Introduction
The glucose-dependent insulinotropic polypeptide (GIP), formerly termed "gastric inhibitory peptide," was first isolated in 1973 from porcine small intestine for its capacity to suppress gastric hydrochloric acid secretion. Comparative studies by John Brown and Raymond Pederson in 1970 provided the first evidence for its existence.[1] Subsequent investigations in 1980 demonstrated that GIP is only a weak inhibitor of acid secretion but a potent stimulator of postprandial insulin release.[2]
The incretin effect is defined as the enhanced insulin secretion observed in response to oral glucose compared to intravenous glucose, despite equivalent plasma glucose levels.[3] GIP is one of the principal incretin hormones and, together with glucagon-like peptide 1 (GLP-1), accounts for 25% to 70% of the postprandial insulin response.[4] For decades, GIP was regarded as an ineffective insulinotropic agent with limited therapeutic potential. The recent success of tirzepatide and emerging triple agonists targeting GLP-1, GIP, and glucagon receptors has renewed interest in GIP physiology and pharmacology.
Issues of Concern
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Issues of Concern
Type 2 diabetes mellitus (T2DM) is associated with a high burden of renal failure and cardiovascular disease. Glucose control frequently requires a personalized approach guided by genetic predisposition and clinical presentation. Resistance to monotherapy with metformin or sulfonylureas has become increasingly common. Incretin-based therapy may be employed as an alternative or combined with standard regimens.[5] Combination therapy with metformin and dipeptidyl peptidase 4 (DPP-4) inhibitors has been associated with lower all-cause mortality and fewer cardiovascular events.[6]
Recent therapeutic strategies that target GIP in addition to other incretin hormones have demonstrated reductions in glycemic levels, greater weight loss, and fewer adverse cardiovascular outcomes. A comprehensive understanding of GIP physiology and DPP-4 inhibition is essential for optimizing the management of T2DM.
Cellular Level
GIP is secreted by enteroendocrine K cells, which are most abundant in the duodenum and proximal jejunum but are distributed throughout the small intestine.[7] The hormone is synthesized as pro-GIP and subsequently cleaved by prohormone convertase 1/3 (PC1/3) into its active form.[8]
Nutrient ingestion is the primary stimulus for GIP release. Glucose, large neutral amino acids, and long-chain fatty acids are potent GIP secretagogues.[9] K cells detect luminal glucose through a mechanism mediated by sodium-coupled glucose transporter 1 (SGLT1). Fructose, which enters cells via glucose transporter 5 (GLUT5), does not effectively stimulate GIP secretion in healthy adults.[10] Insulin contributes to negative feedback regulation by suppressing K-cell activity and thereby limiting GIP release.[11]
GIP exerts its effects through a 7-transmembrane G protein-coupled receptor, activating adenylate cyclase and increasing cyclic adenosine monophosphate (cAMP). This rise in cAMP activates protein kinase A, ultimately leading to insulin secretion from pancreatic β-cells.[12] Transcriptional regulation of GIP involves the GIP promoter region, which contains 2 binding sites for cAMP response element binding protein 1 (CRE-BP1).[13] Additional transcription factors that govern K-cell differentiation and function further modulate GIP gene expression.
Several peptides influence GIP secretion and activity. Gastrin-releasing peptide (GRP) and somatostatin regulate K-cell output, while xenin-25, a 25-amino acid neurotensin-related peptide produced by K cells, enhances GIP-mediated insulin release. The potentiating effect of xenin-25 on glucose-stimulated insulin secretion is attenuated in T2DM.[14] This synergistic action appears to be mediated by cholinergic neural pathways rather than a direct effect on pancreatic β-cells.[15]
Development
GIP was first purified from porcine intestinal extracts in the early 1970s, with John Brown among the key researchers involved in its identification.[16] The gene sequence is highly conserved across mammalian species. GIP is a 42-amino acid peptide hormone generated through posttranslational processing of pre-pro-GIP, a 153–amino acid precursor protein. The molecule shares structural homology with members of the secretin/glucagon peptide family, which includes secretin, glucagon, vasoactive intestinal peptide, GLPs, and growth hormone–releasing factors.[17]
Organ Systems Involved
The principal action of GIP is on the endocrine pancreas, where it potentiates glucose-dependent insulin secretion. A secondary effect is the suppression of gastrin release and gastrin-dependent gastric acid secretion from parietal cells.[18]
GIP receptors are widely distributed and have been identified in adipose tissue, bone, adrenal cortex, heart, pituitary, and brain regions, including the cerebral cortex, hippocampus, and olfactory bulb.[19] In the heart, receptor expression is present at low levels in cardiomyocytes, adipocytes, and pericytes of both atria and ventricles.[20] Low-level expression has also been demonstrated in the immune system, primarily in subsets of T cells and macrophages.[21]
Renal clearance contributes significantly to GIP metabolism.[22][23] Tissues with high metabolic activity, such as hepatocytes and skeletal myocytes, do not express GIP receptors. However, GIP exerts indirect effects on these systems through neural mechanisms and vascular actions of circulating mediators.[24]
Function
The term "enteroinsular axis" refers to the augmentation of insulin secretion by gastrointestinal hormones in response to nutrient intake.[25] Within this axis, GIP functions as an anabolic hormone by enhancing insulin secretion, stimulating glycogen and fatty acid synthesis, and inhibiting lipolysis. GIP receptors are expressed on pancreatic α, β, δ, and PP cells. Oral glucose absorption provides the stimulus for β-cells in the islets of Langerhans to intensify insulin secretion.[26] In healthy adults, GIP increases glucagon release from pancreatic α-cells during hypoglycemia and fasting but has little effect during hyperglycemia.[27]
GIP also exerts extrapancreatic effects. In the stomach, the hormone reduces gastric acid secretion from parietal cells. In bone, GIP promotes osteoblast proliferation and inhibits osteoclast-mediated resorption. The widespread expression of GIP receptors in the brain indicates a role in neurosignaling.[28] Central receptor activity is implicated in the regulation of appetite, satiety, food and energy intake, and energy expenditure. Effects on body weight regulation have contributed to the therapeutic relevance of GIP and related incretin hormones. In experimental models, GIP has demonstrated neuroprotective activity and has been linked to hippocampal neurogenesis in rats and mice (see Image. Tissue-Specific Actions of Glucose-Dependent Insulinotropic Polypeptide).[29]
Mechanism
GIP acts through class II G protein-coupled receptors.[30] The primary signaling pathways involve the activation of adenylate cyclase with subsequent protein kinase A signaling, as well as phospholipase C-mediated activation of protein kinase C. GIP receptors are expressed at high levels in pancreatic β-cells. Ligand binding increases intracellular cAMP, leading to elevated intracellular calcium concentrations and exocytosis of insulin granules.
GIP is rapidly inactivated by DPP-4, the same enzyme responsible for GLP-1 degradation.[31] Inactivation occurs more slowly than with GLP-1, conferring a plasma half-life of 5 to 7 minutes. DPP-4 cleaves alanine or proline residues at the 2nd position of the peptide N-terminus.[32] Substitution of L-alanine with D-alanine at position 2 renders GIP resistant to DPP-4 activity and enhances its incretin effect.[33]
Related Testing
Plasma GIP levels are measured using commercially available sandwich enzyme-linked immunoassay (ELISA) kits. These assays are specific for GIP and do not cross-react with GLP-1 or GLP-2. The biologically active form, GIP-(1-42), is metabolized by DPP-4 to generate GIP-(3-42), which is inactive and exhibits weak antagonistic activity at GIP receptors in rat models.[34] Quantification of active GIP in plasma is achieved with assays targeting the N-terminus of GIP-(1-42). Antibodies directed against the C-terminal region of the peptide are used to measure total GIP secretion.
Pathophysiology
Alterations in GIP secretion and action have been documented in several pathological conditions. Although hypersecretion or hyposecretion is not causally related to disease pathogenesis, changes in GIP regulation are observed in the disorders described below.
Type 2 Diabetes Mellitus
An abnormal incretin effect is a feature of pathological glucose intolerance.[35] Patients with T2DM either have reduced circulating GIP concentrations or β-cell resistance to GIP, in contrast to healthy individuals who exhibit a dose-dependent incretin response to oral glucose. Since incretins contribute to approximately 70% of the postprandial insulin response, a diminished incretin effect accounts for the impaired glucose tolerance observed in diabetes.[36]
The insulinotropic response to GIP is impaired primarily during the late phase of insulin secretion.[37] In contrast, GIP-mediated regulation of glucagon secretion does not differ between patients with T2DM and healthy controls. Following bariatric surgery, an augmented GIP response has been observed in patients with diabetes.[38] Partial restoration of GIP function has also been reported after optimization of glycemic control with insulin, sulfonylureas, or DPP-4 inhibitors.
Obesity
GIP is a key regulator of lipid metabolism and contributes to the pathophysiology of obesity. K-cell hyperplasia and elevated GIP levels are frequently observed in individuals with obesity, as dietary fat is a potent stimulus of GIP secretion. GIP exerts anabolic effects by suppressing lipolysis and promoting lipogenesis. The incretin effect of GIP is attenuated in obesity and shows an inverse correlation with body mass index.[39]
Food-Induced Cushing Syndrome
GIP, similar to ACTH, can cause postprandial hypersecretion of cortisol, resulting in food-induced Cushing syndrome or ACTH-independent macronodular adrenal hyperplasia (AIMAH).[40] GIP receptors are expressed in the zona fasciculata of the adrenal cortex. Circulating GIP concentrations rise after a meal, which can drive cortisol secretion even when ACTH levels are low. ACTH-independent macronodular adrenal hyperplasia arises from a germline mutation or somatic loss of KDM1A expression, leading to cortisol secretion in response to gut-derived GIP.[40] Treatment strategies include the use of somatostatin analogs such as octreotide.[41]
Cystic Fibrosis
In cystic fibrosis, pancreatic exocrine insufficiency leads to digestive abnormalities that can blunt enteroinsular axis activity, in which GIP is a key component. The insulinotropic effect of GIP has been shown to be attenuated in patients with cystic fibrosis and pancreatic insufficiency.[42] This impaired incretin response may contribute to an increased risk of diabetes development in this population.
Insulinoma and Other Neuroendocrine Tumors
Overproduction of GIP and upregulation of GIP receptor expression have been reported in insulinomas and somatotropinomas. Despite the marked hypersecretion of GIP in insulinomas, the insulinotropic effect of GIP appears to be abolished in many cases.[43]
Kidney Disease
Circulating GIP concentrations are elevated because of reduced renal clearance in patients with chronic kidney disease or renal failure. Although GIP receptors have not been detected in renal tissue, no additional biological effects of elevated circulating GIP have been identified to date.
Clinical Significance
DPP-4 inhibitors, including linagliptin, saxagliptin, and sitagliptin, are oral hypoglycemic agents. By inhibiting DPP-4, these agents increase circulating incretin concentrations and enhance glucose-dependent insulin secretion. DPP-4 inhibitors are generally well tolerated, weight-neutral, and do not cause hypoglycemia because of their glucose-dependent mechanism of action. Cardioprotective effects have also been reported, including reductions in systolic blood pressure and endothelial inflammation.[44] However, clinical trials have shown that saxagliptin, but not other DPP-4 inhibitors, is associated with an increased risk of hospitalization for heart failure in patients with T2DM.[45]
Modified Roux-en-Y gastric bypass surgery is increasingly used in managing T2DM. Several studies have demonstrated that this procedure enhances GLP-1 secretion and alters GIP secretion, which collectively contribute to improved glucose tolerance after surgery.[46]
GIP receptors have also become therapeutic targets in the development of new antidiabetic medications. Tirzepatide, a dual GLP-1 and GIP receptor agonist, has demonstrated superior efficacy in weight reduction and glycemic control compared with GLP-1 receptor agonists such as semaglutide, particularly at higher doses of 10 and 15 mg.[47][48] Tirzepatide has also received U.S. Food and Drug Administration approval for the treatment of obstructive sleep apnea, and ongoing clinical studies are investigating its potential benefits in several other conditions, including polycystic ovarian syndrome, psoriasis, cardiovascular disease, and alcohol use disorder. Furthermore, triple-receptor agonists such as reatrutide are in phase III clinical trials and may provide additional therapeutic benefit in weight management and T2DM.
Tirzepatide is associated with adverse effects that are typical of incretin-based therapies, most notably gastrointestinal symptoms such as nausea, vomiting, diarrhea, and constipation. These events are dose-dependent, generally mild to moderate, and arise from delayed gastric emptying and central appetite regulation mediated primarily through GLP-1 receptor activation. Less common but clinically relevant risks include gallbladder and biliary disorders, which have also been reported with other GLP-1 receptor agonists.[49][50] Quantitative partitioning of effects between GIP and GLP-1 in humans has not been definitively established. However, GIP agonism has not been shown to introduce additional adverse effects and may mitigate GLP-1–mediated nausea, thereby improving gastrointestinal tolerability and enabling greater therapeutic exposure.[51]
Media
(Click Image to Enlarge)
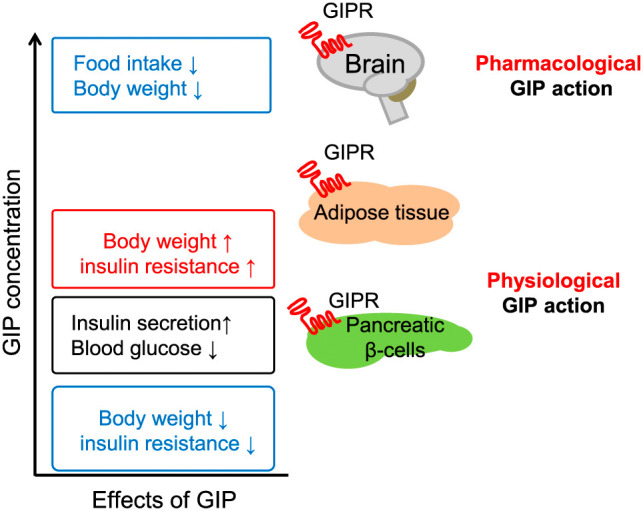
Tissue-Specific Actions of Glucose-Dependent Insulinotropic Polypeptide. The image illustrates how varying levels of glucose-dependent insulinotropic polypeptide (GIP) affect different tissues via GIP receptors (GIPR). Physiological GIP concentrations promote insulin secretion in pancreatic β-cells and regulate blood glucose, while pharmacological levels influence brain function to reduce food intake and body weight. At the same time, adipose tissue responses to GIP can lead to increased body weight and insulin resistance, highlighting the complex, tissue-specific actions of GIP in metabolism regulation.
Yamane S, Harada N, Inagaki N. Physiology and clinical applications of GIP. Endocr J. 2025 Jul 1;72(7):751-764. doi: 10.1507/endocrj.EJ25-0087. Epub 2025 Apr 3. PMID: 40175127; PMCID: PMC12260194.
References
Müller TD, Adriaenssens A, Ahrén B, Blüher M, Birkenfeld AL, Campbell JE, Coghlan MP, D'Alessio D, Deacon CF, DelPrato S, Douros JD, Drucker DJ, Figueredo Burgos NS, Flatt PR, Finan B, Gimeno RE, Gribble FM, Hayes MR, Hölscher C, Holst JJ, Knerr PJ, Knop FK, Kusminski CM, Liskiewicz A, Mabilleau G, Mowery SA, Nauck MA, Novikoff A, Reimann F, Roberts AG, Rosenkilde MM, Samms RJ, Scherer PE, Seeley RJ, Sloop KW, Wolfrum C, Wootten D, DiMarchi RD, Tschöp MH. Glucose-dependent insulinotropic polypeptide (GIP). Molecular metabolism. 2025 May:95():102118. doi: 10.1016/j.molmet.2025.102118. Epub 2025 Feb 28 [PubMed PMID: 40024571]
Maxwell V, Shulkes A, Brown JC, Solomon TE, Walsh JH, Grossman MI. Effect of gastric inhibitory polypeptide on pentagastrin-stimulated acid secretion in man. Digestive diseases and sciences. 1980 Feb:25(2):113-6 [PubMed PMID: 7353457]
Dupre J, Ross SA, Watson D, Brown JC. Stimulation of insulin secretion by gastric inhibitory polypeptide in man. The Journal of clinical endocrinology and metabolism. 1973 Nov:37(5):826-8 [PubMed PMID: 4749457]
Kuhre RE, Wewer Albrechtsen NJ, Hartmann B, Deacon CF, Holst JJ. Measurement of the incretin hormones: glucagon-like peptide-1 and glucose-dependent insulinotropic peptide. Journal of diabetes and its complications. 2015 Apr:29(3):445-50. doi: 10.1016/j.jdiacomp.2014.12.006. Epub 2014 Dec 15 [PubMed PMID: 25623632]
Scheen AJ. Pharmacological management of type 2 diabetes: what's new in 2017? Expert review of clinical pharmacology. 2017 Dec:10(12):1383-1394. doi: 10.1080/17512433.2017.1376652. Epub 2017 Sep 11 [PubMed PMID: 28879786]
Wyncott D, Lyon C, Mounsey A. PURLs: Need an add-on to metformin? Consider this. The Journal of family practice. 2017 Jan:66(1):42-44 [PubMed PMID: 28188318]
Holst JJ. On the physiology of GIP and GLP-1. Hormone and metabolic research = Hormon- und Stoffwechselforschung = Hormones et metabolisme. 2004 Nov-Dec:36(11-12):747-54 [PubMed PMID: 15655703]
Level 3 (low-level) evidenceFujita Y, Asadi A, Yang GK, Kwok YN, Kieffer TJ. Differential processing of pro-glucose-dependent insulinotropic polypeptide in gut. American journal of physiology. Gastrointestinal and liver physiology. 2010 May:298(5):G608-14. doi: 10.1152/ajpgi.00024.2010. Epub 2010 Feb 25 [PubMed PMID: 20185691]
Fehmann HC, Göke R, Göke B. Cell and molecular biology of the incretin hormones glucagon-like peptide-I and glucose-dependent insulin releasing polypeptide. Endocrine reviews. 1995 Jun:16(3):390-410 [PubMed PMID: 7671853]
Level 3 (low-level) evidenceYamane S, Harada N, Inagaki N. Physiology and clinical applications of GIP. Endocrine journal. 2025 Jul 1:72(7):751-764. doi: 10.1507/endocrj.EJ25-0087. Epub 2025 Apr 3 [PubMed PMID: 40175127]
Parker HE, Habib AM, Rogers GJ, Gribble FM, Reimann F. Nutrient-dependent secretion of glucose-dependent insulinotropic polypeptide from primary murine K cells. Diabetologia. 2009 Feb:52(2):289-298. doi: 10.1007/s00125-008-1202-x. Epub 2008 Dec 11 [PubMed PMID: 19082577]
Nauck MA, Müller TD. Incretin hormones and type 2 diabetes. Diabetologia. 2023 Oct:66(10):1780-1795. doi: 10.1007/s00125-023-05956-x. Epub 2023 Jul 11 [PubMed PMID: 37430117]
Someya Y, Inagaki N, Maekawa T, Seino Y, Ishii S. Two 3',5'-cyclic-adenosine monophosphate response elements in the promoter region of the human gastric inhibitory polypeptide gene. FEBS letters. 1993 Feb 8:317(1-2):67-73 [PubMed PMID: 8428636]
Level 3 (low-level) evidenceWice BM, Reeds DN, Tran HD, Crimmins DL, Patterson BW, Dunai J, Wallendorf MJ, Ladenson JH, Villareal DT, Polonsky KS. Xenin-25 amplifies GIP-mediated insulin secretion in humans with normal and impaired glucose tolerance but not type 2 diabetes. Diabetes. 2012 Jul:61(7):1793-800. doi: 10.2337/db11-1451. Epub 2012 Apr 20 [PubMed PMID: 22522617]
Wice BM, Wang S, Crimmins DL, Diggs-Andrews KA, Althage MC, Ford EL, Tran H, Ohlendorf M, Griest TA, Wang Q, Fisher SJ, Ladenson JH, Polonsky KS. Xenin-25 potentiates glucose-dependent insulinotropic polypeptide action via a novel cholinergic relay mechanism. The Journal of biological chemistry. 2010 Jun 25:285(26):19842-53. doi: 10.1074/jbc.M110.129304. Epub 2010 Apr 26 [PubMed PMID: 20421298]
Drucker DJ, Holst JJ. The expanding incretin universe: from basic biology to clinical translation. Diabetologia. 2023 Oct:66(10):1765-1779. doi: 10.1007/s00125-023-05906-7. Epub 2023 Mar 28 [PubMed PMID: 36976349]
Takeda J, Seino Y, Tanaka K, Fukumoto H, Kayano T, Takahashi H, Mitani T, Kurono M, Suzuki T, Tobe T. Sequence of an intestinal cDNA encoding human gastric inhibitory polypeptide precursor. Proceedings of the National Academy of Sciences of the United States of America. 1987 Oct:84(20):7005-8 [PubMed PMID: 2890159]
Villar HV, Fender HR, Rayford PL, Bloom SR, Ramus NI, Thompson JC. Suppression of gastrin release and gastric secretion by gastric inhibitory polypeptide (GIP) and vasoactive intestinal polypeptide (VIP). Annals of surgery. 1976 Jul:184(1):97-102 [PubMed PMID: 938120]
Level 3 (low-level) evidenceUsdin TB, Mezey E, Button DC, Brownstein MJ, Bonner TI. Gastric inhibitory polypeptide receptor, a member of the secretin-vasoactive intestinal peptide receptor family, is widely distributed in peripheral organs and the brain. Endocrinology. 1993 Dec:133(6):2861-70 [PubMed PMID: 8243312]
Level 3 (low-level) evidenceMcLean BA, Wong CK, Kabir MG, Drucker DJ. Glucagon-like Peptide-1 receptor Tie2+ cells are essential for the cardioprotective actions of liraglutide in mice with experimental myocardial infarction. Molecular metabolism. 2022 Dec:66():101641. doi: 10.1016/j.molmet.2022.101641. Epub 2022 Nov 14 [PubMed PMID: 36396031]
Heng TS, Painter MW, Immunological Genome Project Consortium. The Immunological Genome Project: networks of gene expression in immune cells. Nature immunology. 2008 Oct:9(10):1091-4. doi: 10.1038/ni1008-1091. Epub [PubMed PMID: 18800157]
Deacon CF, Nauck MA, Meier J, Hücking K, Holst JJ. Degradation of endogenous and exogenous gastric inhibitory polypeptide in healthy and in type 2 diabetic subjects as revealed using a new assay for the intact peptide. The Journal of clinical endocrinology and metabolism. 2000 Oct:85(10):3575-81 [PubMed PMID: 11061504]
O'Dorisio TM, Sirinek KR, Mazzaferri EL, Cataland S. Renal effects on serum gastric inhibitory polypeptide (GIP). Metabolism: clinical and experimental. 1977 Jun:26(6):651-6 [PubMed PMID: 870794]
Level 3 (low-level) evidenceMcLean BA, Wong CK, Campbell JE, Hodson DJ, Trapp S, Drucker DJ. Revisiting the Complexity of GLP-1 Action from Sites of Synthesis to Receptor Activation. Endocrine reviews. 2021 Mar 15:42(2):101-132. doi: 10.1210/endrev/bnaa032. Epub [PubMed PMID: 33320179]
Unger RH, Eisentraut AM. Entero-insular axis. Archives of internal medicine. 1969 Mar:123(3):261-6 [PubMed PMID: 4885674]
Level 3 (low-level) evidenceNauck MA, Quast DR, Wefers J, Pfeiffer AFH. The evolving story of incretins (GIP and GLP-1) in metabolic and cardiovascular disease: A pathophysiological update. Diabetes, obesity & metabolism. 2021 Sep:23 Suppl 3():5-29. doi: 10.1111/dom.14496. Epub [PubMed PMID: 34310013]
Christensen M, Vedtofte L, Holst JJ, Vilsbøll T, Knop FK. Glucose-dependent insulinotropic polypeptide: a bifunctional glucose-dependent regulator of glucagon and insulin secretion in humans. Diabetes. 2011 Dec:60(12):3103-9. doi: 10.2337/db11-0979. Epub 2011 Oct 7 [PubMed PMID: 21984584]
Irwin N, Gault V, Flatt PR. Therapeutic potential of the original incretin hormone glucose-dependent insulinotropic polypeptide: diabetes, obesity, osteoporosis and Alzheimer's disease? Expert opinion on investigational drugs. 2010 Sep:19(9):1039-48. doi: 10.1517/13543784.2010.513381. Epub [PubMed PMID: 20698813]
Level 3 (low-level) evidenceHammoud R, Drucker DJ. Beyond the pancreas: contrasting cardiometabolic actions of GIP and GLP1. Nature reviews. Endocrinology. 2023 Apr:19(4):201-216. doi: 10.1038/s41574-022-00783-3. Epub 2022 Dec 12 [PubMed PMID: 36509857]
Martin B, Lopez de Maturana R, Brenneman R, Walent T, Mattson MP, Maudsley S. Class II G protein-coupled receptors and their ligands in neuronal function and protection. Neuromolecular medicine. 2005:7(1-2):3-36 [PubMed PMID: 16052036]
Level 3 (low-level) evidenceMayo KE, Miller LJ, Bataille D, Dalle S, Göke B, Thorens B, Drucker DJ. International Union of Pharmacology. XXXV. The glucagon receptor family. Pharmacological reviews. 2003 Mar:55(1):167-94 [PubMed PMID: 12615957]
Level 3 (low-level) evidenceThornberry NA, Gallwitz B. Mechanism of action of inhibitors of dipeptidyl-peptidase-4 (DPP-4). Best practice & research. Clinical endocrinology & metabolism. 2009 Aug:23(4):479-86. doi: 10.1016/j.beem.2009.03.004. Epub [PubMed PMID: 19748065]
Level 3 (low-level) evidenceHinke SA, Gelling RW, Pederson RA, Manhart S, Nian C, Demuth HU, McIntosh CH. Dipeptidyl peptidase IV-resistant [D-Ala(2)]glucose-dependent insulinotropic polypeptide (GIP) improves glucose tolerance in normal and obese diabetic rats. Diabetes. 2002 Mar:51(3):652-61 [PubMed PMID: 11872663]
Level 3 (low-level) evidenceDeacon CF, Plamboeck A, Rosenkilde MM, de Heer J, Holst JJ. GIP-(3-42) does not antagonize insulinotropic effects of GIP at physiological concentrations. American journal of physiology. Endocrinology and metabolism. 2006 Sep:291(3):E468-75 [PubMed PMID: 16608883]
Level 3 (low-level) evidenceCreutzfeldt W. The incretin concept today. Diabetologia. 1979 Feb:16(2):75-85 [PubMed PMID: 32119]
Nauck M, Stöckmann F, Ebert R, Creutzfeldt W. Reduced incretin effect in type 2 (non-insulin-dependent) diabetes. Diabetologia. 1986 Jan:29(1):46-52 [PubMed PMID: 3514343]
Vilsbøll T, Knop FK, Krarup T, Johansen A, Madsbad S, Larsen S, Hansen T, Pedersen O, Holst JJ. The pathophysiology of diabetes involves a defective amplification of the late-phase insulin response to glucose by glucose-dependent insulinotropic polypeptide-regardless of etiology and phenotype. The Journal of clinical endocrinology and metabolism. 2003 Oct:88(10):4897-903 [PubMed PMID: 14557471]
Laferrère B, Heshka S, Wang K, Khan Y, McGinty J, Teixeira J, Hart AB, Olivan B. Incretin levels and effect are markedly enhanced 1 month after Roux-en-Y gastric bypass surgery in obese patients with type 2 diabetes. Diabetes care. 2007 Jul:30(7):1709-16 [PubMed PMID: 17416796]
Aulinger BA, Vahl TP, Prigeon RL, D'Alessio DA, Elder DA. The incretin effect in obese adolescents with and without type 2 diabetes: impaired or intact? American journal of physiology. Endocrinology and metabolism. 2016 May 1:310(9):E774-81. doi: 10.1152/ajpendo.00496.2015. Epub 2016 Mar 15 [PubMed PMID: 26979523]
Reznik Y, Allali-Zerah V, Chayvialle JA, Leroyer R, Leymarie P, Travert G, Lebrethon MC, Budi I, Balliere AM, Mahoudeau J. Food-dependent Cushing's syndrome mediated by aberrant adrenal sensitivity to gastric inhibitory polypeptide. The New England journal of medicine. 1992 Oct 1:327(14):981-6 [PubMed PMID: 1325609]
Level 3 (low-level) evidenceLacroix A. ACTH-independent macronodular adrenal hyperplasia. Best practice & research. Clinical endocrinology & metabolism. 2009 Apr:23(2):245-59. doi: 10.1016/j.beem.2008.10.011. Epub [PubMed PMID: 19500767]
Level 3 (low-level) evidenceNyirjesy SC, Peleckis AJ, Eiel JN, Gallagher K, Doliba A, Tami A, Flatt AJ, De Leon DD, Hadjiliadis D, Sheikh S, Stefanovski D, Gallop R, D'Alessio DA, Rubenstein RC, Kelly A, Rickels MR. Effects of GLP-1 and GIP on Islet Function in Glucose-Intolerant, Pancreatic-Insufficient Cystic Fibrosis. Diabetes. 2022 Oct 1:71(10):2153-2165. doi: 10.2337/db22-0399. Epub [PubMed PMID: 35796669]
Tamburrano G, Lala A, Mauceri M, Leonetti F, Andreani D. Glucose-induced GIP levels in patients with insulinoma. Hormone and metabolic research = Hormon- und Stoffwechselforschung = Hormones et metabolisme. 1984 Dec:16 Suppl 1():200-3 [PubMed PMID: 6099816]
Scheen AJ. Cardiovascular effects of gliptins. Nature reviews. Cardiology. 2013 Feb:10(2):73-84. doi: 10.1038/nrcardio.2012.183. Epub 2013 Jan 8 [PubMed PMID: 23296071]
Level 3 (low-level) evidenceSubrahmanyan NA, Koshy RM, Jacob K, Pappachan JM. Efficacy and Cardiovascular Safety of DPP-4 Inhibitors. Current drug safety. 2021:16(2):154-164. doi: 10.2174/1574886315999200819150544. Epub [PubMed PMID: 32819262]
Xiong SW, Cao J, Liu XM, Deng XM, Liu Z, Zhang FT. Effect of Modified Roux-en-Y Gastric Bypass Surgery on GLP-1, GIP in Patients with Type 2 Diabetes Mellitus. Gastroenterology research and practice. 2015:2015():625196. doi: 10.1155/2015/625196. Epub 2015 Jun 18 [PubMed PMID: 26167177]
Frías JP, Davies MJ, Rosenstock J, Pérez Manghi FC, Fernández Landó L, Bergman BK, Liu B, Cui X, Brown K, SURPASS-2 Investigators. Tirzepatide versus Semaglutide Once Weekly in Patients with Type 2 Diabetes. The New England journal of medicine. 2021 Aug 5:385(6):503-515. doi: 10.1056/NEJMoa2107519. Epub 2021 Jun 25 [PubMed PMID: 34170647]
Vadher K, Patel H, Mody R, Levine JA, Hoog M, Cheng AY, Pantalone KM, Sapin H. Efficacy of tirzepatide 5, 10 and 15 mg versus semaglutide 2 mg in patients with type 2 diabetes: An adjusted indirect treatment comparison. Diabetes, obesity & metabolism. 2022 Sep:24(9):1861-1868. doi: 10.1111/dom.14775. Epub 2022 Jun 13 [PubMed PMID: 35589616]
Xie Z, Liang Z, Xie Y, Zheng G, Cao W. Comparative Safety of GLP-1/GIP Co-Agonists Versus GLP-1 Receptor Agonists for Weight Loss in Patients with Obesity or Overweight: A Systematic Review. Diabetes, metabolic syndrome and obesity : targets and therapy. 2025:18():2837-2849. doi: 10.2147/DMSO.S537229. Epub 2025 Aug 12 [PubMed PMID: 40821754]
Level 1 (high-level) evidenceKim JA, Yoo HJ. Exploring the Side Effects of GLP-1 Receptor Agonist: To Ensure Its Optimal Positioning. Diabetes & metabolism journal. 2025 Jul:49(4):525-541. doi: 10.4093/dmj.2025.0242. Epub 2025 Jul 1 [PubMed PMID: 40631457]
Nauck MA, D'Alessio DA. Tirzepatide, a dual GIP/GLP-1 receptor co-agonist for the treatment of type 2 diabetes with unmatched effectiveness regrading glycaemic control and body weight reduction. Cardiovascular diabetology. 2022 Sep 1:21(1):169. doi: 10.1186/s12933-022-01604-7. Epub 2022 Sep 1 [PubMed PMID: 36050763]