Introduction
The left ventricular ejection fraction (LVEF) is a fundamental measure of left ventricular (LV) systolic function, serving as a surrogate marker of myocardial contractility. LVEF is widely recognized as a crucial parameter for assessing cardiac function and is one of the strongest predictors of cardiovascular outcomes and events across all genders and age groups.
Quantifying and reporting LVEF is a fundamental aspect of assessing cardiac structure and function using modalities such as echocardiography, nuclear ventriculography, cardiac magnetic resonance imaging (CMR), and cardiac computed tomography (CT). Precise measurement of LVEF is crucial for the diagnosis and management of ischemic heart disease, valvular and structural or congenital heart conditions, as well as systemic diseases with cardiac involvement.
LVEF also plays a pivotal role in determining eligibility for device therapies in patients with arrhythmias or those at risk for sudden cardiac death. Over the years, several imaging modalities have been developed to quantify LVEF, with 2-dimensional (2D) echocardiography remaining the most widely used method due to its accessibility and cost-effectiveness.[1]
LVEF is the fraction of blood ejected from the left ventricle during systole (stroke volume [SV]) relative to the volume of blood present at the end of diastole (end-diastolic volume [EDV]). LVEF is expressed as a percentage and represents the proportion of blood pumped out of the left ventricle with each heartbeat.
Stroke volume (SV) is calculated as the difference between EDV and end-systolic volume (ESV). Accordingly, LVEF is determined using the following formula:[2]
LVEF (%) = (SV/EDV) × 100 = [(EDV−ESV)/EDV] × 100
According to the American Society of Echocardiography and the European Association of Cardiovascular Imaging, the reference ranges for LVEF obtained by 2D echocardiography are provided in Table 1 below.[2][3][4]
Table 1. Left Ventricular Ejection Fraction Classification by Sex
| Left Ventricular Ejection Fraction Category | Male (%) | Female (%) |
| Normal | 52-72 | 54-74 |
| Mildly abnormal | 41-51 | 41-53 |
| Moderately abnormal | 30-40 | 30-40 |
| Severely abnormal | <30 |
<30 |
The American College of Cardiology classifies heart failure into distinct categories based on left ventricular ejection fraction LVEF, which helps guide treatment decisions and provides insight into disease severity and underlying cardiac function in affected patients. The categories include:
- Heart failure with preserved ejection fraction (HFpEF): LVEF ≥50%
- Heart failure with reduced ejection fraction (HFrEF): LVEF ≤40%
- Heart failure with mildly reduced ejection fraction (HFmrEF): LVEF 41% to 49%
- Heart failure with improved ejection fraction (HFimpEF): LVEF ≥40% (previously ≤40%) [5]
Anatomy and Physiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Anatomy and Physiology
LVEF reflects the heart's pumping efficiency and is influenced by several key physiological factors, as mentioned below.[6]
- Preload: This refers to the degree of myocardial fiber stretch at the end of diastole, primarily determined by the EDV. According to the Frank-Starling mechanism, increased preload enhances myocardial contractility by optimizing the overlap of actin and myosin filaments, thereby increasing stroke volume up to a physiological limit.
- Afterload: This is defined as the resistance that the left ventricle must overcome to eject blood from the heart. Elevated afterload, as seen in conditions such as hypertension or aortic stenosis, can reduce LVEF by impeding ventricular ejection.
- Myocardial contractility: This is the intrinsic strength of ventricular contraction, independent of preload and afterload. This is increased by sympathetic stimulation, catecholamines, and inotropic agents, and diminished in conditions such as myocardial ischemia or cardiomyopathy.
Indications
Assessing LVEF is crucial for diagnosing and managing heart failure and various cardiovascular conditions. Accurate measurement of LVEF guides treatment decisions, monitors disease progression, and determines eligibility for advanced therapies such as implantable devices. Recognizing the key indications for LVEF assessment ensures that patients with diverse cardiac disorders receive timely and appropriate care.
Heart Failure
- Initial diagnosis and accurate classification based on LVEF.
- Monitoring response to therapy.
- Assessment before device therapy.
Acute Coronary Syndrome
- LVEF assessment should be performed for all patients after myocardial infarction to assist in risk stratification and guide management.
- Consideration for primary prevention implantable cardioverter-defibrillator (ICD) implantation if LVEF remains ≤35% after over 40 days of optimized medical therapy.
Cardiomyopathies
- Evaluation of suspected or confirmed dilated, hypertrophic, restrictive, or other forms of cardiomyopathy.
- Family screening in cases of genetic cardiomyopathies.
Arrhythmias
- Assessment of underlying structural heart disease.
Valvular Heart Diseases
- Risk stratification, monitoring of disease progression, and guidance for therapeutic decision-making.
Hypertension
- LVEF assessment in patients with long-standing hypertension to evaluate for left ventricular hypertrophy or dysfunction.
Chemotherapy
- Baseline and serial LVEF assessments in patients receiving cardiotoxic agents (eg, anthracyclines and trastuzumab) to guide decisions on therapy continuation or interruption.
Preoperative and Postoperative Assessment
- LVEF evaluation as indicated before cardiac or noncardiac surgery.
Congenital Heart Diseases
Technique or Treatment
LVEF can be evaluated using various imaging modalities, either through subjective visual estimation or objective quantitative methods. Quantitative assessment is preferred, as it minimizes interobserver variability and offers greater precision and accuracy in measurement.[6]
Noninvasive Assessment Modalities
Noninvasive imaging techniques play a crucial role in evaluating LVEF, providing important insights into cardiac function without the need for invasive procedures. While these modalities differ in availability, accuracy, and clinical utility, each contributes meaningfully to the comprehensive assessment and management of cardiovascular disease. Commonly used modalities include:
- Echocardiography
- Cardiac magnetic resonance imaging
- Computed tomography
- Gated equilibrium radionuclide angiography (multiple-gated acquisition [MUGA] scan)
- Gated myocardial perfusion imaging with either single-photon emission CT (SPECT) or positron emission tomography (PET)
Invasive Assessment Modalities
Invasive methods for assessing LVEF are generally reserved for situations where noninvasive techniques yield inconclusive results or when additional hemodynamic information is needed. Typically performed during cardiac catheterization, these approaches offer direct and highly accurate measurements of ventricular function, especially in complex or critically ill patients.
Left ventricle contrast ventriculography during invasive catheterization
The assessment of LVEF during cardiac catheterization has largely been supplanted by noninvasive modalities, such as echocardiography and CMR, in routine clinical practice. Invasive evaluation is now primarily reserved for specific situations, such as when performed concurrently with coronary angiography, when noninvasive imaging is inconclusive, or in research settings.
Due to its invasive nature, this method carries certain limitations and risks. Potential complications include arrhythmias, embolism, bleeding, and exposure to ionizing radiation and contrast agents. The use of contrast is especially contraindicated in patients with renal impairment or known contrast allergies. Accuracy may also be compromised in cases of distorted ventricular anatomy or significant mitral regurgitation. Additionally, the procedure requires specialized equipment and experienced operators.
A pigtail catheter is used to inject contrast medium into the ventricular cavity for LVEF assessment. This technique ensures complete opacification of the cavity—from base to apex—without interfering with the mitral subvalvular apparatus, thereby helping to minimize ventricular ectopic activity. The most commonly used imaging views for left ventricular contrast ventriculography are the right anterior oblique (RAO 30°) and left anterior oblique (LAO 60°) projections. The RAO 30° view is often preferred because it provides optimal visualization of the left ventricle and involves lower radiation exposure and procedural cost.
Various geometric methods have been developed to determine LVEF during catheterization by estimating ventricular volumes based on mathematical models that assume a symmetrical ventricular shape. EDV and ESV are calculated using manual or automated border tracing, and LVEF is then derived using the standard formula.
Among geometric techniques, the disk method (Simpson’s rule) and the Dodge-Sandler area-length method are the most commonly used. The Dodge-Sandler method is often preferred when using RAO 30° and LAO 60° views, as the left ventricle in these projections closely resembles an ellipse. This alignment enables the ventricular cavity's longitudinal axis to correspond with that of the ellipsoidal model, allowing for accurate volume estimation using the ellipsoid volume formula.
Echocardiography
Echocardiography is the standard imaging modality for assessing LVEF, favored for its absence of ionizing radiation, broad availability, cost-effectiveness, and ease of use. LVEF can be measured using various echocardiographic techniques, including M-mode, 2D, and 3-dimensional (3D) imaging. These techniques provide linear (1-dimensional [1D]), area-based (2D), or volumetric (3D) measurements for LVEF.
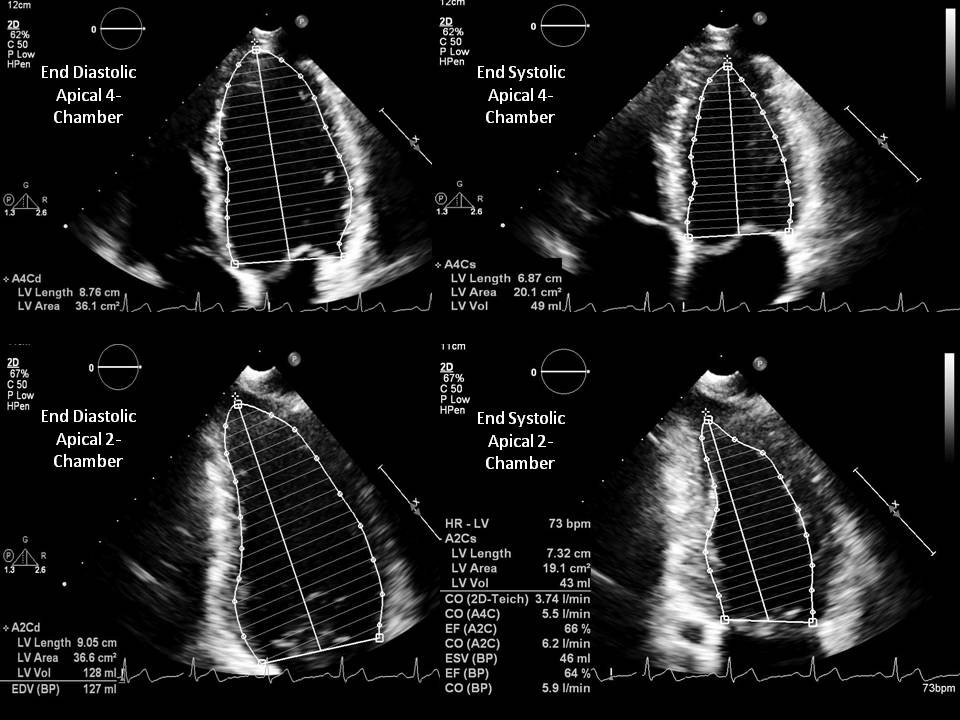
The biplane method of disks, also known as the modified Simpson's rule, is the currently recommended 2D method for LVEF assessment (see Image. Simpson's Biplane Method for LVEF Assessment). Other geometric methods, such as the modified Quinones method, ellipsoid model, and hemisphere-cylinder model, are no longer recommended in clinical practice due to their reliance on simplified geometric assumptions, which limit accuracy.
- M-mode echocardiography: M-mode echocardiography provides a 1D recording of cardiac structures along a single ultrasound line, offering high temporal resolution. Although this technique has largely been replaced by 2D and 3D imaging for more accurate volumetric assessment, it remains useful in certain clinical scenarios, particularly when image quality is suboptimal or for rapid bedside evaluations. This method is based on geometric assumptions and is less accurate in cases of regional wall motion abnormalities or a dilated or irregularly shaped left ventricle. To perform the assessment, the M-mode cursor is positioned perpendicular to the long axis of the left ventricle in the parasternal long-axis (PLAX) view. Measurements are taken at the level of the mitral valve leaflet tips during diastole and systole, including the left ventricular internal diameter in diastole (LVIDd) and systole (LVIDs).
- The Teichholz method is commonly used to estimate LVEF from M-mode measurements.
- Another M-mode–derived parameter of left ventricular systolic function is fractional shortening (FS). Although FS is not a direct measure of LVEF, it correlates well with it and provides a quick estimation of left ventricular systolic function. FS is calculated using the formula: FS (%) = (LVIDd − LVIDs) / LVIDd × 100. Normal FS values typically range from 25% to 45%, which corresponds to an LVEF of 55% to 70%.
- Two-dimensional echocardiography: The Modified Simpson method (also known as the biplane method of disks) is the modality recommended by the American Society of Echocardiography for measuring LVEF. This method involves tracing the endocardial border of the left ventricular cavity in both the apical 4-chamber and 2-chamber views during end-diastole and end-systole. These tracings divide the left ventricle cavity into a predetermined number of disks (typically 20), and the volume of each disk is calculated based on the traced contours. The length of the left ventricle is measured from the mitral annulus to the apex.
- Compared to older methods such as the modified Quinones method, the Modified Simpson's method relies on fewer geometric assumptions and more accurately accounts for variations in left ventricle shape. This modality also directly measures the contribution of longitudinal contraction. However, some geometric assumptions still apply, as the left ventricular cavity border tracing is not completed.
- Other 2-dimensional methods: Besides the biplane method of disks, several other 2D echocardiographic methods are used to estimate LVEF, including eyeball or visual estimation, the area-length method, and the Teicholz or linear method.
- The visual estimation method is a rapid and commonly used approach in urgent settings. This method can be performed using any echocardiographic view. However, it is highly subjective and operator-dependent, relying entirely on the clinician's experience and qualitative assessment.
- The area-length method is typically used when the biplane method is unavailable or not feasible. In this approach, the left ventricle is modeled as an ellipsoid, and either apical or short-axis views are used. Left ventricular volume is calculated using the formula: Left ventricular volume = (5/6) × Area × Length. Because this method assumes a uniform and ventricular shape, it is susceptible to errors from off-axis imaging or asymmetric ventricular remodeling.
- The Teicholz method uses parasternal long-axis view and relies on M-mode or 2D-derived measurements of LVIDd and LVIDs. Left ventricle volume is calculated using the formula: Left ventricle volume = (7 × LVID³) / (2.4 + LVID). Although this method is convenient and easy to perform, it relies on geometric assumptions and is less accurate in patients with regional wall motion abnormalities or irregularly shaped ventricles.
- Three-dimensional echocardiography: 3D echocardiography is a highly accurate and advanced method for assessing LVEF, as it eliminates the geometric assumptions and imaging plane limitations associated with 2D techniques. A full-volume dataset of the left ventricle is acquired from the apical window using a matrix-array transducer. Depending on the patient's heart rate and rhythm, images are captured over multiple cardiac cycles (multi-beat acquisition) or in real time (single-beat acquisition). Endocardial borders are then traced using automatic or semiautomatic methods to reconstruct the left ventricular cavity in 3D. EDV and ESV are directly measured from the complete 3D geometry, and LVEF is calculated using the standard formula.
- The advantages of 3D echocardiography include the elimination of geometric assumptions, independence from imaging FS, higher accuracy in irregularly shaped or dilated ventricles, superior reproducibility for serial monitoring, and enhanced utility in patients with regional wall motion abnormalities.
- The limitations of 3D echocardiography include dependence on good image quality, lower temporal and spatial resolution compared to 2D echocardiography, requirement for 3D-capable equipment and dedicated software, and reduced feasibility in patients with arrhythmias. Table 2 shows the algorithms used to calculate EF from EDV and ESV.
Table 2. Geometric Models for Estimating Left Ventricular Ejection Fraction
|
Model |
Description |
|
Modified Simpson's rule |
The LV is modeled as a composite of 3 geometric shapes: a cylinder (extending from the base to the mitral valve), a truncated cone (from the level of the mitral valve to the papillary muscle), and a cone attributed to the cardiac apex. Each segment is assumed to have equal height. |
|
Ellipsoid model (biplane data) |
Uses 2 perpendicular echocardiographic planes—the apical view and the mitral valve level—as substitutes for angiographic projections. The minor axis of the apical plane (septal to posterolateral) is calculated from the image area and its maximum extended length. The mitral plane is assumed to lie midway between the base and apex, and its minor axis is derived from the area and the septal–posterolateral dimension of the mitral leaflet image. |
|
Ellipsoid model (single-plane data) |
The area and length measurements obtained from a single apical echocardiographic image are used in the standard single-plane area-length equation. |
|
Hemisphere-cylinder model (biplane data) |
The cross-sectional area at the mitral valve level and the long-axis length from the apical view are used to calculate the volume of a cylinder capped at one end by a hemisphere, with both the base area and height corresponding to those of the cylinder. |
|
Modified ellipsoid model (unidimensional data) |
This model utilizes the septal-posterior wall dimension in a formula described by Teichholz, which is based on an ellipsoid model where the major axis is a variable function derived from the measured minor axis, D. Volume = 2 × (7.0/ [2.4+D]) × D. This formula compensates for deviations from the ellipsoid model seen in unusually large and small ventricles. However, the Teichholz method for calculating left ventricular volumes from linear dimensions is no longer recommended for clinical use. |
Cardiac magnetic resonance imaging
CMR is considered the gold standard for estimating LVEF due to its highest accuracy, reproducibility, and freedom from geometric assumptions. CMR is particularly valuable for serial monitoring in patients with cardiomyopathies or those undergoing chemotherapy, as it enables early detection of ventricular dysfunction and allows for simultaneous tissue characterization.
Despite these advantages, CMR has notable limitations. CMR is generally less available and more expensive than echocardiography, and carries specific contraindications, including the presence of certain implanted devices or severe renal dysfunction when gadolinium contrast is used. The procedure also requires patient cooperation and the ability to hold one's breath during image acquisition.
LVEF in CMR can be calculated using manual, semiautomated, or automated methods. The Simpson disk summation technique utilizes short-axis cine steady-state free precession images of the left ventricle, acquired during end-diastole and end-systole, to obtain LVEF. The endocardial borders of the left ventricle are manually traced on each short-axis image to determine the cavity area of each slice. Multiplying the traced area by the slice interval (image gap + slice thickness) yields the volume of each slice. The total left ventricular volume is then calculated by adding the volumes of the individual slices. This method captures the complete shape of the left ventricle, as the entire cavity is included in the tracing. A well-defined endocardial border can be obtained with the use of high contrast. LVEF calculation by CMR does not involve ionizing radiation and does not require contrast agents, making it a safer option in many cases.
Computed tomography
Cardiac CT can be used to calculate LVEF, although it is not a preferred or primary modality for this purpose. Cardiac CT becomes a reasonable option in specific scenarios, such as when coronary CT angiography is already being performed and LVEF assessment is also needed, when echocardiography is nondiagnostic or inconclusive, or when CMR is contraindicated or unavailable.
Noncontrast CT provides poor differentiation between blood and endocardial borders, limiting its utility. The use of iodinated contrast enhances visualization, allowing for more precise delineation of the left ventricular cavity. Automated methods typically rely on Hounsfield unit measurements to distinguish the blood pool from myocardial tissue, making these contrasts essential for accurate differentiation of the left ventricular cavity from the endocardium.
LVEF is commonly calculated using the Simpson method, which involves generating and tracing reconstructed short-axis cine images of the heart. As this technique requires tracing the entire left ventricular cavity, accurate assessment of the left ventricle's shape is essential. The clarity of the endocardial border is directly related to the timing of the contrast bolus.
Unlike CMR, CT imaging can be completed in a single breath hold, offering advantages in patient compliance and scan speed. However, the use of iodinated contrast limits its applicability in patients with renal impairment or contrast allergies, which must be carefully considered before selecting this modality.
Radionuclide angiography
Several techniques are available for calculating LVEF using nuclear cardiac imaging, which offers some of the most established and reproducible methods for this purpose. The 2 most commonly used nuclear cardiac imaging modalities for LVEF assessment are gated equilibrium radionuclide angiography (MUGA scan) and gated myocardial perfusion imaging using either SPECT or PET.
In radionuclide angiography, the patient’s red blood cells are labeled with technetium-99m pertechnetate. Planar images of the left ventricle are then acquired, although SPECT imaging may be used to enhance spatial resolution. For planar imaging, the left anterior oblique projection is typically employed to distinguish the left and right ventricles. After identifying the left ventricular region of interest, the radioactivity counts within that region are analyzed to calculate the LVEF.
Unlike echocardiographic or MRI methods that directly measure ventricular volumes, radionuclide techniques estimate LVEF by assessing changes in radioactivity within the left ventricle between end-diastole and end-systole. This is accomplished through ECG-gated image acquisition over multiple cardiac cycles. Each cycle is divided into a predetermined number of intervals (typically 16-32 frames), with the frame exhibiting the highest count corresponding to end-diastole and the lowest count representing end-systole. LVEF can be calculated using the following equation:
LVEF = (Net counts in the end-diastolic frame − Net counts in the end-systolic frame) / Net counts in the end-diastolic frame.
Net counts are calculated by subtracting the counts from a background region of interest, typically adjacent to the left ventricle, from the measured counts within the left ventricular region. This technique is beneficial in patients whose body habitus limits the accuracy of other imaging modalities. In addition, it is especially valuable for monitoring during cardiotoxic chemotherapy or following radiation therapy to the anterior or left chest when echocardiography yields inconclusive results.
Radionuclide angiography is also recommended in cases where echocardiography is technically inadequate, such as in patients with chronic obstructive pulmonary disease or obesity, or when significant resting wall motion abnormalities or distorted ventricular geometry are present. This modality has no known contraindications.[10]
Gated myocardial perfusion single-photon emission computed tomography or positron emission tomography technique
A radiolabeled myocardial perfusion agent, such as technetium-99m–radiolabelled sestamibi or tetrofosmin, is injected into the patient. Alternative imaging agents such as ammonia, rubidium, or fluorodeoxyglucose can be used, particularly in PET imaging. This technique enables the concurrent assessment of LVEF and myocardial perfusion, allowing for a comprehensive evaluation of myocardial function and perfusion in a single test.
Following radiotracer injection, the myocardium takes up the tracer, and ECG-gated imaging is performed. The cardiac cycle is divided into a predetermined number of frames (images) per cycle (typically 8 or 16). Functional data are then obtained through automated edge-detection software that analyzes the reconstructed 3D dataset to estimate the LVEF. This approach involves geometric assumptions about the left ventricular cavity.
The software identifies the boundary between the radiotracer-avid myocardium and the count-poor left ventricular cavity. EDV and ESV are then derived by measuring the volumes of the left ventricular cavity at various points throughout the cardiac cycle. These values are subsequently used to calculate the LVEF.
Clinical Significance
Given its diagnostic, therapeutic, and prognostic importance, LVEF remains a cornerstone in clinical cardiology. Advancements in imaging technologies and updated definitions of heart failure phenotypes are enhancing the accuracy and clinical significance of LVEF in guiding the treatment and clinical decision-making.
LVEF is a critical marker for risk stratification, therapeutic decision-making, and prognostic evaluation across a range of cardiovascular conditions. However, it should always be interpreted alongside other clinical findings, imaging results, and biomarkers, such as B-type natriuretic peptide (BNP), troponin levels, and functional status (eg, New York Heart Association classification).
Prognostic Indicator
Low LVEF is strongly associated with increased mortality and morbidity. An LVEF of less than 40% is associated with a higher risk of hospitalization, sudden cardiac death, and all-cause mortality.
An LVEF threshold of 35% or less is particularly critical, as it guides the consideration for ICD therapy due to the elevated risk of life-threatening ventricular arrhythmias. Similarly, an LVEF of 40% or less following myocardial infarction indicates a high risk of adverse ventricular remodeling, heart failure, and cardiogenic shock.
LVEF is a central factor in determining prognosis and guiding therapy in patients with dilated cardiomyopathy. Additionally, preoperative LVEF serves as a key predictor of operative risk and long-term outcomes in patients undergoing coronary artery bypass grafting (CABG) or valve surgery.
Heart Failure Assessment and Classification
LVEF is central to the assessment and classification of heart failure, categorizing patients into HFrEF, HFmrEF, or HFpEF. This classification informs treatment strategies, determines eligibility for specific medications and device therapies, and helps estimate prognosis. Accurate LVEF measurement is therefore critical for delivering tailored, evidence-based care.
Therapeutic Decisions
LVEF assessment following a myocardial infarction is essential for identifying patients who may benefit from ICDs or cardiac resynchronization therapy (CRT). This also guides the initiation and optimization of therapies such as beta-blockers, angiotensin-converting enzyme (ACE) inhibitors, and angiotensin receptor–neprilysin inhibitors (ARNIs).
LVEF is a primary criterion for determining the need for defibrillator placement in clinical practice. An LVEF of 35% or less in patients with heart failure or a prior myocardial infarction indicates eligibility for ICD implantation for the primary prevention of sudden cardiac death. CRT is recommended for patients with an LVEF of 35% or less, a QRS duration of 150 ms or more, and left bundle branch block morphology. CRT has been shown to reduce mortality and improve symptoms in patients who are appropriately selected for treatment.[11][12][13][14]
LVEF is a key parameter for monitoring left ventricular function in patients receiving cardiotoxic chemotherapy. Echocardiography is the preferred modality for assessing cardiac function before, during, and after cancer treatment. A baseline LVEF below 53% should prompt referral to a cardiologist to evaluate the risks and benefits of therapy. A comprehensive evaluation often includes global longitudinal strain and troponin measurements. If LVEF decreases by more than 10% from baseline to a final value below 53% during chemotherapy, referral to a cardio-oncology unit is recommended to consider initiation of heart failure therapy and potential modification of the cancer treatment plan.[15]
LVEF is a crucial factor in guiding interventions for patients with valvular heart disease. Aortic valve replacement is recommended for asymptomatic patients with severe aortic stenosis or chronic severe aortic regurgitation who have an LVEF below 50%. Similarly, mitral valve surgery is considered in asymptomatic patients with severe mitral regurgitation and an LVEF of 60% or less.[16]
In advanced heart failure, an LVEF of less than 20% to 25% is commonly used as one of the essential criteria for considering left ventricular assist device implantation or heart transplantation. However, clinical decisions also consider additional factors such as symptom severity, dependence on inotropic support, and functional status, particularly New York Heart Association class IV.
Enhancing Healthcare Team Outcomes
Optimizing healthcare team outcomes in LVEF assessment requires emphasis on clinical accuracy, workflow efficiency, interdisciplinary collaboration, and patient-centered care. Standardizing imaging techniques and measurement protocols, such as using the Simpson’s biplane method in echocardiography, is recommended for consistent and reliable assessment. Contrast agents should be encouraged in technically challenging echocardiograms to enhance image quality. When concerns about accuracy arise, a multimodality imaging approach should be used.
Practical LVEF evaluation relies on coordinated efforts from a multidisciplinary healthcare team, including cardiologists, echocardiographers, radiologists, heart failure specialists, oncologists (particularly in cardio-oncology), nurses, advanced practitioners, and imaging technologists. Ongoing quality improvement should involve routine performance metrics and structured audit and feedback cycles to improve imaging quality, diagnostic accuracy, and timely clinical decision-making.
Media
(Click Image to Enlarge)
References
Dimond MG, Ibrahim NE, Fiuzat M, McMurray JJV, Lindenfeld J, Ahmad T, Bozkurt B, Bristow MR, Butler J, Carson PE, Felker GM, Jessup M, Murillo J, Kondo T, Solomon SD, Abraham WT, O'Connor CM, Psotka MA. Left Ventricular Ejection Fraction and the Future of Heart Failure Phenotyping. JACC. Heart failure. 2024 Mar:12(3):451-460. doi: 10.1016/j.jchf.2023.11.005. Epub 2023 Dec 13 [PubMed PMID: 38099892]
Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, Lancellotti P, Muraru D, Picard MH, Rietzschel ER, Rudski L, Spencer KT, Tsang W, Voigt JU. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Journal of the American Society of Echocardiography : official publication of the American Society of Echocardiography. 2015 Jan:28(1):1-39.e14. doi: 10.1016/j.echo.2014.10.003. Epub [PubMed PMID: 25559473]
Si J, Ding Z, Hu Y, Zhang X, Zhang Y, Cao H, Liu Y. Predictors and prognostic implications of left ventricular ejection fraction trajectory improvement in the spectrum of heart failure with reduced and mildly reduced ejection fraction. Journal of cardiology. 2024 Apr:83(4):250-257. doi: 10.1016/j.jjcc.2023.09.012. Epub 2023 Oct 5 [PubMed PMID: 37802201]
Heidenreich P. What Is a Normal Left Ventricular Ejection Fraction? Circulation. 2023 Aug 29:148(9):750-752. doi: 10.1161/CIRCULATIONAHA.123.065791. Epub 2023 Aug 28 [PubMed PMID: 37639509]
Heidenreich PA, Bozkurt B, Aguilar D, Allen LA, Byun JJ, Colvin MM, Deswal A, Drazner MH, Dunlay SM, Evers LR, Fang JC, Fedson SE, Fonarow GC, Hayek SS, Hernandez AF, Khazanie P, Kittleson MM, Lee CS, Link MS, Milano CA, Nnacheta LC, Sandhu AT, Stevenson LW, Vardeny O, Vest AR, Yancy CW, ACC/AHA Joint Committee Members. 2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2022 May 3:145(18):e895-e1032. doi: 10.1161/CIR.0000000000001063. Epub 2022 Apr 1 [PubMed PMID: 35363499]
Level 1 (high-level) evidenceVancheri F, Longo G, Henein MY. Left ventricular ejection fraction: clinical, pathophysiological, and technical limitations. Frontiers in cardiovascular medicine. 2024:11():1340708. doi: 10.3389/fcvm.2024.1340708. Epub 2024 Feb 7 [PubMed PMID: 38385136]
Bonhorst D, Guerreiro S, Fonseca C, Cardim N, Macedo F, Adragão P. Real-life data on heart failure before and after implantation of resynchronization and/or defibrillation devices - the Síncrone study. Revista portuguesa de cardiologia. 2019 Jan:38(1):33-41. doi: 10.1016/j.repc.2018.04.011. Epub 2019 Jan 23 [PubMed PMID: 30685295]
Song L, Brezden-Masley C, Ramanan V, Ghugre N, Barfett JJ, Chan KKW, Haq R, Petrella T, Dhir V, Jimenez-Juan L, Chacko BR, Kotha V, Connelly KA, Yan AT. Serial Measurements of Left Ventricular Systolic and Diastolic Function by Cardiac Magnetic Resonance Imaging in Patients with Early Stage Breast Cancer on Trastuzumab. The American journal of cardiology. 2019 Apr 1:123(7):1173-1179. doi: 10.1016/j.amjcard.2018.12.046. Epub 2019 Jan 8 [PubMed PMID: 30683420]
Moon YJ, Kim JW, Bang YS, Lim YS, Ki Y, Sang BH. Prediction of all-cause mortality after liver transplantation using left ventricular systolic and diastolic function assessment. PloS one. 2019:14(1):e0209100. doi: 10.1371/journal.pone.0209100. Epub 2019 Jan 25 [PubMed PMID: 30682022]
Plana JC, Galderisi M, Barac A, Ewer MS, Ky B, Scherrer-Crosbie M, Ganame J, Sebag IA, Agler DA, Badano LP, Banchs J, Cardinale D, Carver J, Cerqueira M, DeCara JM, Edvardsen T, Flamm SD, Force T, Griffin BP, Jerusalem G, Liu JE, Magalhães A, Marwick T, Sanchez LY, Sicari R, Villarraga HR, Lancellotti P. Expert consensus for multimodality imaging evaluation of adult patients during and after cancer therapy: a report from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Journal of the American Society of Echocardiography : official publication of the American Society of Echocardiography. 2014 Sep:27(9):911-39. doi: 10.1016/j.echo.2014.07.012. Epub [PubMed PMID: 25172399]
Level 3 (low-level) evidenceDuque ER, Briasoulis A, Alvarez PA. Heart failure with preserved ejection fraction in the elderly: pathophysiology, diagnostic and therapeutic approach. Journal of geriatric cardiology : JGC. 2019 May:16(5):421-428. doi: 10.11909/j.issn.1671-5411.2019.05.009. Epub [PubMed PMID: 31217796]
Kamiya M, Sato N, Matsuda J, Nozaki A, Akiya M, Sato T, Okazaki H, Takahashi Y, Shimizu W. Predictors of responders for low-dose carperitide monotherapy in patients with acute heart failure. Heart and vessels. 2020 Jan:35(1):59-68. doi: 10.1007/s00380-019-01450-w. Epub 2019 Jun 21 [PubMed PMID: 31227874]
Craig JC, Colburn TD, Caldwell JT, Hirai DM, Tabuchi A, Baumfalk DR, Behnke BJ, Ade CJ, Musch TI, Poole DC. Central and peripheral factors mechanistically linked to exercise intolerance in heart failure with reduced ejection fraction. American journal of physiology. Heart and circulatory physiology. 2019 Aug 1:317(2):H434-H444. doi: 10.1152/ajpheart.00164.2019. Epub 2019 Jun 21 [PubMed PMID: 31225988]
Melo X, Abreu A, Santos V, Cunha P, Oliveira M, Pinto R, Carmo M, Fernhall B, Santa-Clara H. A Post hoc analysis on rhythm and high intensity interval training in cardiac resynchronization therapy. Scandinavian cardiovascular journal : SCJ. 2019 Aug:53(4):197-205. doi: 10.1080/14017431.2019.1630747. Epub 2019 Jun 21 [PubMed PMID: 31221002]
Plana JC, Galderisi M, Barac A, Ewer MS, Ky B, Scherrer-Crosbie M, Ganame J, Sebag IA, Agler DA, Badano LP, Banchs J, Cardinale D, Carver J, Cerqueira M, DeCara JM, Edvardsen T, Flamm SD, Force T, Griffin BP, Jerusalem G, Liu JE, Magalhães A, Marwick T, Sanchez LY, Sicari R, Villarraga HR, Lancellotti P. Expert consensus for multimodality imaging evaluation of adult patients during and after cancer therapy: a report from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. European heart journal. Cardiovascular Imaging. 2014 Oct:15(10):1063-93. doi: 10.1093/ehjci/jeu192. Epub [PubMed PMID: 25239940]
Level 3 (low-level) evidenceAortic Stenosis Writing Group, Bonow RO, Brown AS, Gillam LD, Kapadia SR, Kavinsky CJ, Lindman BR, Mack MJ, Thourani VH, Aortic Stenosis Rating Panel, Appropriate Use Criteria Task Force. ACC/AATS/AHA/ASE/EACTS/HVS/SCA/SCAI/SCCT/SCMR/STS 2017 Appropriate use criteria for the treatment of patients with severe aortic stenosis. European journal of cardio-thoracic surgery : official journal of the European Association for Cardio-thoracic Surgery. 2018 Feb 1:53(2):306-308y. doi: 10.1093/ejcts/ezx389. Epub [PubMed PMID: 31089712]