Introduction
Lentigo maligna, a subtype of melanoma, typically presents as an irregular brown macule on chronically sun-damaged skin, most often affecting the head and neck region.[1] Hutchinson first described this lesion in 1890, referring to it as “Hutchinson’s melanotic freckle.”[2] Throughout much of the early 20th century, lentigo maligna was considered benign, infectious, or precancerous due to its slow progression, leading to various names, eg, “junctional nevus,” “infective senile freckles,” and “circumscribed precancerous melanosis.”[2] Widespread recognition of its malignant nature emerged in the late 1970s and 1980s, driven by pivotal research conducted by Silvers, Ackerman, and colleagues.
Current definitions classify lentigo maligna as melanoma in situ (MIS) occurring on chronically sun-damaged skin.[3][4][5] By definition, lentigo maligna remains confined to the epidermis. Once the lesion becomes invasive, the diagnosis changes to lentigo maligna melanoma (LMM) (see Image. Lentigo Maligna Melanoma). An in-depth knowledge of the distinguishing features and distinctive therapeutic approaches of lentigo maligna and LMM is essential to ensure accurate diagnosis, optimal treatment planning, and improved patient outcomes.
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
Lentigo Maligna Melanoma Risk Factors
The major risk factor for developing lentigo maligna and LMM is ultraviolet radiation (UVR), particularly cumulative lifetime exposure to UVR.[6][7][8][9] Several studies have demonstrated that lentigo maligna/LMM is strongly associated with chronic UVR exposure, as opposed to nodular melanoma and superficial spreading melanoma (SSM), which are associated with intense intermittent UVR exposure.[7][8][9][10][11][12][13][14] An Australian study showed an increased risk of lentigo maligna with the number of years lived in Australia, hours of sunlight, amount of actinic damage, and prior history of nonmelanoma skin cancer.[9]
Lentigo maligna/LMM is also most likely to occur on the face, a site of chronic sun damage, whereas SSM and nodular melanoma are more likely to occur on the trunk in men and legs in women, sites which are more often protected.[6] Finally, lentigo maligna tends to occur in older patients compared to SSM and nodular melanoma, presumably due to the increased lifetime sun exposure in older individuals.[15][16] Aside from UVR, x-ray irradiation, estrogen and progesterone, and nonpermanent hair dyes have been suggested as risk factors.[2] Lentigo maligna is also more likely in genetic conditions predisposing to sun sensitivity, including oculocutaneous albinism, xeroderma pigmentosum, Werner syndrome, and porphyria cutanea tarda.[2] Associations with smoking or alcohol have not been demonstrated.[17]
Epidemiology
Lentigo maligna/LMM is the third most common subtype of melanoma, following SSM and nodular melanoma, comprising 4% to 15% of all melanomas and 10% to 26% of melanomas on the head and neck.[2] The mean age of diagnosis is 66 to 72 years, compared to 45 to 57 years for other melanoma subtypes.[14][18][19][20] Women are more often affected than men, with a female-to-male ratio of 1.7 to 1, and are slightly older at diagnosis.[14][21]
The incidence of lentigo maligna and LMM has been rising over the past few decades, with data from Olmstead County, Minnesota, showing an increase from 2.2 cases/100,000 person-years between 1970 and 1989 to 13.7 cases/100,000 person-years between 2004 and 2007.[22] A similar rise in incidence was observed in the Netherlands.[23] In California, a particularly steep 52% increase in incidence occurred among individuals aged 45 to 64 between 1990 and 2000.[18] Whether these trends reflect a true rise in disease occurrence or improved diagnostic accuracy remains uncertain.[24] However, the more rapid increase in MIS compared to invasive melanoma suggests that detection bias likely contributes, at least in part.[25]
Pathophysiology
Melanoma has one of the highest mutational loads among all malignancies [26], and lentigo maligna/LMM has an especially high mutation rate due to chronic UVR exposure.[27][28][29] UVR causes oxidative damage and produces signature C>T and CC>TT mutations, which alter genes involved in the MAPK and PI3K pathways, including BRAF, NRAS, and KIT. Secondary mutations in CCND1, CDKN2A, or p53 then lead to transformation into a malignant tumor.[30][31] Lentigo maligna/LMM is more likely to harbor mutations in KIT than other subtypes of melanoma, in which BRAF mutations are more common.[32][33][34] CCND1, MITF, NRAS, and p53 mutations also play a pathogenic role.[31]
Histopathology
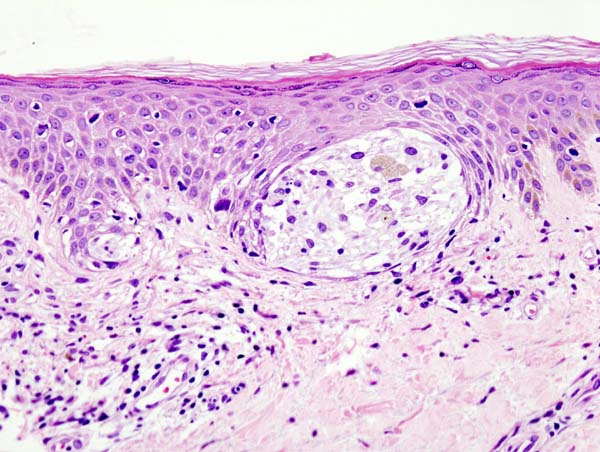
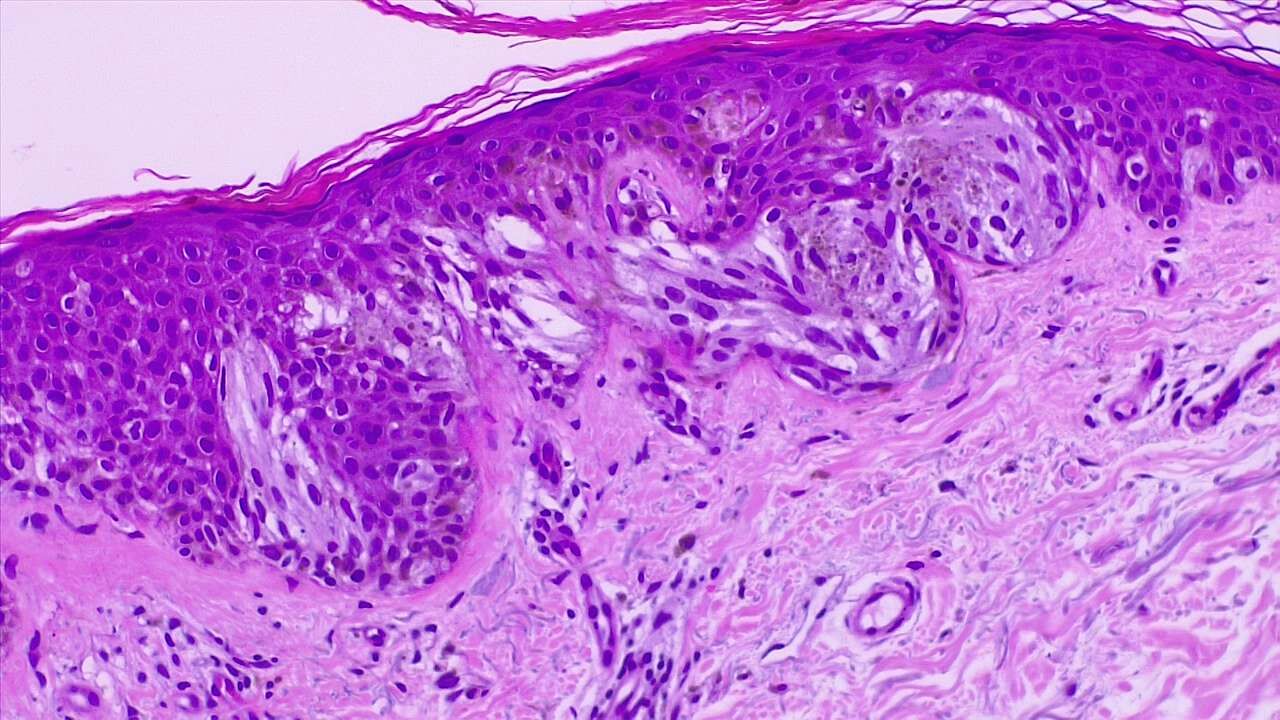
Lentigo maligna is characterized by a proliferation of atypical melanocytes along the dermal-epidermal junction. Early lentigo maligna may be challenging to distinguish from benign changes of chronic solar damage, which also induce melanocytic hyperplasia.[35] For this reason, a control biopsy of non-lesional but sun-damaged skin is sometimes recommended.[36] Melanocytes are typically singly arranged, although nests and multinucleated cells may be seen (see Image. Malignant Melanoma of the Skin). Pagetoid spread may occur, but less frequently than in superficial spreading melanoma. Periadnexal extension is common with the involvement of the follicular outer root sheath and eccrine duct.
The tumor cells often have a conspicuous cytoplasmic retraction artifact, and nuclei are often enlarged, hyperchromatic, and angulated. Background changes of chronic sun damage are usually present, including solar elastosis, epidermal atrophy, and effacement of the rete ridges. Pigmentation levels vary but may be abundant, and melanophages often populate the papillary dermis. Lymphocytes typically localize to the superficial dermis; however, marked lymphocytic infiltration may indicate tumor invasion and warrants closer histopathologic evaluation.
As lentigo maligna becomes more invasive (ie, progresses to LMM), junctional nests become larger and more spindled. Invasion is typically superficial, consisting of isolated or small aggregates of spindled cells in the papillary dermis. Desmoplasia and perineural invasion may occur, and the desmoplastic component may be mistaken for a scar. Rarely, a storiform growth pattern may be present, which may be mistaken for dermatofibrosarcoma protuberans.[2][37][38] Immunohistochemical staining using markers such as MART-1/Melan-A, HMB-45, tyrosinase, MITF, Sox10, and S100 supports accurate diagnosis.[2][36]
History and Physical
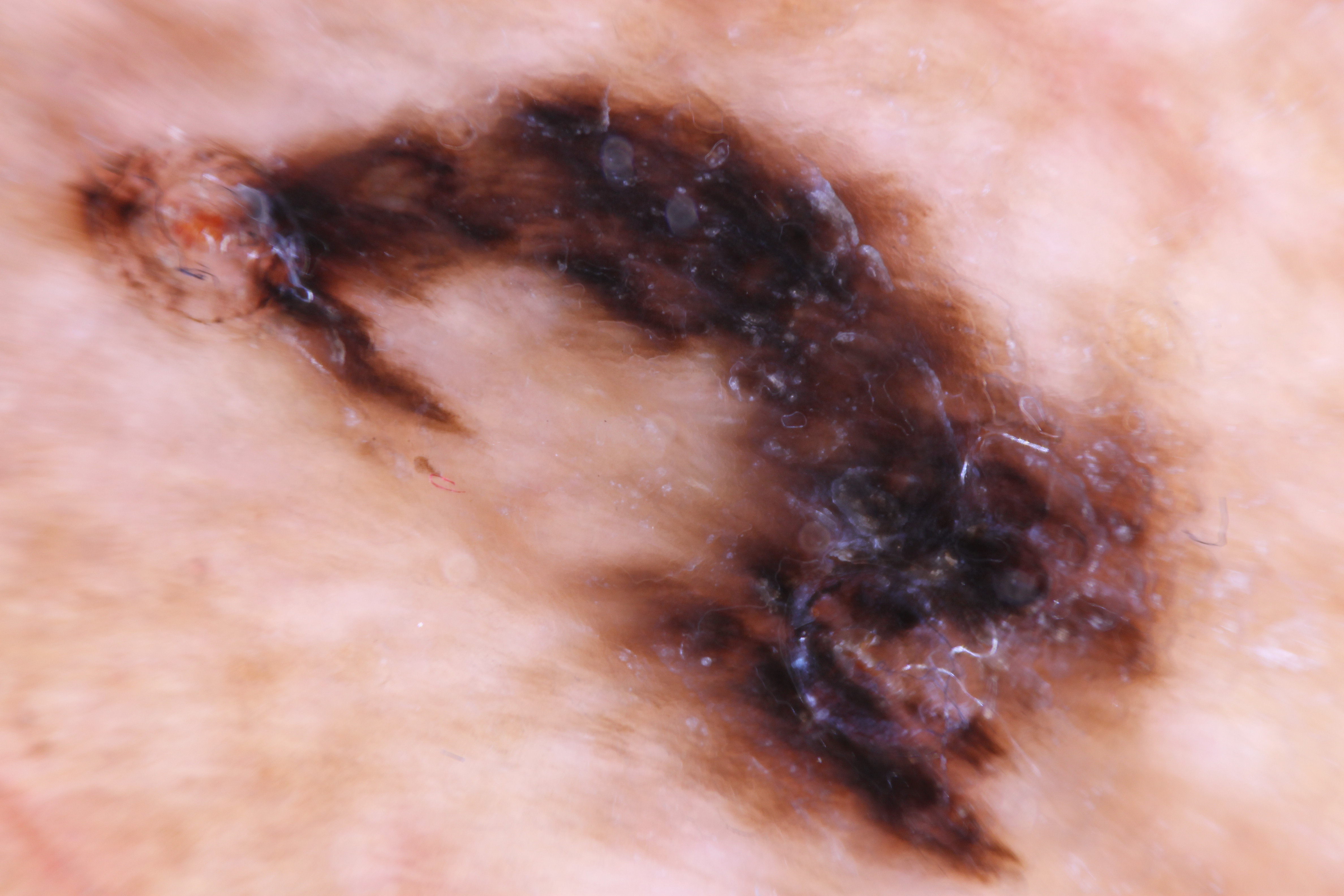
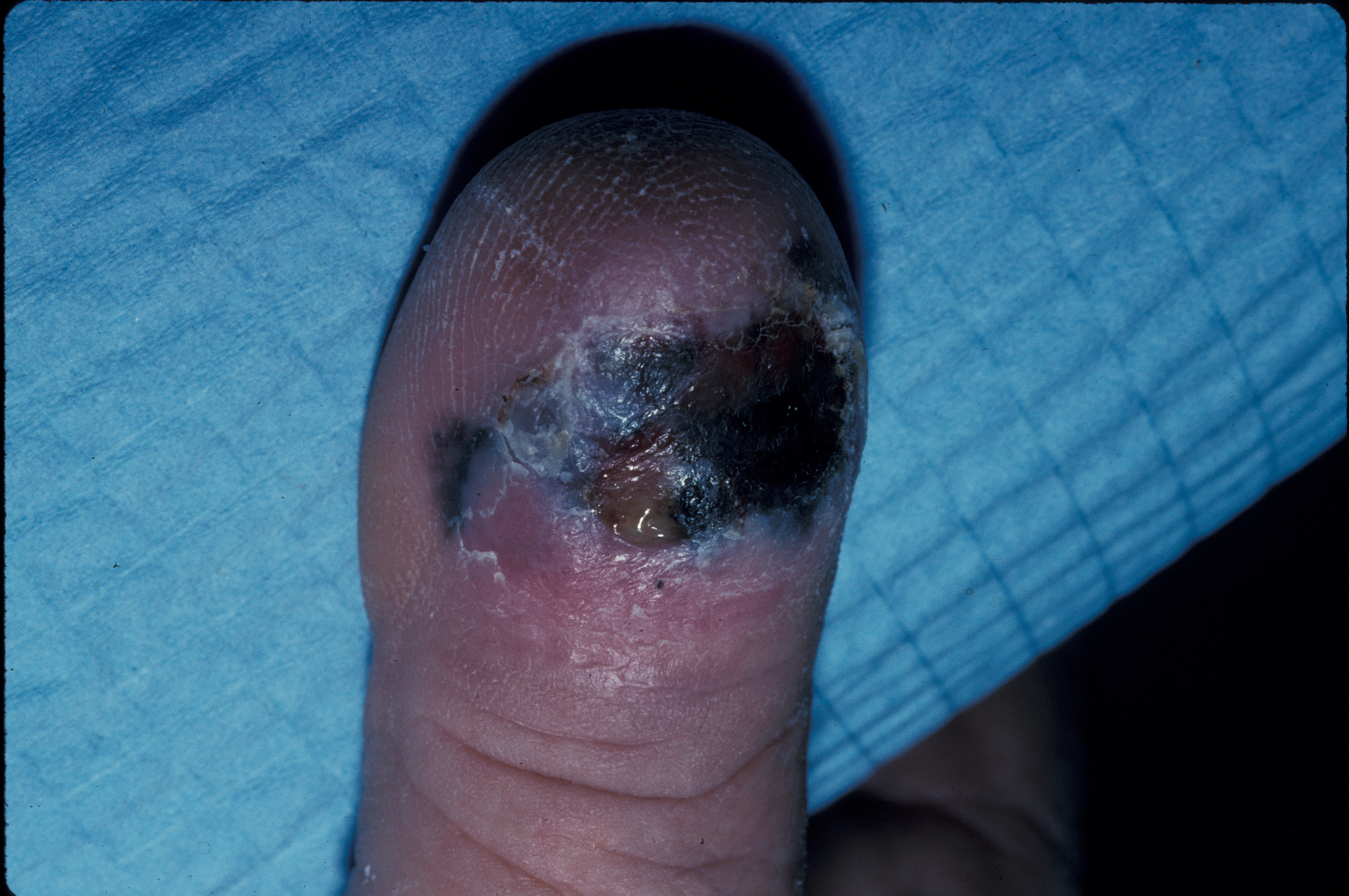
Lentigo maligna often presents as an irregular brown macule or patch on chronically sun-damaged skin. On visual inspection, lesions may appear light-brown to black, display color variegation, appear asymmetric, and tend to have an ill-defined border. As lesions enlarge, they may develop skip areas with a patchy, noncontiguous pattern. Lesions are usually asymptomatic, although advanced tumors may produce pain, burning, itching, or bleeding (see Image. Acral Lentiginous Melanoma). Most cases (86%) occur on the head and neck, with a predilection for the cheek.[2][21]
Extrafacial cases tend to occur in the extremities of women and the back in men.[21][39] Due to its in situ nature, lentigo maligna is typically smooth and nonpalpable. A papular or dermal component may be felt if the lesion becomes invasive (ie, LMM). Lentigo maligna exhibits slow radial growth and might be misdiagnosed for years or even decades as solar lentigo or other benign lesions (Please refer to the Differential Diagnosis section for more information).[5]
The overall lifetime risk of progression from lentigo maligna to LMM has been estimated to be 5% based on a retrospective epidemiologic study.[40] However, the true lifetime risk may be greater, as 10% to 20% of cases initially diagnosed as lentigo maligna on biopsy are later upstaged to LMM after excision.[41][42][43][44] The timeframe of progression to LMM varies widely, from less than 10 years to more than 50 years.[45][46]
Evaluation
Clinical diagnosis of lentigo maligna and LMM can be challenging due to overlapping features with benign pigmented lesions, eg, solar lentigines and pigmented actinic keratoses. To improve diagnostic accuracy, noninvasive optical imaging tools, including dermoscopy and reflectance confocal microscopy (RCM), have been developed.[47]
Dermoscopy Evaluation
Dermoscopy assessment can reveal a stepwise progression of features based on the extent of follicular involvement. Early lesions show pigmentation surrounding follicular openings, appearing as annular-granular structures or blue-grey dots. As the lesion advances, these dots coalesce into short polygonal lines around and between adnexal structures. With further progression, the lines darken and merge into rhomboidal structures. In advanced disease, the lesion may obliterate follicular openings, forming a homogeneous dark brown to black blotch.[48][49][50][51]
RCM is an imaging method that has been shown to have a high correlation to histological assessment. Furthermore, RCM can help better determine surgical margins, thereby optimizing excisional procedures.[52] RCM findings for lentigo maligna/LMM are divided into major and minor criteria.[53] The 2 major criteria include nonedged papillae and round pagetoid cells measuring >20 µm. The 3 minor criteria include atypical cells at the dermal-epidermal junction, follicular localization of atypical cells, and nucleated cells within the dermal papillae. Although dermoscopy and RCM are important tools for diagnosis, skin biopsy for histopathologic examination remains the gold standard (Please refer to the Histopathology section for more information on dermoscopy findings).
Biopsy Evaluation
Biopsy methods for suspected lentigo maligna or LMM include shave biopsy, excisional biopsy with narrow margins, incisional biopsy of the most atypical or thickest portion of the lesion, and broad saucerization of the entire lesion with adequate depth for histopathologic evaluation.[5] The choice of technique depends on lesion size, location, and clinical suspicion. Adjunctive tools such as Wood’s lamp examination, dermoscopy, and RCM may assist in delineating lesion borders and planning surgical margins before definitive excision.[54][55]
Treatment / Management
Surgical Therapies
Surgical excision is the treatment of choice. Numerous studies have shown the traditional 0.5 cm surgical margins for MIS to be inadequate for lentigo maligna, with approximately half of the tumors requiring larger margins for clearance and recurrence rates between 8% and 20%.[36][56] The National Comprehensive Cancer Network (NCCN) in 2014 updated its guidelines to recommend 0.5 to 1 cm margins for MIS, and several single-center studies have called for larger margins up to 1 cm or more.[57][41][58][59][60] A recent study by Zitelli and Brodland, in which they treated over 1500 cases of lentigo maligna with Mohs micrographic surgery (MMS), demonstrated that 1.2 cm margins were required to achieve a 97% clearance rate.[58] (B2)
Wide local excision remains the standard of care among surgical modalities, as recommended by expert panels.[61][62] However, a growing body of literature shows the MMS may be superior.[58][63][47][64] Several institutions that perform MMS and wide local excision for lentigo maligna have demonstrated recurrence rates of 1.8% to 1.9% using MMS and 5.8% to 5.9% using wide local excision.[63][64] In the hands of an experienced practitioner and with the use of immunohistochemistry, recurrence rates with MMS can be as low as 0.3% to 0.5%.[58][65](A1)
Nonsurgical Therapies
For patients who wish to avoid surgery or are otherwise poor surgical candidates, topical imiquimod 5% cream may be a viable alternative, although the data on efficacy are mixed.[66] Clinical and histologic clearance rates for imiquimod range from 46% to 78% and 37% to 76%, respectively.[67][68] (A1)
Definitive radiation therapy may also be an acceptable nonsurgical option.[69] The method and type of radiation vary, but fractionated superficial radiotherapy, also known as Grenz rays, is most commonly delivered. Recurrence rates have been reported to range from 5% to 14%.[70][71][72] Finally, many other nonsurgical modalities have been reported with varying success, including laser ablation, cryotherapy, azelaic acid, 5-fluorouracil cream, and chemical peels. Still, the data are too sparse and inconsistent to draw reliable conclusions.[2](A1)
Differential Diagnosis
The clinical and dermoscopic differential diagnoses include solar lentigo, early/macular seborrheic keratosis, lichen planus-like keratosis, and pigmented actinic keratosis. The histopathologic mimickers include benign melanocytic hyperplasia of sun-damaged skin, invasive lesions, desmoplastic melanoma, and, rarely, dermatofibrosarcoma protuberans (See Image. Ulcerated Acral Lentiginous Melanoma).
Surgical Oncology
Surgical excision remains the mainstay of treatment for both lentigo maligna and LMM. The goal is complete tumor removal with histologically negative margins while preserving functional and cosmetic outcomes, particularly given the common occurrence of these lesions on the face.
Standard wide local excision is commonly performed, with margin recommendations based on tumor depth. For lentigo maligna in situ, a 5 to 10 mm margin is typically used. For invasive LMM, the NCCN and American Joint Committee on Cancer (AJCC) guidelines recommend a margin based on Breslow depth, ranging from 1 cm for tumors up to 2 mm thick to 2 cm for thicker lesions.
MMS, particularly the "slow Mohs" variant using paraffin-embedded sections, offers superior margin control and tissue conservation and is increasingly used for lentigo maligna/LMM in cosmetically sensitive areas. Studies have shown that Mohs can achieve lower recurrence rates compared to standard excision, particularly for lesions with ill-defined borders or those previously treated. Staged excision (also known as the "square technique" or "contoured marginal excision") is another tissue-sparing approach that evaluates peripheral margins before central tumor excision, often used when Mohs is unavailable.
For LMM with invasive components, sentinel lymph node biopsy should be considered in cases with Breslow depth ≥0.8 mm or the presence of ulceration. However, due to the typically older age and lower mitotic rate of these tumors, the yield of sentinel lymph node biopsy may be lower compared to other melanoma subtypes.
Staging
Staging for lentigo maligna/LMM is the same as for all melanomas, using the tumor, node, metastasis (TNM) staging criteria based on the latest AJCC Cancer Staging Manual, Eighth Edition.[73] The stages may be briefly summarized as follows:
- Stage I: Low-risk primary melanomas (T1a, T1b, T2a) without evidence of regional or distant metastases
- Stage II: Primary melanomas at higher risk of recurrence (T2b, T3a, T3b, T4a, T4b), but without evidence of regional or distant metastases
- Stage III: Involvement of regional lymph nodes or in-transit or satellite metastases
- Stage IV: Distant metastases present
Prognosis
The prognosis for lentigo maligna/LMM is excellent. In a study of 270 patients with lentigo maligna/LMM who were completely excised, no disease-related deaths for lentigo maligna and only 1 for LMM were reported.[74] The 5-year and 10-year disease-specific survival rates were 100% and 97.1%, respectively. Thus, lentigo maligna, in itself, does not reduce lifespan.
However, once the tumor becomes invasive, the prognosis is the same as for all other melanomas after controlling for Breslow depth. The prognosis can be potentially quite poor if the disease becomes metastatic (5-year survival, 9% to 27%).[14][75] While mortality is low, morbidity for patients can be significant, given the potentially large surface area on the head/neck that may be involved and the need for extensive surgical excision and reconstruction.[76]
Complications
LMM can eventually metastasize if left untreated; therefore, early diagnosis and intervention are crucial. Surgery to remove LMM may carry cosmetic complications because it often occurs on exposed areas such as the face; modern surgical techniques can help minimize scarring. Potential surgical complications include scarring, infection, delayed wound healing, and poor cosmetic outcome, particularly on the face. Functional impairment may also occur depending on lesion location. Incomplete excision leads to recurrence, with lentigo maligna recurrence rates reported as high as 20% following standard wide local excision without margin control.
Deterrence and Patient Education
Due to the causative nature of chronic UVR damage in inducing lentigo maligna/LMM, diligent sun protection is key to prevention. The American Academy of Dermatology (AAD) periodically publishes guidelines on preventing and treating skin cancer.[61][77] Patients should wear broad-spectrum sunscreen (SPF ≥ 30) whenever outdoors, reapply every 2 hours, and immediately after swimming. They should use a sufficient amount (approximately 1 ounce or equivalent to 1 shot glass) to cover the entire body. Wearing ultraviolet protection factor clothing, avoiding the sun between peak hours of 10 am to 3 pm, and seeking shade provides additional sun protection. Furthermore, patients should be aware of the ABCDE criteria for melanoma (asymmetry, border, color, diameter, and evolving), perform monthly skin self-examinations, and seek professional care by a dermatologist or primary care clinician for any concerning lesions (see Image. Melanoma Detection).
Pearls and Other Issues
Lentigo maligna/LMM presents diagnostic and treatment challenges due to its clinical mimicry of benign lesions, its occurrence on background sun-damaged skin, which confounds histopathologic differentiation between true tumor and benign melanocytic activation, and its occurrence on the head and neck, a cosmetically and functionally sensitive area. Thus, maintaining clinical diligence and having a high index of suspicion are key to early diagnosis, ensuring optimal treatment outcomes, and minimizing patient morbidity and mortality. Surgical excision is the treatment of choice, with MMS emerging as a surgical option that may prove superior to wide local excision.
Enhancing Healthcare Team Outcomes
The management of lentigo maligna and LMM requires a coordinated, interprofessional approach to ensure early diagnosis, effective treatment, and comprehensive follow-up. Physicians across various specialties, including dermatology, pathology, primary care, surgical oncology, plastic surgery, and subspecialties such as oculoplastics and otolaryngology, must clearly communicate regarding biopsy results, staging, and therapeutic strategies. Early recognition by nondermatology clinicians relies on clinical vigilance and ongoing education, particularly given the diagnostic overlap with benign lesions on sun-damaged skin. Each additional family physician per 10,000 population has been associated with a 21% increased odds of early melanoma detection.[78]
Dermatologists, dermatopathologists, and Mohs surgeons collaborate to confirm histopathologic features and determine the most appropriate surgical plan. In cases of complex anatomic involvement, surgical coordination with reconstructive or functional specialists enhances both oncologic safety and cosmetic outcomes. For locally advanced or metastatic disease, oncologists join the care team to guide systemic therapy options in alignment with tumor staging and patient-specific goals.
Team performance and patient-centered outcomes improve when all disciplines remain engaged and informed. Advanced practitioners serve as critical access points for lesion evaluation, patient counseling, and continuity of care, particularly in medically underserved settings. Nurses facilitate education, psychosocial support, wound care, and postoperative recovery, which are especially important for lesions involving cosmetically or functionally sensitive areas. Pharmacists monitor for drug interactions, educate patients on systemic therapies, and assist with adherence, particularly when immunotherapy is prescribed. Regular tumor board discussions, shared electronic records, and proactive communication strategies support seamless care transitions and minimize diagnostic or therapeutic delays. Ethical principles such as shared decision-making and respect for patient autonomy guide treatment planning, especially for older adults who may have surgical contraindications or differing treatment preferences.[79][80] This level of integration ensures that care for patients with lentigo maligna/LMM remains timely, evidence-based, and individualized, ultimately enhancing patient safety, satisfaction, and long-term outcomes.
Media
(Click Image to Enlarge)
(Click Image to Enlarge)
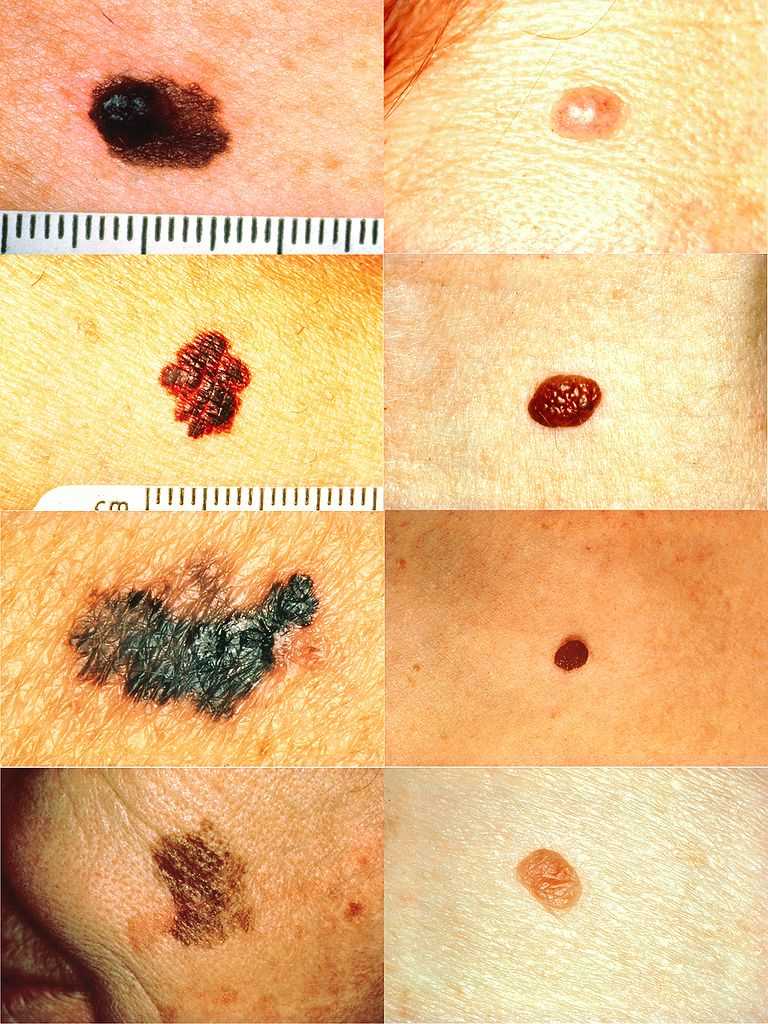
Melanoma Detection. On the left side from top to bottom: melanomas showing (A) asymmetry, (B) a border that is uneven, ragged, or notched, (C) coloring of different shades of brown, black, or tan and, (D) diameter that had changed in size. The normal moles on the right side do not have abnormal characteristics (no asymmetry, even border, even color, no change in diameter).
National Cancer Institute Skin Cancer Foundation
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
References
Kallini JR, Jain SK, Khachemoune A. Lentigo maligna: review of salient characteristics and management. American journal of clinical dermatology. 2013 Dec:14(6):473-80. doi: 10.1007/s40257-013-0044-6. Epub [PubMed PMID: 24019181]
Cohen LM. Lentigo maligna and lentigo maligna melanoma. Journal of the American Academy of Dermatology. 1995 Dec:33(6):923-36; quiz 937-40 [PubMed PMID: 7490362]
Dubow BE, Ackerman AB. Ideas in pathology. Malignant melanoma in situ: the evolution of a concept. Modern pathology : an official journal of the United States and Canadian Academy of Pathology, Inc. 1990 Nov:3(6):734-44 [PubMed PMID: 2263599]
Silvers DN. Focus on melanoma: the therapeutic dilemma of lentigo maligna (Hutchinson's freckle). The Journal of dermatologic surgery. 1976 Sep:2(4):301-3 [PubMed PMID: 1026731]
DeWane ME, Kelsey A, Oliviero M, Rabinovitz H, Grant-Kels JM. Melanoma on chronically sun-damaged skin: Lentigo maligna and desmoplastic melanoma. Journal of the American Academy of Dermatology. 2019 Sep:81(3):823-833. doi: 10.1016/j.jaad.2019.03.066. Epub 2019 Mar 28 [PubMed PMID: 30930085]
Newell GR, Sider JG, Bergfelt L, Kripke ML. Incidence of cutaneous melanoma in the United States by histology with special reference to the face. Cancer research. 1988 Sep 1:48(17):5036-41 [PubMed PMID: 3409232]
Elwood JM, Gallagher RP, Worth AJ, Wood WS, Pearson JC. Etiological differences between subtypes of cutaneous malignant melanoma: Western Canada Melanoma Study. Journal of the National Cancer Institute. 1987 Jan:78(1):37-44 [PubMed PMID: 3467128]
Elwood JM, Hislop TG. Solar radiation in the etiology of cutaneous malignant melanoma in Caucasians. National Cancer Institute monograph. 1982:62():167-71 [PubMed PMID: 7167183]
Level 3 (low-level) evidenceHolman CD, Armstrong BK. Cutaneous malignant melanoma and indicators of total accumulated exposure to the sun: an analysis separating histogenetic types. Journal of the National Cancer Institute. 1984 Jul:73(1):75-82 [PubMed PMID: 6588237]
Holman CD, Armstrong BK, Heenan PJ. A theory of the etiology and pathogenesis of human cutaneous malignant melanoma. Journal of the National Cancer Institute. 1983 Oct:71(4):651-6 [PubMed PMID: 6578359]
Koh HK, Kligler BE, Lew RA. Sunlight and cutaneous malignant melanoma: evidence for and against causation. Photochemistry and photobiology. 1990 Jun:51(6):765-79 [PubMed PMID: 2195564]
Schreiber MM, Moon TE, Bozzo PD. Chronic solar ultraviolet damage associated with malignant melanoma of the skin. Journal of the American Academy of Dermatology. 1984 May:10(5 Pt 1):755-9 [PubMed PMID: 6725671]
Level 2 (mid-level) evidenceArmstrong BK. Epidemiology of malignant melanoma: intermittent or total accumulated exposure to the sun? The Journal of dermatologic surgery and oncology. 1988 Aug:14(8):835-49 [PubMed PMID: 3397443]
Level 2 (mid-level) evidenceCox NH, Aitchison TC, Sirel JM, MacKie RM. Comparison between lentigo maligna melanoma and other histogenetic types of malignant melanoma of the head and neck. Scottish Melanoma Group. British journal of cancer. 1996 Apr:73(7):940-4 [PubMed PMID: 8611411]
McGovern VJ, Shaw HM, Milton GW, Farago GA. Is malignant melanoma arising in a Hutchinson's melanotic freckle a separate disease entity? Histopathology. 1980 May:4(3):235-42 [PubMed PMID: 7390408]
Holman CD, Mulroney CD, Armstrong BK. Epidemiology of pre-invasive and invasive malignant melanoma in Western Australia. International journal of cancer. 1980 Mar 15:25(3):317-23 [PubMed PMID: 7390655]
Osterlind A, Tucker MA, Stone BJ, Jensen OM. The Danish case-control study of cutaneous malignant melanoma. IV. No association with nutritional factors, alcohol, smoking or hair dyes. International journal of cancer. 1988 Dec 15:42(6):825-8 [PubMed PMID: 3192325]
Level 2 (mid-level) evidenceSwetter SM, Boldrick JC, Jung SY, Egbert BM, Harvell JD. Increasing incidence of lentigo maligna melanoma subtypes: northern California and national trends 1990-2000. The Journal of investigative dermatology. 2005 Oct:125(4):685-91 [PubMed PMID: 16185266]
Lemish WM, Heenan PJ, Holman CD, Armstrong BK. Survival from preinvasive and invasive malignant melanoma in Western Australia. Cancer. 1983 Aug 1:52(3):580-5 [PubMed PMID: 6861096]
Gassenmaier M, Keim U, Leiter U, Eigentler TK, Röcken M, Gesierich A, Moritz RKC, Heinzerling L, Tüting T, Wollina U, Garbe C. Age as key factor for pattern, timing, and extent of distant metastasis in patients with cutaneous melanoma: A study of the German Central Malignant Melanoma Registry. Journal of the American Academy of Dermatology. 2019 May:80(5):1299-1307.e7. doi: 10.1016/j.jaad.2019.01.044. Epub 2019 Jan 29 [PubMed PMID: 30703453]
Cox NH, Aitchison TC, MacKie RM. Extrafacial lentigo maligna melanoma: analysis of 71 cases and comparison with lentigo maligna melanoma of the head and neck. The British journal of dermatology. 1998 Sep:139(3):439-43 [PubMed PMID: 9767288]
Level 2 (mid-level) evidenceMirzoyev SA, Knudson RM, Reed KB, Hou JL, Lohse CM, Frohm ML, Brewer JD, Otley CC, Roenigk RK. Incidence of lentigo maligna in Olmsted County, Minnesota, 1970 to 2007. Journal of the American Academy of Dermatology. 2014 Mar:70(3):443-8. doi: 10.1016/j.jaad.2013.11.008. Epub 2013 Dec 24 [PubMed PMID: 24373777]
Level 2 (mid-level) evidenceGreveling K, Wakkee M, Nijsten T, van den Bos RR, Hollestein LM. Epidemiology of Lentigo Maligna and Lentigo Maligna Melanoma in the Netherlands, 1989-2013. The Journal of investigative dermatology. 2016 Oct:136(10):1955-1960. doi: 10.1016/j.jid.2016.06.014. Epub 2016 Jun 24 [PubMed PMID: 27349862]
Higgins HW 2nd, Lee KC, Galan A, Leffell DJ. Melanoma in situ: Part I. Epidemiology, screening, and clinical features. Journal of the American Academy of Dermatology. 2015 Aug:73(2):181-90, quiz 191-2. doi: 10.1016/j.jaad.2015.04.014. Epub [PubMed PMID: 26183967]
Buettner PG, Leiter U, Eigentler TK, Garbe C. Development of prognostic factors and survival in cutaneous melanoma over 25 years: An analysis of the Central Malignant Melanoma Registry of the German Dermatological Society. Cancer. 2005 Feb 1:103(3):616-24 [PubMed PMID: 15630700]
Alexandrov LB, Nik-Zainal S, Wedge DC, Aparicio SA, Behjati S, Biankin AV, Bignell GR, Bolli N, Borg A, Børresen-Dale AL, Boyault S, Burkhardt B, Butler AP, Caldas C, Davies HR, Desmedt C, Eils R, Eyfjörd JE, Foekens JA, Greaves M, Hosoda F, Hutter B, Ilicic T, Imbeaud S, Imielinski M, Jäger N, Jones DT, Jones D, Knappskog S, Kool M, Lakhani SR, López-Otín C, Martin S, Munshi NC, Nakamura H, Northcott PA, Pajic M, Papaemmanuil E, Paradiso A, Pearson JV, Puente XS, Raine K, Ramakrishna M, Richardson AL, Richter J, Rosenstiel P, Schlesner M, Schumacher TN, Span PN, Teague JW, Totoki Y, Tutt AN, Valdés-Mas R, van Buuren MM, van 't Veer L, Vincent-Salomon A, Waddell N, Yates LR, Australian Pancreatic Cancer Genome Initiative, ICGC Breast Cancer Consortium, ICGC MMML-Seq Consortium, ICGC PedBrain, Zucman-Rossi J, Futreal PA, McDermott U, Lichter P, Meyerson M, Grimmond SM, Siebert R, Campo E, Shibata T, Pfister SM, Campbell PJ, Stratton MR. Signatures of mutational processes in human cancer. Nature. 2013 Aug 22:500(7463):415-21. doi: 10.1038/nature12477. Epub 2013 Aug 14 [PubMed PMID: 23945592]
Mar VJ, Wong SQ, Li J, Scolyer RA, McLean C, Papenfuss AT, Tothill RW, Kakavand H, Mann GJ, Thompson JF, Behren A, Cebon JS, Wolfe R, Kelly JW, Dobrovic A, McArthur GA. BRAF/NRAS wild-type melanomas have a high mutation load correlating with histologic and molecular signatures of UV damage. Clinical cancer research : an official journal of the American Association for Cancer Research. 2013 Sep 1:19(17):4589-98. doi: 10.1158/1078-0432.CCR-13-0398. Epub 2013 Jul 5 [PubMed PMID: 23833303]
Whiteman DC, Whiteman CA, Green AC. Childhood sun exposure as a risk factor for melanoma: a systematic review of epidemiologic studies. Cancer causes & control : CCC. 2001 Jan:12(1):69-82 [PubMed PMID: 11227927]
Level 2 (mid-level) evidenceDavis EJ, Johnson DB, Sosman JA, Chandra S. Melanoma: What do all the mutations mean? Cancer. 2018 Sep 1:124(17):3490-3499. doi: 10.1002/cncr.31345. Epub 2018 Apr 17 [PubMed PMID: 29663336]
Sanchez MI, Grichnik JM. Melanoma's high C}T mutation rate: is deamination playing a role? Experimental dermatology. 2014 Aug:23(8):551-2. doi: 10.1111/exd.12436. Epub 2014 Aug 1 [PubMed PMID: 24815223]
Bastian BC. The molecular pathology of melanoma: an integrated taxonomy of melanocytic neoplasia. Annual review of pathology. 2014:9():239-71. doi: 10.1146/annurev-pathol-012513-104658. Epub [PubMed PMID: 24460190]
Level 3 (low-level) evidenceLee JH, Choi JW, Kim YS. Frequencies of BRAF and NRAS mutations are different in histological types and sites of origin of cutaneous melanoma: a meta-analysis. The British journal of dermatology. 2011 Apr:164(4):776-84. doi: 10.1111/j.1365-2133.2010.10185.x. Epub 2011 Mar 21 [PubMed PMID: 21166657]
Level 1 (high-level) evidenceLang J, MacKie RM. Prevalence of exon 15 BRAF mutations in primary melanoma of the superficial spreading, nodular, acral, and lentigo maligna subtypes. The Journal of investigative dermatology. 2005 Sep:125(3):575-9 [PubMed PMID: 16117801]
Curtin JA, Busam K, Pinkel D, Bastian BC. Somatic activation of KIT in distinct subtypes of melanoma. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2006 Sep 10:24(26):4340-6 [PubMed PMID: 16908931]
Weyers W, Bonczkowitz M, Weyers I, Bittinger A, Schill WB. Melanoma in situ versus melanocytic hyperplasia in sun-damaged skin. Assessment of the significance of histopathologic criteria for differential diagnosis. The American Journal of dermatopathology. 1996 Dec:18(6):560-6 [PubMed PMID: 8989926]
McGuire LK, Disa JJ, Lee EH, Busam KJ, Nehal KS. Melanoma of the lentigo maligna subtype: diagnostic challenges and current treatment paradigms. Plastic and reconstructive surgery. 2012 Feb:129(2):288e-299e. doi: 10.1097/PRS.0b013e31823aeb72. Epub [PubMed PMID: 22286443]
Green A, Little JH, Weedon D. The diagnosis of Hutchinson's melanotic freckle (lentigo maligna) in Queensland. Pathology. 1983 Jan:15(1):33-5 [PubMed PMID: 6856341]
Smoller BR. Histologic criteria for diagnosing primary cutaneous malignant melanoma. Modern pathology : an official journal of the United States and Canadian Academy of Pathology, Inc. 2006 Feb:19 Suppl 2():S34-40 [PubMed PMID: 16446714]
Martínez-Leboráns L, Garcías-Ladaria J, Oliver-Martínez V, Alegre de Miquel V. Extrafacial Lentigo Maligna: A Report on 14 Cases and a Review of the Literature. Actas dermo-sifiliograficas. 2016 Oct:107(8):e57-63. doi: 10.1016/j.ad.2015.10.018. Epub 2016 May 11 [PubMed PMID: 27180003]
Level 3 (low-level) evidenceWeinstock MA, Sober AJ. The risk of progression of lentigo maligna to lentigo maligna melanoma. The British journal of dermatology. 1987 Mar:116(3):303-10 [PubMed PMID: 3567069]
Bosbous MW, Dzwierzynski WW, Neuburg M. Staged excision of lentigo maligna and lentigo maligna melanoma: a 10-year experience. Plastic and reconstructive surgery. 2009 Dec:124(6):1947-1955. doi: 10.1097/PRS.0b013e3181bcf002. Epub [PubMed PMID: 19952650]
Level 2 (mid-level) evidenceHazan C, Dusza SW, Delgado R, Busam KJ, Halpern AC, Nehal KS. Staged excision for lentigo maligna and lentigo maligna melanoma: A retrospective analysis of 117 cases. Journal of the American Academy of Dermatology. 2008 Jan:58(1):142-8 [PubMed PMID: 18029055]
Level 2 (mid-level) evidencePenneys NS. Microinvasive lentigo maligna melanoma. Journal of the American Academy of Dermatology. 1987 Oct:17(4):675-80 [PubMed PMID: 3312317]
Level 2 (mid-level) evidenceSomach SC, Taira JW, Pitha JV, Everett MA. Pigmented lesions in actinically damaged skin. Histopathologic comparison of biopsy and excisional specimens. Archives of dermatology. 1996 Nov:132(11):1297-302 [PubMed PMID: 8915306]
Jackson R, Williamson GS, Beattie WG. Lentigo maligna and malignant melanoma. Canadian Medical Association journal. 1966 Oct 22:95(17):846-51 [PubMed PMID: 5922502]
Clark WH Jr, Mihm MC Jr. Lentigo maligna and lentigo-maligna melanoma. The American journal of pathology. 1969 Apr:55(1):39-67 [PubMed PMID: 5776171]
Ungureanu L, Vasilovici AF, Trufin II, Apostu AP, Halmágyi SR. Lentigo Maligna Treatment-An Update. Journal of clinical medicine. 2024 Apr 25:13(9):. doi: 10.3390/jcm13092527. Epub 2024 Apr 25 [PubMed PMID: 38731056]
Cognetta AB Jr, Stolz W, Katz B, Tullos J, Gossain S. Dermatoscopy of lentigo maligna. Dermatologic clinics. 2001 Apr:19(2):307-18 [PubMed PMID: 11556239]
Bollea-Garlatti LA, Galimberti GN, Galimberti RL. Lentigo Maligna: Keys to Dermoscopic Diagnosis. Actas dermo-sifiliograficas. 2016 Jul-Aug:107(6):489-97. doi: 10.1016/j.ad.2016.01.001. Epub 2016 Feb 11 [PubMed PMID: 26875792]
Annessi G, Bono R, Abeni D. Correlation between digital epiluminescence microscopy parameters and histopathological changes in lentigo maligna and solar lentigo: A dermoscopic index for the diagnosis of lentigo maligna. Journal of the American Academy of Dermatology. 2017 Feb:76(2):234-243. doi: 10.1016/j.jaad.2016.08.032. Epub 2016 Oct 26 [PubMed PMID: 28341252]
Mataca E, Migaldi M, Cesinaro AM. Impact of Dermoscopy and Reflectance Confocal Microscopy on the Histopathologic Diagnosis of Lentigo Maligna/Lentigo Maligna Melanoma. The American Journal of dermatopathology. 2018 Dec:40(12):884-889. doi: 10.1097/DAD.0000000000001212. Epub [PubMed PMID: 29933314]
Navarrete-Dechent C, Cordova M, Aleissa S, Liopyris K, Dusza SW, Kose K, Busam KJ, Hollman T, Lezcano C, Pulitzer M, Chen CJ, Lee EH, Rossi AM, Nehal KS. Lentigo maligna melanoma mapping using reflectance confocal microscopy correlates with staged excision: A prospective study. Journal of the American Academy of Dermatology. 2023 Feb:88(2):371-379. doi: 10.1016/j.jaad.2019.11.058. Epub 2019 Dec 5 [PubMed PMID: 31812621]
Guitera P, Pellacani G, Crotty KA, Scolyer RA, Li LX, Bassoli S, Vinceti M, Rabinovitz H, Longo C, Menzies SW. The impact of in vivo reflectance confocal microscopy on the diagnostic accuracy of lentigo maligna and equivocal pigmented and nonpigmented macules of the face. The Journal of investigative dermatology. 2010 Aug:130(8):2080-91. doi: 10.1038/jid.2010.84. Epub 2010 Apr 15 [PubMed PMID: 20393481]
Chen CS, Elias M, Busam K, Rajadhyaksha M, Marghoob AA. Multimodal in vivo optical imaging, including confocal microscopy, facilitates presurgical margin mapping for clinically complex lentigo maligna melanoma. The British journal of dermatology. 2005 Nov:153(5):1031-6 [PubMed PMID: 16225620]
Level 3 (low-level) evidenceRobinson JK. Use of digital epiluminescence microscopy to help define the edge of lentigo maligna. Archives of dermatology. 2004 Sep:140(9):1095-100 [PubMed PMID: 15381550]
Level 2 (mid-level) evidenceOsborne JE, Hutchinson PE. A follow-up study to investigate the efficacy of initial treatment of lentigo maligna with surgical excision. British journal of plastic surgery. 2002 Dec:55(8):611-5 [PubMed PMID: 12550112]
Coit DG, Thompson JA, Andtbacka R, Anker CJ, Bichakjian CK, Carson WE 3rd, Daniels GA, Daud A, Dimaio D, Fleming MD, Gonzalez R, Guild V, Halpern AC, Hodi FS Jr, Kelley MC, Khushalani NI, Kudchadkar RR, Lange JR, Martini MC, Olszanski AJ, Ross MI, Salama A, Swetter SM, Tanabe KK, Trisal V, Urist MM, McMillian NR, Ho M, National Comprehensive Cancer Network. Melanoma, version 4.2014. Journal of the National Comprehensive Cancer Network : JNCCN. 2014 May:12(5):621-9 [PubMed PMID: 24812131]
Kunishige JH, Doan L, Brodland DG, Zitelli JA. Comparison of surgical margins for lentigo maligna versus melanoma in situ. Journal of the American Academy of Dermatology. 2019 Jul:81(1):204-212. doi: 10.1016/j.jaad.2019.01.051. Epub 2019 Apr 20 [PubMed PMID: 31014825]
Jejurikar SS, Borschel GH, Johnson TM, Lowe L, Brown DL. Immediate, optimal reconstruction of facial lentigo maligna and melanoma following total peripheral margin control. Plastic and reconstructive surgery. 2007 Oct:120(5):1249-1255. doi: 10.1097/01.prs.0000279324.35616.72. Epub [PubMed PMID: 17898597]
Bosbous MW, Dzwierzynski WW, Neuburg M. Lentigo maligna: diagnosis and treatment. Clinics in plastic surgery. 2010 Jan:37(1):35-46. doi: 10.1016/j.cps.2009.08.006. Epub [PubMed PMID: 19914456]
Bichakjian CK, Halpern AC, Johnson TM, Foote Hood A, Grichnik JM, Swetter SM, Tsao H, Barbosa VH, Chuang TY, Duvic M, Ho VC, Sober AJ, Beutner KR, Bhushan R, Smith Begolka W, American Academy of Dermatology. Guidelines of care for the management of primary cutaneous melanoma. American Academy of Dermatology. Journal of the American Academy of Dermatology. 2011 Nov:65(5):1032-47. doi: 10.1016/j.jaad.2011.04.031. Epub 2011 Aug 25 [PubMed PMID: 21868127]
Coit DG, Thompson JA, Algazi A, Andtbacka R, Bichakjian CK, Carson WE 3rd, Daniels GA, DiMaio D, Ernstoff M, Fields RC, Fleming MD, Gonzalez R, Guild V, Halpern AC, Hodi FS Jr, Joseph RW, Lange JR, Martini MC, Materin MA, Olszanski AJ, Ross MI, Salama AK, Skitzki J, Sosman J, Swetter SM, Tanabe KK, Torres-Roca JF, Trisal V, Urist MM, McMillian N, Engh A. Melanoma, Version 2.2016, NCCN Clinical Practice Guidelines in Oncology. Journal of the National Comprehensive Cancer Network : JNCCN. 2016 Apr:14(4):450-73 [PubMed PMID: 27059193]
Level 1 (high-level) evidenceNosrati A, Berliner JG, Goel S, McGuire J, Morhenn V, de Souza JR, Yeniay Y, Singh R, Lee K, Nakamura M, Wu RR, Griffin A, Grimes B, Linos E, Chren MM, Grekin R, Wei ML. Outcomes of Melanoma In Situ Treated With Mohs Micrographic Surgery Compared With Wide Local Excision. JAMA dermatology. 2017 May 1:153(5):436-441. doi: 10.1001/jamadermatol.2016.6138. Epub [PubMed PMID: 28241261]
Hou JL, Reed KB, Knudson RM, Mirzoyev SA, Lohse CM, Frohm ML, Brewer JD, Otley CC, Roenigk RK. Five-year outcomes of wide excision and Mohs micrographic surgery for primary lentigo maligna in an academic practice cohort. Dermatologic surgery : official publication for American Society for Dermatologic Surgery [et al.]. 2015 Feb:41(2):211-8. doi: 10.1097/DSS.0000000000000248. Epub [PubMed PMID: 25590473]
Level 2 (mid-level) evidenceEtzkorn JR, Sobanko JF, Elenitsas R, Newman JG, Goldbach H, Shin TM, Miller CJ. Low recurrence rates for in situ and invasive melanomas using Mohs micrographic surgery with melanoma antigen recognized by T cells 1 (MART-1) immunostaining: tissue processing methodology to optimize pathologic staging and margin assessment. Journal of the American Academy of Dermatology. 2015 May:72(5):840-50. doi: 10.1016/j.jaad.2015.01.007. Epub 2015 Mar 13 [PubMed PMID: 25774012]
Level 2 (mid-level) evidenceVaienti S, Calzari P, Nazzaro G. Topical Treatment of Melanoma In Situ, Lentigo Maligna, and Lentigo Maligna Melanoma with Imiquimod Cream: A Systematic Review of the Literature. Dermatology and therapy. 2023 Oct:13(10):2187-2215. doi: 10.1007/s13555-023-00993-1. Epub 2023 Aug 24 [PubMed PMID: 37615838]
Level 1 (high-level) evidenceMarsden JR, Fox R, Boota NM, Cook M, Wheatley K, Billingham LJ, Steven NM, NCRI Skin Cancer Clinical Studies Group, the U.K. Dermatology Clinical Trials Network and the LIMIT-1 Collaborative Group. Effect of topical imiquimod as primary treatment for lentigo maligna: the LIMIT-1 study. The British journal of dermatology. 2017 May:176(5):1148-1154. doi: 10.1111/bjd.15112. Epub 2017 Apr 10 [PubMed PMID: 27714781]
Mora AN, Karia PS, Nguyen BM. A quantitative systematic review of the efficacy of imiquimod monotherapy for lentigo maligna and an analysis of factors that affect tumor clearance. Journal of the American Academy of Dermatology. 2015 Aug:73(2):205-12. doi: 10.1016/j.jaad.2015.05.022. Epub 2015 Jun 16 [PubMed PMID: 26088690]
Level 1 (high-level) evidenceHendrickx A, Cozzio A, Plasswilm L, Panje CM. Radiotherapy for lentigo maligna and lentigo maligna melanoma - a systematic review. Radiation oncology (London, England). 2020 Jul 14:15(1):174. doi: 10.1186/s13014-020-01615-2. Epub 2020 Jul 14 [PubMed PMID: 32664998]
Level 1 (high-level) evidenceFogarty GB, Hong A, Scolyer RA, Lin E, Haydu L, Guitera P, Thompson J. Radiotherapy for lentigo maligna: a literature review and recommendations for treatment. The British journal of dermatology. 2014 Jan:170(1):52-8. doi: 10.1111/bjd.12611. Epub [PubMed PMID: 24032599]
Farshad A, Burg G, Panizzon R, Dummer R. A retrospective study of 150 patients with lentigo maligna and lentigo maligna melanoma and the efficacy of radiotherapy using Grenz or soft X-rays. The British journal of dermatology. 2002 Jun:146(6):1042-6 [PubMed PMID: 12072074]
Level 2 (mid-level) evidenceTsang RW, Liu FF, Wells W, Payne DG. Lentigo maligna of the head and neck. Results of treatment by radiotherapy. Archives of dermatology. 1994 Aug:130(8):1008-12 [PubMed PMID: 8053696]
Gershenwald JE, Scolyer RA. Melanoma Staging: American Joint Committee on Cancer (AJCC) 8th Edition and Beyond. Annals of surgical oncology. 2018 Aug:25(8):2105-2110. doi: 10.1245/s10434-018-6513-7. Epub 2018 May 30 [PubMed PMID: 29850954]
Gambichler T, Kempka J, Kampilafkos P, Bechara FG, Altmeyer P, Stücker M. Clinicopathological characteristics of 270 patients with lentigo maligna and lentigo maligna melanoma: data from a German skin cancer centre. The British journal of dermatology. 2014 Dec:171(6):1605-7. doi: 10.1111/bjd.13204. Epub 2014 Oct 22 [PubMed PMID: 24958545]
Level 3 (low-level) evidenceBalch CM, Gershenwald JE, Soong SJ, Thompson JF, Atkins MB, Byrd DR, Buzaid AC, Cochran AJ, Coit DG, Ding S, Eggermont AM, Flaherty KT, Gimotty PA, Kirkwood JM, McMasters KM, Mihm MC Jr, Morton DL, Ross MI, Sober AJ, Sondak VK. Final version of 2009 AJCC melanoma staging and classification. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2009 Dec 20:27(36):6199-206. doi: 10.1200/JCO.2009.23.4799. Epub 2009 Nov 16 [PubMed PMID: 19917835]
Walling HW, Scupham RK, Bean AK, Ceilley RI. Staged excision versus Mohs micrographic surgery for lentigo maligna and lentigo maligna melanoma. Journal of the American Academy of Dermatology. 2007 Oct:57(4):659-64 [PubMed PMID: 17870430]
Level 2 (mid-level) evidenceSwetter SM, Tsao H, Bichakjian CK, Curiel-Lewandrowski C, Elder DE, Gershenwald JE, Guild V, Grant-Kels JM, Halpern AC, Johnson TM, Sober AJ, Thompson JA, Wisco OJ, Wyatt S, Hu S, Lamina T. Guidelines of care for the management of primary cutaneous melanoma. Journal of the American Academy of Dermatology. 2019 Jan:80(1):208-250. doi: 10.1016/j.jaad.2018.08.055. Epub 2018 Nov 1 [PubMed PMID: 30392755]
Roetzheim RG, Pal N, van Durme DJ, Wathington D, Ferrante JM, Gonzalez EC, Krischer JP. Increasing supplies of dermatologists and family physicians are associated with earlier stage of melanoma detection. Journal of the American Academy of Dermatology. 2000 Aug:43(2 Pt 1):211-8 [PubMed PMID: 10906640]
Kantor J, Kantor DE. Routine dermatologist-performed full-body skin examination and early melanoma detection. Archives of dermatology. 2009 Aug:145(8):873-6. doi: 10.1001/archdermatol.2009.137. Epub [PubMed PMID: 19687416]
Gerbert B, Maurer T, Berger T, Pantilat S, McPhee SJ, Wolff M, Bronstone A, Caspers N. Primary care physicians as gatekeepers in managed care. Primary care physicians' and dermatologists' skills at secondary prevention of skin cancer. Archives of dermatology. 1996 Sep:132(9):1030-8 [PubMed PMID: 8795541]