Anatomy, Shoulder and Upper Limb, Hand Intrinsic Muscles
Anatomy, Shoulder and Upper Limb, Hand Intrinsic Muscles
Introduction
The skeletal muscles of the hand enable movement of the hand and fingers. These muscles are categorized into extrinsic and intrinsic groups. The extrinsic muscle bellies originate in the forearm, whereas the intrinsic muscles are smaller and located entirely within the hand, extending from the wrist proximally to the phalanges distally. The intrinsic muscles are essential for fine motor control and contribute significantly to pinch and grip strength (see Image. Intrinsic Hand Muscles).[1]
Intrinsic muscle atrophy or weakness is an early clinical sign in conditions such as ulnar neuropathy, lower motor neuron lesions, and certain myopathies. Knowledge of intrinsic muscle anatomy is critical during procedures such as tendon transfers, nerve decompressions, and compartment releases to preserve hand function and optimize outcomes. Understanding the anatomy and physiology of the intrinsic hand muscles enables clinicians to localize neuromuscular pathology, assess functional deficits accurately, and guide targeted interventions.
Structure and Function
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Structure and Function
The intrinsic muscles of the hand are categorized by regional distribution and specialized function (see Image. Palmar View of the Hand Muscles, Tendons, and Fascia). The 4 groups include the thenar, hypothenar, interossei, and lumbrical muscles, explained below.
Thenar Muscles
The thenar muscle group, collectively referred to as the "thenar eminence," consists of 3 muscles located at the fleshy base of the thumb on the palmar aspect. These muscles, which include the abductor pollicis brevis (APB), flexor pollicis brevis (FPB), and opponens pollicis (OPP), are primarily responsible for thumb abduction, flexion, and opposition, respectively.
Although anatomically adjacent, the adductor pollicis is not part of the thenar eminence. This muscle functions independently to adduct the thumb.[2]
The APB abducts the 1st digit and originates from the flexor retinaculum and scaphoid tubercle, inserting on the lateral aspect of the proximal phalanx. The flexor pollicis brevis flexes the thumb and arises from the flexor retinaculum and trapezium tubercle, with insertion on the lateral aspect of the proximal phalanx. The opponens pollicis facilitates opposition, originating from the flexor retinaculum and trapezium and inserting along the lateral aspect of the thumb metacarpal. All 3 muscles receive innervation from the recurrent motor branch of the median nerve.
With origins at the 2nd and 3rd metacarpals and the capitate, the adductor pollicis inserts on the medial base of the thumb’s proximal phalanx and its extensor mechanism. This muscle receives motor input from the deep branch of the ulnar nerve.
Hypothenar Muscles
The hypothenar muscle group comprises 3 muscles situated at the fleshy base of the 5th digit on the palmar aspect. These muscles, which include the abductor digiti minimi (ADM), flexor digiti minimi brevis (FDMB), and opponens digiti minimi (ODM), coordinate movement of the little finger.
The ADM abducts the 5th digit. This muscle originates from the pisiform and inserts on the medial portion of the proximal phalanx of the 5th digit. The flexor digiti minimi brevis is responsible for flexing the 5th digit. This muscle arises from the flexor retinaculum and the hamate, inserting on the medial aspect of the proximal phalanx. The opponens digiti minimi facilitates opposition of the 5th digit. This muscle originates from the flexor retinaculum and hamate and inserts along the medial aspect of the 5th metacarpal. The deep branch of the ulnar nerve transmits motor impulses to all 3 muscles.
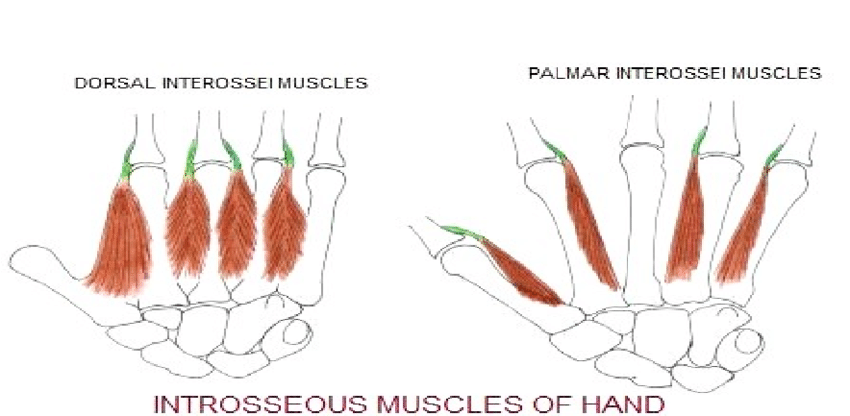
Interossei
The interossei consist of 4 dorsal and 3 palmar interossei, located between the metacarpals. A commonly used mnemonic to recall their actions is “DAB and PAD.”[3] “DAB” refers to the dorsal interossei, which abduct the digits, while “PAD” refers to the palmar interossei, which adduct the digits.[4]
The dorsal interossei abduct the 2nd, 3rd, and 4th digits. These muscles originate from adjacent sides of the metacarpals and insert on the extensor hood and proximal phalanges of the corresponding digits. The palmar interossei adduct the 2nd, 4th, and 5th digits. These muscles arise from the palmar surfaces of the corresponding metacarpals and insert on the extensor hood and proximal phalanges. All interossei receive innervation from the deep branch of the ulnar nerve.
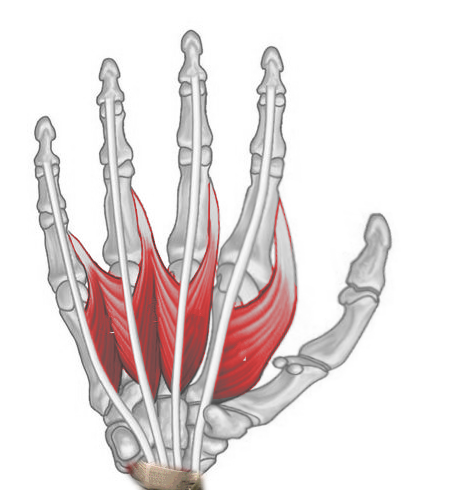
Lumbricals
The lumbrical muscle group consists of 4 slender muscles that flex the metacarpophalangeal (MCP) joints while simultaneously extending the proximal and distal interphalangeal joints of the 2nd to 5th digits (see Image. Right Hand Lumbricals, Anterior View). The lumbricals emanate from the tendons of the flexor digitorum profundus and insert on the extensor hood of the 2nd, 3rd, 4th, and 5th digits. The 1st and 2nd lumbricals are innervated by the median nerve, whereas the deep branch of the ulnar nerve supplies the 3rd and 4th.[5]
Embryology
Understanding the genetics of limb development is essential in both medical and surgical contexts, as disruptions in embryological signaling may result in congenital deformities. While the full genetic mechanisms remain incompletely characterized, recent studies in both invertebrate and vertebrate models have identified key stages in the complex signaling pathways that govern limb formation.[6]
Limb development begins after the formation of the 3 germ layers—ectoderm, mesoderm, and endoderm—during gastrulation. Within the mesoderm, undifferentiated cells known as mesenchyme initiate limb formation by secreting and responding to specific biochemical signals that establish patterning along spatial axes. Interactions between the surface ectoderm and the underlying mesoderm stimulate proliferation and lateral expansion of mesenchymal cells in the peripheral embryo. These cells, derived from the lateral plate mesoderm, give rise to the initial limb bud.[7]
This section provides a brief overview of both the macroscopic and microscopic development of the limb bud that forms the upper extremity and hand. Although the lower extremity shares similar embryological pathways, it is beyond the scope of this discussion.
Macroscopic Embryological Development of the Upper Extremity and Hand
The limb bud consists of undifferentiated mesodermal cells covered by an overlying ectoderm. Two key mesodermal tissues contribute to limb development: the lateral plate mesoderm, which gives rise to cartilage and bone, and the somites, which give rise to skeletal muscle.[8] The upper extremity first becomes visible during week 4 of embryonic development, approximately day 26, as a lateral projection emerging from the body wall.
By week 5, approximately day 36, the arm buds enlarge and assume a paddle-like appearance. During this period, nerves and vasculature enter the upper extremity in a proximodistal pattern, chondrification begins, and muscle development is initiated. Myogenic progenitor cells divide into superficial and deep layers, with the deep layers giving rise to the intrinsic muscles of the hand.
During weeks 6 to 7, skeletal elongation and muscle formation progress proximodistally, and the process of ossification begins. In week 8, the digits of the hand become distinct following apoptosis of the interdigital web spaces. By week 9, all upper extremity muscles have developed except for the intrinsic muscles of the hand. By week 12, the musculature of the upper extremity is complete, including the intrinsic hand muscles.[9]
Microscopic Embryological Development of the Upper Extremity and Hand
In the developing limb, the orderly formation of macroscopic structures such as muscle, bone, and connective tissue depends on tightly regulated genetic signaling. This process also determines limb identity and coordinates limb outgrowth in a stepwise manner. Genes and protein factors govern the microscopic mechanisms that drive upper extremity and hand development through a process known as pattern formation, or patterning.
Patterning of the limb bud begins with upregulation of the Hox gene family, a group of genes located in the lateral plate mesoderm that initiate downstream signaling to establish limb bud location. These signals generate 3 organizing centers that regulate growth along the proximal-to-distal, anterior-to-posterior, and dorsal-to-ventral axes.[10]
When the limb bud first appears during week 4 of development, approximately day 26, activation of mesenchymal cells in the lateral plate mesoderm initiates proximal-to-distal outgrowth through formation of the apical ectodermal ridge (AER), a thickened region of ectoderm overlying the mesoderm. This process begins with Hox gene expression, which activates the T-box transcription factors Tbx5 and Tbx4. These proteins establish limb identity and stimulate the expression of fibroblast growth factor (FGF) ligands.
The T-box proteins induce FGF10 expression in the proliferating mesoderm, which in turn promotes formation of the AER. Once established, the AER secretes its own FGFs, sustaining continued mesodermal proliferation and limb outgrowth. In terms of limb identity, Tbx5 directs upper extremity development, whereas Tbx4 specifies lower extremity structures. The essential role of the AER and FGFs in limb development is demonstrated by experimental models in which disruption of either structure results in severe limb truncation.[11]
The 2nd axis, anterior-posterior, is regulated by the zone of polarizing activity (ZPA), a group of mesenchymal cells situated at the posterior margin of the limb bud. This signaling center is established through the combined action of Hox genes and retinoic acid, which induce expression of the sonic hedgehog (Shh) protein. Proper Shh expression is essential for establishing digit number and identity in the developing hand. The critical role of the ZPA and Shh signaling is evident in experimental models. Duplication of the ZPA leads to mirror-image digit formation, while removal of Shh results in the failure of digit development.
The 3rd axis, dorsal-ventral, is regulated by Wnt7a, a signaling protein expressed in the ectoderm overlying the limb bud. Wnt7a mediates interactions between dorsalizing and ventralizing factors to ensure appropriate dorsal patterning. Experimental deletion of WNT7A leads to defective or absent dorsal hand structures, underscoring its essential role in dorsal-ventral axis specification.[12]
Blood Supply and Lymphatics
Blood supply to the intrinsic hand muscles is derived from the ulnar and radial arteries, along with their collateral and anastomotic branches. These arteries cross the wrist and distribute branches both superficially and deeply, forming the superficial palmar arch and the deep palmar arch. The ulnar artery primarily contributes to the superficial palmar arch, while the radial artery contributes most to the deep palmar arch. Distal branches arising from the superficial palmar arch include the common palmar digital arteries, which further divide into proper palmar digital arteries supplying the digits.[13]
The thenar muscles receive their primary vascular supply from the superficial palmar arch. The hypothenar muscle group is supplied predominantly by the ulnar artery. Blood supply to the dorsal interossei arises from dorsal metacarpal arteries. The 1st dorsal metacarpal artery, a direct branch of the radial artery, supplies the 1st dorsal interosseous muscle. The 2nd, 3rd, and 4th dorsal interossei are perfused by their respective dorsal metacarpal arteries, each of which originates from the dorsal carpal arch.
The palmar metacarpal arteries, which branch from the deep palmar arch, supply the palmar interossei. The lumbrical muscles receive blood primarily from the superficial palmar arch, with additional contributions from the deep palmar arch, dorsal digital arteries, and common palmar digital arteries.[14]
Lymphatic drainage of the upper limb and hand occurs through superficial and deep lymphatic systems. The superficial system drains the skin of the palm and dorsum of the hand through plexuses that follow the cephalic and basilic veins toward the axillary and cubital lymph nodes.[15] The deep system drains skeletal muscle via lymphatic vessels accompanying the deep veins and terminates in the humeral group of axillary lymph nodes.[16]
Nerves
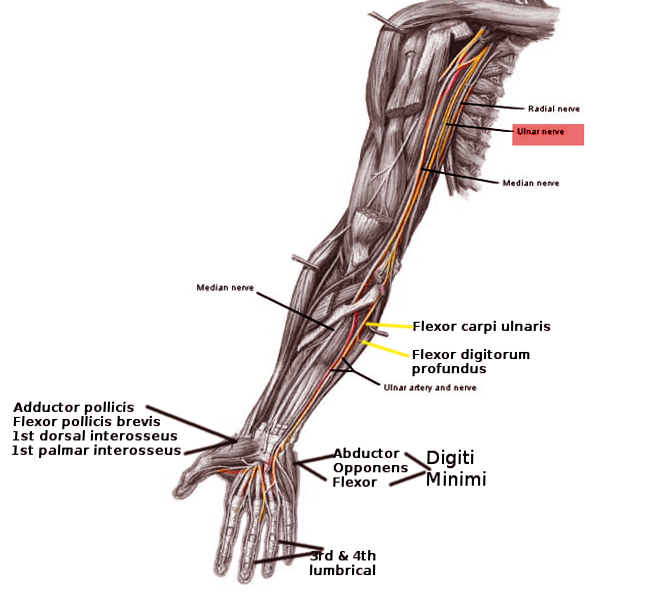
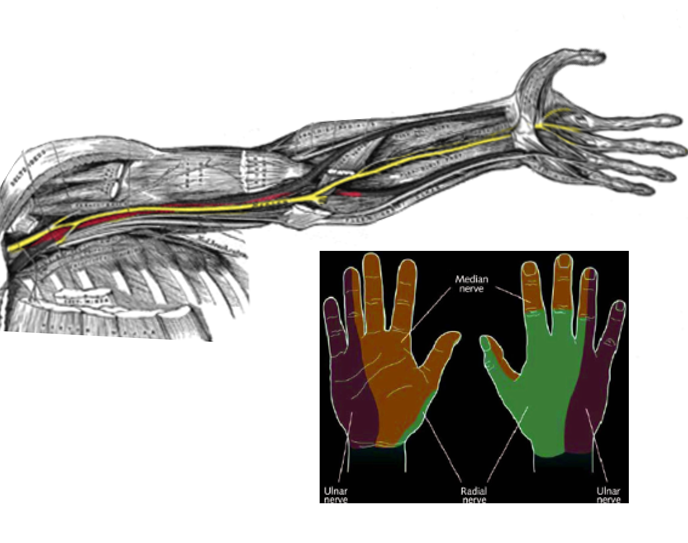
Intrinsic hand muscles are innervated by branches of the brachial plexus, a neural network that arises from spinal nerve roots C5 to T1 via their ventral rami (see Image. Nerve Distribution in the Arm, Forearm, and Hand). The median and ulnar nerves, both terminal branches of the brachial plexus, provide motor innervation to these muscles. The median nerve contains fibers from C5 to C8 and enters the hand through the carpal tunnel, where it divides into a recurrent motor branch and a cutaneous branch. The ulnar nerve, composed of fibers from C8 to T1, enters the hand through the Guyon canal and diverges into superficial and deep branches (see Image. Ulnar Nerve Pathway).[17][18][19]
The median nerve's recurrent motor branch innervates the thenar muscle group. The adductor pollicis receives motor innervation from the deep branch of the ulnar nerve. The hypothenar muscle group is also innervated by the deep branch of the ulnar nerve. The dorsal and palmar interossei receive motor supply exclusively from the deep branch of the ulnar nerve. The 1st and 2nd lumbricals are innervated by the median nerve, whereas the 3rd and 4th lumbricals are supplied by the ulnar nerve's deep branch.[20]
Physiologic Variants
Physiologic variants of the upper extremity may occur within or adjacent to the intrinsic hand muscles. Anomalous muscles or neurovascular structures may distort expected anatomical landmarks or contribute to compressive neuropathies. Although these variants occur in a minority of individuals, distinguishing them from both normal anatomy and pathological findings is essential to avoid misdiagnosis or overdiagnosis.
Among the most studied are variants of the lumbrical muscles.[21] The lumbricals typically originate from the tendons of the flexor digitorum profundus distal to the carpal tunnel. However, in a recent study, intraoperative assessment demonstrated that lumbrical muscle excursion into or at the level of the carpal tunnel occurred in 13% to 47% of individuals during finger flexion. (Source: Clarnette et al, 2025) While often asymptomatic, this variant may contribute to carpal tunnel syndrome (CTS) if the muscle hypertrophies and occupies space within the already confined tunnel.[22]
Studies have also documented neurovascular variants within the hand. In a series of 526 elective carpal tunnel releases, anatomical variations were identified in 6% of cases. With regard to the median nerve, a key structure for intrinsic muscle innervation, variations were noted in its course through the carpal tunnel, including alignment, shape, and relationship to adjacent flexor tendons. As for the ulnar nerve, another critical nerve supplying the intrinsic muscles, an aberrant branch was observed traversing the carpal tunnel.[23][24] The clinical relevance of these variants lies in the potential for deviations from expected anatomical landmarks during surgical procedures, which may hinder accurate identification of critical structures.
Additional variants of anatomic or functional significance include insertional deviations of the 1st dorsal interosseous, which may attach directly to the proximal phalanx in addition to the extensor expansion. This variant augments MCP flexion strength and contributes to lateral pinch stability, with implications for tendon transfer selection.[25] Innervation anomalies such as the Martin-Gruber anastomosis, or median-to-ulnar nerve crossover in the forearm, may result in unexpected sparing or preservation of intrinsic hand muscle function, especially in ulnar neuropathies.[26] Recognition of these patterns is essential, as they can obscure lesion localization and alter surgical planning.
Surgical Considerations
Surgical planning should account for physiologic variants. Awareness of anomalous muscles and nerves is critical since numerous anatomic differences may alter expected landmarks during hand imaging and operative procedures.
The use of intrinsic hand muscles in muscle flap procedures has been described in the treatment of CTS and nerve palsies. The 1st and 2nd lumbrical muscles have been used to create flaps that provide coverage and protection in patients with exposed or compressed median nerves. A study also demonstrated the use of thenar muscle flaps, in conjunction with lumbricals, for managing painful neuromas.[27]
Tendon transfers for median and ulnar nerve palsies, both of which impair intrinsic hand muscle function, are well described in the literature.[28] The primary surgical goals for ulnar nerve palsy are to restore pinch and grip strength and to correct clawing. Pinch strength depends on the 1st dorsal interosseous and adductor pollicis muscles, both innervated by the ulnar nerve. Transfers using the wrist and finger extensors, finger flexors, or the brachioradialis have successfully restored pinch function without creating new deficits at the donor sites. Claw correction involves rerouting tendons to counteract MCP hyperextension.
Meanwhile, the surgical priority for median nerve palsy is to restore thumb opposition. Transfers that direct force toward the insertion of the APB most closely replicate native opposition, an action that requires palmar abduction, pronation, and flexion of the thumb. Several operative techniques have been developed to achieve this restoration.
In addition to tendon transfers and muscle flaps, nerve transfer techniques have expanded surgical options for restoring intrinsic muscle function. Recent cadaveric and clinical research supports the use of anterior interosseous nerve transfers to reinnervate muscles such as the 1st dorsal interosseous and ADM.[29][30] These procedures enhance fine motor control and pinch strength in patients with proximal nerve injuries by selectively reestablishing neural input to key intrinsic targets.
Clinical Significance
When the hand is at rest, a balance exists between the intrinsic and extrinsic muscle forces, particularly those exerted by the lumbricals and the interossei (see Image. Bones of the Left Hand). The lumbricals flex the MCP joints while extending the proximal and distal interphalangeal joints. Loss of innervation to these muscles disrupts this equilibrium, allowing the extrinsic muscles to dominate.
Recent literature underscores the role of the interossei in supporting lumbrical function. With unopposed extrinsic action, the hand adopts a posture of MCP extension and interphalangeal joint flexion. Neuropathies affecting the median or ulnar nerves, whether compressive, traumatic, or systemic, commonly produce such distortions. Recognized clinical patterns include ulnar clawing, the so-called "Pope’s hand of benediction," and Klumpke palsy.[31] Early recognition is essential, as untreated nerve injury may lead to persistent deficits that impair activities of daily living.
Ulnar clawing and the Pope’s hand of benediction may appear similar but result from distinct nerve injuries. Ulnar clawing arises from a distal ulnar nerve lesion at the level of the wrist, leading to paralysis of all interossei and the 3rd and 4th lumbricals while sparing the extrinsic flexors (see Image. Claw Hand). Loss of intrinsic muscle function disrupts the normal flexion-extension balance across the digits, resulting in exaggerated MCP joint flexion and interphalangeal joint extension at rest. This deformity impairs the ability to cup the hand around objects.
In contrast, the Pope’s hand of benediction arises from a proximal median nerve lesion at the level of the elbow. Because the intrinsic muscles remain intact, the deformity reflects paralysis of the extrinsic flexors in the radial half of the forearm. When attempting to make a fist, the 2nd and 3rd digits fail to flex, whereas the 4th and 5th digits, innervated by the ulnar nerve, retain some flexion. Unlike ulnar clawing, this deformity becomes apparent only during active flexion.[32]
Klumpke palsy, also referred to as "total hand claw," results from injury to the lower trunk of the brachial plexus (C8 to T1). This neuropathy causes paralysis of all intrinsic hand muscles, leading to hyperextension of the MCP joints and flexion of the interphalangeal joints. Involvement of the thenar and hypothenar muscles produces palmar atrophy and weakness in thumb and 5th-digit abduction, flexion, and opposition. The most common mechanism of injury is forced hyperabduction of the upper extremity. This occurs most frequently in neonates subjected to excessive traction during delivery and in adults with traumatic upward pulling injuries.
Other Issues
Wide-Awake Local Anesthesia No Tourniquet (WALANT) procedures have transformed hand surgery by allowing real-time intraoperative assessment of intrinsic muscle function, particularly in reconstructive procedures such as tendon transfers.[33] Since patients remain awake and able to move their hands during surgery, surgeons can evaluate and adjust tension in transferred tendons targeting muscles like the APB and the lumbricals. This intraoperative feedback is especially important in opponensplasty and tendon-based reconstructions for nerve palsies, such as median or radial nerve injuries, where precise reestablishment of thumb opposition or finger flexion is essential.
Clinical outcomes using WALANT are favorable, with studies confirming its safety for flexor tendon repairs without increasing the risk of rupture and adhesions or reducing range of motion and functional outcomes.[34][35] A multicenter cohort of 226 office-based WALANT-assisted carpal tunnel releases confirmed sustained symptom relief, rapid recovery, and high satisfaction in both unilateral and bilateral procedures.[36] In a 2023 case series, Wakasugi et al demonstrated that 2-portal endoscopic carpal tunnel release under WALANT led to significant functional improvement without major complications, supporting the procedure's safety and efficacy for CTS. (Source: Wakasugi, 2023) Overall, WALANT improves precision in handling intrinsic muscle reconstructions while enhancing patient safety and surgical efficiency.
Media
(Click Image to Enlarge)
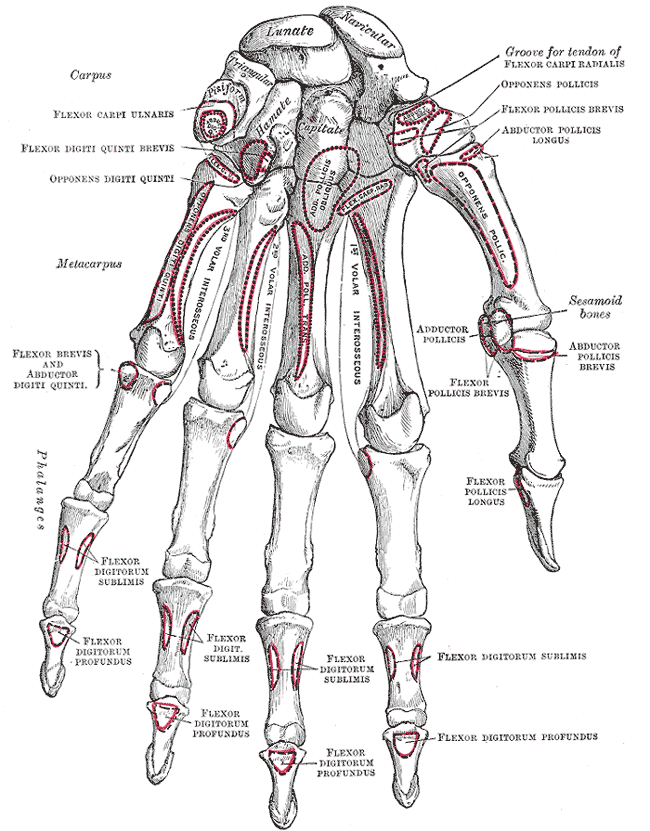
Bones of the Left Hand. Palmar view of the skeletal anatomy of the left hand, illustrating the carpal, metacarpal, and phalangeal bones. Labels include relevant bony landmarks and insertions of intrinsic and extrinsic hand muscles, providing anatomical orientation for clinical or surgical reference.
Henry Vandyke Carter, Public Domain, via Wikimedia Commons
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)

Claw Hand. Clinical presentation of claw hand deformity in a patient with ulnar nerve injury, characterized by hyperextension at the 4th and 5th metacarpophalangeal joints and flexion at the corresponding proximal interphalangeal and distal interphalangeal joints.
Mcstrothe, Public Domain, via Wikimedia Commons
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
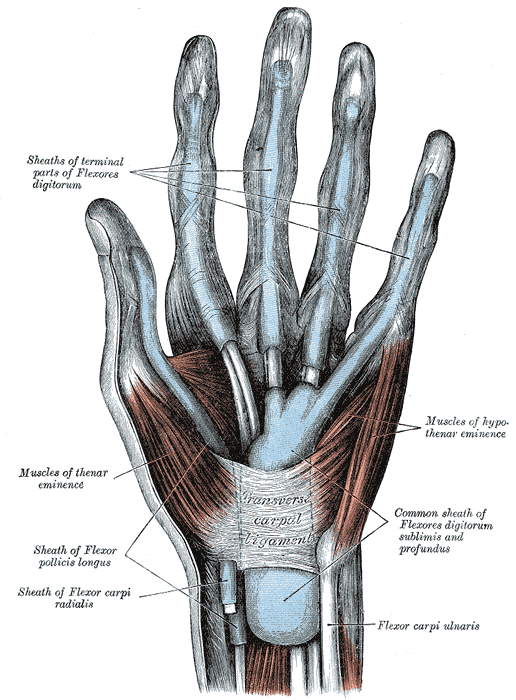
Palmar View of the Hand Muscles, Tendons, and Fascia. The image depicts the superficial structures of the palm, including the thenar and hypothenar eminences, digital tendon sheaths, and the transverse carpal ligament. The flexor tendons of flexor digitorum profundus and flexor digitorum superficialis are enclosed in a common sheath proximal to the carpal tunnel.
Henry Vandyke Carter, Public Domain, via Wikimedia Commons
References
Kooi K, Legerstee IWF, van Vliet E, Freundt LA, Reikersdorfer K, Chen NC, Eberlin KR. Anatomy of the Lumbrical Muscles: Implications for Mechanical Advantage. Hand (New York, N.Y.). 2024 Mar 29:():15589447241235340. doi: 10.1177/15589447241235340. Epub 2024 Mar 29 [PubMed PMID: 38551109]
Picasso R, Zaottini F, Pistoia F, Perez MM, Macciò M, Bianco D, Rinaldi S, Pansecchi M, Rossi G, Tovt L, Martinoli C. Ultrasound of the palmar aspect of the hand: normal anatomy and clinical applications of intrinsic muscles imaging. Journal of ultrasonography. 2023 Sep:23(94):e122-e130. doi: 10.15557/jou.2023.0021. Epub 2023 Sep 11 [PubMed PMID: 37732107]
Liss FE. The interosseous muscles: the foundation of hand function. Hand clinics. 2012 Feb:28(1):9-12. doi: 10.1016/j.hcl.2011.09.005. Epub [PubMed PMID: 22117919]
Eladoumikdachi F, Valkov PL, Thomas J, Netscher DT. Anatomy of the intrinsic hand muscles revisited: part I. Interossei. Plastic and reconstructive surgery. 2002 Oct:110(5):1211-24 [PubMed PMID: 12360058]
Eladoumikdachi F, Valkov PL, Thomas J, Netscher DT. Anatomy of the intrinsic hand muscles revisited: part II. Lumbricals. Plastic and reconstructive surgery. 2002 Oct:110(5):1225-31 [PubMed PMID: 12360059]
Basson CT, Bachinsky DR, Lin RC, Levi T, Elkins JA, Soults J, Grayzel D, Kroumpouzou E, Traill TA, Leblanc-Straceski J, Renault B, Kucherlapati R, Seidman JG, Seidman CE. Mutations in human TBX5 [corrected] cause limb and cardiac malformation in Holt-Oram syndrome. Nature genetics. 1997 Jan:15(1):30-5 [PubMed PMID: 8988165]
Level 3 (low-level) evidenceTickle C. How the embryo makes a limb: determination, polarity and identity. Journal of anatomy. 2015 Oct:227(4):418-30. doi: 10.1111/joa.12361. Epub 2015 Aug 7 [PubMed PMID: 26249743]
Valenzuela M, Varacallo MA. Anatomy, Shoulder and Upper Limb, Hand Interossei Muscles. StatPearls. 2025 Jan:(): [PubMed PMID: 30521193]
Hita-Contreras F, Martínez-Amat A, Ortiz R, Caba O, Alvarez P, Prados JC, Lomas-Vega R, Aránega A, Sánchez-Montesinos I, Mérida-Velasco JA. Development and morphogenesis of human wrist joint during embryonic and early fetal period. Journal of anatomy. 2012 Jun:220(6):580-90. doi: 10.1111/j.1469-7580.2012.01496.x. Epub 2012 Mar 19 [PubMed PMID: 22428933]
Barham G, Clarke NM. Genetic regulation of embryological limb development with relation to congenital limb deformity in humans. Journal of children's orthopaedics. 2008 Feb:2(1):1-9. doi: 10.1007/s11832-008-0076-2. Epub 2008 Feb 7 [PubMed PMID: 19308596]
Cole P, Kaufman Y, Hatef DA, Hollier LH Jr. Embryology of the hand and upper extremity. The Journal of craniofacial surgery. 2009 Jul:20(4):992-5. doi: 10.1097/SCS.0b013e3181abb18e. Epub [PubMed PMID: 19553860]
Woods CG, Stricker S, Seemann P, Stern R, Cox J, Sherridan E, Roberts E, Springell K, Scott S, Karbani G, Sharif SM, Toomes C, Bond J, Kumar D, Al-Gazali L, Mundlos S. Mutations in WNT7A cause a range of limb malformations, including Fuhrmann syndrome and Al-Awadi/Raas-Rothschild/Schinzel phocomelia syndrome. American journal of human genetics. 2006 Aug:79(2):402-8 [PubMed PMID: 16826533]
Level 3 (low-level) evidenceEpperson TN, Varacallo MA. Anatomy, Shoulder and Upper Limb, Brachial Artery. StatPearls. 2025 Jan:(): [PubMed PMID: 30725830]
Tan RES, Lahiri A. Vascular Anatomy of the Hand in Relation to Flaps. Hand clinics. 2020 Feb:36(1):1-8. doi: 10.1016/j.hcl.2019.08.001. Epub [PubMed PMID: 31757342]
Granoff MD, Pardo JA, Johnson AR, Fleishman A, Tillotson E, Thomson S, Lee BT, Singhal D. Superficial and Functional Lymphatic Anatomy of the Upper Extremity. Plastic and reconstructive surgery. 2022 Oct 1:150(4):900-907. doi: 10.1097/PRS.0000000000009555. Epub 2022 Aug 4 [PubMed PMID: 35939638]
Ma CX, Pan WR, Liu ZA, Zeng FQ, Qiu ZQ, Liu MY. Deep lymphatic anatomy of the upper limb: An anatomical study and clinical implications. Annals of anatomy = Anatomischer Anzeiger : official organ of the Anatomische Gesellschaft. 2019 May:223():32-42. doi: 10.1016/j.aanat.2019.01.005. Epub 2019 Feb 1 [PubMed PMID: 30716466]
Level 2 (mid-level) evidenceBayot ML, Nassereddin A, Varacallo MA. Anatomy, Shoulder and Upper Limb, Brachial Plexus. StatPearls. 2025 Jan:(): [PubMed PMID: 29763192]
Aleksenko D, Varacallo MA. Guyon Canal Syndrome. StatPearls. 2025 Jan:(): [PubMed PMID: 28613717]
Sammer DM, Chung KC. Tendon transfers: Part II. Transfers for ulnar nerve palsy and median nerve palsy. Plastic and reconstructive surgery. 2009 Sep:124(3):212e-221e. doi: 10.1097/PRS.0b013e3181b037c7. Epub [PubMed PMID: 19730287]
Borges ÁVRM, Souza SAL. Anatomy of the nerves, vessels, and muscular compartments of the forearm, as revealed by high-resolution ultrasound. Part 1: overall structure and forearm compartments. Radiologia brasileira. 2021 Nov-Dec:54(6):388-397. doi: 10.1590/0100-3984.2021.0030. Epub [PubMed PMID: 34866699]
Pfirrmann CW, Zanetti M. Variants, pitfalls and asymptomatic findings in wrist and hand imaging. European journal of radiology. 2005 Dec:56(3):286-95 [PubMed PMID: 16298674]
Middleton WD, Kneeland JB, Kellman GM, Cates JD, Sanger JR, Jesmanowicz A, Froncisz W, Hyde JS. MR imaging of the carpal tunnel: normal anatomy and preliminary findings in the carpal tunnel syndrome. AJR. American journal of roentgenology. 1987 Feb:148(2):307-16 [PubMed PMID: 3492109]
Lindley SG, Kleinert JM. Prevalence of anatomic variations encountered in elective carpal tunnel release. The Journal of hand surgery. 2003 Sep:28(5):849-55 [PubMed PMID: 14507518]
Mannerfelt L. Studies on the hand in ulnar nerve paralysis. A clinical-experimental investigation in normal and anomalous innervation. Acta orthopaedica Scandinavica. 1966:():Suppl 87:1+ [PubMed PMID: 4287179]
Low ZX, McGrouther DA. First dorsal interosseous muscle reconstruction: a systematic review of its attachments. Surgical and radiologic anatomy : SRA. 2023 Jul:45(7):901-909. doi: 10.1007/s00276-023-03149-0. Epub 2023 May 12 [PubMed PMID: 37169993]
Level 1 (high-level) evidenceTran P, Bennett D, Mendoza M, Sarpong A, Ayala M, Sadacharan C, Tippen SP. A Cadaveric Study of the Martin-Gruber Anastomosis Morphology. Cureus. 2025 Jan:17(1):e78139. doi: 10.7759/cureus.78139. Epub 2025 Jan 28 [PubMed PMID: 40018488]
Rose J, Belsky MR, Millender LH, Feldon P. Intrinsic muscle flaps: the treatment of painful neuromas in continuity. The Journal of hand surgery. 1996 Jul:21(4):671-4 [PubMed PMID: 8842964]
Level 3 (low-level) evidenceSchwarz RJ, Brandsma JW, Giurintano DJ. A review of the biomechanics of intrinsic replacement in ulnar palsy. The Journal of hand surgery, European volume. 2010 Feb:35(2):94-102. doi: 10.1177/1753193408091569. Epub 2009 Jul 10 [PubMed PMID: 19592605]
Abaskhron M, Ezzat M, Boulis AG, Safoury YE. Supercharged end-to-side anterior interosseous nerve transfer to restore intrinsic function in high ulnar nerve injury: a prospective cohort study. BMC musculoskeletal disorders. 2024 Jul 20:25(1):566. doi: 10.1186/s12891-024-07650-4. Epub 2024 Jul 20 [PubMed PMID: 39033290]
Mitchell EC, Mansouri M, Miller T, Ross D, Gillis J. Early and Late Intrinsic Hand Muscle Reinnervation After End-to-Side AIN to Ulnar Motor Nerve Transfer. Hand (New York, N.Y.). 2024 Nov 6:():15589447241286263. doi: 10.1177/15589447241286263. Epub 2024 Nov 6 [PubMed PMID: 39503214]
Rosen IM, Koznarsky MJ. Nerve damage from soft tissue injury to the forearm. American family physician. 2009 May 1:79(9):793-4 [PubMed PMID: 20141099]
Level 3 (low-level) evidenceGoldman SB, Brininger TL, Schrader JW, Koceja DM. A review of clinical tests and signs for the assessment of ulnar neuropathy. Journal of hand therapy : official journal of the American Society of Hand Therapists. 2009 Jul-Sep:22(3):209-19; quiz 220. doi: 10.1016/j.jht.2008.10.010. Epub 2009 Feb 1 [PubMed PMID: 19188042]
Nolan GS, Kiely AL, Madura T, Karantana A. Wide-awake local anaesthesia no tourniquet (WALANT) vs regional or general anaesthesia for flexor tendon repair in adults: protocol for a systematic review and meta-analysis. Systematic reviews. 2020 Nov 21:9(1):264. doi: 10.1186/s13643-020-01532-1. Epub 2020 Nov 21 [PubMed PMID: 33220705]
Level 1 (high-level) evidenceTownsend CB, Henry TW, Matzon JL, Seigerman D, Sodha SC, Beredjiklian PK. Functional Outcomes of Flexor Tendon Repair in the Fingers: A Comparison of Wide-Awake Local Anesthesia No Tourniquet Versus Traditional Anesthesia. Hand (New York, N.Y.). 2023 Jun:18(4):635-640. doi: 10.1177/15589447211064364. Epub 2022 Jan 7 [PubMed PMID: 34991396]
El-Gammal TA, Saleh WR, Ragheb YF, Morsy M, Ibrahim MA, Fekry MS. Outcomes of Zone II Flexor Tendon Repair Under General Versus Wide Awake Local Anesthesia: A Randomized Controlled Trial. The Journal of hand surgery. 2024 Nov:49(11):1095-1103. doi: 10.1016/j.jhsa.2024.06.008. Epub 2024 Aug 8 [PubMed PMID: 39115486]
Level 1 (high-level) evidencePistorio AL, Marwin VM, Paterson PD, Alexander RD, Nelson JT, Miller LE. Office-Based Carpal Tunnel Release With Ultrasound Guidance: 6-month Outcomes From the Multicenter ROBUST Trial. Journal of hand surgery global online. 2024 May:6(3):268-274. doi: 10.1016/j.jhsg.2023.12.005. Epub 2024 Feb 19 [PubMed PMID: 38817765]