Introduction
Gastrointestinal stromal tumors (GISTs) are rare mesenchymal neoplasms of the gastrointestinal tract, most frequently driven by activating mutations in the KIT or platelet-derived growth factor receptor alpha (PDGFRAgenes. The estimated incidence is approximately 10 to 15 cases per million individuals annually, with a predilection for presentation between the fifth and seventh decades of life. GISTs originate from the interstitial cells of Cajal and are characterized by constitutive activation of receptor tyrosine kinases, resulting in unregulated cellular proliferation and survival.[1][2]
Histopathologically, GISTs demonstrate spindle cell, epithelioid, or mixed morphology and typically exhibit immunohistochemical positivity for KIT (CD117) and DOG1. Clinical manifestations are often nonspecific and may include vague abdominal discomfort, occult or overt gastrointestinal bleeding, early satiety, or detection of a palpable abdominal mass.[3]
Diagnostic evaluation encompasses contrast-enhanced computed tomography (CT) or magnetic resonance imaging (MRI), endoscopic ultrasonography with tissue acquisition, and mutational profiling to inform targeted therapeutic strategies. Management is dictated by tumor size, anatomical location, mitotic index, and molecular alterations. Complete surgical resection with negative margins remains the cornerstone of treatment for localized disease. Although radiotherapy has a limited role, it may be used for palliation in select cases of advanced disease.[4]
Systemic therapy has been revolutionized by the advent of tyrosine kinase inhibitors (TKIs), with imatinib serving as first-line treatment for advanced, unresectable, or metastatic GIST, significantly improving survival outcomes. Risk stratification for recurrence and the need for adjuvant therapy is based on a combination of tumor size, mitotic rate, and location. The integration of molecular diagnostics, precision therapeutics, and interprofessional oncologic care has markedly improved the prognosis for patients with GIST.[5][6]
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
GISTs originate from the interstitial cells of Cajal, specialized pacemaker cells situated within the muscularis propria layer of the gastrointestinal tract. These cells help regulate gut motility by coordinating smooth muscle contractions. GISTs most commonly originate in the stomach (60%) and small intestine (30%), but they can also develop—less frequently—in the colon, rectum, esophagus, or even outside the gastrointestinal tract (eg, mesentery or omentum), in which case they are called extraintestinal GISTs.[1][2]
Mutations of the KIT (CD117) or PDGFRA have been shown to lead to the activation of their encoded tyrosine kinase receptors, causing constitutive activation in approximately 85% of sporadic cases of GISTs. This activation leads to hyperplasia, which ultimately progresses to neoplasia. In addition to being responsible for the majority of sporadic cases of GISTs, KIT and PDGFRA mutations can also be inherited, leading to the much rarer familial GISTs.[3] GISTs without KIT or PDGFRA are collectively known as wild-type. This group comprises a heterogeneous mix of mutations, including NF1, BRAF, HRAS, and can be seen in tumor syndromes, eg, neurofibromatosis type 1, Carney triad (GIST, paraganglioma, and pulmonary chondroma), and Carney-Stratakis syndrome (GIST and paraganglioma).[4][5][6][7][8]
Epidemiology
GISTs have an estimated annual incidence in the United States of approximately 0.68 to 0.78 cases per 100,000 individuals. Although GISTs can develop throughout the gastrointestinal tract, the stomach accounts for the majority of cases (approximately 60%), followed by the small intestine (around 30%). Less commonly, they may arise in the duodenum (4% to 5%) or rectum (4%), with rare occurrences in the colon, appendix (1% to 2%), or esophagus (<1%). On rare occasions, GISTs have also been identified in extraintestinal locations.[9][10][11][12][13][14]
Pathophysiology
GISTs are typically slow-growing mesenchymal neoplasms that arise from the interstitial cells of Cajal, with approximately 70% considered benign at the time of diagnosis. However, all GISTs carry malignant potential, and their biological behavior depends on factors, eg, tumor size, mitotic rate, and anatomic location. Most cases are driven by activating mutations in the KIT proto-oncogene (approximately 75% to 80%) or PDGFRA (approximately 5% to 10%), resulting in constitutive tyrosine kinase signaling and unchecked cellular proliferation.[1][15]
GISTs most commonly arise in the stomach and small intestine but may also occur anywhere along the gastrointestinal tract or, in rare instances, in extraintestinal locations. Histologically, they exhibit spindle cell or epithelioid morphology and frequently stain positive for KIT (CD117) and DOG1, which aid in diagnosis. While lymphatic spread is rare, malignant GISTs most often metastasize hematogenously, especially to the liver or peritoneum. Tumors larger than 5 cm, those with high mitotic activity (>5 mitoses/50 high-power fields [HPF]), and those arising outside the stomach (eg, small intestine) are associated with a higher risk of recurrence or metastasis.[16][17] Recognizing these features is essential for risk stratification, prognosis, and guiding decisions regarding adjuvant therapy.
Histopathology
Histologic Diagnostic Studies
On gross examination, GISTs typically appear as well-circumscribed, submucosal masses arising from the muscularis propria, often with a smooth, lobulated surface. Cut sections may reveal a tan to gray-white, fleshy appearance with areas of hemorrhage, cystic degeneration, or necrosis in larger or more aggressive tumors. Microscopically, GISTs are categorized into 3 major histologic subtypes: spindle cell (the most common), epithelioid, or mixed types.[14] Spindle cell type GISTs are described as having cells arranged in short fascicles or whirls, whereas epithelioid cell GISTs have cells arranged in a diffuse or nested pattern. Mixed cell-type GISTs incorporate both spindle cell and epithelioid cell histologic patterns.[5]
The mitotic index, assessed as the number of mitoses per 5 mm² (approximately 50 HPFs), is a key prognostic feature, with a value greater than 5 mitoses per 5 mm² associated with an increased risk of recurrence.[18] Immunohistochemically, GISTs typically express KIT (CD117), which is a highly sensitive and specific diagnostic marker. Approximately 95% of GISTs are positive for KIT staining. For the remaining 5%, anoctamin 1, also known as diagnosed on GIST 1 (DOG1), along with CD34, is considered diagnostic, provided the appropriate morphologic features are present.[4]
Histologic Risk Stratification and Staging
Risk stratification and staging are based on a combination of tumor size, mitotic rate, and anatomic location.[3] Unlike most gastrointestinal malignancies, GISTs rarely metastasize to lymph nodes, and TNM (tumor, node, metastasis) staging is less commonly employed. Instead, recurrence risk stratification guides decisions on adjuvant therapy. Tumor size and mitotic index are key histopathologic features used to assess the risk of malignancy in GISTs. However, these factors alone do not fully capture the tumor’s biological behavior.
In a large cohort study of 1,765 patients with gastric GISTs, Miettinen et al found that tumors larger than 10 cm with high mitotic activity (>5 mitoses/50 HPF) had an 86% risk of metastasis. In contrast, those of the same size but with lower mitotic activity (<5 mitoses/50 HPFs) showed a significantly lower metastatic rate of 11%.[19] Similarly, in small intestinal GISTs, even those with low mitotic activity exhibited a higher risk of metastasis—approximately 50% for tumors larger than 10 cm—highlighting the more aggressive nature of GISTs outside the stomach.[14][20] As a result, Miettinen and colleagues developed a risk stratification model that incorporates not only tumor size and mitotic count but also the primary site of the tumor (see Table. Risk Classification of GISTs Using Modified National Institute of Health Criteria). According to these guidelines, small gastric GISTs (≤2 cm) typically behave benignly, regardless of mitotic rate, whereas tumors of similar size in the small intestine or rectum exhibit greater malignant potential. Notably, even rectal GISTs less than 2 cm with elevated mitotic activity carry a higher risk of recurrence and aggressive behavior.
Table. Risk Classification of GISTs Using Modified National Institute of Health Criteria
| Tumor Size | Mitotic Rate | Anatomic Location | Risk Stratification |
| ≤2 | ≤5 | Any | Very Low |
| 2–5 | ≤5 | Gastric | Low |
| 2–5 | ≤5 | Small intestine/Rectum | Intermediate |
| 5–10 | ≤5 | Gastric | Intermediate |
| 5–10 | ≤5 | Small intestine/Rectum | High |
| >10 | ≤5 | Any | High |
| Any size | >5 | Gastric | High |
| Any size | >5 | Small intestine/Rectum | High |
History and Physical
Clinical History
Patients with a suspected GIST can present with a range of nonspecific symptoms, depending on the tumor’s size, location, and extent of progression. Complaints may include early satiety, abdominal fullness, or discomfort due to mass effect, gastrointestinal bleeding, or fatigue from chronic anemia. Some individuals may exhibit signs of acute intra-abdominal events, eg, hemorrhage or obstruction, and in rare cases, may present emergently with severe abdominal pain resembling peritonitis, requiring urgent evaluation.
Physical Examination
On physical examination, findings may be minimal; however, a palpable abdominal mass or signs of anemia (eg, pallor or tachycardia) may be present in symptomatic patients. While GISTs primarily spread to the liver and peritoneum, lymphatic metastases are uncommon, except in rare histologic variants of GIST. Involvement of the lungs, bones, or other distant sites typically occurs only in advanced stages of the disease. Notably, in 15% to 30% of cases, GISTs are found incidentally on surgery, imaging, or autopsy.[6][7]
Evaluation
The diagnostic workup for GISTs involves an interprofessional approach that integrates imaging, tissue sampling, and histopathologic analysis. Imaging plays a pivotal role not only in the initial detection and staging of GISTs but also in monitoring response to therapy, evaluating for recurrence, and guiding long-term surveillance strategies. Ultimately, the interpretation of imaging findings must be integrated with clinical presentation, cytologic or histologic analysis, and immunohistochemical staining—particularly for KIT (CD117) and DOG1—to establish a definitive diagnosis and guide therapeutic decision-making within an interprofessional care framework.[7]
Computed Tomography
Contrast-enhanced computed tomography (CT) remains the first-line imaging modality for evaluating suspected or biopsy-confirmed GISTs. It offers high spatial resolution and is particularly effective in delineating tumor size, location, and morphology, while also enabling detection of metastatic spread, most commonly to the liver and peritoneum. CT is the modality of choice for perioperative planning and follow-up, given its reproducibility and ability to assess extraintestinal disease.
Magnetic Resonance Imaging
Magnetic resonance imaging (MRI) serves as an essential adjunct, especially for the characterization of hepatic metastases, where it offers superior contrast resolution compared to CT. MRI is also preferred in specific clinical scenarios, eg, pelvic GISTs, iodinated contrast allergy, or impaired renal function. Although CT remains more sensitive and specific for detecting peritoneal and extrahepatic metastases in the context of initial diagnosis and staging, MRI provides greater sensitivity in assessing hepatic involvement.
Positron Emission Tomography
Positron emission tomography, typically combined with CT (PET/CT), may be employed selectively to distinguish viable tumor from necrotic or fibrotic tissue, to clarify indeterminate findings on conventional imaging, or to detect occult disease. PET/CT is particularly valuable in assessing early treatment response, as metabolic changes often precede morphologic alterations in tumor burden. Nevertheless, the routine use of PET/CT is limited, and this study is not considered a substitute for standard CT imaging. PET/CT may be most beneficial in patients with contraindications to intravenous contrast or in those with diffuse peritoneal involvement.
Endoscopic Studies
Endoscopic evaluation, particularly upper gastrointestinal endoscopy, can facilitate the detection of GISTs in the stomach or proximal small intestine. Despite their origin in the muscularis propria, these tumors often manifest as subepithelial masses with overlying mucosal ulceration or intact mucosa. However, superficial mucosal biopsies are frequently nondiagnostic due to the deep location of the tumor.
Endoscopic ultrasound (EUS) enhances diagnostic yield by providing high-resolution imaging of the gastrointestinal wall layers and enabling fine-needle aspiration or core needle biopsy. On EUS, GISTs typically appear as hypoechoic lesions arising from the fourth (muscularis propria) or occasionally the second (muscularis mucosa) layer. This modality aids in differentiating GISTs from other subepithelial lesions, eg, leiomyomas, lipomas, or neuroendocrine tumors.[21]
Treatment / Management
A combination of tumor size, location, mitotic activity, and presence or risk of metastasis guides the management of GISTs. Treatment decisions are typically determined by an interprofessional team and incorporate both surgical and medical strategies. For localized, resectable tumors, complete surgical excision with negative margins remains the primary treatment modality. However, due to the risk of recurrence—particularly in tumors with high-risk features—adjuvant therapy with TKIs (eg, imatinib) is often employed. In cases of unresectable, metastatic, or recurrent disease, systemic therapy becomes the cornerstone of treatment, with the choice of agent influenced by the tumor’s mutational profile.[1][22](A1)
Differential Diagnosis
The differential diagnosis for gastrointestinal stromal tumors is quite broad and is largely determined by a patient’s signs and symptoms as well as the diagnostic information obtained previously. In patients presenting with gastrointestinal bleeding or anemia, the differential diagnoses include other malignancies of the alimentary tract, diverticular bleed, vascular lesions, inflammatory bowel disease, peptic ulcer disease, gastritis, or esophagitis. In patients whose presentation is consistent with the mass effects of an advanced tumor, the differential diagnoses would include other causes of intra-abdominal malignancy. In patients found to have subepithelial mass lesions on endoscopy, the differential diagnosis would include leiomyomas, leiomyosarcomas, schwannomas, lipomas, and melanomas.[5][7][8] Differential diagnoses that should be considered when evaluating patients with potential GISTs include:
- Epithelioid hemangioendothelioma
- Fibromatosis
- Metastatic melanoma
- Lymphoma
- Schwannoma
- Leiomyoma
- Benign neoplasms of the small intestine
- Dermatofibroma
- Gastric cancer
- Leiomyosarcoma
- Lipomas
- Solitary fibrous tumor
- Inflammatory bowel disease
- Diverticulosis
- Gastritis
- Peptic ulcer disease
Surgical Oncology
Surgical resection remains the cornerstone of curative-intent treatment for localized GISTs. The primary goal of surgery is complete resection (R0) of the tumor without rupturing the pseudocapsule or leaving microscopic residual disease, as tumor rupture increases the risk of recurrence and is related to poorer prognosis.
Evaluation for Surgery
Patients with localized, resectable GISTs, particularly those without evidence of metastatic spread on imaging, should be evaluated for surgery as the initial therapeutic approach. Preoperative staging with contrast-enhanced CT or MRI helps determine tumor size, relationship to adjacent organs, and potential for complete resection. As stated earlier in this review, EUS can be helpful for tumors near the gastroesophageal junction or rectum. In patients with borderline resectable or high-risk tumors in anatomically challenging locations, neoadjuvant imatinib may be considered to reduce tumor size, facilitate resection, and preserve organ function. Please refer to the Medical Oncology section for more information.
Surgical Techniques and Considerations
Unlike many gastrointestinal cancers, GISTs rarely metastasize to lymph nodes, so routine lymphadenectomy is not required. Surgical management typically involves segmental or wedge resections with a focus on achieving clear margins while avoiding tumor rupture. For small, favorably located gastric GISTs, laparoscopic resection has become an accepted approach, offering comparable oncologic outcomes with lower perioperative morbidity. Minimally invasive surgery is generally reserved for tumors 5 cm or less in size and not adjacent to critical anatomical structures.[7][8][23][24]
For GISTs located in more complex regions, eg, the duodenum, rectum, or esophagogastric junction, open or hybrid approaches may be necessary. In some cases, more extensive resections, eg, pancreaticoduodenectomy or transanal excision, may be required, and careful interprofessional planning is essential. Tumor rupture or positive margins during surgery significantly increase the risk of recurrence and peritoneal dissemination, and may warrant adjuvant imatinib, even in tumors that would otherwise be considered low-risk.[25]
Role of Surgery in Metastatic Disease
Although systemic therapy with TKIs remains the mainstay for metastatic GISTs, surgery may be considered in select cases to improve disease control or palliate symptoms. Resection of limited metastatic disease, particularly isolated liver or peritoneal metastases, may benefit carefully selected patients who have demonstrated a favorable response to imatinib or other systemic agents. The rationale is to remove resistant tumor clones and reduce tumor burden, potentially prolonging progression-free survival. Surgery in this setting is typically considered after at least 6 to 12 months of TKI therapy, once disease stability is confirmed.
Additionally, metastasectomy may be warranted in cases of complications, eg, hemorrhage, obstruction, or tumor rupture. Complete resection (R0) is the goal; however, R1 resection may still offer benefits when gross disease is removed. Importantly, surgery for metastatic GIST should be performed within the context of an interprofessional team, and ongoing systemic therapy is typically continued postoperatively to suppress residual microscopic disease. Routine surgery is not recommended for widespread or rapidly progressive disease, where outcomes are generally poor despite intervention.[26][27]
Radiation Oncology
Radiation therapy is not a standard component of GIST treatment due to the tumor’s general radioresistance and the heightened sensitivity of surrounding abdominal organs to radiation. However, in selected advanced or metastatic cases, especially involving painful bone or fixed abdominal metastases, radiotherapy can provide effective palliation of symptoms, pain control, and local tumor volume reduction. Radiation therapy does not currently have a proven impact on overall survival in GIST. Its use ultimately remains palliative or adjunctive, especially in the context of refractory disease or when additional local control is needed.[28][29]
Medical Oncology
Tyrosine Kinase Inhibitors
The advent of TKIs has markedly transformed the therapeutic landscape of GISTs, shifting management from the limited efficacy of conventional cytotoxic chemotherapy to targeted molecular therapies guided by tumor-specific mutational profiles. Imatinib, a selective inhibitor of KIT and PDGFRA genes that encode receptor tyrosine kinases frequently mutated in GIST, remains the cornerstone of systemic therapy. Clinical practice guidelines from the European Society for Medical Oncology (ESMO), the National Comprehensive Cancer Network (NCCN), and the Japanese Society of Clinical Oncology (JSCO) uniformly endorse imatinib as the standard first-line treatment for patients with metastatic, recurrent, or unresectable GIST.[30][31]
First-Line Therapy
Imatinib serves as the standard initial treatment for unresectable, metastatic, or recurrent GISTs. The pivotal B2222 trial demonstrated that imatinib induced partial responses or disease stabilization in over 80% of patients, resulting in significantly prolonged progression-free survival (PFS) and prompting its accelerated approval by the United States Food and Drug Administration (FDA) in 2002.[32] The EORTC 62005 phase 3 trial subsequently compared daily doses of 400 mg versus 800 mg of imatinib, revealing no significant difference in overall survival. However, the higher dose was associated with a modest improvement in PFS.[33]
With the increasing availability of molecular diagnostics, mutational analysis has become an integral part of treatment planning. For instance, tumors harboring the PDGFRA D842V mutation exhibit primary resistance to imatinib, whereas those with KIT exon 11 mutations demonstrate superior response rates. Accordingly, routine mutational testing is now recommended before initiating therapy.[16]
Adjuvant Therapy
In patients with resected high-risk primary GISTs, adjuvant imatinib has been shown to improve both recurrence-free survival (RFS) and overall survival. The ACOSOG Z9001 randomized, double-blind, placebo-controlled trial established that 1 year of adjuvant imatinib significantly improved RFS in patients with tumors 3 cm or larger.[34] This benefit was extended by the SSG XVIII/AIO trial, which demonstrated that 3 years of adjuvant therapy conferred superior RFS and overall survival compared to 1 year in patients with high-risk features.[35]
Neoadjuvant Therapy
Neoadjuvant imatinib is a valuable option in cases of locally advanced or anatomically complex GISTs where primary surgical resection would result in significant morbidity or organ loss. Neoadjuvant therapy aims to downsize the tumor, facilitate complete resection, and preserve organ function. The RTOG 0132/ACRIN 6665 phase 2 trial provided early evidence that preoperative imatinib administered for 8 to 12 weeks was safe and associated with disease stabilization or partial response in most patients.[36] Importantly, neoadjuvant treatment enabled less extensive resections.
Given the imatinib resistance of PDGFRA D842V-mutant tumors, mutational profiling is essential before initiating neoadjuvant therapy to avoid unnecessary delays in definitive surgical intervention.[37] Tumors located in surgically challenging regions, eg, the rectum, duodenum, or gastroesophageal junction, are particularly well-suited for this approach, as resection in these areas can result in significant functional impairments (eg, a permanent colostomy or pancreaticoduodenectomy). The duration of therapy typically ranges from 6 months to 1 year, with interval imaging performed every 2 to 3 months to guide surgical timing at the point of maximal or plateaued tumor response.
Second- and Third-Line Therapies
For patients who develop resistance to or cannot tolerate imatinib, sunitinib, a multitargeted TKI, represents the second-line standard. A phase 3 trial demonstrated that sunitinib significantly improved time to progression (27.3 weeks versus 6.4 weeks with placebo).[38] Regorafenib, approved as a third-line therapy, was shown in the GRID trial to substantially improve PFS (4.8 months versus 0.9 months with placebo) in patients who had progressed on both imatinib and sunitinib.[39]
Fourth-Line and Subsequent Therapies
Ripretinib, a switch-control TKI targeting a broad range of KIT and PDGFRA mutations, is approved for use as a fourth-line therapy. In the INVICTUS phase 3 trial, ripretinib improved median PFS (6.3 months versus 1 month) and OS (15.1 months versus 6.6 months) compared to placebo in patients with advanced GIST who were refractory to prior therapies.[40] Avapritinib, a selective PDGFRA inhibitor, has shown remarkable activity against the PDGFRA D842V mutation subtype, which is resistant to most TKIs, including imatinib. The NAVIGATOR trial reported an overall response rate of 88% and a manageable safety profile in this population, leading to the drug’s accelerated FDA approval in 2020 for this molecular subset.[37]
Staging
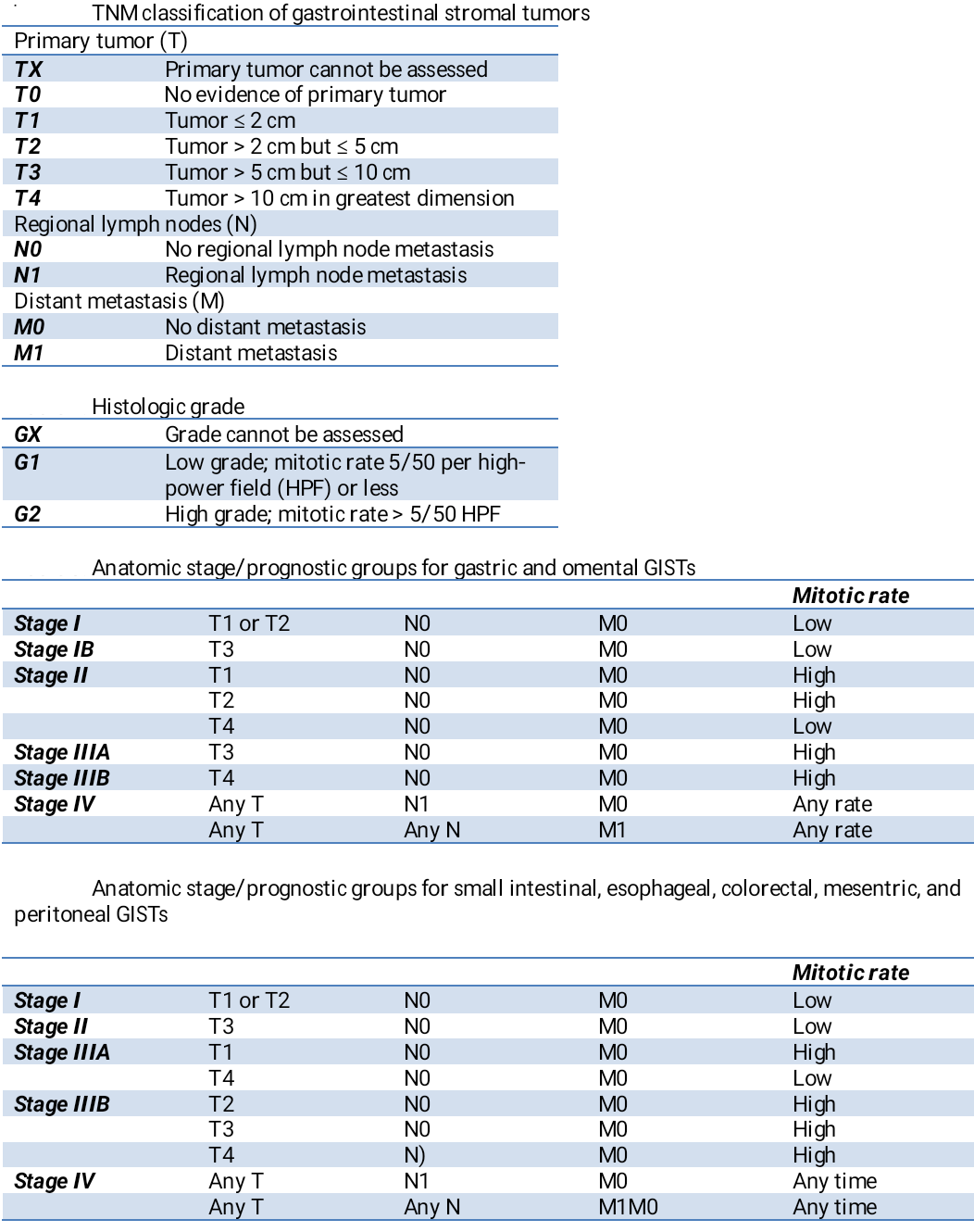
A tumor-node-metastasis (TNM) staging classification system has been developed for gastrointestinal stromal tumors by the Union for International Cancer Control/American Joint Committee on Cancer; however, this classification system is not typically used for GISTs, due to its limitations in capturing the factors shown to impact prognosis (see Table. Tumor, Node, Metastasis Classification of Gastrointestinal Stromal Tumors).[41]
Prognosis
The prognosis for patients with gastrointestinal stromal tumors is based on several characteristics, including:
-
Tumor size and mitotic rate: These are the most powerful predictors of recurrence and survival. Larger tumors (>5 cm) and those with high mitotic activity (>5 mitoses/50 HPF) are associated with worse outcomes.[7]
-
Tumor location: GISTs of the stomach tend to have a more favorable prognosis than those of the small intestine, rectum, or mesentery, even when matched for size and mitotic index.
-
Molecular subtype: Genetic mutations influence both treatment response and prognosis. Tumors with KIT exon 11 mutations generally respond well to imatinib and carry an intermediate risk. In contrast, PDGFRA D842V mutations confer resistance to imatinib and are associated with variable outcomes.[41]
-
R0 resection (complete resection with negative margins): Complete resection significantly improves recurrence-free and overall survival, whereas tumor rupture or incomplete resection markedly increases the risk of peritoneal recurrence.[42]
Complications
General Risks and Complications
The most common complications of GISTs relate to gastrointestinal bleeding or the mass effect of the tumors. These tumors may present with acute gastrointestinal bleeding in the form of melena or hematemesis, or with chronic gastrointestinal bleeding leading to anemia. These tumors can also lead to intestinal obstruction, intraperitoneal hemorrhage, and rupture with peritonitis.[43]
Surgical Complications and Adverse Events
Postoperative complications after GIST resection consist of those related to any operative intervention and can vary depending on tumor location and surgical approach. Some complications may include bleeding, infection, anastomotic leak, or delayed gastric emptying. Tumors near the rectum or gastroesophageal junction may also result in functional morbidity, such as bowel dysfunction or reflux, which can impact quality of life. The most significant oncologic complications include intraoperative tumor rupture and positive surgical margins, both of which are associated with a markedly increased risk of recurrence and are considered high-risk features for adjuvant therapy consideration.[7]
Tyrosine Kinase Inhibitor Adverse Effects
Treatment with TKIs is associated with a wide variety of adverse effects, which are most commonly seen in the first 8 weeks of therapy. The most common adverse effects of imatinib include anemia, edema, fatigue, nausea, pleuritic pain, diarrhea, granulocytopenia, and rash.
Deterrence and Patient Education
Deterrence of GISTs remains limited, as no established preventive strategies exist due to the sporadic nature of most cases and the lack of modifiable risk factors. Unlike other gastrointestinal malignancies, GISTs rarely arise from precursor lesions, and hereditary syndromes account for only a minority of cases. Therefore, deterrence primarily focuses on reducing morbidity and mortality through early recognition of clinical signs, timely diagnostic evaluation, and appropriate management to prevent complications, eg, hemorrhage, obstruction, or tumor rupture. Clinicians play a crucial role in maintaining vigilance, particularly when nonspecific symptoms (eg, abdominal discomfort, anemia, or gastrointestinal bleeding) are present.
Patient education is essential to empower individuals with GIST to participate actively in their care and long-term surveillance. Education should emphasize the importance of reporting new or worsening symptoms, adhering to prescribed TKI therapy, and attending follow-up imaging or laboratory monitoring to assess treatment response and detect recurrence. Patients should be counseled regarding potential adverse effects of therapy, strategies to manage them, and the need for open communication with their care team. By enhancing understanding of the disease, treatment expectations, and follow-up requirements, patient education fosters adherence, reduces anxiety, and supports shared decision-making in the overall management of GIST.
Enhancing Healthcare Team Outcomes
GISTs are rare mesenchymal neoplasms of the gastrointestinal tract, most often driven by mutations in the KIT or PDGFRA genes. They arise from the interstitial cells of Cajal and frequently present with nonspecific symptoms, eg, abdominal pain, gastrointestinal bleeding, or early satiety, although many are discovered incidentally. Diagnosis relies on imaging, endoscopic ultrasound, histopathology, and molecular profiling, while management depends on factors, eg, tumor size, location, mitotic index, and genetic alterations. Surgical resection remains the cornerstone of treatment for localized disease, with tyrosine kinase inhibitors like imatinib transforming outcomes for advanced or unresectable tumors.
Providing patient-centered care for GISTs requires clinicians to integrate specialized skills, including interpretation of diagnostic studies, risk stratification, and precise selection of therapeutic strategies. Physicians, advanced practitioners, and nurses must collaborate with radiologists, pathologists, pharmacists, and genetic counselors to ensure accurate diagnosis, effective treatment, and ongoing surveillance. Responsibilities include monitoring for recurrence, managing treatment-related toxicities, and addressing ethical considerations surrounding therapeutic choices.
Interprofessional communication forms the backbone of effective GIST care. Seamless coordination ensures timely sharing of diagnostic findings, consensus in treatment planning, and continuity in follow-up care. Pharmacists optimize systemic therapy by managing adverse effects and drug interactions, while nurses and advanced practitioners provide symptom management, education, and psychosocial support. Coordinated care strategies that emphasize early recurrence detection, rapid intervention, and supportive management ultimately enhance patient safety, improve outcomes, and strengthen team performance, aligning clinical decisions with patients’ values and goals.
Media
(Click Image to Enlarge)
References
El-Menyar A, Mekkodathil A, Al-Thani H. Diagnosis and management of gastrointestinal stromal tumors: An up-to-date literature review. Journal of cancer research and therapeutics. 2017 Oct-Dec:13(6):889-900. doi: 10.4103/0973-1482.177499. Epub [PubMed PMID: 29237949]
Schaefer IM, Mariño-Enríquez A, Fletcher JA. What is New in Gastrointestinal Stromal Tumor? Advances in anatomic pathology. 2017 Sep:24(5):259-267. doi: 10.1097/PAP.0000000000000158. Epub [PubMed PMID: 28632504]
Level 3 (low-level) evidenceMiettinen M, Lasota J. Histopathology of gastrointestinal stromal tumor. Journal of surgical oncology. 2011 Dec:104(8):865-73. doi: 10.1002/jso.21945. Epub [PubMed PMID: 22069171]
Nishida T, Blay JY, Hirota S, Kitagawa Y, Kang YK. The standard diagnosis, treatment, and follow-up of gastrointestinal stromal tumors based on guidelines. Gastric cancer : official journal of the International Gastric Cancer Association and the Japanese Gastric Cancer Association. 2016 Jan:19(1):3-14. doi: 10.1007/s10120-015-0526-8. Epub 2015 Aug 15 [PubMed PMID: 26276366]
Demetri GD, von Mehren M, Antonescu CR, DeMatteo RP, Ganjoo KN, Maki RG, Pisters PW, Raut CP, Riedel RF, Schuetze S, Sundar HM, Trent JC, Wayne JD. NCCN Task Force report: update on the management of patients with gastrointestinal stromal tumors. Journal of the National Comprehensive Cancer Network : JNCCN. 2010 Apr:8 Suppl 2(0 2):S1-41; quiz S42-4 [PubMed PMID: 20457867]
Joensuu H, Hohenberger P, Corless CL. Gastrointestinal stromal tumour. Lancet (London, England). 2013 Sep 14:382(9896):973-83. doi: 10.1016/S0140-6736(13)60106-3. Epub 2013 Apr 24 [PubMed PMID: 23623056]
Miettinen M, Lasota J. Gastrointestinal stromal tumors: review on morphology, molecular pathology, prognosis, and differential diagnosis. Archives of pathology & laboratory medicine. 2006 Oct:130(10):1466-78 [PubMed PMID: 17090188]
Akahoshi K, Oya M, Koga T, Shiratsuchi Y. Current clinical management of gastrointestinal stromal tumor. World journal of gastroenterology. 2018 Jul 14:24(26):2806-2817. doi: 10.3748/wjg.v24.i26.2806. Epub [PubMed PMID: 30018476]
Hirota S, Isozaki K, Moriyama Y, Hashimoto K, Nishida T, Ishiguro S, Kawano K, Hanada M, Kurata A, Takeda M, Muhammad Tunio G, Matsuzawa Y, Kanakura Y, Shinomura Y, Kitamura Y. Gain-of-function mutations of c-kit in human gastrointestinal stromal tumors. Science (New York, N.Y.). 1998 Jan 23:279(5350):577-80 [PubMed PMID: 9438854]
Level 3 (low-level) evidenceMa GL, Murphy JD, Martinez ME, Sicklick JK. Epidemiology of gastrointestinal stromal tumors in the era of histology codes: results of a population-based study. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2015 Jan:24(1):298-302. doi: 10.1158/1055-9965.EPI-14-1002. Epub 2014 Oct 2 [PubMed PMID: 25277795]
Tran T, Davila JA, El-Serag HB. The epidemiology of malignant gastrointestinal stromal tumors: an analysis of 1,458 cases from 1992 to 2000. The American journal of gastroenterology. 2005 Jan:100(1):162-8 [PubMed PMID: 15654796]
Level 3 (low-level) evidencePerez EA, Livingstone AS, Franceschi D, Rocha-Lima C, Lee DJ, Hodgson N, Jorda M, Koniaris LG. Current incidence and outcomes of gastrointestinal mesenchymal tumors including gastrointestinal stromal tumors. Journal of the American College of Surgeons. 2006 Apr:202(4):623-9 [PubMed PMID: 16571433]
Patel N, Benipal B. Incidence of Gastrointestinal Stromal Tumors in the United States from 2001-2015: A United States Cancer Statistics Analysis of 50 States. Cureus. 2019 Feb 22:11(2):e4120. doi: 10.7759/cureus.4120. Epub 2019 Feb 22 [PubMed PMID: 31037234]
Miettinen M, Lasota J. Gastrointestinal stromal tumors: pathology and prognosis at different sites. Seminars in diagnostic pathology. 2006 May:23(2):70-83 [PubMed PMID: 17193820]
PDGFRA activating mutations in gastrointestinal stromal tumors., Heinrich MC,Corless CL,Duensing A,McGreevey L,Chen CJ,Joseph N,Singer S,Griffith DJ,Haley A,Town A,Demetri GD,Fletcher CD,Fletcher JA,, Science (New York, N.Y.), 2003 Jan 31 [PubMed PMID: 12522257]
Level 3 (low-level) evidenceCorless CL, Barnett CM, Heinrich MC. Gastrointestinal stromal tumours: origin and molecular oncology. Nature reviews. Cancer. 2011 Nov 17:11(12):865-78. doi: 10.1038/nrc3143. Epub 2011 Nov 17 [PubMed PMID: 22089421]
Joensuu H. Risk stratification of patients diagnosed with gastrointestinal stromal tumor. Human pathology. 2008 Oct:39(10):1411-9. doi: 10.1016/j.humpath.2008.06.025. Epub [PubMed PMID: 18774375]
Dematteo RP, Gold JS, Saran L, Gönen M, Liau KH, Maki RG, Singer S, Besmer P, Brennan MF, Antonescu CR. Tumor mitotic rate, size, and location independently predict recurrence after resection of primary gastrointestinal stromal tumor (GIST). Cancer. 2008 Feb 1:112(3):608-15 [PubMed PMID: 18076015]
Miettinen M, Sobin LH, Lasota J. Gastrointestinal stromal tumors of the stomach: a clinicopathologic, immunohistochemical, and molecular genetic study of 1765 cases with long-term follow-up. The American journal of surgical pathology. 2005 Jan:29(1):52-68 [PubMed PMID: 15613856]
Level 3 (low-level) evidenceMiettinen M, Makhlouf H, Sobin LH, Lasota J. Gastrointestinal stromal tumors of the jejunum and ileum: a clinicopathologic, immunohistochemical, and molecular genetic study of 906 cases before imatinib with long-term follow-up. The American journal of surgical pathology. 2006 Apr:30(4):477-89 [PubMed PMID: 16625094]
Level 3 (low-level) evidenceDeprez PH, Moons LMG, OʼToole D, Gincul R, Seicean A, Pimentel-Nunes P, Fernández-Esparrach G, Polkowski M, Vieth M, Borbath I, Moreels TG, Nieveen van Dijkum E, Blay JY, van Hooft JE. Endoscopic management of subepithelial lesions including neuroendocrine neoplasms: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy. 2022 Apr:54(4):412-429. doi: 10.1055/a-1751-5742. Epub 2022 Feb 18 [PubMed PMID: 35180797]
Casali PG, Blay JY, Abecassis N, Bajpai J, Bauer S, Biagini R, Bielack S, Bonvalot S, Boukovinas I, Bovee JVMG, Boye K, Brodowicz T, Buonadonna A, De Álava E, Dei Tos AP, Del Muro XG, Dufresne A, Eriksson M, Fedenko A, Ferraresi V, Ferrari A, Frezza AM, Gasperoni S, Gelderblom H, Gouin F, Grignani G, Haas R, Hassan AB, Hindi N, Hohenberger P, Joensuu H, Jones RL, Jungels C, Jutte P, Kasper B, Kawai A, Kopeckova K, Krákorová DA, Le Cesne A, Le Grange F, Legius E, Leithner A, Lopez-Pousa A, Martin-Broto J, Merimsky O, Messiou C, Miah AB, Mir O, Montemurro M, Morosi C, Palmerini E, Pantaleo MA, Piana R, Piperno-Neumann S, Reichardt P, Rutkowski P, Safwat AA, Sangalli C, Sbaraglia M, Scheipl S, Schöffski P, Sleijfer S, Strauss D, Strauss SJ, Hall KS, Trama A, Unk M, van de Sande MAJ, van der Graaf WTA, van Houdt WJ, Frebourg T, Gronchi A, Stacchiotti S, ESMO Guidelines Committee, EURACAN and GENTURIS. Electronic address: clinicalguidelines@esmo.org. Gastrointestinal stromal tumours: ESMO-EURACAN-GENTURIS Clinical Practice Guidelines for diagnosis, treatment and follow-up. Annals of oncology : official journal of the European Society for Medical Oncology. 2022 Jan:33(1):20-33. doi: 10.1016/j.annonc.2021.09.005. Epub 2021 Sep 21 [PubMed PMID: 34560242]
Level 1 (high-level) evidenceCasali PG, Abecassis N, Aro HT, Bauer S, Biagini R, Bielack S, Bonvalot S, Boukovinas I, Bovee JVMG, Brodowicz T, Broto JM, Buonadonna A, De Álava E, Dei Tos AP, Del Muro XG, Dileo P, Eriksson M, Fedenko A, Ferraresi V, Ferrari A, Ferrari S, Frezza AM, Gasperoni S, Gelderblom H, Gil T, Grignani G, Gronchi A, Haas RL, Hassan B, Hohenberger P, Issels R, Joensuu H, Jones RL, Judson I, Jutte P, Kaal S, Kasper B, Kopeckova K, Krákorová DA, Le Cesne A, Lugowska I, Merimsky O, Montemurro M, Pantaleo MA, Piana R, Picci P, Piperno-Neumann S, Pousa AL, Reichardt P, Robinson MH, Rutkowski P, Safwat AA, Schöffski P, Sleijfer S, Stacchiotti S, Sundby Hall K, Unk M, Van Coevorden F, van der Graaf WTA, Whelan J, Wardelmann E, Zaikova O, Blay JY, ESMO Guidelines Committee and EURACAN. Gastrointestinal stromal tumours: ESMO-EURACAN Clinical Practice Guidelines for diagnosis, treatment and follow-up. Annals of oncology : official journal of the European Society for Medical Oncology. 2018 Oct 1:29(Suppl 4):iv267. doi: 10.1093/annonc/mdy320. Epub [PubMed PMID: 30188977]
Level 1 (high-level) evidenceOhtani H, Maeda K, Noda E, Nagahara H, Shibutani M, Ohira M, Muguruma K, Tanaka H, Kubo N, Toyokawa T, Sakurai K, Yamashita Y, Yamamoto A, Hirakawa K. Meta-analysis of laparoscopic and open surgery for gastric gastrointestinal stromal tumor. Anticancer research. 2013 Nov:33(11):5031-41 [PubMed PMID: 24222147]
Level 1 (high-level) evidenceHohenberger P, Ronellenfitsch U, Oladeji O, Pink D, Ströbel P, Wardelmann E, Reichardt P. Pattern of recurrence in patients with ruptured primary gastrointestinal stromal tumour. The British journal of surgery. 2010 Dec:97(12):1854-9. doi: 10.1002/bjs.7222. Epub 2010 Aug 20 [PubMed PMID: 20730857]
Park SJ, Ryu MH, Ryoo BY, Park YS, Sohn BS, Kim HJ, Kim CW, Kim KH, Yu CS, Yook JH, Kim BS, Kang YK. The role of surgical resection following imatinib treatment in patients with recurrent or metastatic gastrointestinal stromal tumors: results of propensity score analyses. Annals of surgical oncology. 2014 Dec:21(13):4211-7. doi: 10.1245/s10434-014-3866-4. Epub 2014 Jul 1 [PubMed PMID: 24980089]
Yonkus JA, Alva-Ruiz R, Grotz TE. Surgical Management of Metastatic Gastrointestinal Stromal Tumors. Current treatment options in oncology. 2021 Mar 20:22(5):37. doi: 10.1007/s11864-021-00837-0. Epub 2021 Mar 20 [PubMed PMID: 33743084]
Corbin KS, Kindler HL, Liauw SL. Considering the role of radiation therapy for gastrointestinal stromal tumor. OncoTargets and therapy. 2014:7():713-8. doi: 10.2147/OTT.S36873. Epub 2014 May 12 [PubMed PMID: 24872712]
Zhang H, Jiang T, Mu M, Zhao Z, Yin X, Cai Z, Zhang B, Yin Y. Radiotherapy in the Management of Gastrointestinal Stromal Tumors: A Systematic Review. Cancers. 2022 Jun 28:14(13):. doi: 10.3390/cancers14133169. Epub 2022 Jun 28 [PubMed PMID: 35804945]
Level 1 (high-level) evidenceNishida T, Hirota S, Yanagisawa A, Sugino Y, Minami M, Yamamura Y, Otani Y, Shimada Y, Takahashi F, Kubota T, GIST Guideline Subcommittee. Clinical practice guidelines for gastrointestinal stromal tumor (GIST) in Japan: English version. International journal of clinical oncology. 2008 Oct:13(5):416-30. doi: 10.1007/s10147-008-0798-7. Epub 2008 Oct 23 [PubMed PMID: 18946752]
Level 1 (high-level) evidenceCasali PG, Abecassis N, Aro HT, Bauer S, Biagini R, Bielack S, Bonvalot S, Boukovinas I, Bovee JVMG, Brodowicz T, Broto JM, Buonadonna A, De Álava E, Dei Tos AP, Del Muro XG, Dileo P, Eriksson M, Fedenko A, Ferraresi V, Ferrari A, Ferrari S, Frezza AM, Gasperoni S, Gelderblom H, Gil T, Grignani G, Gronchi A, Haas RL, Hassan B, Hohenberger P, Issels R, Joensuu H, Jones RL, Judson I, Jutte P, Kaal S, Kasper B, Kopeckova K, Krákorová DA, Le Cesne A, Lugowska I, Merimsky O, Montemurro M, Pantaleo MA, Piana R, Picci P, Piperno-Neumann S, Pousa AL, Reichardt P, Robinson MH, Rutkowski P, Safwat AA, Schöffski P, Sleijfer S, Stacchiotti S, Sundby Hall K, Unk M, Van Coevorden F, van der Graaf WTA, Whelan J, Wardelmann E, Zaikova O, Blay JY, ESMO Guidelines Committee and EURACAN. Gastrointestinal stromal tumours: ESMO-EURACAN Clinical Practice Guidelines for diagnosis, treatment and follow-up. Annals of oncology : official journal of the European Society for Medical Oncology. 2018 Oct 1:29(Suppl 4):iv68-iv78. doi: 10.1093/annonc/mdy095. Epub [PubMed PMID: 29846513]
Level 1 (high-level) evidenceDemetri GD, von Mehren M, Blanke CD, Van den Abbeele AD, Eisenberg B, Roberts PJ, Heinrich MC, Tuveson DA, Singer S, Janicek M, Fletcher JA, Silverman SG, Silberman SL, Capdeville R, Kiese B, Peng B, Dimitrijevic S, Druker BJ, Corless C, Fletcher CD, Joensuu H. Efficacy and safety of imatinib mesylate in advanced gastrointestinal stromal tumors. The New England journal of medicine. 2002 Aug 15:347(7):472-80 [PubMed PMID: 12181401]
Verweij J, Casali PG, Zalcberg J, LeCesne A, Reichardt P, Blay JY, Issels R, van Oosterom A, Hogendoorn PC, Van Glabbeke M, Bertulli R, Judson I. Progression-free survival in gastrointestinal stromal tumours with high-dose imatinib: randomised trial. Lancet (London, England). 2004 Sep 25-Oct 1:364(9440):1127-34 [PubMed PMID: 15451219]
Level 1 (high-level) evidenceDematteo RP, Ballman KV, Antonescu CR, Maki RG, Pisters PW, Demetri GD, Blackstein ME, Blanke CD, von Mehren M, Brennan MF, Patel S, McCarter MD, Polikoff JA, Tan BR, Owzar K, American College of Surgeons Oncology Group (ACOSOG) Intergroup Adjuvant GIST Study Team. Adjuvant imatinib mesylate after resection of localised, primary gastrointestinal stromal tumour: a randomised, double-blind, placebo-controlled trial. Lancet (London, England). 2009 Mar 28:373(9669):1097-104. doi: 10.1016/S0140-6736(09)60500-6. Epub 2009 Mar 18 [PubMed PMID: 19303137]
Level 1 (high-level) evidenceJoensuu H, Eriksson M, Sundby Hall K, Reichardt A, Hartmann JT, Pink D, Ramadori G, Hohenberger P, Al-Batran SE, Schlemmer M, Bauer S, Wardelmann E, Nilsson B, Sihto H, Bono P, Kallio R, Junnila J, Alvegård T, Reichardt P. Adjuvant Imatinib for High-Risk GI Stromal Tumor: Analysis of a Randomized Trial. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2016 Jan 20:34(3):244-50. doi: 10.1200/JCO.2015.62.9170. Epub 2015 Nov 2 [PubMed PMID: 26527782]
Level 1 (high-level) evidenceWang D, Zhang Q, Blanke CD, Demetri GD, Heinrich MC, Watson JC, Hoffman JP, Okuno S, Kane JM, von Mehren M, Eisenberg BL. Phase II trial of neoadjuvant/adjuvant imatinib mesylate for advanced primary and metastatic/recurrent operable gastrointestinal stromal tumors: long-term follow-up results of Radiation Therapy Oncology Group 0132. Annals of surgical oncology. 2012 Apr:19(4):1074-80. doi: 10.1245/s10434-011-2190-5. Epub 2011 Dec 28 [PubMed PMID: 22203182]
Heinrich MC, Jones RL, von Mehren M, Schöffski P, Serrano C, Kang YK, Cassier PA, Mir O, Eskens F, Tap WD, Rutkowski P, Chawla SP, Trent J, Tugnait M, Evans EK, Lauz T, Zhou T, Roche M, Wolf BB, Bauer S, George S. Avapritinib in advanced PDGFRA D842V-mutant gastrointestinal stromal tumour (NAVIGATOR): a multicentre, open-label, phase 1 trial. The Lancet. Oncology. 2020 Jul:21(7):935-946. doi: 10.1016/S1470-2045(20)30269-2. Epub [PubMed PMID: 32615108]
Demetri GD, van Oosterom AT, Garrett CR, Blackstein ME, Shah MH, Verweij J, McArthur G, Judson IR, Heinrich MC, Morgan JA, Desai J, Fletcher CD, George S, Bello CL, Huang X, Baum CM, Casali PG. Efficacy and safety of sunitinib in patients with advanced gastrointestinal stromal tumour after failure of imatinib: a randomised controlled trial. Lancet (London, England). 2006 Oct 14:368(9544):1329-38 [PubMed PMID: 17046465]
Level 1 (high-level) evidenceDemetri GD, Reichardt P, Kang YK, Blay JY, Rutkowski P, Gelderblom H, Hohenberger P, Leahy M, von Mehren M, Joensuu H, Badalamenti G, Blackstein M, Le Cesne A, Schöffski P, Maki RG, Bauer S, Nguyen BB, Xu J, Nishida T, Chung J, Kappeler C, Kuss I, Laurent D, Casali PG, GRID study investigators. Efficacy and safety of regorafenib for advanced gastrointestinal stromal tumours after failure of imatinib and sunitinib (GRID): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet (London, England). 2013 Jan 26:381(9863):295-302. doi: 10.1016/S0140-6736(12)61857-1. Epub 2012 Nov 22 [PubMed PMID: 23177515]
Level 1 (high-level) evidenceBlay JY, Serrano C, Heinrich MC, Zalcberg J, Bauer S, Gelderblom H, Schöffski P, Jones RL, Attia S, D'Amato G, Chi P, Reichardt P, Meade J, Shi K, Ruiz-Soto R, George S, von Mehren M. Ripretinib in patients with advanced gastrointestinal stromal tumours (INVICTUS): a double-blind, randomised, placebo-controlled, phase 3 trial. The Lancet. Oncology. 2020 Jul:21(7):923-934. doi: 10.1016/S1470-2045(20)30168-6. Epub 2020 Jun 5 [PubMed PMID: 32511981]
Level 1 (high-level) evidenceGheorghe M, Predescu D, Iosif C, Ardeleanu C, Băcanu F, Constantinoiu S. Clinical and therapeutic considerations of GIST. Journal of medicine and life. 2014 Jun 15:7(2):139-49 [PubMed PMID: 25408717]
Gold JS, Gönen M, Gutiérrez A, Broto JM, García-del-Muro X, Smyrk TC, Maki RG, Singer S, Brennan MF, Antonescu CR, Donohue JH, DeMatteo RP. Development and validation of a prognostic nomogram for recurrence-free survival after complete surgical resection of localised primary gastrointestinal stromal tumour: a retrospective analysis. The Lancet. Oncology. 2009 Nov:10(11):1045-52. doi: 10.1016/S1470-2045(09)70242-6. Epub 2009 Sep 28 [PubMed PMID: 19793678]
Level 2 (mid-level) evidenceSorour MA, Kassem MI, Ghazal Ael-H, El-Riwini MT, Abu Nasr A. Gastrointestinal stromal tumors (GIST) related emergencies. International journal of surgery (London, England). 2014:12(4):269-80. doi: 10.1016/j.ijsu.2014.02.004. Epub 2014 Feb 12 [PubMed PMID: 24530605]
Level 2 (mid-level) evidence