Introduction
Acute stroke is characterized by the sudden onset of focal neurological deficits within a vascular territory affecting the brain, retina, or spinal cord, caused by underlying cerebrovascular diseases.[1] Stroke is prevalent across patient populations and is a leading cause of morbidity and mortality. Strokes are classified as ischemic or hemorrhagic, with hemorrhagic strokes further divided into intracerebral and subarachnoid hemorrhages. Among ischemic strokes, the Trial of Org 10172 in Acute Stroke Treatment (TOAST) classification system is commonly used to categorize subtypes, which include:
- Cardioembolism
- Small vessel occlusion
- Large artery atherosclerosis
- Stroke of undetermined etiology
- Stroke of other determined etiology (possible or probable, depending on the results of ancillary studies) [2]
A diagnosis is considered "probable" when clinical findings, neuroimaging data, and diagnostic study results consistently support a specific subtype, and other potential causes have been ruled out. On the other hand, a diagnosis is labeled "possible" when clinical findings and neuroimaging data suggest a specific subtype, but further studies have not been conducted to confirm it. As many patients undergo limited testing, these categories enable physicians to assign the most accurate subtype diagnosis possible.
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
The etiology of ischemic stroke involves either a thrombotic or embolic event that impairs blood flow to a region of the brain. In a thrombotic event, the obstruction occurs within the affected vessel due to a thrombus (blood clot), typically resulting from atherosclerotic disease, arterial dissection, fibromuscular dysplasia, or inflammatory conditions. In an embolic event, circulating debris originating from another part of the body travels to and occludes a cerebral vessel, disrupting blood flow. Emboli can originate from a proximal artery, such as an atherosclerotic plaque in the internal carotid artery, causing artery-to-artery embolic stroke to more distal cerebral vessels, or commonly from the heart. Occasionally, emboli arise from the right side of the circulation and pass through a right-to-left shunt—such as a patent foramen ovale—into the cerebral arterial system. The etiology of stroke affects both prognosis and outcomes.[3][4]
Cardioembolism
This category includes patients with arterial occlusions likely caused by emboli originating from the heart. Cardiac sources are classified as high-risk or medium-risk based on their potential to cause embolism. At least one cardiac source of an embolus must be identified to consider a possible or probable diagnosis of cardioembolic stroke. Clinical and brain neuroimaging findings resemble those described for large-artery atherosclerosis. A history of transient ischemic attack (TIA) or stroke involving multiple vascular territories, or evidence of systemic embolism, supports the diagnosis of cardiogenic stroke. Potential large-artery atherosclerotic sources of thrombosis or embolism should be excluded. A stroke occurring in a patient with a medium-risk cardiac source and no other identifiable cause is classified as a possible cardioembolic stroke.[5]
Large artery atherosclerosis
Patients in this category typically present with clinical and brain neuroimaging findings indicating significant (>50%) stenosis or complete occlusion of a major cerebral artery or a branch cortical artery, most likely due to atherosclerosis. Clinical features may include signs of cortical impairment (eg, aphasia, neglect, or limited motor function) or dysfunction of the brainstem or cerebellum. A history of intermittent claudication, TIAs in the same vascular territory, carotid bruits, or diminished peripheral pulses can support the clinical diagnosis. Infarcts involving the cortex, cerebellum, brainstem, or subcortical regions larger than 1.5 cm in diameter on computed tomography (CT) or magnetic resonance imaging (MRI) suggest a large artery atherosclerotic etiology.
Additional supportive evidence from duplex ultrasonography or arteriography demonstrating more than 50% stenosis in a relevant intracranial or extracranial artery is essential. Diagnostic evaluations should also exclude potential sources of cardiogenic embolism. A diagnosis of stroke due to large artery atherosclerosis cannot be made if duplex or arteriographic findings are normal or show only minimal abnormalities.[6]
Small vessel occlusion
This category includes patients with strokes typically classified as lacunar infarcts in other classification systems. These patients should present with one of the classic lacunar syndromes and should not exhibit signs of cerebral cortical dysfunction. A history of hypertension or diabetes mellitus supports the clinical diagnosis. Additionally, patients should have normal CT or MRI findings or exhibit a relevant brainstem or subcortical lesion smaller than 1.5 cm in diameter. Cardiac sources of embolism should be absent, and evaluation of the major extracranial arteries should show no stenosis greater than 50% in an artery on the same side.[7]
Stroke of undetermined etiology
In numerous cases, determining the precise cause of a stroke remains challenging. Some patients undergo extensive evaluation without revealing a likely etiology, while others receive only limited assessment, resulting in an undetermined cause. This category also includes patients with 2 or more potential stroke causes, making a definitive diagnosis challenging. For instance, a patient presenting with a medium-risk cardiac source of embolism alongside another possible cause would be classified as having a stroke of undetermined etiology. Similarly, patients with atrial fibrillation and ipsilateral carotid stenosis of 50%, or those with a classic lacunar syndrome and ipsilateral carotid stenosis of 50%, fall into this category.[8]
Stroke of other determined etiology
This category includes patients with less common causes of stroke, such as nonatherosclerotic vasculopathies, hypercoagulable states, or hematological disorders. These patients should present with clinical symptoms and CT or MRI findings consistent with acute ischemic stroke, regardless of lesion size or location. Diagnostic tests, such as blood work or arteriography, should identify one of these uncommon etiologies. Additional evaluations should be performed to exclude cardiac sources of embolism and large artery atherosclerosis.[9]
Epidemiology
In 2021, stroke accounted for 1 in 6 deaths from cardiovascular disease, with a stroke occurring in an individual every 40 seconds and a stroke-related death every 3 minutes and 14 seconds.[10] Annually, over 795,000 Americans experience a stroke, of which approximately 610,000 are first-time events.[10] Nearly one-quarter of strokes (about 185,000 cases) occur in individuals with a history of prior stroke. Ischemic strokes, which block blood flow to the brain, make up approximately 87% of all strokes. According to the Framingham Heart Study, stroke incidence has declined, though the study population was predominantly White.[11][12][13]
The financial burden of stroke in the United States totaled nearly $56.5 billion between 2018 and 2019, encompassing healthcare costs, medications, and lost productivity from missed workdays. Stroke remains a leading cause of severe long-term disability, with more than half of survivors aged 65 and older experiencing mobility limitations.[14]
Significant disparities in stroke incidence and outcomes exist across racial and ethnic groups. Non-Hispanic Black adults face nearly twice the risk of a first stroke compared to White adults, and both non-Hispanic Black and Pacific Islander adults have the highest stroke-related mortality rates. Additionally, the stroke death rate increased from 38.8 per 100,000 in 2020 to 41.1 per 100,000 in 2021.[15]
Pathophysiology
In cerebral thrombosis, an obstructive process impedes blood flow to specific areas of the brain. The most common underlying risk factor is large-vessel atherosclerosis. Other risk factors include vasculitides and arterial dissection.
Embolic events occur when a clot forms in another part of the body and travels to the brain. The most common source is the heart’s chambers or valves—for instance, a thrombus may form in the atria during atrial fibrillation and subsequently dislodge into the arterial vascular supply. Less frequent sources include venous, septic, air, or fat emboli.
Lacunar infarcts typically occur in the subcortical regions of the brain supplied by small penetrating or perforating arteries, which generally lack collateral circulation. These arteries include the lenticulostriate branches of the middle cerebral artery (MCA), thalamic perforators from the posterior cerebral artery (PCA), and paramedian branches of the basilar artery. The underlying pathology is small vessel arteriolosclerosis, often caused by hypertension, aging, smoking, diabetes, and other conventional vascular risk factors.[16]
Cerebral Autoregulation
Under normal physiological conditions, cerebral blood flow is primarily regulated by the resistance within the cerebral blood vessels, which is directly correlated to their diameter. Vasodilation results in increased blood volume within the brain and heightened cerebral blood flow, while vasoconstriction produces the opposite effect.[17] Additionally, cerebral blood flow is influenced by fluctuations in cerebral perfusion pressure.
Cerebral autoregulation refers to the brain’s ability to maintain relatively stable cerebral blood flow despite moderate changes in perfusion pressure.[17][18] Although the precise mechanisms underlying autoregulation remain incompletely understood, multiple pathways are likely involved. Evidence suggests that smooth muscle in cerebral vessels responds directly to changes in perfusion pressure by contracting when pressure rises and relaxing when it falls. Additionally, reductions in cerebral blood flow may trigger blood vessel dilation by releasing vasoactive substances, although the specific molecules responsible have yet to be identified. Nitric oxide released by endothelial cells also appears to have a role in autoregulation.
Under normal conditions, cerebral blood flow is regulated by autoregulation within a mean arterial pressure (MAP) range of approximately 60 to 150 mmHg, although individual upper and lower limits may vary. Outside this range, the brain’s capacity to compensate for changes in perfusion pressure decreases, causing cerebral blood flow to passively increase or decrease in response to pressure fluctuations. This passive response raises the risk of ischemia at low pressures and edema at high pressures.
During certain pathological conditions, such as ischemic stroke, cerebral autoregulation becomes impaired. As cerebral perfusion pressure decreases, cerebral blood vessels dilate in an attempt to augment cerebral blood flow.[19] However, if the reduction in perfusion pressure surpasses the brain’s compensatory ability, cerebral blood flow decreases. Initially, the brain increases its oxygen extraction fraction to maintain oxygen delivery. As cerebral blood flow continues to fall, additional compensatory mechanisms are activated.
Protein synthesis becomes inhibited when cerebral blood flow falls below 50 mL/100 g/min and ceases entirely at 35 mL/100 g/min, accompanied by a transient increase in glucose utilization. As blood flow decreases further to 25 mL/100 g/min, glucose utilization drops significantly, triggering anaerobic glycolysis and resulting in tissue acidosis due to the accumulation of lactic acid. Neuronal electrical activity fails at cerebral blood flow rates of 16 to 18 mL/100 g/min, followed by failure of membrane ion homeostasis at cerebral blood flow rates of 10 to 12 mL/100 g/min.[19] This threshold typically marks the onset of infarction.
In individuals with hypertension, cerebral autoregulation adapts to function at higher arterial pressures. Reducing blood pressure (BP) to normal levels in these patients during a stroke may worsen autoregulatory dysfunction, causing further decreases in cerebral blood flow.
Concept of the Ischemic Penumbra
During an acute ischemic stroke, brain tissue that depends solely on one artery for blood supply undergoes infarction, forming the infarct core.[20] Surrounding this core is the ischemic penumbra, an area of brain tissue that retains partial blood flow through collateral circulation. However, as swelling from the infarct increases, the penumbra gradually shrinks, and the infarct core expands.
Under normal conditions, cerebral perfusion is approximately 50 mL/100 g/min. Brain cells begin to die when perfusion falls below 30% of this level, equivalent to less than 15 mL/100 g/min. Hence, when blood flow is reduced but remains above 30% of the normal rate, the brain tissue is ischemic but not infarcted, highlighting the critical principle that "time is brain." This principle underscores the importance of timely revascularization treatments in acute ischemic stroke, given the distinct time windows for intervention based on these physiological insights.[21][22][23]
Ischemic Stroke Syndromes
Ischemic strokes often present as specific syndromes caused by reduced blood flow to distinct brain regions. These patterns correlate with clinical examination findings, enabling clinicians to predict the affected areas of the cerebral vasculature.
Middle Cerebral Artery Infarction
The MCA is the most commonly affected artery in ischemic stroke (see Images. Left Middle Cerebral Artery Territory Infarction and Right Middle Cerebral Artery "Cord Sign" Indicative of Acute Infarction). The MCA is divided into 4 segments—M1, M2, M3, and M4—and supplies a large area of the lateral surface of the brain, as well as parts of the basal ganglia and internal capsule. The M1 (horizontal) segment gives rise to the lenticulostriate arteries, which supply the basal ganglia and internal capsule. The M1 segment continues into the M2 (Sylvian) segment, which supplies the insula, superior temporal lobe, parietal lobe, and inferolateral frontal lobe. Please see StatPearls' companion resource, "Middle Cerebral Artery Stroke," for more information.
The MCA distribution involves the lateral aspect of the cerebral cortex. MCA syndrome is best understood through the homunculus of the cerebral cortex, where the lateral portion of the cerebral cortex corresponds to motor and sensory functions that involve the face and upper extremities. Infarctions in the MCA territory typically present with contralateral hemiparesis, facial paralysis, and sensory loss affecting the face and upper extremities. Although the lower extremities may also be involved—particularly when deep brain structures are affected—symptoms in the upper extremities generally predominate.
In addition, patients may exhibit gaze preference toward the side of the lesion. Additional symptoms include:
- Dysarthria, which is characterized by difficulty in phonation due to weakness of the facial muscles, lacks strong localizing value in acute stroke because it can result from both cortical and subcortical infarcts. Dysarthria may occur in strokes affecting either the dominant or nondominant hemisphere. The most severe form, anarthria, results in complete loss of speech output. Dysarthria is often mistaken for aphasia.
- Neglect occurs when a patient appears to “ignore” one side of their environment due to an inability to perceive that area. This is a sensory dysfunction associated with the nondominant cerebral cortex. Extinction, or the failure to detect simultaneous stimuli on both sides, is a common bedside test used to assess nondominant cortical lesions.
- Visual field loss can occur in MCA infarcts because branches of the MCA supply the optic radiations. A more localized ischemic stroke in the parietal lobe may cause contralateral inferior quadrantanopia, while a temporal lobe infarct typically results in contralateral superior quadrantanopia, often referred to as "pie-in-the-sky."
- Aphasia, the inability to produce or comprehend language, results from injury to the language areas of the brain’s dominant hemisphere, which are supplied by the dominant MCA.
Anterior Cerebral Artery Infarction
The anterior cerebral artery (ACA) supplies blood to the medial areas of the frontal, prefrontal, primary motor, primary sensory, and supplemental motor cortices. These are areas corresponding to the lower extremities in the cortical homunculus. Pure ACA infarcts are relatively rare due to the presence of a good collateral blood supply. The sensory and motor cortices process sensory information and control the movement of the contralateral lower extremity. The ACA territory primarily involves the medial cerebral cortex. Clinically, an ACA infarction presents with contralateral sensory and motor deficits affecting the lower extremity, while the upper extremity and face are typically spared. Please see StatPearls' companion resource, "Anterior Cerebral Artery Stroke," for more information.
Posterior Cerebral Artery Infarction
The superficial branches of the PCA supply the occipital lobe and the medial portion of the temporal lobe. In contrast, the deep PCA branches supply the thalamus and other posterior deep brain structures. The occipital lobe houses the visual cortex, and the thalamus serves as the primary relay center for ascending and descending neural pathways. The most common cause of a PCA infarct is an atherothrombotic lesion in the vertebral artery. Please see StatPearls' companion resource, "Posterior Cerebral Artery Stroke," for more information.
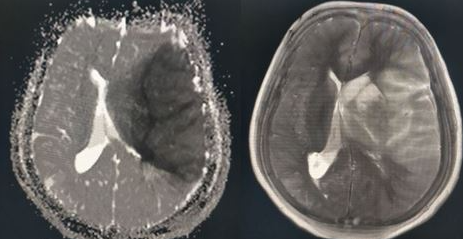
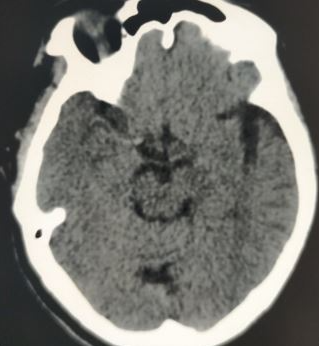
PCA infarctions can be categorized as deep or superficial based on the vascular territories supplied by the PCA. If the deep segments of the PCA are involved, the symptoms primarily reflect thalamic dysfunction and may include hypersomnolence, cognitive impairment, ocular findings, hypoesthesia, and ataxia. Larger infarcts that involve deep structures can lead to hemisensory loss and hemiparesis due to the involvement of the thalamus and the adjacent internal capsule. Superficial PCA infarcts typically present with contralateral homonymous hemianopia, often with macular sparing. In rare cases, bilateral PCA infarcts may lead to amnesia and cortical blindness.[24][25] As symptoms of posterior circulation strokes are often subtle, healthcare providers should maintain a low threshold for suspicion and promptly pursue imaging and neurology consultation (see Image. Axial Noncontrast CT Scan of the Head Reveals Left PCA Ischemic Stroke Without Hemorrhage).[26]
Vertebrobasilar Infarction
The vertebrobasilar region of the brain is supplied by the 2 vertebral arteries and the basilar artery as they course along the anterior surface of the pons. The vertebral arteries arise from their respective subclavian artery, course through the transverse foramina of the upper 6 cervical vertebrae, and enter the skull through the foramen magnum. The main branches of each vertebral artery include the PCA and the anterior spinal artery. The vertebral arteries terminate by merging to form the basilar artery, which then bifurcates into the 2 PCAs that connect the circle of Willis. In addition to the paramedian penetrating branches, the basilar artery gives rise to the anterior inferior cerebellar artery and the superior cerebellar artery, which supply the cerebellum and the brainstem.[27]
The clinical presentation includes ataxia, vertigo, headache, vomiting, oropharyngeal dysfunction, visual field deficits, and abnormal oculomotor findings. The patterns of clinical presentation vary depending on the location and the infarction pattern, as well as whether the underlying cause is an embolism or atherosclerosis.[28][29]
Cerebellar Infarction
Patients may present with ataxia, nausea, vomiting, headache, dysarthria, and vertigo. Cerebellar infarction may also be complicated by edema and rapid clinical deterioration.[28]
Lacunar Infarction
Lacunar infarcts result from the occlusion of small perforating or penetrating arteries and are defined as being less than 1 centimeter in diameter. They may present with pure motor or sensory deficits, a combination of sensorimotor loss, or ataxic hemiparesis.[30][31][32] Please see StatPearls' companion resource, "Lacunar Stroke," for more information.
History and Physical
Ischemic strokes usually manifest suddenly, making it crucial to determine the exact time when symptoms first appeared. If the exact onset of symptoms is unknown, clinicians use the time the patient was last observed in their usual state of health, without new neurological symptoms, as the reference point. This established timeline is crucial for determining the eligibility of administering intravenous (IV) thrombolytic therapy.
Another essential component of the clinical assessment is identifying potential underlying causes to help determine the stroke mechanism. Key factors to consider include common vascular risk factors such as hypertension, a history of stroke or TIAs, smoking, and diabetes. A detailed cardiac history is also important, particularly atrial fibrillation, recent myocardial infarction, and cardiomyopathy. Additional considerations include a history of neck trauma, recent chiropractic manipulation, and signs of a hypercoagulable state.[33]
A neurological examination is essential for all patients suspected of having a stroke. Monitoring vital signs and cardiac rhythm is also critical, as is listening for a neck bruit, which may indicate underlying vascular abnormalities. The National Institutes of Health Stroke Scale (NIHSS) is the standard tool used to assess the severity of a stroke. This includes 11 categories, with scores ranging from 0 to 42 (refer to NIH Stroke Scale).[34][35] These categories assess the level of consciousness (LOC) through instructions, questions, commands, gaze direction, vision, facial symmetry, motor function of the arms and legs, limb coordination, sensory response, language, speech clarity, and attention to both sides of the body (see Table below). The NIHSS should be administered in the specified sequence, and scoring should reflect the patient’s actual performance during the examination, rather than on predicted abilities.
Table. National Institutes of Health Stroke Scale
| Category | Score Meaning | Score |
| 1a. Level of Consciousness (instructions) | 0: Fully alert; 1: Not fully alert, but not drowsy; 2: Obtunded or requires minor stimulation to stay alert; 3: Unresponsive or requires repeated stimulation | |
| 1b. Level of Consciousness (questions) | 0: Answers both questions correctly; 1: Answers 1 question correctly; 2: Answers neither question correctly | |
| 1c. Level of Consciousness (commands) | 0: Performs both tasks correctly; 1: Performs 1 task correctly; 2: Performs neither task correctly | |
| 2. Best Gaze | 0: Normal; 1: Partial gaze palsy; 2: Forced deviation | |
| 3. Visual Field Testing | 0: No visual loss; 1: Partial hemianopia; 2: Complete hemianopia; 3: Bilateral hemianopia (blind including cortical blindness) | |
| 4. Facial Palsy | 0: Normal symmetrical movements; 1: Minor paralysis; 2: Partial paralysis; 3: Complete paralysis of 1 or both sides | |
| 5. Motor Arm (score both left and right) | 0: No drift; 1: Drifts down; 2: Some effort against gravity; 3: No effort against gravity; 4: No movement | |
| 6. Motor Leg (score both left and right) | 0: No drift; 1: Drifts down; 2: Some effort against gravity; 3: No effort against gravity; 4: No movement | |
| 7. Limb Ataxia | 0: Absent; 1: Present in 1 limb; 2: Present in 2 limbs | |
| 8. Sensory | 0: Normal; 1: Mild-to-moderate sensory loss; 2: Severe-to-total sensory loss | |
| 9. Best Language | 0: Normal; 1: Mild-to-moderate aphasia; 2: Severe aphasia; 3: Mute or global aphasia | |
| 10. Dysarthria | 0: Normal; 1: Mild-to-moderate dysarthria; 2: Severe dysarthria or anarthria | |
| 11. Extinction and Inattention | 0: No abnormality; 1: Visual, tactile, auditory, spatial, or personal inattention; 2: Profound hemi-inattention or neglect to more than 1 modality | |
| Total: |
Evaluation
An organized stroke protocol is highly recommended to expedite patient evaluation and treatment.[34] A door-to-needle time of 60 minutes is advised for acute ischemic stroke patients eligible for thrombolytic therapy.[34]
The goals in the initial phase include:
- Ensuring medical stability, with particular attention to airway, breathing, and circulation.
- Promptly addressing and reversing any conditions that contribute to the patient's problem.
- Determining the patient’s eligibility for IV thrombolytic therapy or endovascular thrombectomy.
- Initiating efforts to identify the underlying pathophysiological cause of the patient's neurological symptoms.
The initial evaluation of any patient should prioritize airway, breathing, circulation, and vital signs. Patients with elevated intracranial pressure (ICP) may exhibit respiratory abnormalities and are at risk for aspiration and asphyxiation. Endotracheal intubation may be required to secure the airway and ensure adequate oxygenation and ventilation.
A fingerstick glucose test should be performed promptly, as hypoglycemia is a readily reversible cause of neurological abnormalities.
A plain CT scan of the head is recommended within 20 minutes of patient presentation to exclude hemorrhage. In stroke centers or facilities equipped for emergency care, vascular imaging should be considered to evaluate eligibility for endovascular intervention. However, endovascular procedures must not delay the administration of thrombolytic therapy.[34]
Diffusion-weighted imaging (DWI) is a specialized MRI technique that measures the diffusion of water molecules within tissue. DWI is particularly sensitive for identifying acute ischemic strokes, as it can reveal cytotoxic edema—an early marker of infarction—within minutes of stroke onset. DWI can indicate a brain infarction much earlier than other MRI sequences, such as fluid-attenuated inversion recovery (FLAIR). While DWI scan may show abnormalities within minutes of stroke onset, FLAIR changes typically appear around 4.5 hours later to reveal signs of a brain infarction. If DWI shows signs of stroke but the FLAIR sequence does not, it suggests the ischemic event occurred less than 4.5 hours prior. This timing is critical, as patients within this window may be eligible for early intravenous thrombolysis, which can potentially reverse neurological deficits.[36]
Other diagnostic tests include an electrocardiogram (ECG), troponin levels, complete blood count (CBC), electrolytes, blood urea nitrogen (BUN), creatinine (Cr), and coagulation studies. ECG and troponin testing are essential, as stroke is frequently associated with underlying coronary artery disease. A CBC may reveal anemia or signs of infection. Electrolyte imbalances should be corrected, as they can cause altered mental status and confound the diagnosis of ischemic stroke. Monitoring BUN and creatinine is important, especially if contrast imaging is planned, due to the risk of nephrotoxicity. Coagulation studies—including PT, PTT, and INR—should be obtained, as elevated values may indicate a hemorrhagic stroke or influence treatment decisions.[34]
For institutions lacking expert imaging interpretation, the US Food and Drug Administration (FDA) strongly recommends using a teleradiology system to interpret images for patients suspected of having a stroke. Rapid image review facilitates timely decisions regarding the administration of IV alteplase. Collaboration and agreement between telestroke neurologists and radiologists are highly encouraged. In settings without an in-house stroke team or established telestroke protocol, telephone consultation may be considered to guide thrombolytic therapy. However, the supporting evidence for this approach is limited.[37][38][39]
Treatment / Management
The goal of therapy in acute ischemic stroke is to preserve tissue in areas where perfusion is reduced but still sufficient to prevent infarction. This region of oligemia can be preserved by restoring blood flow to the compromised regions and improving collateral flow. Recanalization strategies include the use of IV recombinant tissue-type plasminogen activator (tPA) and mechanical thrombectomy. Timely restoration of blood flow is critical to minimize ischemic damage. Endovascular techniques have been successfully used in selected patients for acute ischemic stroke treatment. Neuroprotective agents have also been explored, but none have demonstrated a clear benefit in improving clinical outcomes. Essential treatments proven effective in controlled trials for acute ischemic stroke across various patient groups are mentioned below.
Acute Reperfusion Therapy
Intravenous alteplase (within 4.5 hours of stroke onset)
The American Heart Association (AHA) and American Stroke Association (ASA) recommend IV alteplase (tPA) for patients who meet the inclusion criteria and have symptom onset or last known well time within 3 hours.[40] IV tPA should be administered at a dose of 0.9 mg/kg, with a maximum dose of 90 mg. The first 10% of the dose is given as an initial bolus over 1 minute, followed by the remaining 90% infused over 60 minutes. The treatment window has been extended to 4.5 hours for selected candidates.
Inclusion criteria include a diagnosis of ischemic stroke with a measurable neurological deficit, symptom onset within 3 hours before treatment, and age 18 years or older.[40]
Healthcare providers must review the exclusion criteria for thrombolytic agents before administering tPA. According to the FDA, contraindications to IV thrombolysis include active internal bleeding, recent intracranial surgery or severe head trauma, intracranial conditions that increase bleeding risk, bleeding diathesis, severe uncontrolled hypertension, current intracranial hemorrhage, subarachnoid hemorrhage, and a recent history of stroke.
Healthcare providers must weigh the benefits and risks of treatment for patients presenting between 3 and 4.5 hours after symptom onset. Additional relative exclusion criteria in this time window include patients aged 80 or older, an NIHSS score greater than 25, use of oral anticoagulants, and a history of both diabetes and prior ischemic stroke.[40]
Magnetic resonance imaging–guided thrombolysis for stroke with unknown time of onset
Many patients wake up with symptoms of an acute stroke, rendering them ineligible for IV thrombolytic therapy because their last known normal state was at bedtime. The WAKE-UP Stroke Trial utilized the mismatch between a positive DWI MRI sequence, indicating an acute ischemic infarction, and a negative FLAIR MRI sequence, which suggests that the infarct occurred within 4.5 hours of the MRI. DWI becomes positive within 30 minutes of an acute infarct, while the FLAIR sequence typically remains negative until about 4.5 hours after onset. This mismatch indicates the stroke occurred within the 4.5-hour window, making the patient eligible for IV thrombolytic therapy. The WAKE-UP Stroke Trial confirmed the positive result.[36][41]
Intravenous tenecteplase (within 4.5 hours of stroke onset)
Tenecteplase (TNK), another fibrinolytic agent, may be considered an alternative to alteplase. TNK offers advantages over alteplase, including a longer half-life and the ability to be administered as a single IV bolus. TNK is typically given as a weight-based IV bolus of 30 to 50 mg over 5 seconds. TNK has increasingly become the fibrinolytic agent of choice at many stroke centers, particularly during the COVID-19 pandemic.
Recent studies indicate that TNK has a comparable efficacy and safety profile to tPA.[42][43] According to the 2023 AHA guidelines, it may be reasonable to choose TNK over alteplase in patients who have no contraindications to IV fibrinolytics and are also eligible for mechanical thrombectomy.[34] Notably, a dose of 0.4 mg/kg has not demonstrated any advantage over the 0.25 mg/kg dose.(A1)
Mechanical thrombectomy (within 6 hours of stroke onset)
Mechanical thrombectomy should be considered for all eligible patients, including those who have received fibrinolytic therapy. The AHA/ASA guidelines recommend proceeding with mechanical thrombectomy without waiting to observe the response to IV tPA in patients who qualify for the procedure.[40]
In recent years, acute stroke care has seen significant advancements. Multiple trials conducted in 2015 demonstrated that endovascular thrombectomy within the first 6 hours significantly outperforms standard medical care in patients with large vessel occlusion (LVO) affecting the proximal anterior circulation. These benefits are consistent across various geographic regions and patient populations.[44] Please see StatPearls' companion resource, "Acute Stroke," for more information.(B3)
Mechanical thrombectomy with perfusion study (within 16 to 24 hours of stroke onset)
Perfusion imaging studies (eg, CT perfusion or MR perfusion) can define the areas of the brain that are ischemic but not yet infarcted, known as the ischemic penumbra. Depending on the size of the penumbra relative to the ischemic core, a significant amount of brain tissue can be saved by restoring blood flow in cases of LVO identified on CT or MR angiography, leading to improved clinical outcomes.
In 2018, a significant paradigm shift occurred in stroke care. The DAWN trial showed significant benefits of endovascular thrombectomy in patients with LVO in the arteries of the proximal anterior circulation. This trial extended the stroke window to 24 hours in selected patients using perfusion imaging. Subsequently, more patients can be treated, even up to 24 hours.[45]
Mechanical thrombectomy is recommended within 6 to 16 hours of the last known normal in selected patients with LVO acute ischemic stroke in the anterior circulation who meet the DAWN and DEFUSE 3 criteria. For selected patients meeting DAWN criteria, mechanical thrombectomy may be considered reasonable up to 24 hours after the last known normal.[45][46]
Endovascular therapy (thrombectomy) for acute ischemic stroke with a large infarct
Two major trials published in 2023—the ANGEL-ASPECT and SELECT 2 trials—demonstrated favorable outcomes with endovascular thrombectomy in patients with large ischemic strokes due to LVO and an Alberta Stroke Program Early CT Score (ASPECTS) of 3 to 5. These patients presented within 24 hours of their last known normal and had an NIHSS score greater than 6. Outcomes were significantly improved in those treated with thrombectomy compared to those who received standard medical therapy alone.[47][48]
- Definition of large vessel occlusion: LVO refers to occlusions in major cerebral arteries, including the intracranial internal carotid artery, MCA (M1 segment), basilar artery, and PCA (P1 segment) occlusion.[49]
- Determining Alberta Stroke Program Early CT Score: ASPECTS is a 10-point scoring system used to assess acute ischemic stroke in the MCA territory. One point is subtracted from 10 for each region showing infarction on CT imaging. Thus, a lower score indicates a larger infarct size. The evaluated regions include the caudate, putamen, internal capsule, insular cortex, and cortical areas M1 through M6.[50]
- Basilar artery occlusion: This rare type of stroke carries a poor prognosis, with approximately 80% of patients experiencing unfavorable outcomes. The AHA guidelines recommend mechanical thrombectomy within 6 hours of symptom onset. Two large trials published in 2022 demonstrated the benefits of endovascular treatment over conventional therapy. The ATTENTION trial showed positive outcomes for thrombectomy within 12 hours,[51] whereas the BAOCHE trial supported the benefits of thrombectomy performed 6 to 24 hours after stroke onset due to basilar artery occlusion.[52] In conclusion, mechanical thrombectomy should be considered for eligible patients up to 24 hours after symptom onset. (B2)
Acute Hospital Management
Blood pressure
Guidelines recommend maintaining BP below 180/105 mmHg for the first 24 hours following IV tPA administration.[34] The 2023 AHA guidelines recommend maintaining BP at or below 185/110 mmHg before the procedure in patients scheduled for mechanical thrombectomy who have not received IV fibrinolytic therapy.[40] Additionally, a new recommendation advises an initial 15% reduction in blood pressure for patients with comorbidities such as acute heart failure or aortic dissection.
Antihypertensive management has not been shown to reduce death or dependency in patients with BP less than 220/120 mmHg who have not received IV tPA and have no comorbid conditions necessitating BP reduction. Theoretical concerns include reduced perfusion to ischemic brain tissue, which may be pressure-dependent in the acute phase. This risk is most relevant during the first 48 to 72 hours after an acute ischemic stroke. For patients with BP 220/120 mmHg or higher who have not received IV tPA, guidelines suggest it may be reasonable to reduce BP by 15% within the first 24 hours, although the benefit remains uncertain. A recent large trial demonstrated a U-shaped relationship between acute MAP and stroke outcomes, identifying an ideal mean systolic BP range of 135 to 150 mmHg.[40]
Antihypertensive options include:
- Labetalol: Can be initiated at 10 to 20 mg IV; may repeat once if needed.
- Nicardipine: Can be started at 5 mg/h IV and increased by 2.5 mg/h every 5 to 15 minutes as needed. The maximum dose is 15 mg/h.
- Clevidipine: Can be initiated at 1 to 2 mg/h IV. The dosage can be doubled every 15 minutes, but should not exceed 21 mg/h.
- Hydralazine and enalaprilat: May be considered based on the clinical scenario.
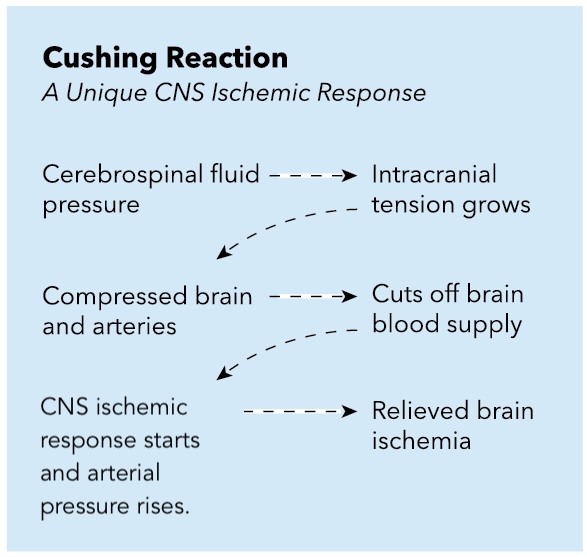
Hypotension and hypovolemia should be avoided, as cerebral perfusion pressure relies on maintaining an adequate MAP, especially when ICP increases due to an ischemic event (see Image. Cushing Reaction: Central Nervous System Ischemic Response).
Temperature
Hyperthermia (>38 °C) should be avoided and managed appropriately using antipyretics such as acetaminophen. Common sources of infection, including pneumonia and urinary tract infections, should be identified and treated. Currently, there is insufficient evidence to support the routine use of therapeutic hypothermia in acute ischemic stroke. A recent retrospective study found that a peak temperature exceeding 39 °C (102.2 °F) within the first 24 hours was associated with an increased risk of in-hospital mortality.
Glucose
Blood glucose should be maintained in the range of 140 to 180 mg/dL during the first 24 hours following an acute ischemic stroke. Levels below 60 mg/dL should be promptly treated to achieve normoglycemia. The brain relies on oxidative pathways that require glucose for metabolism, and its high metabolic demand makes it particularly vulnerable to hypoglycemia, which can impair the brain's ability to repair. However, hyperglycemia is thought to impair reperfusion by oxidizing nitric oxide–dependent pathways, leading to a loss of vascular tone. Additionally, increased acidosis may contribute, possibly from injury to lactic acid–sensing channels. Capes et al demonstrated that hyperglycemia in ischemic stroke patients increases 30-day mortality and independently raises the risk of hemorrhagic stroke conversion.[53](A1)
Nutrition
Early enteral feeding is recommended. For patients with dysphagia, a nasogastric tube should be used to facilitate enteral nutrition. If swallowing difficulties are expected to persist beyond 2 to 3 weeks, placement of a percutaneous gastrostomy tube is advised. Early feeding has been shown to significantly reduce the risk of death.[54](A1)
Deep vein thrombosis prophylaxis
Intermittent pneumatic compression is recommended for all immobile patients, unless contraindicated. The European Stroke Organization specifically recommends the use of intermittent pneumatic compression in the acute phase for immobile stroke patients. Additionally, low-dose heparin or low-molecular-weight heparin may be considered for deep vein thrombosis (DVT) prophylaxis when the potential benefits outweigh the risk of bleeding.[55][56](A1)
Depression screening
Screening for depression should be considered following an acute ischemic stroke, as post-stroke depression affects approximately 18% to 33% of patients. Identified risk factors include female sex, large infarcts, strokes involving the frontal lobes, and limited social support. Selective serotonin reuptake inhibitors (SSRIs) are the preferred pharmacologic treatment for post-stroke depression.[57][58](A1)
Cerebellar or cerebral edema
Cerebral edema following acute ischemic stroke initially results from cytotoxic edema due to cellular swelling, followed by vasogenic edema as the blood-brain barrier becomes compromised. The extent of edema generally correlates with the size of the infarct and is typically not clinically significant in lacunar strokes. However, in larger strokes, edema can become symptomatic, leading to worsening neurological deficits and impaired consciousness due to herniation. Cerebral edema typically peaks between 3 and 5 days after the onset of ischemic stroke.[59]
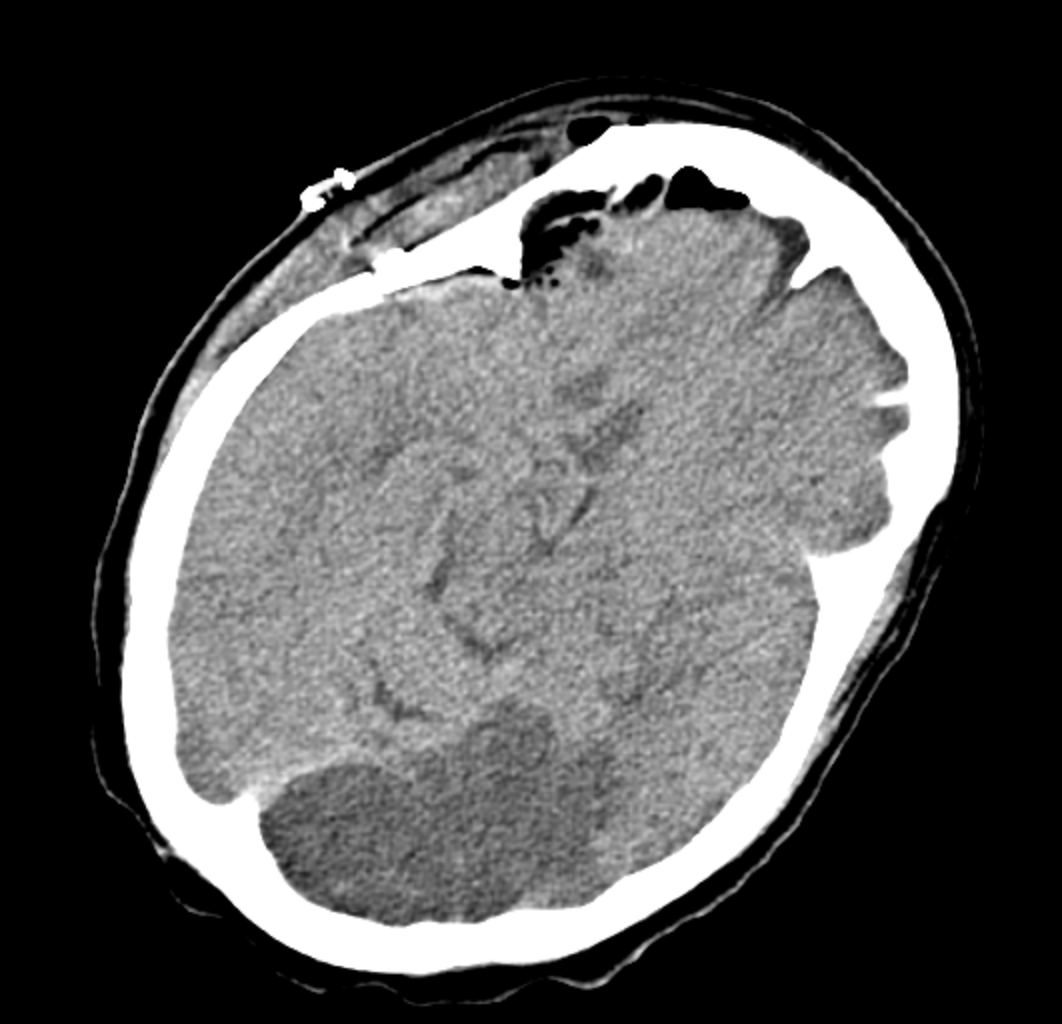
Cerebellar edema complicates cerebellar infarctions and can lead to rapid clinical deterioration. Increased ICP may cause obstructive hydrocephalus of the fourth ventricle or transtentorial herniation of the superior vermis and downward cerebellar tonsillar herniation. Signs include altered or worsening mental status, decreased consciousness, respiratory abnormalities, pupillary changes, posturing, and death.
Early recognition and diagnosis of intracranial hypertension due to cerebral edema are critical for improving outcomes in acute stroke patients. Prompt neurosurgical consultation is advised. Ventriculostomy is indicated for obstructive hydrocephalus following a cerebellar infarct, and decompressive suboccipital craniectomy is strongly recommended in cases of cerebellar edema with mass effect.[60][61]
Seizures
Post-stroke seizures occur in approximately 10% of patients, most commonly in those with hemorrhagic strokes or cortical infarcts. If a patient experiences a seizure within the first 2 weeks, antiepileptic drugs are indicated for a short period, generally 1 month. Long-term anticonvulsant therapy may be necessary for seizures occurring weeks or months after a stroke. However, routine prophylactic use of antiepileptic drugs is not recommended according to the latest AHA guidelines for hemorrhagic stroke management and the Emergency Neurological Life Support (ENLS) guidelines.[62][63]
Cardiac evaluation
Continuous cardiac monitoring is recommended during the first 24 hours to detect atrial fibrillation or other arrhythmias. The benefit of extended monitoring beyond this period remains uncertain. An initial troponin test is advised due to the established association between stroke and underlying coronary artery disease.
Antiplatelet treatment
Aspirin is recommended within 24 to 48 hours of symptom onset in patients with ischemic stroke. A Cochrane review found that initiating aspirin within 48 hours reduces the risk of recurrent ischemic stroke and improves long-term outcomes, without a significant increase in early intracranial hemorrhage risk.[64](A1)
Antithrombotic treatment
Full-dose anticoagulation is not recommended during the acute phase of ischemic stroke. The primary exception is the use of low-dose anticoagulation for DVT prophylaxis.
In patients with atrial fibrillation, guidelines recommend initiating oral anticoagulation within 4 to 14 days after neurological symptom onset. The timing of anticoagulation initiation is often challenging and depends on factors such as the size of the stroke and comorbidities. For small to moderate strokes, anticoagulation is typically started between 7 and 14 days.[65](B3)
In some patients with small hemorrhagic transformation following an acute stroke, delaying anticoagulation is warranted. This delay is not associated with an increased risk of stroke recurrence.[66](B3)
Statins
High-intensity statins (atorvastatin 80 mg daily or rosuvastatin 20 mg daily) are recommended for patients under 75 years old with clinical atherosclerotic cardiovascular disease. Patients already taking statins before their ischemic stroke may continue their therapy.[67] (A1)
Differential Diagnosis
The differential diagnosis of ischemic stroke includes:
- Complicated migraine
- Drug toxicity
- Intracranial abscess
- Intracranial hemorrhage
- Intracranial tumor
- Hyperglycemia
- Hypoglycemia
- Hypertensive encephalopathy
- Metabolic abnormalities
- Movement disorders
- Multiple sclerosis
- Seizure
- Sepsis
- Syncope
- Wernicke encephalopathy
Prognosis
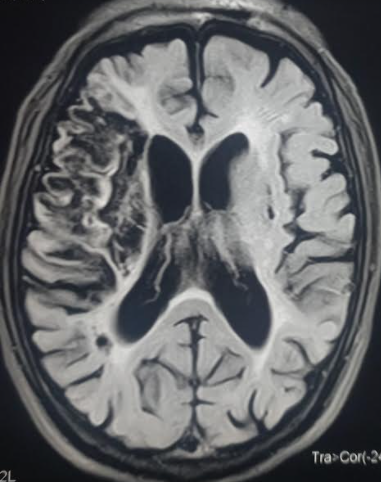
Prognosis in stroke plays a crucial role in guiding treatment decisions and counseling patients and caregivers about expected outcomes. This involves evaluating factors such as stroke type, severity, extent of neurological deficits, comorbid conditions, and treatment response. Predictive tools, including clinical scales and imaging studies, help estimate outcomes such as functional impairment, mortality, and risk of recurrence (see Image. Encephalomalacia Following Ischemic Stroke).
Early intervention and rehabilitation play a critical role in determining stroke prognosis, emphasizing the importance of timely medical attention and individualized care. While some individuals may fully recover, others may experience long-term disabilities or complications. Stroke prognosis highlights the importance of a multidisciplinary approach, ongoing monitoring, and comprehensive support to optimize outcomes and improve the quality of life for individuals affected by stroke and their families.[68][69]
Complications
The complications of acute ischemic stroke are many and common.[70] These include, but are not limited to:
- Deep vein thrombosis and pulmonary embolism: DVT prophylaxis is recommended.
- Aspiration and pneumonia: A swallowing evaluation before feeding is always indicated and is a requirement for stroke center accreditation.
- Seizures
- Depression
- Cerebral edema and increased ICP
Postoperative and Rehabilitation Care
Early rehabilitation benefits stroke patients; however, initiating rehabilitation within the first 24 hours should be avoided. The AVERT trial, which randomized patients to receive very early rehabilitation (within 24 hours) versus usual stroke-unit care, found that very early mobilization was associated with less favorable outcomes based on the modified Rankin score.[71]
Deterrence and Patient Education
Deterrence and prevention strategies are essential in reducing the incidence and impact of ischemic stroke. Addressing modifiable risk factors—such as hypertension, diabetes, hyperlipidemia, and smoking—through lifestyle modifications and pharmacological interventions can significantly lower an individual’s risk of stroke. Additionally, increasing awareness of stroke warning signs and encouraging prompt medical evaluation for symptoms like sudden weakness, numbness, or difficulty speaking can expedite treatment and reduce neurological damage. Community-based education campaigns that promote healthy behaviors, regular physical activity, and balanced diets play a key role in stroke prevention. By addressing both individual lifestyle choices and broader societal factors, these comprehensive efforts can help reduce the burden of ischemic stroke and improve overall public health.
Pearls and Other Issues
Key clinical pearls that provide valuable insights into the management of ischemic stroke include:
- Maintain a low threshold for stroke evaluation, particularly in high-risk populations—remember: time is brain!
- Stroke symptoms vary depending on the specific area of the brain affected by ischemia.
- A noncontrast head CT is the initial imaging modality for evaluating acute stroke, primarily to exclude intracranial hemorrhage.
- tPA or TNK should be considered if a thrombotic cerebrovascular accident (CVA) is identified within 4.5 hours of symptom onset.
- When indicated, an early CT angiogram or MR angiogram with a perfusion study should be obtained to identify LVO and facilitate endovascular thrombectomy within 6 to 24 hours of symptom onset.
- Clinicians should be aware of the patient's presenting BP. Management strategies may involve either aggressive BP control or permissive hypertension, depending on the type of stroke and the indication for IV fibrinolytic therapy.
- An antiplatelet agent should be initiated within 24 hours of patient presentation.
- Clinicians should address additional risk factors, including hyperlipidemia, hyperglycemia, and cardiac arrhythmias, which may elevate the risk of vascular disease or thrombotic events.
- Early and aggressive physical and occupational therapy should be considered following the onset of CVA.
Enhancing Healthcare Team Outcomes
Effective management of ischemic stroke requires a cohesive, interprofessional healthcare team committed to delivering patient-centered care, improving outcomes, and maximizing safety and team performance. The healthcare team comprises physicians, advanced practice providers, nurses, pharmacists, neuroimaging technicians, rehabilitation therapists, and stroke specialists who work collaboratively.
Prompt recognition and treatment of ischemic stroke are essential. Emergency medicine providers must be skilled at identifying early signs of stroke to initiate immediate care. Neuroimaging technicians and radiologists play a crucial role in rapidly obtaining and interpreting CT and MRI scans to confirm diagnoses. Neurointerventionalists may be required to perform procedures such as thrombectomy. Pharmacists ensure the timely administration of thrombolytic agents when indicated and help manage medications to prevent complications and recurrent strokes. Nurses provide continuous monitoring and implement care protocols, while rehabilitation therapists support recovery beginning in the acute phase and continuing through long-term rehabilitation.
The prognosis for patients treated with tPA is generally favorable; however, outcomes are more guarded for those who do not receive thrombolytic therapy.[72] Effective communication among all healthcare team members is crucial for prompt diagnosis, informed decision-making, and the implementation of effective treatment plans. This collaborative approach supports comprehensive care, spanning acute intervention, rehabilitation, and secondary prevention. All clinical decisions should be guided by ethical principles, including informed consent and respect for patient autonomy. Shared decision-making is prioritized, ensuring that patient preferences are honored while upholding the principles of beneficence and non-maleficence.
An interprofessional healthcare team approach is essential for a timely and effective response, minimizing complications, and prioritizing patient safety and quality of care in managing ischemic stroke. Continuous education and professional development ensure that the healthcare team stays current with the latest evidence-based practices, from acute management to stroke recurrence prevention. Through dedicated collaboration, the healthcare team delivers patient-centered care across the continuum—from emergency treatment to rehabilitation—ultimately improving outcomes and enhancing the quality of life for affected patients.
Media
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
References
Sacco RL, Kasner SE, Broderick JP, Caplan LR, Connors JJ, Culebras A, Elkind MS, George MG, Hamdan AD, Higashida RT, Hoh BL, Janis LS, Kase CS, Kleindorfer DO, Lee JM, Moseley ME, Peterson ED, Turan TN, Valderrama AL, Vinters HV, American Heart Association Stroke Council, Council on Cardiovascular Surgery and Anesthesia, Council on Cardiovascular Radiology and Intervention, Council on Cardiovascular and Stroke Nursing, Council on Epidemiology and Prevention, Council on Peripheral Vascular Disease, Council on Nutrition, Physical Activity and Metabolism. An updated definition of stroke for the 21st century: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2013 Jul:44(7):2064-89. doi: 10.1161/STR.0b013e318296aeca. Epub 2013 May 7 [PubMed PMID: 23652265]
Adams HP Jr, Bendixen BH, Kappelle LJ, Biller J, Love BB, Gordon DL, Marsh EE 3rd. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke. 1993 Jan:24(1):35-41 [PubMed PMID: 7678184]
Level 1 (high-level) evidenceNtaios G. Embolic Stroke of Undetermined Source: JACC Review Topic of the Week. Journal of the American College of Cardiology. 2020 Jan 28:75(3):333-340. doi: 10.1016/j.jacc.2019.11.024. Epub [PubMed PMID: 31976872]
Pierik R, Algra A, van Dijk E, Erasmus ME, van Gelder IC, Koudstaal PJ, Luijckx GR, Nederkoorn PJ, van Oostenbrugge RJ, Ruigrok YM, Scheeren TWL, Uyttenboogaart M, Visser MC, Wermer MJH, van den Bergh WM, on behalf of the Parelsnoer Institute-Cerebrovascular Accident Study Group. Distribution of Cardioembolic Stroke: A Cohort Study. Cerebrovascular diseases (Basel, Switzerland). 2020:49(1):97-104. doi: 10.1159/000505616. Epub 2020 Jan 21 [PubMed PMID: 31962331]
Spence JD. Cardioembolic stroke: everything has changed. Stroke and vascular neurology. 2018 Jun:3(2):76-83. doi: 10.1136/svn-2018-000143. Epub 2018 Mar 9 [PubMed PMID: 30022801]
Cole JW. Large Artery Atherosclerotic Occlusive Disease. Continuum (Minneapolis, Minn.). 2017 Feb:23(1, Cerebrovascular Disease):133-157. doi: 10.1212/CON.0000000000000436. Epub [PubMed PMID: 28157748]
Li Q, Yang Y, Reis C, Tao T, Li W, Li X, Zhang JH. Cerebral Small Vessel Disease. Cell transplantation. 2018 Dec:27(12):1711-1722. doi: 10.1177/0963689718795148. Epub 2018 Sep 25 [PubMed PMID: 30251566]
Kamel H, Merkler AE, Iadecola C, Gupta A, Navi BB. Tailoring the Approach to Embolic Stroke of Undetermined Source: A Review. JAMA neurology. 2019 Jul 1:76(7):855-861. doi: 10.1001/jamaneurol.2019.0591. Epub [PubMed PMID: 30958521]
Kim H, Kim JT, Lee JS, Kim BJ, Kang J, Lee KJ, Park JM, Kang K, Lee SJ, Kim JG, Cha JK, Kim DH, Park TH, Lee KB, Lee J, Hong KS, Cho YJ, Park HK, Lee BC, Yu KH, Oh MS, Kim DE, Ryu WS, Choi JC, Kwon JH, Kim WJ, Shin DI, Yum KS, Sohn SI, Hong JH, Lee SH, Park MS, Choi KH, Lee J, Bae HJ. Stroke of Other Determined Etiology: Results From the Nationwide Multicenter Stroke Registry. Stroke. 2022 Aug:53(8):2597-2606. doi: 10.1161/STROKEAHA.121.037582. Epub 2022 May 9 [PubMed PMID: 35531778]
Tsao CW, Aday AW, Almarzooq ZI, Anderson CAM, Arora P, Avery CL, Baker-Smith CM, Beaton AZ, Boehme AK, Buxton AE, Commodore-Mensah Y, Elkind MSV, Evenson KR, Eze-Nliam C, Fugar S, Generoso G, Heard DG, Hiremath S, Ho JE, Kalani R, Kazi DS, Ko D, Levine DA, Liu J, Ma J, Magnani JW, Michos ED, Mussolino ME, Navaneethan SD, Parikh NI, Poudel R, Rezk-Hanna M, Roth GA, Shah NS, St-Onge MP, Thacker EL, Virani SS, Voeks JH, Wang NY, Wong ND, Wong SS, Yaffe K, Martin SS, American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee. Heart Disease and Stroke Statistics-2023 Update: A Report From the American Heart Association. Circulation. 2023 Feb 21:147(8):e93-e621. doi: 10.1161/CIR.0000000000001123. Epub 2023 Jan 25 [PubMed PMID: 36695182]
Writing Group Members, Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, Das SR, de Ferranti S, Després JP, Fullerton HJ, Howard VJ, Huffman MD, Isasi CR, Jiménez MC, Judd SE, Kissela BM, Lichtman JH, Lisabeth LD, Liu S, Mackey RH, Magid DJ, McGuire DK, Mohler ER 3rd, Moy CS, Muntner P, Mussolino ME, Nasir K, Neumar RW, Nichol G, Palaniappan L, Pandey DK, Reeves MJ, Rodriguez CJ, Rosamond W, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Woo D, Yeh RW, Turner MB, American Heart Association Statistics Committee, Stroke Statistics Subcommittee. Executive Summary: Heart Disease and Stroke Statistics--2016 Update: A Report From the American Heart Association. Circulation. 2016 Jan 26:133(4):447-54. doi: 10.1161/CIR.0000000000000366. Epub [PubMed PMID: 26811276]
Benjamin EJ, Blaha MJ, Chiuve SE, Cushman M, Das SR, Deo R, de Ferranti SD, Floyd J, Fornage M, Gillespie C, Isasi CR, Jiménez MC, Jordan LC, Judd SE, Lackland D, Lichtman JH, Lisabeth L, Liu S, Longenecker CT, Mackey RH, Matsushita K, Mozaffarian D, Mussolino ME, Nasir K, Neumar RW, Palaniappan L, Pandey DK, Thiagarajan RR, Reeves MJ, Ritchey M, Rodriguez CJ, Roth GA, Rosamond WD, Sasson C, Towfighi A, Tsao CW, Turner MB, Virani SS, Voeks JH, Willey JZ, Wilkins JT, Wu JH, Alger HM, Wong SS, Muntner P, American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart Disease and Stroke Statistics-2017 Update: A Report From the American Heart Association. Circulation. 2017 Mar 7:135(10):e146-e603. doi: 10.1161/CIR.0000000000000485. Epub 2017 Jan 25 [PubMed PMID: 28122885]
White H, Boden-Albala B, Wang C, Elkind MS, Rundek T, Wright CB, Sacco RL. Ischemic stroke subtype incidence among whites, blacks, and Hispanics: the Northern Manhattan Study. Circulation. 2005 Mar 15:111(10):1327-31 [PubMed PMID: 15769776]
Level 2 (mid-level) evidenceJackson G, Chari K. National Hospital Care Survey Demonstration Projects: Stroke Inpatient Hospitalizations. National health statistics reports. 2019 Nov:(132):1-11 [PubMed PMID: 32510306]
Level 3 (low-level) evidenceJaiswal V, Hanif M, Ang SP, Suresh V, Ruchika F, Momi NK, Naz S, Rajak K, Halder A, Kumar T, Naz H, Alvarez VHA. The Racial Disparity Among the Clinical Outcomes Post Stroke and its Intervention Outcomes: A Systematic Review and Meta-analysis. Current problems in cardiology. 2023 Sep:48(9):101753. doi: 10.1016/j.cpcardiol.2023.101753. Epub 2023 Apr 21 [PubMed PMID: 37088178]
Level 1 (high-level) evidenceMarkus HS, de Leeuw FE. Cerebral small vessel disease: Recent advances and future directions. International journal of stroke : official journal of the International Stroke Society. 2023 Jan:18(1):4-14. doi: 10.1177/17474930221144911. Epub [PubMed PMID: 36575578]
Level 3 (low-level) evidenceMarkus HS. Cerebral perfusion and stroke. Journal of neurology, neurosurgery, and psychiatry. 2004 Mar:75(3):353-61 [PubMed PMID: 14966145]
Atkins ER, Brodie FG, Rafelt SE, Panerai RB, Robinson TG. Dynamic cerebral autoregulation is compromised acutely following mild ischaemic stroke but not transient ischaemic attack. Cerebrovascular diseases (Basel, Switzerland). 2010 Feb:29(3):228-35. doi: 10.1159/000267845. Epub 2009 Dec 18 [PubMed PMID: 20029195]
Aries MJ, Elting JW, De Keyser J, Kremer BP, Vroomen PC. Cerebral autoregulation in stroke: a review of transcranial Doppler studies. Stroke. 2010 Nov:41(11):2697-704. doi: 10.1161/STROKEAHA.110.594168. Epub 2010 Oct 7 [PubMed PMID: 20930158]
Chalet L, Boutelier T, Christen T, Raguenes D, Debatisse J, Eker OF, Becker G, Nighoghossian N, Cho TH, Canet-Soulas E, Mechtouff L. Clinical Imaging of the Penumbra in Ischemic Stroke: From the Concept to the Era of Mechanical Thrombectomy. Frontiers in cardiovascular medicine. 2022:9():861913. doi: 10.3389/fcvm.2022.861913. Epub 2022 Mar 9 [PubMed PMID: 35355966]
Desowska A, Turner DL. Dynamics of brain connectivity after stroke. Reviews in the neurosciences. 2019 Jul 26:30(6):605-623. doi: 10.1515/revneuro-2018-0082. Epub [PubMed PMID: 30768425]
Liu S, Levine SR, Winn HR. Targeting ischemic penumbra: part I - from pathophysiology to therapeutic strategy. Journal of experimental stroke & translational medicine. 2010 Mar 15:3(1):47-55 [PubMed PMID: 20607107]
Astrup J, Siesjö BK, Symon L. Thresholds in cerebral ischemia - the ischemic penumbra. Stroke. 1981 Nov-Dec:12(6):723-5 [PubMed PMID: 6272455]
Brandt T, Steinke W, Thie A, Pessin MS, Caplan LR. Posterior cerebral artery territory infarcts: clinical features, infarct topography, causes and outcome. Multicenter results and a review of the literature. Cerebrovascular diseases (Basel, Switzerland). 2000 May-Jun:10(3):170-82 [PubMed PMID: 10773642]
Cereda C, Carrera E. Posterior cerebral artery territory infarctions. Frontiers of neurology and neuroscience. 2012:30():128-31. doi: 10.1159/000333610. Epub 2012 Feb 14 [PubMed PMID: 22377879]
Nouh A, Remke J, Ruland S. Ischemic posterior circulation stroke: a review of anatomy, clinical presentations, diagnosis, and current management. Frontiers in neurology. 2014:5():30. doi: 10.3389/fneur.2014.00030. Epub 2014 Apr 7 [PubMed PMID: 24778625]
Carvalho V, Cruz VT. Clinical presentation of vertebrobasilar stroke. Porto biomedical journal. 2020 Nov-Dec:5(6):e096. doi: 10.1097/j.pbj.0000000000000096. Epub 2020 Nov 24 [PubMed PMID: 33283066]
Jensen MB, St Louis EK. Management of acute cerebellar stroke. Archives of neurology. 2005 Apr:62(4):537-44 [PubMed PMID: 15824250]
Level 3 (low-level) evidenceAldrich MS, Alessi AG, Beck RW, Gilman S. Cortical blindness: etiology, diagnosis, and prognosis. Annals of neurology. 1987 Feb:21(2):149-58 [PubMed PMID: 3827223]
Regenhardt RW, Das AS, Lo EH, Caplan LR. Advances in Understanding the Pathophysiology of Lacunar Stroke: A Review. JAMA neurology. 2018 Oct 1:75(10):1273-1281. doi: 10.1001/jamaneurol.2018.1073. Epub [PubMed PMID: 30167649]
Level 3 (low-level) evidenceWardlaw JM. What causes lacunar stroke? Journal of neurology, neurosurgery, and psychiatry. 2005 May:76(5):617-9 [PubMed PMID: 15834013]
Bamford JM, Warlow CP. Evolution and testing of the lacunar hypothesis. Stroke. 1988 Sep:19(9):1074-82 [PubMed PMID: 3046071]
Goldstein LB, Simel DL. Is this patient having a stroke? JAMA. 2005 May 18:293(19):2391-402 [PubMed PMID: 15900010]
Level 1 (high-level) evidencePowers WJ, Rabinstein AA, Ackerson T, Adeoye OM, Bambakidis NC, Becker K, Biller J, Brown M, Demaerschalk BM, Hoh B, Jauch EC, Kidwell CS, Leslie-Mazwi TM, Ovbiagele B, Scott PA, Sheth KN, Southerland AM, Summers DV, Tirschwell DL. Guidelines for the Early Management of Patients With Acute Ischemic Stroke: 2019 Update to the 2018 Guidelines for the Early Management of Acute Ischemic Stroke: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke. 2019 Dec:50(12):e344-e418. doi: 10.1161/STR.0000000000000211. Epub 2019 Oct 30 [PubMed PMID: 31662037]
Kothari R, Hall K, Brott T, Broderick J. Early stroke recognition: developing an out-of-hospital NIH Stroke Scale. Academic emergency medicine : official journal of the Society for Academic Emergency Medicine. 1997 Oct:4(10):986-90 [PubMed PMID: 9332632]
Thomalla G, Simonsen CZ, Boutitie F, Andersen G, Berthezene Y, Cheng B, Cheripelli B, Cho TH, Fazekas F, Fiehler J, Ford I, Galinovic I, Gellissen S, Golsari A, Gregori J, Günther M, Guibernau J, Häusler KG, Hennerici M, Kemmling A, Marstrand J, Modrau B, Neeb L, Perez de la Ossa N, Puig J, Ringleb P, Roy P, Scheel E, Schonewille W, Serena J, Sunaert S, Villringer K, Wouters A, Thijs V, Ebinger M, Endres M, Fiebach JB, Lemmens R, Muir KW, Nighoghossian N, Pedraza S, Gerloff C, WAKE-UP Investigators. MRI-Guided Thrombolysis for Stroke with Unknown Time of Onset. The New England journal of medicine. 2018 Aug 16:379(7):611-622. doi: 10.1056/NEJMoa1804355. Epub 2018 May 16 [PubMed PMID: 29766770]
Cassella CR, Jagoda A. Ischemic Stroke: Advances in Diagnosis and Management. Emergency medicine clinics of North America. 2017 Nov:35(4):911-930. doi: 10.1016/j.emc.2017.07.007. Epub [PubMed PMID: 28987436]
Level 3 (low-level) evidenceDemaerschalk BM, Bobrow BJ, Raman R, Ernstrom K, Hoxworth JM, Patel AC, Kiernan TE, Aguilar MI, Ingall TJ, Dodick DW, Meyer BC, Stroke Team Remote Evaluation Using a Digital Observation Camera (STRokE DOC) in Arizona—The Initial Mayo Clinic Experience (AZ TIME) Investigators. CT interpretation in a telestroke network: agreement among a spoke radiologist, hub vascular neurologist, and hub neuroradiologist. Stroke. 2012 Nov:43(11):3095-7. doi: 10.1161/STROKEAHA.112.666255. Epub 2012 Sep 13 [PubMed PMID: 22984007]
Level 1 (high-level) evidenceJohnston KC, Worrall BB, Teleradiology Assessment of Computerized Tomographs Online Reliability Study. Teleradiology Assessment of Computerized Tomographs Online Reliability Study (TRACTORS) for acute stroke evaluation. Telemedicine journal and e-health : the official journal of the American Telemedicine Association. 2003 Fall:9(3):227-33 [PubMed PMID: 14611689]
Level 3 (low-level) evidenceHoh BL, Ko NU, Amin-Hanjani S, Chou SH-Y, Cruz-Flores S, Dangayach NS, Derdeyn CP, Du R, Hänggi D, Hetts SW, Ifejika NL, Johnson R, Keigher KM, Leslie-Mazwi TM, Lucke-Wold B, Rabinstein AA, Robicsek SA, Stapleton CJ, Suarez JI, Tjoumakaris SI, Welch BG. 2023 Guideline for the Management of Patients With Aneurysmal Subarachnoid Hemorrhage: A Guideline From the American Heart Association/American Stroke Association. Stroke. 2023 Jul:54(7):e314-e370. doi: 10.1161/STR.0000000000000436. Epub 2023 May 22 [PubMed PMID: 37212182]
Zhang J, Ta N, Fu M, Tian FH, Wang J, Zhang T, Wang B. Use of DWI-FLAIR Mismatch to Estimate the Onset Time in Wake-Up Strokes. Neuropsychiatric disease and treatment. 2022:18():355-361. doi: 10.2147/NDT.S351943. Epub 2022 Feb 21 [PubMed PMID: 35228801]
Kvistad CE, Næss H, Helleberg BH, Idicula T, Hagberg G, Nordby LM, Jenssen KN, Tobro H, Rörholt DM, Kaur K, Eltoft A, Evensen K, Haasz J, Singaravel G, Fromm A, Thomassen L. Tenecteplase versus alteplase for the management of acute ischaemic stroke in Norway (NOR-TEST 2, part A): a phase 3, randomised, open-label, blinded endpoint, non-inferiority trial. The Lancet. Neurology. 2022 Jun:21(6):511-519. doi: 10.1016/S1474-4422(22)00124-7. Epub 2022 May 4 [PubMed PMID: 35525250]
Level 1 (high-level) evidenceRehman AU, Mohsin A, Cheema HA, Zahid A, Ebaad Ur Rehman M, Ameer MZ, Ayyan M, Ehsan M, Shahid A, Aemaz Ur Rehman M, Shah J, Khawaja A. Comparative efficacy and safety of tenecteplase and alteplase in acute ischemic stroke: A pairwise and network meta-analysis of randomized controlled trials. Journal of the neurological sciences. 2023 Feb 15:445():120537. doi: 10.1016/j.jns.2022.120537. Epub 2022 Dec 29 [PubMed PMID: 36630803]
Level 1 (high-level) evidenceWidimsky P, Snyder K, Sulzenko J, Hopkins LN, Stetkarova I. Acute ischaemic stroke: recent advances in reperfusion treatment. European heart journal. 2023 Apr 7:44(14):1205-1215. doi: 10.1093/eurheartj/ehac684. Epub [PubMed PMID: 36477996]
Level 3 (low-level) evidenceNogueira RG, Jadhav AP, Haussen DC, Bonafe A, Budzik RF, Bhuva P, Yavagal DR, Ribo M, Cognard C, Hanel RA, Sila CA, Hassan AE, Millan M, Levy EI, Mitchell P, Chen M, English JD, Shah QA, Silver FL, Pereira VM, Mehta BP, Baxter BW, Abraham MG, Cardona P, Veznedaroglu E, Hellinger FR, Feng L, Kirmani JF, Lopes DK, Jankowitz BT, Frankel MR, Costalat V, Vora NA, Yoo AJ, Malik AM, Furlan AJ, Rubiera M, Aghaebrahim A, Olivot JM, Tekle WG, Shields R, Graves T, Lewis RJ, Smith WS, Liebeskind DS, Saver JL, Jovin TG, DAWN Trial Investigators. Thrombectomy 6 to 24 Hours after Stroke with a Mismatch between Deficit and Infarct. The New England journal of medicine. 2018 Jan 4:378(1):11-21. doi: 10.1056/NEJMoa1706442. Epub 2017 Nov 11 [PubMed PMID: 29129157]
Albers GW, Marks MP, Kemp S, Christensen S, Tsai JP, Ortega-Gutierrez S, McTaggart RA, Torbey MT, Kim-Tenser M, Leslie-Mazwi T, Sarraj A, Kasner SE, Ansari SA, Yeatts SD, Hamilton S, Mlynash M, Heit JJ, Zaharchuk G, Kim S, Carrozzella J, Palesch YY, Demchuk AM, Bammer R, Lavori PW, Broderick JP, Lansberg MG, DEFUSE 3 Investigators. Thrombectomy for Stroke at 6 to 16 Hours with Selection by Perfusion Imaging. The New England journal of medicine. 2018 Feb 22:378(8):708-718. doi: 10.1056/NEJMoa1713973. Epub 2018 Jan 24 [PubMed PMID: 29364767]
Huo X, Ma G, Tong X, Zhang X, Pan Y, Nguyen TN, Yuan G, Han H, Chen W, Wei M, Zhang J, Zhou Z, Yao X, Wang G, Song W, Cai X, Nan G, Li D, Wang AY, Ling W, Cai C, Wen C, Wang E, Zhang L, Jiang C, Liu Y, Liao G, Chen X, Li T, Liu S, Li J, Gao F, Ma N, Mo D, Song L, Sun X, Li X, Deng Y, Luo G, Lv M, He H, Liu A, Zhang J, Mu S, Liu L, Jing J, Nie X, Ding Z, Du W, Zhao X, Yang P, Liu L, Wang Y, Liebeskind DS, Pereira VM, Ren Z, Wang Y, Miao Z, ANGEL-ASPECT Investigators. Trial of Endovascular Therapy for Acute Ischemic Stroke with Large Infarct. The New England journal of medicine. 2023 Apr 6:388(14):1272-1283. doi: 10.1056/NEJMoa2213379. Epub 2023 Feb 10 [PubMed PMID: 36762852]
Sarraj A, Hassan AE, Abraham MG, Ortega-Gutierrez S, Kasner SE, Hussain MS, Chen M, Blackburn S, Sitton CW, Churilov L, Sundararajan S, Hu YC, Herial NA, Jabbour P, Gibson D, Wallace AN, Arenillas JF, Tsai JP, Budzik RF, Hicks WJ, Kozak O, Yan B, Cordato DJ, Manning NW, Parsons MW, Hanel RA, Aghaebrahim AN, Wu TY, Cardona-Portela P, Pérez de la Ossa N, Schaafsma JD, Blasco J, Sangha N, Warach S, Gandhi CD, Kleinig TJ, Sahlein D, Elijovich L, Tekle W, Samaniego EA, Maali L, Abdulrazzak MA, Psychogios MN, Shuaib A, Pujara DK, Shaker F, Johns H, Sharma G, Yogendrakumar V, Ng FC, Rahbar MH, Cai C, Lavori P, Hamilton S, Nguyen T, Fifi JT, Davis S, Wechsler L, Pereira VM, Lansberg MG, Hill MD, Grotta JC, Ribo M, Campbell BC, Albers GW, SELECT2 Investigators. Trial of Endovascular Thrombectomy for Large Ischemic Strokes. The New England journal of medicine. 2023 Apr 6:388(14):1259-1271. doi: 10.1056/NEJMoa2214403. Epub 2023 Feb 10 [PubMed PMID: 36762865]
Nicholls JK, Ince J, Minhas JS, Chung EML. Emerging Detection Techniques for Large Vessel Occlusion Stroke: A Scoping Review. Frontiers in neurology. 2021:12():780324. doi: 10.3389/fneur.2021.780324. Epub 2022 Jan 6 [PubMed PMID: 35095726]
Level 2 (mid-level) evidencePop NO, Tit DM, Diaconu CC, Munteanu MA, Babes EE, Stoicescu M, Popescu MI, Bungau S. The Alberta Stroke Program Early CT score (ASPECTS): A predictor of mortality in acute ischemic stroke. Experimental and therapeutic medicine. 2021 Dec:22(6):1371. doi: 10.3892/etm.2021.10805. Epub 2021 Sep 27 [PubMed PMID: 34659517]
Tao C, Nogueira RG, Zhu Y, Sun J, Han H, Yuan G, Wen C, Zhou P, Chen W, Zeng G, Li Y, Ma Z, Yu C, Su J, Zhou Z, Chen Z, Liao G, Sun Y, Ren Y, Zhang H, Chen J, Yue X, Xiao G, Wang L, Liu R, Liu W, Liu Y, Wang L, Zhang C, Liu T, Song J, Li R, Xu P, Yin Y, Wang G, Baxter B, Qureshi AI, Liu X, Hu W, ATTENTION Investigators. Trial of Endovascular Treatment of Acute Basilar-Artery Occlusion. The New England journal of medicine. 2022 Oct 13:387(15):1361-1372. doi: 10.1056/NEJMoa2206317. Epub [PubMed PMID: 36239644]
Jovin TG, Li C, Wu L, Wu C, Chen J, Jiang C, Shi Z, Gao Z, Song C, Chen W, Peng Y, Yao C, Wei M, Li T, Wei L, Xiao G, Yang H, Ren M, Duan J, Liu X, Yang Q, Liu Y, Zhu Q, Shi W, Zhu Q, Li X, Guo Z, Yang Q, Hou C, Zhao W, Ma Q, Zhang Y, Jiao L, Zhang H, Liebeskind DS, Liang H, Jadhav AP, Wen C, Brown S, Zhu L, Ye H, Ribo M, Chang M, Song H, Chen J, Ji X, BAOCHE Investigators. Trial of Thrombectomy 6 to 24 Hours after Stroke Due to Basilar-Artery Occlusion. The New England journal of medicine. 2022 Oct 13:387(15):1373-1384. doi: 10.1056/NEJMoa2207576. Epub [PubMed PMID: 36239645]
Capes SE, Hunt D, Malmberg K, Pathak P, Gerstein HC. Stress hyperglycemia and prognosis of stroke in nondiabetic and diabetic patients: a systematic overview. Stroke. 2001 Oct:32(10):2426-32 [PubMed PMID: 11588337]
Level 1 (high-level) evidenceDennis M, Lewis S, Cranswick G, Forbes J, FOOD Trial Collaboration. FOOD: a multicentre randomised trial evaluating feeding policies in patients admitted to hospital with a recent stroke. Health technology assessment (Winchester, England). 2006 Jan:10(2):iii-iv, ix-x, 1-120 [PubMed PMID: 16409880]
Level 1 (high-level) evidenceAVERT Trial Collaboration group. Efficacy and safety of very early mobilisation within 24 h of stroke onset (AVERT): a randomised controlled trial. Lancet (London, England). 2015 Jul 4:386(9988):46-55. doi: 10.1016/S0140-6736(15)60690-0. Epub 2015 Apr 16 [PubMed PMID: 25892679]
Level 1 (high-level) evidenceDennis M, Caso V, Kappelle LJ, Pavlovic A, Sandercock P, European Stroke Organisation. European Stroke Organisation (ESO) guidelines for prophylaxis for venous thromboembolism in immobile patients with acute ischaemic stroke. European stroke journal. 2016 Mar:1(1):6-19. doi: 10.1177/2396987316628384. Epub 2016 Mar 1 [PubMed PMID: 31008263]
Medeiros GC, Roy D, Kontos N, Beach SR. Post-stroke depression: A 2020 updated review. General hospital psychiatry. 2020 Sep-Oct:66():70-80. doi: 10.1016/j.genhosppsych.2020.06.011. Epub 2020 Jun 27 [PubMed PMID: 32717644]
Legg LA, Rudberg AS, Hua X, Wu S, Hackett ML, Tilney R, Lindgren L, Kutlubaev MA, Hsieh CF, Barugh AJ, Hankey GJ, Lundström E, Dennis M, Mead GE. Selective serotonin reuptake inhibitors (SSRIs) for stroke recovery. The Cochrane database of systematic reviews. 2021 Nov 15:11(11):CD009286. doi: 10.1002/14651858.CD009286.pub4. Epub 2021 Nov 15 [PubMed PMID: 34780067]
Level 1 (high-level) evidenceDostovic Z, Dostovic E, Smajlovic D, Ibrahimagic OC, Avdic L. Brain Edema After Ischaemic Stroke. Medical archives (Sarajevo, Bosnia and Herzegovina). 2016 Oct:70(5):339-341 [PubMed PMID: 27994292]
Neugebauer H, Witsch J, Zweckberger K, Jüttler E. Space-occupying cerebellar infarction: complications, treatment, and outcome. Neurosurgical focus. 2013 May:34(5):E8. doi: 10.3171/2013.2.FOCUS12363. Epub [PubMed PMID: 23634927]
Wijdicks EF, Sheth KN, Carter BS, Greer DM, Kasner SE, Kimberly WT, Schwab S, Smith EE, Tamargo RJ, Wintermark M, American Heart Association Stroke Council. Recommendations for the management of cerebral and cerebellar infarction with swelling: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2014 Apr:45(4):1222-38. doi: 10.1161/01.str.0000441965.15164.d6. Epub 2014 Jan 30 [PubMed PMID: 24481970]
Silverman IE, Restrepo L, Mathews GC. Poststroke seizures. Archives of neurology. 2002 Feb:59(2):195-201 [PubMed PMID: 11843689]
Xu MY. Poststroke seizure: optimising its management. Stroke and vascular neurology. 2019 Mar:4(1):48-56. doi: 10.1136/svn-2018-000175. Epub 2018 Dec 9 [PubMed PMID: 31105979]
Sandercock PA, Counsell C, Tseng MC, Cecconi E. Oral antiplatelet therapy for acute ischaemic stroke. The Cochrane database of systematic reviews. 2014 Mar 26:2014(3):CD000029. doi: 10.1002/14651858.CD000029.pub3. Epub 2014 Mar 26 [PubMed PMID: 24668137]
Level 1 (high-level) evidencePaciaroni M, Agnelli G, Falocci N, Tsivgoulis G, Vadikolias K, Liantinioti C, Chondrogianni M, Bovi P, Carletti M, Cappellari M, Zedde M, Ntaios G, Karagkiozi E, Athanasakis G, Makaritsis K, Silvestrelli G, Lanari A, Ciccone A, Putaala J, Tomppo L, Tatlisumak T, Abdul-Rahim AH, Lees KR, Alberti A, Venti M, Acciarresi M, D'Amore C, Becattini C, Mosconi MG, Cimini LA, Soloperto R, Masotti L, Vannucchi V, Lorenzini G, Tassi R, Guideri F, Acampa M, Martini G, Sohn SI, Marcheselli S, Mumoli N, De Lodovici ML, Bono G, Furie KL, Tadi P, Yaghi S, Toni D, Letteri F, Tassinari T, Kargiotis O, Lotti EM, Flomin Y, Mancuso M, Maccarrone M, Giannini N, Bandini F, Pezzini A, Poli L, Padovani A, Scoditti U, Denti L, Consoli D, Galati F, Sacco S, Carolei A, Tiseo C, Gourbali V, Orlandi G, Giuntini M, Chiti A, Giorli E, Gialdini G, Corea F, Ageno W, Bellesini M, Colombo G, Monaco S, Maimone Baronello M, Karapanayiotides T, Caso V. Early Recurrence and Major Bleeding in Patients With Acute Ischemic Stroke and Atrial Fibrillation Treated With Non-Vitamin-K Oral Anticoagulants (RAF-NOACs) Study. Journal of the American Heart Association. 2017 Nov 29:6(12):. doi: 10.1161/JAHA.117.007034. Epub 2017 Nov 29 [PubMed PMID: 29220330]
Level 3 (low-level) evidencePaciaroni M, Bandini F, Agnelli G, Tsivgoulis G, Yaghi S, Furie KL, Tadi P, Becattini C, Zedde M, Abdul-Rahim AH, Lees KR, Alberti A, Venti M, Acciarresi M, D'Amore C, Mosconi MG, Cimini LA, Altavilla R, Volpi G, Bovi P, Carletti M, Rigatelli A, Cappellari M, Putaala J, Tomppo L, Tatlisumak T, Marcheselli S, Pezzini A, Poli L, Padovani A, Masotti L, Vannucchi V, Sohn SI, Lorenzini G, Tassi R, Guideri F, Acampa M, Martini G, Ntaios G, Athanasakis G, Makaritsis K, Karagkiozi E, Vadikolias K, Liantinioti C, Chondrogianni M, Mumoli N, Consoli D, Galati F, Sacco S, Carolei A, Tiseo C, Corea F, Ageno W, Bellesini M, Colombo G, Silvestrelli G, Ciccone A, Lanari A, Scoditti U, Denti L, Mancuso M, Maccarrone M, Ulivi L, Orlandi G, Giannini N, Gialdini G, Tassinari T, De Lodovici ML, Bono G, Rueckert C, Baldi A, D'Anna S, Toni D, Letteri F, Giuntini M, Lotti EM, Flomin Y, Pieroni A, Kargiotis O, Karapanayiotides T, Monaco S, Maimone Baronello M, Csiba L, Szabó L, Chiti A, Giorli E, Del Sette M, Imberti D, Zabzuni D, Doronin B, Volodina V, Michel P, Vanacker P, Barlinn K, Pallesen LP, Barlinn J, Deleu D, Melikyan G, Ibrahim F, Akhtar N, Gourbali V, Caso V. Hemorrhagic Transformation in Patients With Acute Ischemic Stroke and Atrial Fibrillation: Time to Initiation of Oral Anticoagulant Therapy and Outcomes. Journal of the American Heart Association. 2018 Nov 20:7(22):e010133. doi: 10.1161/JAHA.118.010133. Epub [PubMed PMID: 30571487]
Level 3 (low-level) evidenceChou R, Cantor A, Dana T, Wagner J, Ahmed AY, Fu R, Ferencik M. Statin Use for the Primary Prevention of Cardiovascular Disease in Adults: Updated Evidence Report and Systematic Review for the US Preventive Services Task Force. JAMA. 2022 Aug 23:328(8):754-771. doi: 10.1001/jama.2022.12138. Epub [PubMed PMID: 35997724]
Level 1 (high-level) evidenceSeshadri S, Beiser A, Kelly-Hayes M, Kase CS, Au R, Kannel WB, Wolf PA. The lifetime risk of stroke: estimates from the Framingham Study. Stroke. 2006 Feb:37(2):345-50 [PubMed PMID: 16397184]
Level 2 (mid-level) evidenceCarandang R, Seshadri S, Beiser A, Kelly-Hayes M, Kase CS, Kannel WB, Wolf PA. Trends in incidence, lifetime risk, severity, and 30-day mortality of stroke over the past 50 years. JAMA. 2006 Dec 27:296(24):2939-46 [PubMed PMID: 17190894]
Level 2 (mid-level) evidenceChohan SA, Venkatesh PK, How CH. Long-term complications of stroke and secondary prevention: an overview for primary care physicians. Singapore medical journal. 2019 Dec:60(12):616-620. doi: 10.11622/smedj.2019158. Epub [PubMed PMID: 31889205]
Level 3 (low-level) evidenceConnell LA, Lincoln NB, Radford KA. Somatosensory impairment after stroke: frequency of different deficits and their recovery. Clinical rehabilitation. 2008 Aug:22(8):758-67. doi: 10.1177/0269215508090674. Epub [PubMed PMID: 18678576]
Puri I, Bhatia R, Vibha D, Singh MB, Padma MV, Aggarwal P, Prasad K. Stroke-related education to emergency department staff: An acute stroke care quality improvement initiative. Neurology India. 2019 Jan-Feb:67(1):129-133. doi: 10.4103/0028-3886.253636. Epub [PubMed PMID: 30860110]
Level 2 (mid-level) evidence