hospitalizations in resource-limited countries and mortality rates up to 20%.[2][13] Malnutrition-associated mortality exhibits seasonal fluctuations, with the highest incidence occurring during the pre-harvest rainy season, primarily due to food scarcity and an increased burden of infectious diseases.[2]
SAM primarily affects children younger than 5, most often in low- and middle-income countries. There is an estimation that 155 million children younger than 5 experienced chronic malnutrition with growth stunting in 2016, 52 million experienced wasting, and of these, 17 million children exhibited severe wasting.[2] Globally, the prevalence of marasmus (wasting) is estimated at 6 to 7% among children younger than 5, with higher rates in South Asia and sub-Saharan Africa. The prevalence of SAM varies by geographic region, ranging from less than 1% in many Western countries to nearly 10% in some Asian and African settings.
SAM is responsible for approximately 1 to 2 million children dying every year.[14] Individuals aged 6 to 24 months, particularly those with comorbid medical conditions such as HIV or tuberculosis or living in resource-limited settings, are at the highest risk.[15] SAM is strongly associated with poverty, food insecurity, infectious diseases, low birth weight, low maternal education, and regions affected by armed conflict.[16] The war in Gaza that began in October 2023 illustrates the effects on children. The United Nations Relief and Works Agency (UNRWA) for Palestine Refugees has documented widespread food insecurity in Gaza over the past 2 decades.
A study conducted in the summer of 2023 assessed 3229 children entering first grade at UNRWA schools, revealing that 4% to 5% of these children experienced wasting, significant food insecurity, and widespread dependence on food assistance.[17] The military escalation of October 2023 exacerbated preexisting conditions. Results from a study conducted a year later showed that 98% of families experienced severe food insecurity, and estimates indicated that 25% to 30% of Gazan children were affected by SAM due to a lack of food and the collapse of the healthcare system.[18]
Pathophysiology
Inadequate energy intake leads to complex physiological changes encompassing adaptive and maladaptive processes, affecting metabolism, endocrine function, immune status, and various organ systems. In response to insufficient dietary intake of calories, the body conserves energy and protects vital functions through several mechanisms:
- Reduced metabolic rate and total energy expenditure, leading to growth restriction
- Hormonal adaptations, including decreased insulin, insulin-like growth factor-1, and triiodothyronine, and increased cortisol and growth hormone secretion (elevated cortisol levels due to reduced cortisol binding to albumin secondary to hypoalbuminemia)
- Mobilization of fat and skeletal muscle stores for gluconeogenesis as glycogen stores are depleted within a day of caloric deprivation
- Suppressed immune function and increased infection risk with atrophy of the thymus, lymph nodes, and tonsils
- Impaired glucose metabolism, including decreased insulin secretion and β-cell dysfunction, resulting in muscle and hepatic reliance on partially oxidized fatty acids for energy
- Intestinal villous atrophy with resulting malabsorption [2]
- Impaired cardiac contractility with decreased cardiac output, bradycardia, hypotension, and elevated risk of dysrhythmias
- Decreased chest wall musculature, impairing ventilation
- Reliance on ketones, not glucose, as fuel for the central nervous system, with reduced brain growth and delays in global function and development [19]
SAM manifests primarily as marasmus, kwashiorkor, or a combination of both. Although they arise from profound nutritional deprivation and share features such as metabolic disturbances, immune dysfunction, and heightened vulnerability to infection and mortality, their underlying pathophysiological mechanisms differ. Marasmus is characterized by a severe total energy (calorie) deficiency, resulting in chronic adaptation to insufficient caloric intake, as the body mobilizes its fat and protein stores for energy and employs compensatory mechanisms to adapt to starvation.[19] Marasmus is classified as a form of nonedematous severe acute malnutrition characterized by wasting due to prolonged deficiency in energy intake.
The pathogenesis of kwashiorkor is multifactorial and not solely attributable to protein deficiency, as previously thought. The descriptive term is edematous malnutrition since the condition is characterized by bilateral pitting edema. Key mechanisms include profound deficiencies in antioxidants (notably glutathione), essential amino acids (particularly methionine and cysteine), and micronutrients. These deficiencies contribute to oxidative stress, impaired hepatic protein synthesis, and alterations in gut microbiota, including an overrepresentation of pathogenic Proteobacteria. These changes disrupt the gut-liver axis, leading to profound hypoalbuminemia, which causes increased vascular permeability, decreased plasma osmotic pressure, and edema.[20] Environmental factors such as aflatoxin exposure and infections further exacerbate metabolic dysfunction.[20][21] The edema, hepatic dysfunction (fatty liver), and multisystem involvement make kwashiorkor even more dangerous than marasmus.
History and Physical
Marasmus is the result of prolonged calorie deprivation, often in the context of food insecurity, underlying illness, or severe neglect. The medical and dietary history typically reveals a sustained period of insufficient energy intake, sometimes exacerbated by recurrent infections, poor feeding practices, or early weaning. Caregivers may report a history of failure to grow, progressive weight loss, increased appetite, irritability, or prior episodes of dehydration.[https://doi.org/10.1542/9781610027700] Infants may exhibit apathy, fatigue, and pallor due to deficiencies in micronutrients.
On physical examination, children with marasmus exhibit marked muscle wasting and a near-complete loss of subcutaneous fat as the body mobilizes muscle protein and fat stores to meet metabolic demands.[2] Their vital signs may show hypotension, hypothermia, and bradycardia.[https://doi.org/10.1542/9781610027700] Their bodies appear emaciated, with loose skin folds, sunken cheeks, and visible ribs. Weight loss is often most pronounced in the groin and axillae early on and later in the buttocks, face, and thighs, resulting in a characteristically aged or "old man" appearance due to the loss of facial adipose tissue.
Other signs include sunken fontanelles in infants due to dehydration and stunted linear growth, which may falsely normalize the weight-for-height ratio. Findings related to specific micronutrient deficiencies are common, including dry eyes and Bitot spots (vitamin A deficiency), koilonychia and pallor (iron deficiency anemia), and signs of hypocalcemia such as Chvostek or Trousseau signs. Long-term calcium and vitamin D deficiency can progress to rickets and bony deformities. Notably, children with marasmus lack the hallmark features of kwashiorkor, such as edema, dermatosis, cheilosis, and depigmentation of hair or skin.[1]
Kwashiorkor develops in a protein-deficient but energy-sufficient diet, often following the abrupt introduction of carbohydrate-rich foods after weaning. The medical and dietary history typically includes a recent stressor, such as infection or trauma, in a child who was previously nursing. These children often have a poor-quality diet lacking essential amino acids and antioxidants. Caregivers may report swelling of the legs or face, poor appetite, lethargy, or skin breakdown. Unlike children with marasmus, those with kwashiorkor usually have little or no history of hunger or increased food-seeking behavior.
The physical examination in kwashiorkor reveals bilateral pitting edema, initially in the lower extremities and potentially progressing to generalized edema (anasarca). Despite their swollen appearance, these children are usually profoundly malnourished. The skin may show "flaky-paint" dermatosis—patches of hyperpigmentation and desquamation, especially over pressure points or areas of trauma.[22] Sores at the corners of the mouth and pale, thinning, or easily pluckable hair are common. Hepatomegaly due to hepatic steatosis is frequently observed. These children are typically apathetic and listless, often with little interest in eating or interacting with their environment.[https://doi.org/10.1542/9781610027700]
Evaluation
Evaluating a child for SAM involves a combination of anthropometric measurements, clinical assessment, and laboratory investigations. Meeting any of the following criteria establishes a diagnosis of SAM:
- Weight-for-height z-score (WHZ) <–3 standard deviation (SD)
- Mid-upper arm circumference (MUAC) <115 mm (or MUAC-for-age z-score <–3 SD)
- Bilateral pitting edema of nutritional origin [2][23]
Anthropometric measurements involve the accurate measurement of vertical length to a precision of 0.5 cm, weight to a precision of 0.1 kg (100 g), and MUAC to a precision of 2 mm or less.[24] In resource-limited or high-edema settings, MUAC-for-age z-score (MUACZ) and WHZ are used together to improve diagnostic sensitivity, particularly for detecting kwashiorkor. Importantly, although commonly used, weight-for-age less than the third percentile is not a reliable indicator for SAM because it does not consider the effects of stunting on a child's linear growth.[25]
Children with SAM require a comprehensive clinical assessment to distinguish marasmus from kwashiorkor, identify associated micronutrient deficiencies, and screen for complications or comorbidities. The presence of ascites should prompt careful evaluation for underlying conditions, including tuberculosis (TB), HIV, sepsis, and malaria.[26] The physical examination may reveal signs of vitamin A deficiency (eg, Bitot spots), iron deficiency anemia (eg, koilonychia or pallor), or rickets, which can be confirmed through specific testing.[2][27] Evaluation for possible TB includes inquiring about exposure, performing a physical examination, and, where available, obtaining chest imaging and microbiologic testing (eg, gastric aspirate, sputum, or urine lipoarabinomannan testing). Ascites may suggest abdominal TB, prompting a diagnostic paracentesis.
Additional laboratory evaluation can identify nutritional deficiencies, systemic illness, and complications of SAM. The WHO recommends a core set of tests, including:
- Hemoglobin and blood smear to assess for anemia and parasitic infections
- Blood glucose to detect hypoglycemia
- Serum albumin and electrolytes to evaluate fluid and protein status
- Stool microscopy and culture to identify parasitic or bacterial infections
- HIV testing
- Urine microscopy and culture [1]
Additional investigations to identify micronutrient deficiencies include vitamin D levels, a complete blood count, iron studies, and folate and vitamin B12 levels, which further characterize anemia. Plasma protein levels, including transferrin, albumin, and thyroxine-binding prealbumin, are biomarkers to assess nutritional status and determine response to treatment.[28]
Treatment / Management
The 3 phases of SAM treatment are stabilization, rehabilitation, and follow-up. SAM is categorized as complicated or uncomplicated, a distinction guiding treatment and management. Children with uncomplicated SAM have a good appetite and no clinical signs, such as edema or acute medical conditions, that place them at greater risk and necessitate inpatient treatment. Evidence suggests that community-based management of uncomplicated SAM yields better outcomes than inpatient hospital care and is considered the standard for most children with SAM.[29][30](A1)
Community-based treatment requires a structured program with trained staff, ready-to-use therapeutic foods (RUTFs), and regular follow-up care.[https://iris.who.int/handle/10665/44295] RUTFs are energy-dense with therapeutic micronutrients for severely malnourished children. The most frequently used RUTF contains peanut paste; other ingredients include milk powder and vegetable oils.[19] The calorie goal when treating SAM is 175 kcal/kg/day.[31] Both weight-based and MUAC-based dosing protocols exist for treatment with RUTFs. Typical regimens include 2 packets (or sachets) per day for children with MUAC less than 115 mm or edema and 1 per day for those with MUAC between 115 and 125 mm. However, RUTFs may be inaccessible in low-resource settings due to their high cost and the absence of reliable food aid programs.[32](A1)
Because children with SAM often have underlying subclinical bacterial infections, the WHO recommends routine treatment with broad-spectrum antibiotics such as amoxicillin. This guideline is controversial due to the risks of growing antibiotic resistance and the potential adverse effects on the gut microbiome.[33][34] All children should be screened and treated for coexisting infections as indicated. The WHO also recommends vitamin A supplementation because deficiencies are common and associated with a higher risk of viral and bacterial infections, blindness, and overall mortality.[35](A1)
Children with complicated SAM require inpatient treatment for rehydration to prevent infections that may progress to sepsis and to avoid complications of treatment such as refeeding syndrome. Refeeding syndrome is a potentially life-threatening condition that occurs when nutrition is reintroduced too rapidly after a period of severe malnutrition; it is characterized by electrolyte shifts, particularly hypophosphatemia, and disturbances in potassium, magnesium, and fluid balance, which can result in cardiac, neurological, and respiratory complications.
Initial management involves a stabilization phase, during which hypoglycemia, hypothermia, dehydration, and electrolyte imbalances are carefully corrected, along with prompt treatment of infections. The standard intravenous normal saline solution contains too much sodium and too little potassium for rehydration. ReSoMal (Rehydration Solution for Malnutrition) is a specialized oral rehydration solution formulated for managing dehydration in children with SAM that can be administered by mouth or nasogastric tube. This solution contains less sodium and more potassium compared to standard WHO-oral rehydration solution, aiming to address the unique electrolyte disturbances in SAM, such as hypokalemia and the risk of sodium overload.[36]
Nutritional rehabilitation for children with severe acute malnutrition begins with cautious refeeding using therapeutic milk such as F-75, designed for the stabilization phase (typically the first 2–7 days). F-75 provides approximately 75 kcal and 0.9 g of protein per 100 mL, with low fat, protein, and sodium content, to reduce the risk of refeeding syndrome and support digestion in severely ill children. These milks are not intended for weight gain but rather to stabilize metabolism, correct electrolyte imbalances, and allow for the treatment of underlying infections. This formula is easy to digest and is specifically formulated to meet the metabolic needs during the early phase of treatment.[24][32](A1)
Dehydration is treated with intravenous isotonic solutions, and if hypovolemia is present, plasma or blood may also be administered to restore volume. The child should be in a warm environment, as they are susceptible to hypothermia. Furthermore, as there is an atypical response to infections, a child with SAM may not exhibit overt signs indicating sepsis, and antibiotics may be given after obtaining blood cultures when sepsis is suspected.[37]
To prevent the development of refeeding syndrome, nutrition should be delivered slowly and carefully, with caloric intake between 60% and 80% of the recommended daily calorie requirement for the individual's age. A potential risk of refeeding is the development of hypoglycemia, which can be mitigated by continuous nasogastric feeding at night or by consuming frequent small meals throughout the day and at night.[1] Supplements, including thiamine and oral phosphate, should be administered to prevent the development of hypophosphatemia, which is commonly associated with refeeding syndrome.
Once the child becomes clinically stable, shows signs of appetite return, and has no significant medical complications, nutritional support transitions to F-100 or RUTF. The F-100 formula is used in the rehabilitation phase and provides 100 kcal and approximately 2.9 g of protein per 100 mL, supporting weight gain and the recovery of lean body mass. This transition marks the shift from metabolic stabilization to active nutritional rehabilitation. During the stabilization and rehabilitation phases, close clinical monitoring and supportive care are crucial for identifying and managing complications, ensuring tolerance of feeds, and facilitating a full recovery.[24] The rehabilitation phase may last from 2 to 6 weeks, during which children may require 120% to 140% of the recommended daily caloric intake to maintain a growth rate similar to that of their healthy peers.
The follow-up phase in managing SAM is crucial for ensuring sustained recovery, preventing relapse, and monitoring for complications.[1] After initial treatment and discharge from either inpatient or outpatient care, children are typically monitored through scheduled weekly clinic visits, anthropometric assessments (such as MUAC and weight-for-height z-scores), and clinical evaluations for new or ongoing illnesses, including diarrhea and respiratory infections. The healthcare team should follow children for at least 3 to 6 months after initial treatment. Health workers educate caregivers about signs and symptoms that indicate a possible relapse, distribute therapeutic foods, provide access to clean drinking water, and connect families to social service programs during these visits.[37] Community-based strategies to ensure close follow-up are essential for achieving high recovery rates and reducing adverse outcomes in affected children.
Differential Diagnosis
The differential diagnosis of SAM includes a range of conditions that can mimic the features of marasmus (wasting) or kwashiorkor (edematous malnutrition). Wasting, defined as low weight-for-height, can also result from chronic infections such as tuberculosis, HIV/AIDS, and intestinal parasitic diseases.[38] Wasting is seen in malabsorption syndromes as well, including celiac disease and cystic fibrosis, and in childhood malignancies such as leukemia and lymphoma. Additionally, severe psychosocial deprivation or neglect can lead to inadequate nutritional intake and growth failure.
Children presenting with severe edema require evaluation for conditions that may resemble kwashiorkor, including nephrotic syndrome, hepatic disease with hypoalbuminemia, congestive heart failure, and severe anemia. Distinguishing these conditions from SAM is essential, as management strategies differ significantly. In most cases of SAM, a thorough history and physical examination, including anthropometric measurements, will help differentiate between malnutrition and other medical conditions.
Additionally, children with SAM often have associated medical conditions that must be identified and treated, including tuberculosis, HIV/AIDS, other infections such as measles and malaria, and micronutrient deficiencies. In particular, the presence of both ascites and kwashiorkor increases the clinical suspicion of coexisting tuberculosis and merits diagnostic evaluation with ascitic fluid analysis and imaging.[39][40][41] A systematic approach to the differential diagnosis, starting with a thorough history and physical examination, is crucial for distinguishing SAM from other medical conditions and coexisting diseases with overlapping features, thereby ensuring that children receive appropriate and targeted treatment.
Prognosis
The prognosis for SAM remains guarded, even with appropriate inpatient or outpatient management. Reported recovery rates in outpatient programs range from 65% to 80%, with a median time to recovery of 8 to 9 weeks, provided compliance and no complications occur. However, a significant proportion of children remain stunted in height or underweight, with persistent deficits in height-for-age, weight-for-age, and muscle mass observed up to 5 years after discharge.[42] Relapse rates range from 3% to as high as 37% within 6 to 12 months, depending on the child's environment, follow-up care, and definition of relapse. Children who have recovered from SAM remain at higher risk of recurrent malnutrition and other illnesses compared to their peers, with a 14-fold increased risk of relapse in the year following discharge.[43]
Factors associated with worse outcomes include low anthropometric measurements during treatment, early discharge before meeting the WHO criteria, and the occurrence of intercurrent illnesses and infections during the follow-up period. In a long-term study, approximately half of the children treated for SAM had reached international growth standards in weight and height 10 years later, and no significant anthropometric differences were noted between former patients and their control siblings at the 10-year follow-up examination.[44] This study's results highlight the importance of long-term monitoring, sustained nutritional support, and comprehensive follow-up care to improve outcomes and reduce the risk of relapse in children recovering from SAM.
Complications
The complications of SAM can be categorized into short-term medical complications, long-term health and developmental consequences, and the broader impact of SAM on global child health. Acute complications can be life-threatening and include electrolyte abnormalities, cardiac dysrhythmias, sepsis, urinary tract infections, malabsorption, hypothermia, and endocrine dysfunction, such as impaired glucose metabolism.[1] During the initial resuscitation, fluid overload may result in acute heart failure and edema.[45]
Preventing and recognizing refeeding syndrome is particularly important during nutritional rehabilitation. Refeeding syndrome occurs when nutritional therapy is reintroduced too rapidly and results in sudden shifts of fluids and electrolytes, leading to life-threatening complications. Despite normal serum levels, children with SAM frequently exhibit depleted total body stores of phosphate, potassium, and magnesium. Refeeding increases insulin secretion in response to a carbohydrate load, promoting cellular uptake of electrolytes and serum electrolyte depletion, leading to dysrhythmias, rhabdomyolysis, confusion, and even sudden death.[45][46] Thiamine deficiency may also occur due to increased metabolic demand during glucose metabolism, increasing the risk of lactic acidosis and neurologic complications.[45]
Even after initial recovery, children with SAM may experience persistent health and developmental challenges. SAM causes immune and metabolic dysfunction with subsequent increased risk of morbidity and mortality. Mortality rates after discharge can be as high as nearly 10% in the first year, and children with SAM are almost 12 times more likely to perish than their adequately nourished peers.[47] SAM causes susceptibility to serious infections such as measles, tuberculosis, diarrheal illnesses, and malaria, which are often the end cause of death.[3] Surviving individuals may exhibit long-lasting alterations in their lipid and glucose metabolism even as adults, with an increased risk of diabetes and hepatocellular steatosis.[48] Neurodevelopmental impairment sometimes occurs, particularly for children with complicated SAM or comorbidities such as HIV infection. Additionally, SAM is strongly associated with shorter adult height and lower birth weight in offspring.[49]
Globally, malnutrition contributes significantly to child mortality, especially in low- and middle-income countries. There is an estimation that 45% of all deaths in children younger than 5—most of which occur in those younger than 2—are associated with some form of malnutrition.[4][47] Beyond its impact on mortality, SAM hinders educational attainment, economic productivity, and socioeconomic advancement in affected populations.[50] The long-term burden of malnutrition perpetuates intergenerational cycles of poverty and poor health, making it both a public health and socioeconomic crisis in low-resource settings worldwide.
Deterrence and Patient Education
Education in low-resource settings plays a vital role in preventing SAM by targeting key underlying causes of early childhood undernutrition, particularly suboptimal breastfeeding practices and inadequate access to clean water and sanitation. Health workers should encourage exclusive breastfeeding for all infants younger than 6 months of age and counsel caregivers to avoid unsafe formula feeding, especially in settings where clean water is not reliably available. Breastfeeding significantly lowers the risk of diarrheal disease, respiratory infections, and growth faltering, major contributors to SAM. HIV-positive mothers can still breastfeed their infants if safe and nutritionally adequate alternatives are unavailable. Mothers can introduce appropriate complementary foods to infants aged 6 months while continuing to breastfeed.[https://doi.org/10.1542/9781610027700] Community-based education on safe food preparation, hand hygiene, and latrine use is equally important in preventing infections, particularly diarrheal illnesses that can lead to dehydration and nutrient loss. Household water safety practices such as boiling, chlorination, and safe storage enhance child and family health by reducing exposure to waterborne pathogens.
Enhancing Healthcare Team Outcomes
Improving outcomes for children with SAM depends on the coordinated efforts of an interprofessional healthcare team. This team typically includes physicians, nurses, dietitians, community health workers, and social workers, each playing a vital role in health screens, diagnosis, treatment, and follow-up care. Effective communication, shared decision-making, and culturally sensitive education are crucial for ensuring adherence to treatment protocols and promoting long-term recovery. Strengthening collaboration across disciplines improves clinical outcomes and supports families and communities in sustaining nutrition and health beyond the clinical setting.
Media
(Click Image to Enlarge)
References
Grover Z, Ee LC. Protein energy malnutrition. Pediatric clinics of North America. 2009 Oct:56(5):1055-68. doi: 10.1016/j.pcl.2009.07.001. Epub [PubMed PMID: 19931063]
Bhutta ZA, Berkley JA, Bandsma RHJ, Kerac M, Trehan I, Briend A. Severe childhood malnutrition. Nature reviews. Disease primers. 2017 Sep 21:3():17067. doi: 10.1038/nrdp.2017.67. Epub 2017 Sep 21 [PubMed PMID: 28933421]
Ibrahim MK, Zambruni M, Melby CL, Melby PC. Impact of Childhood Malnutrition on Host Defense and Infection. Clinical microbiology reviews. 2017 Oct:30(4):919-971. doi: 10.1128/CMR.00119-16. Epub [PubMed PMID: 28768707]
Karlsson O, Kim R, Hasman A, Subramanian SV. Age Distribution of All-Cause Mortality Among Children Younger Than 5 Years in Low- and Middle-Income Countries. JAMA network open. 2022 May 2:5(5):e2212692. doi: 10.1001/jamanetworkopen.2022.12692. Epub 2022 May 2 [PubMed PMID: 35587349]
Sachs JD, McArthur JW. The Millennium Project: a plan for meeting the Millennium Development Goals. Lancet (London, England). 2005 Jan 22-28:365(9456):347-53 [PubMed PMID: 15664232]
Müller O, Krawinkel M. Malnutrition and health in developing countries. CMAJ : Canadian Medical Association journal = journal de l'Association medicale canadienne. 2005 Aug 2:173(3):279-86 [PubMed PMID: 16076825]
de Waal A, Whiteside A. New variant famine: AIDS and food crisis in southern Africa. Lancet (London, England). 2003 Oct 11:362(9391):1234-7 [PubMed PMID: 14568749]
Abuya BA, Onsomu EO, Kimani JK, Moore D. Influence of maternal education on child immunization and stunting in Kenya. Maternal and child health journal. 2011 Nov:15(8):1389-99. doi: 10.1007/s10995-010-0670-z. Epub [PubMed PMID: 20848172]
Level 2 (mid-level) evidenceArthur SS, Nyide B, Soura AB, Kahn K, Weston M, Sankoh O. Tackling malnutrition: a systematic review of 15-year research evidence from INDEPTH health and demographic surveillance systems. Global health action. 2015:8():28298. doi: 10.3402/gha.v8.28298. Epub 2015 Oct 29 [PubMed PMID: 26519130]
Level 1 (high-level) evidenceKimani-Murage EW, Norris SA, Pettifor JM, Tollman SM, Klipstein-Grobusch K, Gómez-Olivé XF, Dunger DB, Kahn K. Nutritional status and HIV in rural South African children. BMC pediatrics. 2011 Mar 25:11():23. doi: 10.1186/1471-2431-11-23. Epub 2011 Mar 25 [PubMed PMID: 21439041]
Level 2 (mid-level) evidencePapathakis PC, Rollins NC, Chantry CJ, Bennish ML, Brown KH. Micronutrient status during lactation in HIV-infected and HIV-uninfected South African women during the first 6 mo after delivery. The American journal of clinical nutrition. 2007 Jan:85(1):182-92 [PubMed PMID: 17209195]
Nyakeriga AM, Troye-Blomberg M, Dorfman JR, Alexander ND, Bäck R, Kortok M, Chemtai AK, Marsh K, Williams TN. Iron deficiency and malaria among children living on the coast of Kenya. The Journal of infectious diseases. 2004 Aug 1:190(3):439-47 [PubMed PMID: 15243915]
Level 3 (low-level) evidenceMaitland K, Berkley JA, Shebbe M, Peshu N, English M, Newton CR. Children with severe malnutrition: can those at highest risk of death be identified with the WHO protocol? PLoS medicine. 2006 Dec:3(12):e500 [PubMed PMID: 17194194]
Level 2 (mid-level) evidenceAhmed T, Hossain M, Mahfuz M, Choudhury N, Hossain MM, Bhandari N, Lin MM, Joshi PC, Angdembe MR, Wickramasinghe VP, Hossain SM, Shahjahan M, Irianto SE, Soofi S, Bhutta Z. Severe acute malnutrition in Asia. Food and nutrition bulletin. 2014 Jun:35(2 Suppl):S14-26 [PubMed PMID: 25069289]
Chama GC, Siame L, Kapoma C, Hamooya BM, Masenga SK. Severe acute malnutrition among children under the age of 5 years. PloS one. 2024:19(8):e0309122. doi: 10.1371/journal.pone.0309122. Epub 2024 Aug 26 [PubMed PMID: 39186515]
Belay DG, Chilot D, Alem AZ, Aragaw FM, Asratie MH. Spatial distribution and associated factors of severe malnutrition among under-five children in Ethiopia: further analysis of 2019 mini EDHS. BMC public health. 2023 Apr 28:23(1):791. doi: 10.1186/s12889-023-15639-2. Epub 2023 Apr 28 [PubMed PMID: 37118793]
Horino M, Zaqqout R, Habash R, Albaik S, Abed Y, Al-Jadba G, West KP Jr, Seita A. Food insecurity, dietary inadequacy, and malnutrition in the Gaza Strip: a cross-sectional nutritional assessment of refugee children entering the first grade of UNRWA schools and their households before the conflict of 2023-24. The Lancet. Global health. 2024 Nov:12(11):e1871-e1880. doi: 10.1016/S2214-109X(24)00320-6. Epub 2024 Sep 23 [PubMed PMID: 39326434]
Level 2 (mid-level) evidenceFaris M, Abutair AS, Elfarra RM, Barqawi NA, Firwana AM, Firwana RM, AbuHajjaj MM, Shamaly SA, AbuSamra SS, Bashir HS, Abedalrahim NA, Nofal NA, Alshawaf MK, Al Shatali RM, Ghaben KI, Alron MI, Alqeeq SS, Al-Nabahin AO, Badawi RA. Catastrophic famine in Gaza: Unprecedented levels of hunger post-October 7th. A real population-based study from the Gaza Strip. PloS one. 2025:20(5):e0309854. doi: 10.1371/journal.pone.0309854. Epub 2025 May 28 [PubMed PMID: 40435059]
Dipasquale V, Cucinotta U, Romano C. Acute Malnutrition in Children: Pathophysiology, Clinical Effects and Treatment. Nutrients. 2020 Aug 12:12(8):. doi: 10.3390/nu12082413. Epub 2020 Aug 12 [PubMed PMID: 32806622]
Pham TP, Alou MT, Golden MH, Million M, Raoult D. Difference between kwashiorkor and marasmus: Comparative meta-analysis of pathogenic characteristics and implications for treatment. Microbial pathogenesis. 2021 Jan:150():104702. doi: 10.1016/j.micpath.2020.104702. Epub 2021 Jan 3 [PubMed PMID: 33359074]
Level 1 (high-level) evidenceDi Giovanni V, Bourdon C, Wang DX, Seshadri S, Senga E, Versloot CJ, Voskuijl W, Semba RD, Trehan I, Moaddel R, Ordiz MI, Zhang L, Parkinson J, Manary MJ, Bandsma RH. Metabolomic Changes in Serum of Children with Different Clinical Diagnoses of Malnutrition. The Journal of nutrition. 2016 Dec:146(12):2436-2444 [PubMed PMID: 27807038]
Maltos AL, Portari GV, Moraes GV, Monteiro MC, Vannucchi H, da Cunha DF. Niacin metabolism and indoleamine 2,3-dioxygenase activation in malnourished patients with flaky paint dermatosis. Nutrition (Burbank, Los Angeles County, Calif.). 2015 Jun:31(6):890-2. doi: 10.1016/j.nut.2014.12.023. Epub 2014 Dec 31 [PubMed PMID: 25933499]
Ngaboyeka G, Bisimwa G, Neven A, Mwene-Batu P, Kambale R, Ongezi E, Chimanuka C, Ntagerwa J, Balolebwami S, Mulume F, Battisti O, Dramaix M, Donnen P. Arm circumference for age, arm circumference and weight-for-height z-score for the evaluation of severe acute malnutrition: a retrospective cohort study in eastern Democratic Republic of Congo. BMC public health. 2024 Feb 23:24(1):587. doi: 10.1186/s12889-024-18083-y. Epub 2024 Feb 23 [PubMed PMID: 38395784]
Level 2 (mid-level) evidenceTrehan I, Manary MJ. Management of severe acute malnutrition in low-income and middle-income countries. Archives of disease in childhood. 2015 Mar:100(3):283-7. doi: 10.1136/archdischild-2014-306026. Epub 2014 Nov 24 [PubMed PMID: 25421910]
Briend A, Khara T, Dolan C. Wasting and stunting--similarities and differences: policy and programmatic implications. Food and nutrition bulletin. 2015 Mar:36(1 Suppl):S15-23 [PubMed PMID: 25902610]
Asare H, Carboo J, Nel ED, Dolman RC, Conradie C, Lombard MJ, Ricci C. Mortality in relation to profiles of clinical features in Ghanaian severely undernourished children aged 0-59 months: an observational study. The British journal of nutrition. 2021 May 28:125(10):1157-1165. doi: 10.1017/S0007114520003396. Epub 2020 Sep 2 [PubMed PMID: 32873346]
Level 2 (mid-level) evidenceNgari MM, Thitiri J, Mwalekwa L, Timbwa M, Iversen PO, Fegan GW, Berkley JA. The impact of rickets on growth and morbidity during recovery among children with complicated severe acute malnutrition in Kenya: A cohort study. Maternal & child nutrition. 2018 Apr:14(2):e12569. doi: 10.1111/mcn.12569. Epub 2017 Nov 27 [PubMed PMID: 29178404]
Burritt MF, Anderson CF. Laboratory assessment of nutritional status. Human pathology. 1984 Feb:15(2):130-3 [PubMed PMID: 6421715]
Ciliberto MA, Sandige H, Ndekha MJ, Ashorn P, Briend A, Ciliberto HM, Manary MJ. Comparison of home-based therapy with ready-to-use therapeutic food with standard therapy in the treatment of malnourished Malawian children: a controlled, clinical effectiveness trial. The American journal of clinical nutrition. 2005 Apr:81(4):864-70 [PubMed PMID: 15817865]
Level 1 (high-level) evidenceLinneman Z, Matilsky D, Ndekha M, Manary MJ, Maleta K, Manary MJ. A large-scale operational study of home-based therapy with ready-to-use therapeutic food in childhood malnutrition in Malawi. Maternal & child nutrition. 2007 Jul:3(3):206-15 [PubMed PMID: 17539889]
Maleta K, Amadi B. Community-based management of acute malnutrition (CMAM) in sub-Saharan Africa: case studies from Ghana, Malawi, and Zambia. Food and nutrition bulletin. 2014 Jun:35(2 Suppl):S34-8 [PubMed PMID: 25069291]
Level 3 (low-level) evidenceDas JK, Salam RA, Saeed M, Kazmi FA, Bhutta ZA. Effectiveness of Interventions for Managing Acute Malnutrition in Children under Five Years of Age in Low-Income and Middle-Income Countries: A Systematic Review and Meta-Analysis. Nutrients. 2020 Jan 1:12(1):. doi: 10.3390/nu12010116. Epub 2020 Jan 1 [PubMed PMID: 31906272]
Level 1 (high-level) evidenceAlcoba G, Kerac M, Breysse S, Salpeteur C, Galetto-Lacour A, Briend A, Gervaix A. Do children with uncomplicated severe acute malnutrition need antibiotics? A systematic review and meta-analysis. PloS one. 2013:8(1):e53184. doi: 10.1371/journal.pone.0053184. Epub 2013 Jan 9 [PubMed PMID: 23326395]
Level 1 (high-level) evidenceIsanaka S, Langendorf C, Berthé F, Gnegne S, Li N, Ousmane N, Harouna S, Hassane H, Schaefer M, Adehossi E, Grais RF. Routine Amoxicillin for Uncomplicated Severe Acute Malnutrition in Children. The New England journal of medicine. 2016 Feb 4:374(5):444-53. doi: 10.1056/NEJMoa1507024. Epub [PubMed PMID: 26840134]
Iannotti LL, Trehan I, Manary MJ. Review of the safety and efficacy of vitamin A supplementation in the treatment of children with severe acute malnutrition. Nutrition journal. 2013 Sep 12:12():125. doi: 10.1186/1475-2891-12-125. Epub 2013 Sep 12 [PubMed PMID: 24028603]
Alam NH, Hamadani JD, Dewan N, Fuchs GJ. Efficacy and safety of a modified oral rehydration solution (ReSoMaL) in the treatment of severely malnourished children with watery diarrhea. The Journal of pediatrics. 2003 Nov:143(5):614-9 [PubMed PMID: 14615732]
Barltrop D, Sandhu BK. Marasmus--1985. Postgraduate medical journal. 1985 Oct:61(720):915-23 [PubMed PMID: 3932990]
Coodley GO, Loveless MO, Merrill TM. The HIV wasting syndrome: a review. Journal of acquired immune deficiency syndromes. 1994 Jul:7(7):681-94 [PubMed PMID: 8207646]
Adler H, Archary M, Mahabeer P, LaRussa P, Bobat RA. Tuberculosis in HIV-infected South African children with complicated severe acute malnutrition. The international journal of tuberculosis and lung disease : the official journal of the International Union against Tuberculosis and Lung Disease. 2017 Apr 1:21(4):438-445. doi: 10.5588/ijtld.16.0753. Epub [PubMed PMID: 28284260]
Sartoris G, Seddon JA, Rabie H, Nel ED, Losurdo G, Schaaf HS. Abdominal Involvement in Children With Bacteriologically Confirmed Tuberculosis: A Five-year Experience From Cape Town, South Africa. The Pediatric infectious disease journal. 2020 Oct:39(10):914-919. doi: 10.1097/INF.0000000000002749. Epub [PubMed PMID: 32496408]
Lukosiute-Urboniene A, Dekeryte I, Donielaite-Anise K, Kilda A, Barauskas V. Challenging diagnosis of abdominal tuberculosis in children: case report. International journal of infectious diseases : IJID : official publication of the International Society for Infectious Diseases. 2022 Mar:116():130-132. doi: 10.1016/j.ijid.2021.12.342. Epub 2021 Dec 23 [PubMed PMID: 34954096]
Level 3 (low-level) evidenceGizaw G, Bahwere P, Argaw A, Wells JCK, Friis H, Olsen MF, Abdissa A, Wibaek R, Abera M, Sadler K, Boyd E, Collins S, Girma T. Growth and Body Composition 5 y After Treatment for Severe Acute Malnutrition: A 5-y Prospective Matched Cohort Study in Ethiopian Children. The American journal of clinical nutrition. 2023 Nov:118(5):1029-1041. doi: 10.1016/j.ajcnut.2023.07.020. Epub 2023 Sep 18 [PubMed PMID: 37923494]
Bliznashka L, Rattigan SM, Sudfeld CR, Isanaka S. Analysis of Postdischarge Interventions for Children Treated for Moderate or Severe Wasting, Growth Faltering or Failure, or Edema: A Systematic Review. JAMA network open. 2023 May 1:6(5):e2315077. doi: 10.1001/jamanetworkopen.2023.15077. Epub 2023 May 1 [PubMed PMID: 37223898]
Level 1 (high-level) evidenceKeet MP, Moodie AD, Wittmann W, Hansen JD. Kwashiorkor: a prospective ten-year follow-up study. South African medical journal = Suid-Afrikaanse tydskrif vir geneeskunde. 1971 Dec 25:45(49):1427-49 [PubMed PMID: 4334564]
Pulcini CD, Zettle S, Srinath A. Refeeding Syndrome. Pediatrics in review. 2016 Dec:37(12):516-523 [PubMed PMID: 27909106]
Cederholm T, Bosaeus I. Malnutrition in Adults. The New England journal of medicine. 2024 Jul 11:391(2):155-165. doi: 10.1056/NEJMra2212159. Epub [PubMed PMID: 38986059]
Kassaw A, Amare D, Birhanu M, Tesfaw A, Zeleke S, Arage G, Kefale D. Survival and predictors of mortality among severe acute malnourished under-five children admitted at Felege-Hiwot comprehensive specialized hospital, northwest, Ethiopia: a retrospective cohort study. BMC pediatrics. 2021 Apr 16:21(1):176. doi: 10.1186/s12887-021-02651-x. Epub 2021 Apr 16 [PubMed PMID: 33863303]
Level 2 (mid-level) evidenceThompson DS, Bourdon C, Massara P, Boyne MS, Forrester TE, Gonzales GB, Bandsma RHJ. Childhood severe acute malnutrition is associated with metabolomic changes in adulthood. JCI insight. 2020 Dec 17:5(24):. pii: 141316. doi: 10.1172/jci.insight.141316. Epub 2020 Dec 17 [PubMed PMID: 33201860]
Victora CG, Adair L, Fall C, Hallal PC, Martorell R, Richter L, Sachdev HS, Maternal and Child Undernutrition Study Group. Maternal and child undernutrition: consequences for adult health and human capital. Lancet (London, England). 2008 Jan 26:371(9609):340-57. doi: 10.1016/S0140-6736(07)61692-4. Epub [PubMed PMID: 18206223]
Level 2 (mid-level) evidenceWalker SP, Chang SM, Vera-Hernández M, Grantham-McGregor S. Early childhood stimulation benefits adult competence and reduces violent behavior. Pediatrics. 2011 May:127(5):849-57. doi: 10.1542/peds.2010-2231. Epub 2011 Apr 25 [PubMed PMID: 21518715]
Level 2 (mid-level) evidence