Introduction
First introduced in 1974 at the University of Glasgow by neurosurgery professors Graham Teasdale and Bryan Jennett, the Glasgow Coma Scale (GCS) offers an objective method for describing the extent of impaired consciousness in patients with acute medical conditions or trauma.[1] The scale evaluates 3 aspects of responsiveness—eye-opening, verbal, and motor responses. Reporting each component separately allows clinicians to communicate a clear and detailed picture of the patient’s neurologic status. Individual findings from each component may also be aggregated into a total Glasgow Coma Score, offering a concise, though less detailed, summary of overall severity.[2] For example, a score of 10 may be documented as GCS10=E3V4M3, indicating the specific values for each response category.
The widespread adoption of the GCS began in the 1980s, following the endorsement of its use for trauma patients in the first edition of the Advanced Trauma and Life Support (ATLS) course. In 1988, the World Federation of Neurosurgical Societies (WFNS) incorporated it into its grading scale for subarachnoid hemorrhage.[3] The GCS has since become embedded in numerous clinical guidelines and scoring systems for trauma and critical illness, extending across all age groups, including preverbal children. Required by the NIH Common Data Elements for head injury studies and included in the ICD-11, the GCS is now used in over 75 countries.[4][5][6] Since 1974, publications referencing the GCS have increased globally at an average annual rate of 16.7%, accumulating over 37,000 citations.[7]
Function
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Function
Scoring and Parameters
The GCS evaluates the following 3 parameters of responsiveness: best eye response (E), best verbal response (V), and best motor response (M). The levels of response in the components of the GSC are scored from 1, for no response, up to normal values of 4 (eye-opening response), 5 (verbal response), and 6 (motor response). The total GCS score thus ranges from 3 to 15, with 3 being the lowest and 15 being the highest.
The score is the sum of the scores as well as the individual elements. For example, a score of 10 might be expressed as GCS10 = E3V4M3. Clinicians should denote any untestable component of the GCS as “NT” (not testable) and avoid using the total score when a component is not testable. The following scores are assigned for each response parameter:
- Best eye response
- No eye opening
- Eyes opening to pain
- Eyes opening to sound
- Eyes open spontaneously
- Best verbal response
- No verbal response
- Incomprehensible sounds
- Inappropriate words
- Confused
- Orientated
- Best motor response
- No motor response
- Abnormal extension with pain
- Abnormal flexion to pain
- Withdrawal from pain
- Localizing pain
- Obeys commands
Motor response
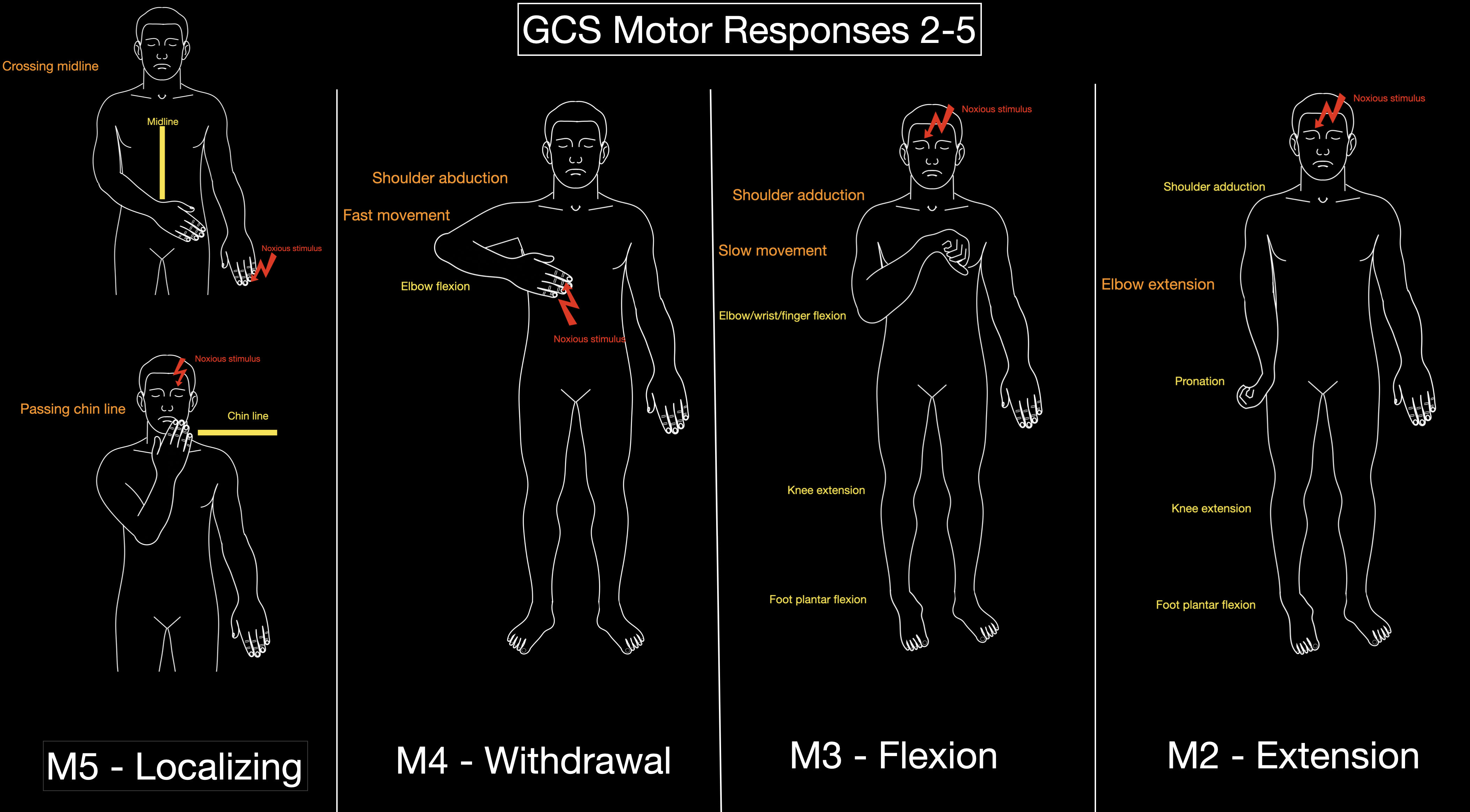
According to the GCS Aid [GCS AID], noxious stimulation should first be applied by pressing on the fingernail. If this fails to elicit any response, firm pressure is then applied to either the trapezius muscle or the supraorbital notch (see Image. Glasgow Coma Scale Responses).
M5 is scored when, following a supra-orbital stimulus, the hand crosses the chin, or after a nail-bed stimulus, the opposite hand crosses the midline.[8] These 2 criteria help clearly distinguish localization (M5) from withdrawal (M4), as M4 responses do not involve crossing the midline or the chin. Variability exists in the literature regarding the characterization of M3. Teasdale and Jennett differentiated withdrawal (M4) from abnormal flexion (M3) based on shoulder movement—abduction for M4 and adduction for M3—and described M3 as a “slower, stereotyped” response.[1] In 2004, Van der Naalt defined M3 as the presence of at least 2 of the following: forearm extension or internal rotation, thumb positioned among the fingers, maximal wrist flexion, or simultaneous leg extension/internal rotation.[8] Matis and Birbilis described M3 as involving arm adduction, flexion at the elbow, wrist, and fingers, along with leg extension and internal rotation with plantar-flexed feet.[9] They also described M2 as characterized by arm adduction and pronation, leg extension, and plantar flexion of the feet.
Application of the Glasgow Coma Scale in Pediatric Patients
The GSC can be used in children older than 5 years without modification. However, younger children and infants are unable to provide the necessary verbal responses for a clinician to use the scale to assess their orientation or obey commands to evaluate their motor response. Since the pediatric GCS was initially described in Adelaide, several modifications have been made, but no single method has become universally accepted.[10] The following versions are derived from those of James and the Pediatric Emergency Care Applied Research Network in preverbal children younger than 2 years and verbal children older than 2 years (see Table. Modified Pediatric Glasgow Coma Scale).[11][6]
Table. Modified Pediatric Glasgow Coma Scale
| Children Younger Than 2 Years (Preverbal) | Children Older Than 2 Years (Verbal) | |
| Best Eye Response |
|
|
| Best Verbal Response |
|
|
| Best Motor Response |
|
|
Disorders of Consciousness
The Glasgow Coma Scale (GCS) serves as a tool for classifying disorders of consciousness. A decision tree based on sequential evaluation of GCS subscores enables systematic categorization. This approach designates a recovered posttraumatic confusional state (rPTCS) when the verbal score (V) equals 5 (oriented) and a posttraumatic confusional state (PTCS) when V equals 4 (confused). A minimally conscious state with preserved language (MCS+) is assigned when V equals 3 or motor score (M) equals 6, while a minimally conscious state without preserved language (MCS−) is assigned when M equals 5 and V is less than 3. When none of these criteria apply, the classification assigns a vegetative state/unresponsive wakefulness syndrome if V equals 2 or if eye opening (E) exceeds 1. In the absence of these findings, a coma is designated.[12]
Issues of Concern
The following factors may interfere with the GSC assessment:
- Preexisting factors
- Language barriers
- Intellectual or neurological deficit
- Hearing loss or speech impediment
- Effects of current treatment
- Physical (eg, intubation): If a patient is intubated and unable to speak, they are evaluated only on motor and eye-opening responses, and the suffix "T" is added to their score to indicate intubation.
- Pharmacological (eg, sedation) or paralysis: If possible, the clinician should obtain the score before sedating the patient.
- Effects of other injuries or lesions
- Orbital and cranial fracture
- Spinal cord damage
- Hypoxic-ischemic encephalopathy after cold exposure
In some instances, the GCS is unobtainable despite efforts to overcome these interfering factors. The total score should not be reported without testing and including all components, as it may be low and cause confusion.
Intoxicating substances can confound initial GCS assessments. The effect is more pronounced with benzodiazepines and polysubstance use, which can produce an artificially low score that increases by up to a mean of 1.7 points on later reassessment. Screening for intoxication is highly recommended in the appropriate clinical setting.[13]
Clinicians often encounter situations in which the verbal component of the GCS cannot be assessed, most commonly due to intubation or sedation. A simple imputation strategy based on the combined eye–motor score can reliably estimate the missing verbal score. For eye–motor totals of 2 to 6, add 1 point; for eye–motor equaling 7, add 2 points; for an eye–motor score of 8 or 9, add 4 points; and for eye–motor equaling 10, add 5 points. This method preserves prognostic accuracy and supports consistent communication when the verbal component is not testable.[14] In patients with stroke who cannot be scored on the verbal subscale due to aphasia or intubation, omitting the verbal component and using the eye-motor sum predicts early mortality with accuracy equivalent to the full GCS sum.[15]
The GCS total score may misclassify the level of consciousness because identical total scores can correspond to different clinical states. A GCS total of 8 can reflect vegetative state, minimally conscious state, or posttraumatic confusional state, and scores of 7 to 11 are associated with 5 distinct disorders of consciousness.[12]
Clinical Significance
Assessment of responsiveness with the GCS is widely used to guide early management of patients with a head injury or other kind of acute brain injury. Decisions in more severely impaired patients include emergent management, eg, securing the airway and triage to determine patient transfer. Decisions in less severely impaired patients include the need for neuroimaging, admission for observation, or discharge. Serial GSC assessments are also crucial in monitoring a patient's clinical course and guiding adjustments in management.
The information gained from the 3 components of the GCS varies across the spectrum of responsiveness (see Image. Glasgow Coma Scale Responses).[16] Changes in motor response are the predominant factor in patients with more severe impairments, whereas eye and verbal responses are more useful in those with lesser degrees of impairment. In individual patients, the clinical findings in the 3 components should, therefore, be reported separately. The total score communicates a useful summary overall index, but with some loss of information.
In both preverbal and verbal pediatric patients, the GCS is an accurate marker for clinically significant traumatic brain injury (ie, injury requiring neurosurgical intervention, intubation for over 24 hours, hospitalization for more than 2 nights, or causing death).[6] The GCS has been incorporated into numerous guidelines and assessment scores, including trauma guidelines (eg, Advanced Trauma Life Support), Brain Trauma Foundation guidelines for severe traumatic brain injury, intensive care scoring systems (eg, APACHE II, SOFA), and Advanced Cardiac Life Support.
A motor score of 3 reflects dysfunction/injury to the cerebral hemispheres, where loss of corticospinal input above the midbrain disinhibits reflexive flexor responses, producing the decorticate posturing. In contrast, a score of 2 indicates a dysfunction/lesion from the midbrain through the upper pons, where interruption of midbrain reticular (and possibly red nucleus) inhibition leads to disinhibition of vestibulospinal and pontine reticular pathways, resulting in decerebrate posturing.[9]
Relation to Outcome
A relationship between assessments of the GCS (typically reported as the total GCS Score) and the outcome was shown clearly by Gennarelli et al [17], who demonstrated the existence of a continuous, progressive association between increasing mortality after a head injury and decreases in GCS Score from 15 to 3. This association has been observed in numerous subsequent studies. The findings for the eye, verbal, and motor responses also relate to the outcome but in distinctive ways, so that assessment of each separately yields more information than the aggregate total score.[16]
Although widely recognized as one of the most powerful clinical prognostic indicators, the GCS score—or any single clinical feature—should not be used in isolation to predict an individual patient's outcome. Several factors influence the prognostic value of the GCS score, including the underlying diagnosis, the mechanism of injury in trauma cases, and the presence of extracranial injuries.[18] Patient-specific variables, eg, age, pupillary abnormalities, and imaging results, also affect outcome predictions. The GCS score plays a central role in multifactorial prognostic models, eg, those developed in the IMPACT and CRASH trials, which integrate these additional clinical indicators.[18][19]
Glasgow Coma Scale Pupils Score
The Glasgow Coma Scale Pupils Score (GCS-P) was described by Paul Brennan, Gordon Murray, and Graham Teasdale in 2018 as a strategy to combine the 2 key indicators of the severity of traumatic brain injury into a single, simple index.[20][21]
The GCS-P score is calculated by subtracting the Pupil Reactivity Score (PRS) from the total GCS score: GCS-P = GCS – PRS. The PRS reflects the number of unreactive pupils in response to light as follows:
-
Both pupils unreactive: PRS = 2
-
One pupil unreactive: PRS = 1
-
Neither pupil unreactive: PRS = 0
The GCS-P score can range from 1 to 15, extending the range over which early severity can be shown to relate to outcomes, including mortality or independent recovery.
The MOST (mortality score for traumatic brain injury) integrates the GCS components, pupillary reactivity, and age. Specifically, MOST assigns weighted values to each component, and based on the sum of the values, a conversion table predicts the mortality risk. The AUC of the model in the United States trauma population was 0.875, outperforming CRASH-Basic (AUC = 0.837) and IMPACT-Core (AUC = 0.821) models.[22]
Classification of Severity of Traumatic Brain Injury
The relationship between the GCS score and outcome is the basis for the following common classification of acute traumatic brain injury:
- Severe: GCS 3 to 8
- Moderate: GCS 9 to 12
- Mild: GCS 13 to 15
The GCS-P score values between 1 and 8 denote a severe injury.
In pediatric head injury, a GCS threshold of ≤5 identifies more accurately the severe injury than the adult cutoff of 8, because pediatric TBI patients with scores of 3 to 5 exhibit markedly higher mortality and morbidity.[23]
Other Issues
The reliability of the GCS has been extensively studied. Although its reproducibility has been questioned in a small number of reports, these have proved to be exceptions. Thus, a systematic review of all 53 published reports in 2016 concluded that 85% of the findings in higher-quality studies showed substantial reliability, as judged by the standard criterion of a kappa statistic (k) >0.6.[15] The reproducibility of the total GCS score was also high, with a kappa >0.6 in 77% of observations. An apparent beneficial effect on reliability was observed as a result of education and training. To promote this initiative, a standardized, structured approach to assessment has been established.[5]
Alternatives to the GCS have been proposed, typically by simplifying its components or incorporating additional features. The Simplified Motor Scale, for example, recognizes only 3 levels of motor response. While this may suffice for binary clinical decisions—such as whether to intubate—in prehospital and emergency settings, it does not outperform the GCS in predicting early mortality.[24][25] Scales with fewer components inevitably provide less information and cannot match the GCS or GCS-P in distinguishing degrees of early severity, tracking individual patient progress, or correlating with diverse long-term outcomes.
More complex alternatives include the FOUR score, developed for neurological intensive care.[26] In addition to eye and motor responses adapted from the GCS, the FOUR score incorporates brainstem and respiratory components. While pupil reactivity—part of the brainstem evaluation—is a well-established prognostic indicator, the contributions of corneal and cough reflexes remain unclear. The respiratory subscale evaluates breathing patterns, though this measure lacks consistency due to influences such as extracranial factors, sedation, and ventilatory technique. No systematic review has yet compared the reliability and prognostic accuracy of the FOUR score with the GCS. However, most studies have found no significant differences, and supplementing the GCS with pupil reactivity improves its performance compared to the FOUR score. Nevertheless, FOUR offers a broader neurological assessment, especially valuable for intubated patients in intensive care.
A systematic review has not compared the reliability and prognostic yield of the FOUR Score and the GCS score. Nevertheless, most studies have not shown a significant difference [27], and the addition of information about pupil response to the GCS is expected to increase its performance relative to the FOUR score.[25] However, FOUR provides a more comprehensive neurological assessment, particularly in intubated patients in intensive care units.[28]
The GCS-PA charts combine prognostic information from the GCS, pupil response, imaging findings, and the patient’s age in a simple, visual format that is easy to understand.[26] They provide a user-friendly predictive tool that balances between the simplicity but limited information in a ‘score’ and the more precise but more complex calculations of multivariate models.
The GCS does not assess brainstem reflexes, which may delay recognition of herniation syndromes and brainstem injury. Subjectivity in applying painful stimuli and equal weighting of component scores can obscure critical neurologic changes. Awareness of these limitations can prompt supplemental assessments and training to improve reliability and patient safety.[29]
The GCS demonstrates face validity by formalizing responsiveness assessments that clinicians routinely perform in practice. Criterion validity remains lacking, as no universally accepted reference standard exists to confirm its accuracy; in fact, the GCS often serves as the reference against which other scales are compared. The scale shows moderate construct validity, evidenced by consistent correlations with both mortality and functional recovery outcomes. However, its content validity is limited, as the GCS does not capture the full range of central nervous system functions.[30]
Enhancing Healthcare Team Outcomes
Effective use of the GCS in clinical settings demands coordinated interprofessional strategies, clearly defined responsibilities, and consistent communication among healthcare professionals. Physicians, advanced practitioners, nurses, pharmacists, emergency medical technicians, and allied health staff all play critical roles in patient-centered care, especially when managing noncommunicative patients. Variability in pain stimulus techniques and inconsistent reporting formats compromise the reliability of GCS scores. International surveys have shown that at least 5 different painful stimuli are commonly used to assess motor responses, leading to reduced comparability between assessments. Standardizing these practices through interprofessional education significantly enhances inter-rater reliability and clinician confidence in GCS use, thereby improving patient safety and outcome tracking.[31]
Every team member must understand the GCS and its clinical implications. Emergency medical technicians assess and communicate initial scores, setting the stage for urgent decision-making. Nurses and advanced practitioners document the eye, verbal, and motor components individually, followed by the total score, and record all findings in the electronic health record, including the date and time, for trend monitoring. Physicians interpret patterns, correlate GCS trends with diagnostic findings, and initiate appropriate airway, imaging, and transfer protocols. Pharmacists may contribute by advising on sedation regimens that could influence consciousness levels. Routine and consistent scoring facilitates timely neurosurgical consultations, escalates care when necessary, and informs discussions with patients’ families about prognosis. This collaborative, protocol-driven approach not only enhances team performance but also ensures safe, transparent, and patient-centered care.
Media
(Click Image to Enlarge)
References
Teasdale G, Jennett B. Assessment of coma and impaired consciousness. A practical scale. Lancet (London, England). 1974 Jul 13:2(7872):81-4 [PubMed PMID: 4136544]
Teasdale G, Murray G, Parker L, Jennett B. Adding up the Glasgow Coma Score. Acta neurochirurgica. Supplementum. 1979:28(1):13-6 [PubMed PMID: 290137]
Teasdale GM, Drake CG, Hunt W, Kassell N, Sano K, Pertuiset B, De Villiers JC. A universal subarachnoid hemorrhage scale: report of a committee of the World Federation of Neurosurgical Societies. Journal of neurology, neurosurgery, and psychiatry. 1988 Nov:51(11):1457 [PubMed PMID: 3236024]
Level 3 (low-level) evidenceGrinnon ST, Miller K, Marler JR, Lu Y, Stout A, Odenkirchen J, Kunitz S. National Institute of Neurological Disorders and Stroke Common Data Element Project - approach and methods. Clinical trials (London, England). 2012 Jun:9(3):322-9. doi: 10.1177/1740774512438980. Epub 2012 Feb 27 [PubMed PMID: 22371630]
Teasdale G, Maas A, Lecky F, Manley G, Stocchetti N, Murray G. The Glasgow Coma Scale at 40 years: standing the test of time. The Lancet. Neurology. 2014 Aug:13(8):844-54. doi: 10.1016/S1474-4422(14)70120-6. Epub [PubMed PMID: 25030516]
Borgialli DA, Mahajan P, Hoyle JD Jr, Powell EC, Nadel FM, Tunik MG, Foerster A, Dong L, Miskin M, Dayan PS, Holmes JF, Kuppermann N, Pediatric Emergency Care Applied Research Network (PECARN). Performance of the Pediatric Glasgow Coma Scale Score in the Evaluation of Children With Blunt Head Trauma. Academic emergency medicine : official journal of the Society for Academic Emergency Medicine. 2016 Aug:23(8):878-84. doi: 10.1111/acem.13014. Epub 2016 Aug 1 [PubMed PMID: 27197686]
Zhang JK, Dinh TU, Teasdale G, Mercier P, Mattei TA. The Message of the Glasgow Coma Scale: A Comprehensive Bibliometric Analysis and Systematic Review of Clinical Practice Guidelines Spanning the Past 50 years. World neurosurgery. 2024 May:185():393-402.e27. doi: 10.1016/j.wneu.2024.02.139. Epub 2024 Mar 2 [PubMed PMID: 38437980]
Level 1 (high-level) evidencevan der Naalt J. [Physical diagnosis--the Glasgow coma scale for the measurement of disturbances of consciousness]. Nederlands tijdschrift voor geneeskunde. 2004 Mar 6:148(10):472-6 [PubMed PMID: 15042892]
Matis G, Birbilis T. The Glasgow Coma Scale--a brief review. Past, present, future. Acta neurologica Belgica. 2008 Sep:108(3):75-89 [PubMed PMID: 19115670]
Reilly PL, Simpson DA, Sprod R, Thomas L. Assessing the conscious level in infants and young children: a paediatric version of the Glasgow Coma Scale. Child's nervous system : ChNS : official journal of the International Society for Pediatric Neurosurgery. 1988 Feb:4(1):30-3 [PubMed PMID: 3135935]
James HE. Neurologic evaluation and support in the child with an acute brain insult. Pediatric annals. 1986 Jan:15(1):16-22 [PubMed PMID: 3951884]
Bodien YG, Barra A, Temkin NR, Barber J, Foreman B, Vassar M, Robertson C, Taylor SR, Markowitz AJ, Manley GT, Giacino JT, Edlow BL, TRACK-TBI Investigators. Diagnosing Level of Consciousness: The Limits of the Glasgow Coma Scale Total Score. Journal of neurotrauma. 2021 Dec:38(23):3295-3305. doi: 10.1089/neu.2021.0199. Epub [PubMed PMID: 34605668]
DiGiorgio AM, Wittenberg BA, Crutcher CL 2nd, Kennamer B, Greene CS, Velander AJ, Wilson JD, Tender GC, Culicchia F, Hunt JP. The Impact of Drug and Alcohol Intoxication on Glasgow Coma Scale Assessment in Patients with Traumatic Brain Injury. World neurosurgery. 2020 Mar:135():e664-e670. doi: 10.1016/j.wneu.2019.12.095. Epub 2019 Dec 24 [PubMed PMID: 31881342]
Brennan PM, Murray GD, Teasdale GM. A practical method for dealing with missing Glasgow Coma Scale verbal component scores. Journal of neurosurgery. 2021 Jul 1:135(1):214-219. doi: 10.3171/2020.6.JNS20992. Epub 2020 Sep 8 [PubMed PMID: 32898843]
Prasad K, Menon GR. Comparison of the three strategies of verbal scoring of the Glasgow Coma Scale in patients with stroke. Cerebrovascular diseases (Basel, Switzerland). 1998 Mar-Apr:8(2):79-85 [PubMed PMID: 9548004]
Reith FCM, Lingsma HF, Gabbe BJ, Lecky FE, Roberts I, Maas AIR. Differential effects of the Glasgow Coma Scale Score and its Components: An analysis of 54,069 patients with traumatic brain injury. Injury. 2017 Sep:48(9):1932-1943. doi: 10.1016/j.injury.2017.05.038. Epub 2017 Jun 1 [PubMed PMID: 28602178]
Gennarelli TA, Champion HR, Copes WS, Sacco WJ. Comparison of mortality, morbidity, and severity of 59,713 head injured patients with 114,447 patients with extracranial injuries. The Journal of trauma. 1994 Dec:37(6):962-8 [PubMed PMID: 7996612]
Steyerberg EW, Mushkudiani N, Perel P, Butcher I, Lu J, McHugh GS, Murray GD, Marmarou A, Roberts I, Habbema JD, Maas AI. Predicting outcome after traumatic brain injury: development and international validation of prognostic scores based on admission characteristics. PLoS medicine. 2008 Aug 5:5(8):e165; discussion e165. doi: 10.1371/journal.pmed.0050165. Epub [PubMed PMID: 18684008]
Level 1 (high-level) evidenceMRC CRASH Trial Collaborators, Perel P, Arango M, Clayton T, Edwards P, Komolafe E, Poccock S, Roberts I, Shakur H, Steyerberg E, Yutthakasemsunt S. Predicting outcome after traumatic brain injury: practical prognostic models based on large cohort of international patients. BMJ (Clinical research ed.). 2008 Feb 23:336(7641):425-9. doi: 10.1136/bmj.39461.643438.25. Epub 2008 Feb 12 [PubMed PMID: 18270239]
Level 2 (mid-level) evidenceBrennan PM, Murray GD, Teasdale GM. Simplifying the use of prognostic information in traumatic brain injury. Part 1: The GCS-Pupils score: an extended index of clinical severity. Journal of neurosurgery. 2018 Jun:128(6):1612-1620. doi: 10.3171/2017.12.JNS172780. Epub 2018 Apr 10 [PubMed PMID: 29631516]
Reith FC, Van den Brande R, Synnot A, Gruen R, Maas AI. The reliability of the Glasgow Coma Scale: a systematic review. Intensive care medicine. 2016 Jan:42(1):3-15. doi: 10.1007/s00134-015-4124-3. Epub 2015 Nov 12 [PubMed PMID: 26564211]
Level 1 (high-level) evidenceKarabacak M, Jagtiani P, Dams-O'Connor K, Legome E, Hickman ZL, Margetis K. The MOST (Mortality Score for TBI): A novel prediction model beyond CRASH-Basic and IMPACT-Core for isolated traumatic brain injury. Injury. 2025 Jan:56(1):111956. doi: 10.1016/j.injury.2024.111956. Epub 2024 Oct 15 [PubMed PMID: 39428266]
Ghaffarpasand F, Razmkon A, Dehghankhalili M. Glasgow Coma Scale Score in Pediatric Patients with Traumatic Brain Injury; Limitations and Reliability. Bulletin of emergency and trauma. 2013 Oct:1(4):135-6 [PubMed PMID: 27162843]
Haukoos JS, Gill MR, Rabon RE, Gravitz CS, Green SM. Validation of the Simplified Motor Score for the prediction of brain injury outcomes after trauma. Annals of emergency medicine. 2007 Jul:50(1):18-24 [PubMed PMID: 17113193]
Level 1 (high-level) evidenceTeasdale GM, Stocchetti N, Maas AI, Murray GD. Predicting Mortality in Critically Ill Patients. Critical care medicine. 2015 Oct:43(10):e471-2. doi: 10.1097/CCM.0000000000001153. Epub [PubMed PMID: 26376275]
Murray GD, Brennan PM, Teasdale GM. Simplifying the use of prognostic information in traumatic brain injury. Part 2: Graphical presentation of probabilities. Journal of neurosurgery. 2018 Jun:128(6):1621-1634. doi: 10.3171/2017.12.JNS172782. Epub 2018 Apr 10 [PubMed PMID: 29631517]
Ghelichkhani P, Esmaeili M, Hosseini M, Seylani K. Glasgow Coma Scale and FOUR Score in Predicting the Mortality of Trauma Patients; a Diagnostic Accuracy Study. Emergency (Tehran, Iran). 2018:6(1):e42 [PubMed PMID: 30584558]
Brun FK, Fagertun VH, Larsen MH, Solberg MT. Comparison of Glasgow Coma Scale and Full Outline of UnResponsiveness score to assess the level of consciousness in patients admitted to intensive care units and emergency departments: A quantitative systematic review. Australian critical care : official journal of the Confederation of Australian Critical Care Nurses. 2025 Jan:38(1):101057. doi: 10.1016/j.aucc.2024.03.012. Epub 2024 May 22 [PubMed PMID: 38777642]
Level 1 (high-level) evidenceAndraos C, Siddiqi A, Brazdzionis J, Siddiqi J. Limitations of the Glasgow Coma Scale: Challenges and Considerations. Cureus. 2025 Feb:17(2):e78900. doi: 10.7759/cureus.78900. Epub 2025 Feb 12 [PubMed PMID: 40091938]
Level 2 (mid-level) evidenceGill MR, Reiley DG, Green SM. Interrater reliability of Glasgow Coma Scale scores in the emergency department. Annals of emergency medicine. 2004 Feb:43(2):215-23 [PubMed PMID: 14747811]
Reith FC, Brennan PM, Maas AI, Teasdale GM. Lack of Standardization in the Use of the Glasgow Coma Scale: Results of International Surveys. Journal of neurotrauma. 2016 Jan 1:33(1):89-94. doi: 10.1089/neu.2014.3843. Epub 2015 Aug 12 [PubMed PMID: 25951090]
Level 3 (low-level) evidence