Introduction
Filariasis refers to a group of parasitic infections caused by thread-like nematodes transmitted to humans through insect vectors such as mosquitoes, blackflies, and deerflies. Only a few of the hundreds of known filarial species naturally infect humans. The primary clinical forms include lymphatic filariasis, onchocerciasis, loiasis, and mansonelliasis. Lymphatic filariasis, caused by Wuchereria bancrofti, Brugia malayi, or Brugia timori, is the most widespread and is recognized by the World Health Organization as a leading cause of disability worldwide.
Symptoms of filariasis vary by species. Lymphatic filariasis is typically acquired during childhood and may remain asymptomatic for years, causing progressive damage to the lymphatic and renal systems while impairing immune function. Over time, repeated inflammatory episodes can lead to chronic lymphedema and elephantiasis, affecting the limbs, breasts, vulva, and scrotum.[1][2][3] Acute syndromes include adenolymphangitis, filarial fever, acute dermatolymphangioadenitis, and tropical pulmonary eosinophilia. Onchocerciasis, or river blindness, presents a triad of dermatologic, ocular, and nodular symptoms. Skin manifestations include pruritus, papules, lichenification, and altered pigmentation. Subcutaneous nodules, or onchocercomas, form near bony prominences. Ocular damage from microfilarial migration may lead to progressive visual impairment. Loiasis presents with Calabar swellings, transient, localized, nonerythematous subcutaneous edema caused by hypersensitivity to migrating worms, commonly near joints. Additionally, migration of adult worms across the conjunctiva can cause eye irritation. Mansonellosis is usually asymptomatic but may present with symptoms such as fever, pruritus, lymphadenitis, skin lumps, and abdominal pain.
Diagnosis relies on identifying microfilariae in blood, skin, or ocular tissues. Clinicians commonly use blood smears to diagnose lymphatic filariasis, Loa loa, Mansonella perstans, and M ozzardi; skin snips are preferred for diagnosing Onchocerca volvulus and M streptocerca. For W bancrofti, circulating filarial antigen assays are the preferred diagnostic method. A slit-lamp examination may detect O volvulus microfilariae in the eye. Imaging techniques such as ultrasonography and lymphoscintigraphy support the diagnosis and identify early complications by visualizing lymphatic obstruction or the presence of living adult worms. Chest radiography may reveal diffuse infiltrates in patients with tropical pulmonary eosinophilia and nodules or cavities, often described as "coin lesions," in dirofilariasis.[4]
Management includes antiparasitic therapy tailored to the infecting species. Medications include diethylcarbamazine, ivermectin, albendazole, and doxycycline (targeting Wolbachia endosymbionts). In lymphatic filariasis, surgical excision may be performed for hydrocele, lymphedema, and elephantiasis, though limb surgery is often less successful.[5] Nodullectomy may reduce skin and ocular complications in patients with onchocerciasis, and extraction or surgical excision of the worm may be performed in dirofilariasis. Early diagnosis, appropriate medical and surgical interventions, and widespread participation in mass drug administration programs have significantly reduced the disability associated with filarial diseases.[1][6][7]
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
Filariasis is caused by nematodes (roundworms) that infect the lymphatic or subcutaneous tissues. Lymphatic filariasis, transmitted by mosquitoes, is primarily caused by Wuchereria bancrofti, Brugia malayi, and Brugia timori. (See Images. Wuchereria bancrofti and Brugia malayi).[1] W bancrofti accounts for approximately 90% of all cases of lymphatic filariasis, with the remainder due to B malayi. Subcutaneous filariasis includes Loa loa, transmitted by deerflies and causing loiasis (African eye worm); Onchocerca volvulus, transmitted by blackflies and responsible for river blindness; and 3 species of Mansonella that cause human infections: M streptocerca, M perstans, and M ozzardi, which are transmitted by biting midges and cause cutaneous symptoms. Other filarial species, such as Dirofilaria immitis (dog heartworm) and Dirofilaria tenuis (raccoon heartworm), and related species, occasionally infect humans.
Epidemiology
Lymphatic Filariasis
Due to widespread drug administration programs, the global incidence of lymphatic filariasis has declined from 199 million cases in 2000 to approximately 50 million in 2018.[8][9] According to the World Health Organization (WHO), around 36 million people have manifestations of filariasis, including 25 million men with hydrocele and over 15 million people with lymphedema.[8] Although many countries have eliminated filariasis, over 657 million people in 39 countries remain at risk of infection. Areas that lack mass drug administration or face disruptions from challenges like the COVID-19 pandemic or insufficient funding continue to experience ongoing infections.[10]
The WHO estimates an annual economic loss of $1 billion in endemic countries due to the debilitating effects of the disease, resulting in reduced productivity and increased healthcare costs.[11] The prevalence of lymphatic filariasis increases with age, with most people in endemic areas exposed by midlife. However, study results indicate that nearly one-third of children may be infected before age 5, often remaining asymptomatic aside from possible lymphadenopathy until after puberty.[12][13][14] Filariasis is more common in men than women, and nearly two-thirds of affected patients live in Asia. Travelers to endemic regions rarely develop chronic symptoms but may experience acute disease.
Loiasis
Loiasis is endemic in West and Central Africa, with prevalence estimates ranging from 3 to 13 million people infected globally.[15] As with lymphatic filariasis, loiasis also has a global economic impact, estimated as high as $3 million annually.[16] Loiasis is associated with increased morbidity and mortality, with a recent study revealing a mortality rate of 20 deaths per 1000 person-years.[17] An additional study's results reported that the median survival time is 58 years for individuals with amicrofilaremic disease and 39 years for those with microfilaremic disease.[15]
Onchocerciasis
Most patients with onchocerciasis live in Africa and Yemen, with approximately 1% of cases in Latin America. The Global Burden of Disease Study in 2017 estimates 20.9 million O volvulus infections worldwide, with 14.6 million infected individuals experiencing skin disease and 1.15 million experiencing vision loss.[18] Oncocerciasis is the second leading cause of blindness due to infection worldwide.[19] Due to 2 strains of O volvulus, the epidemiologic disease pattern differs by geographic location. Ocular onchocerciasis is more common in West African savannah areas where O volvulus contains higher levels of Wolbachia endosymbiotic bacteria. In contrast, skin disease is more prevalent in African forests.
Mansonellosis
M perstans is endemic in sub-Saharan Africa, Central America, and South America. M ozzardi is endemic in Central and South America and the Caribbean islands, while M streptocerca is endemic in West and Central Africa. The true prevalence of Mansonella infections is unknown; however, study results reveal that infection rates increase with age. Some study results indicate a higher prevalence in men, particularly with M ozardi, while other studies reveal minimal or no difference when focusing on M perstans.[20][21]
Dirofilariasis
Dirofilariasis is most common in the Mediterranean region, but cases are also present in the United States, Eastern Europe, and Central Asia.[22][23] D immitis is most commonly encountered in the Americas, while D repens is prevalent in Europe, Africa, and Asia.
Pathophysiology
Lymphatic Filariasis
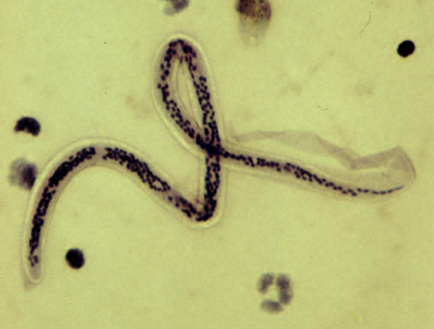
Humans are the only definitive hosts for W bancrofti, while B malayi and B timori can also infect domestic and wild animals. When an infected mosquito bites a human, it deposits third-stage larvae into the skin. These larvae migrate to the lymphatic system, mature into adults, and reproduce. Female worms release microfilariae with nocturnal periodicity, which circulate in the blood (see Image. Microfilaria of Wuchereria bancrofti). A mosquito ingests the microfilariae during a subsequent blood meal. Inside the mosquito, microfilariae develop into infectious third-stage larvae, which are transmitted to another human during the mosquito's next feeding. This cycle takes approximately 12 months, with adult worms living up to 15 years and producing microfilariae for about 5 years.[13] See Image. W Bancrofti Lifecycle.
The clinical presentation varies depending on the host immune response, exposure intensity, genetic factors, and the infecting species. Many individuals with circulating microfilariae remain asymptomatic, whereas some with severe lymphedema often have no detectable microfilariae. Early infection elicits a T helper 2–type immune response, characterized by elevated levels of interleukin (IL)-4, IL-5, and IL-13, as well as increased eosinophils, immunoglobulin (Ig) E, and IgG4. The balance between protective immunity and inflammatory injury remains unclear.[24] Chronic infection promotes immune downregulation through impaired T-cell function and increased regulatory T-cell activity, enabling prolonged infection and altering responses to other pathogens or vaccines.
Lymphatic injury results from the mechanical injury of adult worms and inflammation triggered by antigens released upon worm death. Endosymbiotic Wolbachia bacteria are key in triggering these inflammatory responses, particularly after antifilarial therapy. Progressive lymphatic obstruction leads to edema, chronic inflammation, fibrosis, and lymphatic remodeling.[25][26][27][28][29] These changes impair lymphatic function, contribute to dermal thickening associated with elephantiasis, and increase susceptibility to secondary infections caused by streptococci and fungi.[13][30]
Loiasis
The bite of an infected deerfly transmits L loa third-stage larvae. Once in the body, the larvae migrate to the subcutaneous tissues and mature into adult worms over 3 to 6 months. The adult worms migrate through subcutaneous tissue, including the subconjunctiva of the eye. Female worms release microfilariae into the bloodstream after 6 to 12 months, exhibiting diurnal periodicity; their concentration peaks during the day, which aligns with the feeding behavior of the deerfly vector, facilitating transmission. Microfilariae, taken up by the deerfly, mature into infective third-stage larvae within 7 to 10 days.
Mansonellosis
Following a bite from infected midges, third-stage larvae enter the skin through the bite, mature into adults, and inhabit the subcutaneous tissue, peritoneal cavity, pleural cavity, or occasionally, the pericardium, depending on the species. Symptoms result from an inflammatory response to adult worm migration. Adult worms release microfilariae, which are ingested by midges. The microfilariae migrate from the midgut through the hemocoel to the thoracic muscles, developing into first- and third-stage larvae, returning to the midge's proboscis and infecting another human with their next blood meal. Please see StatPearls' companion topic, "Onchocerciasis," for a complete discussion of the pathophysiology of O volvulus infection.
Dirofilariasis
An infected mosquito injects a third-stage larva into a definitive host, typically a dog in the United States. Other hosts include cats, raccoons, coyotes, horses, and aquatic mammals. The larvae mature into adults in the pulmonary arteries, right ventricle, or subcutaneous tissue. Adult females release microfilariae into the host's blood, which mosquitoes ingest during their next feeding. In the mosquito, microfilariae migrate from the midgut to the Malpighian tubules, developing into first- and third-stage infective larvae. These larvae migrate to the proboscis, and the mosquito transmits them to the next human during feeding. In humans, D repens typically localizes in subcutaneous or ocular tissues, while D immitis may lodge in pulmonary vessels, causing infarction or pneumonitis with granuloma formation.
History and Physical
Lymphatic Filariasis
While most infected individuals are asymptomatic, some develop acute or chronic disease. Study results suggest that only about one-third develop noticeable symptoms, typically during adolescence or adulthood, as the worm burden increases. Common subclinical findings include lymphatic dilatation, impaired lymphatic drainage, scrotal lymphangiectasia, and microscopic hematuria or proteinuria.
Acute Disease
Acute adenolymphangitis
Acute adenolymphangitis develops from the host's immune response to the antigens released by dying worms and presents as repeated bouts of sudden-onset painful inguinal lymphadenopathy, inflammation of the testis, spermatic cord, and epididymis, as well as lymphedema.[31] A characteristic feature is the spread of inflammation distal from the lymph nodes, distinguishing filariasis from bacterial lymphadenitis. The skin around the affected body part exfoliates after the episode resolves. Patients can experience 1 to 4 episodes annually, with inflammation resolving after 4 to 7 days. Each episode significantly impacts routine daily activities and may lead to lost income. Additionally, recurrent episodes can lead to progression of lymphedema and elephantiasis.
Filarial fever
Filarial fever is a fever without the associated lymphadenitis.
Tropical pulmonary eosinophilia
The inflammatory effects of microfilariae trapped in the lungs cause tropical pulmonary eosinophilia. Common symptoms include dry cough, nocturnal paroxysmal cough, wheezing, dyspnea, anorexia, malaise, and weight loss. Rare findings, observed in approximately 15% of patients, are lymphadenopathy, hepatomegaly, and splenomegaly.[13]
Acute dermatolymphangioadenitis
A superficial bacterial infection resulting from breaks in the skin is likely the cause of acute dermatolymphangioadenitis. Clinical findings include edematous, inflammatory plaques, fever, chills, myalgia, and headache. Recurrent episodes can worsen lymphedema.
Chronic Disease
Chronic manifestations of filariasis include lymphedema, hydrocele, and renal involvement. See StatPearls companion topic, "Filarial Hydrocele," for further information regarding hydrocele in patients with lymphatic filariasis. Clinically, patients progress from pitting edema to brawny, nonpitting edema, which results in tissue hardening, hyperpigmentation, and hyperkeratosis. Adenopathy occurs in the inguinal and axillary lymph nodes. Patients can also develop unilateral or bilateral hydrocele, most often associated with W bancrofti. Involvement of the ovary, mesosalpinx, and vulva may occur. Chyluria occurs when intestinal lymph passes into the renal pelvis and is excreted in the urine.
Other Forms of Filariasis
Onchocerciasis, caused by O volvulus, presents with ocular changes, pruritus, subcutaneous nodules, and skin disease. Please see StatPearls' companion topic, "Onchocerciasis," for an in-depth discussion of the clinical presentation of onchocerciasis. Further, many individuals with loiasis are asymptomatic; still, some have Calabar swellings, which are transient, nonerythematous subcutaneous swellings most commonly on the face or extremities, caused by hypersensitivity to migrating adult worms or microfilariae. These lesions generally resolve in 2 to 4 days, and pain, pruritus, and urticaria may precede the swelling. Patients may report seeing a worm migrate across the eye, which can cause temporary edema and inflammation. Subconjunctival migration generally takes 10 to 20 minutes, and ocular symptoms typically resolve once the worm exits the eye. Less common complications include arthritis, endomyocardial fibrosis, meningoencephalopathy, and various other organ-specific pathologies.
Most patients with Mansonella infections are also asymptomatic. When present, symptoms may include fever, pruritus, skin nodules, lymphadenitis, and abdominal pain. Dirofilaria causes heartworm infection in dogs, cats, and other mammals. Adult worms can mature in human hosts but cannot achieve sexual maturity or produce microfilariae, as humans are not their primary host. While clinical disease in humans is rare, Dirofilaria may cause either pulmonary, subcutaneous, or ocular disease. Pulmonary dirofilariasis, caused by D immitis, the dog heartworm, presents as chest pain, cough, fever, and hemoptysis. Subcutaneous dirofilariasis caused by D repens (dogs and cats) and D tenuis (raccoons) typically features an erythematous, tender nodule, sometimes associated with abscess formation, and located near the eye, genital region, or limbs. Allergic symptoms such as urticaria and fever may also occur. The nodule typically contains a degenerating worm surrounded by dense fibrous tissue. D repens usually causes ocular dirofilariasis, classically presenting with conjunctival nodules. Common symptoms include ocular pain, a gritty sensation, and eye redness.
Evaluation
Clinicians should consider lymphatic filariasis in patients residing in endemic areas exhibiting classic clinical symptoms. To establish the diagnosis, the detection of circulating filarial antigens in W bancrofti infection, microfilariae or filarial DNA in the blood, or the identification of adult worms in the lymphatic system is required. Occasionally, microfilariae or adult worms are found incidentally in tissue biopsies or cytological specimens. Additional laboratory findings include eosinophilia, often exceeding 3000/µL, elevated serum IgE levels, proteinuria, and hematuria.
Laboratory Evaluation
Antibody testing
Serologic tests detecting IgG and IgG4 antibodies are available through the National Institutes of Health, the United States Centers for Disease Control and Prevention, and some commercial laboratories. However, these tests are limited because they use crude antigens that cannot distinguish among filarial species and may cross-react with other helminths. While a negative test result can exclude recent infection, the presence of antibodies after successful treatment cannot differentiate between active infection and past exposure.
The following rapid IgG4 antibody detection tests use recombinant antigens and have improved the sensitivity of antibody testing:
- Antibodies to the recombinant protein BmR1, specific for B malayi and B timori
- Antibodies to the recombinant proteins BmR1 and BmSXP test for W bancrofti, B malayi, and B timori
- Antibodies to recombinant proteins Wb123 and Ov16 detect infections of both O volvulus and W bancrofti
- Antibodies to the recombinant L loa antigen LLSXP-1 (currently research only) [32][33][34]
Circulating filarial antigen assay
Circulating filarial antigen assays detect antigens produced by adult worms, making them useful in patients without microfilaremia, and are currently available only for W bancrofti. The Og4C3 (a monoclonal antibody) enzyme-linked immunosorbent assay from TropBio offers quantitative results correlating with worm burden. Additionally, the WHO provides 2 immunochromatographic tests: a card-based assay that delivers both qualitative and semiquantitative results, and a test strip that provides qualitative results. These assays are more sensitive than traditional microscopy and are considered the diagnostic test of choice for W bancrofti. However, patients with high levels of circulating L loa microfilariae may have false-positive test results for W bancrofti.[35] However, despite lymphatic damage, circulating filarial antigen testing may not exclude filariasis in chronic disease or treated infections. Filarial antigens eventually become undetectable, as adult worms produce microfilariae that perpetuate the infection cycle, and die or cease to release microfilariae into the bloodstream.
Blood smear
When clinicians test for Brugian filariasis, M perstans, M ozzardi, or if circulating filarial antigen testing is unavailable, a blood smear stained with Wright or Giemsa stains can be diagnostic. Due to their nocturnal periodicity, the ideal time to draw blood is between 10 pm and 2 am for patients with Bancroftian and Brugian filariasis. In patients with L loa infection, the ideal time is between 10 am and 2 pm, given its diurnal periodicity.
Additional testing
Clinicians diagnose O volvulus and M streptocera infections by identifying microfilariae in multiple skin snip specimens. The preferred site for skin sampling depends on the pathogen. With suspected African onchocerciasis, clinicians should obtain skin snips from the gluteal and thigh regions. The scapula and iliac crest areas are the preferred sites for American onchocerciasis. Skin snips are ineffective diagnostic tools in early infection, as it takes 9 to 15 months for a worm to mature enough to release detectable microfilariae. Clinicians can differentiate M streptocera from O volvulus based on the appearance of the microfilariae. (See Table. Summary of Filarial Species: Characteristic Morphologic Features and Preferred Diagnostic Tests).
Additionally, slit lamp examination may reveal microfilariae associated with O volvulus in the cornea or anterior chamber of the eye. Patch testing with topical diethylcarbamazine is a reasonable alternative to skin snipping for diagnosing onchocerciasis, particularly in areas with low prevalence. Unlike the systemic Mazzotti test, which can trigger severe reactions by killing microfilariae in the body, patch testing applies diethylcarbamazine to a small area of skin to provoke a localized reaction. This method is noninvasive, inexpensive, and often more sensitive than skin snipping, making it a practical screening tool when disease burden is low. Chylous urine and hydrocele fluid are additional sites used to identify microfilariae. Clinicians establish the diagnosis of L loa either by identifying a migrating adult worm in the subcutaneous tissue or conjunctiva or by detecting microfilariae in the blood.
Imaging
Ultrasound and lymphoscintigraphy are the preferred imaging techniques for evaluating lymphatic filariasis. Ultrasound can directly visualize living adult worms within lymphatic vessels by detecting continual movement known as the "filarial dance."[36] This finding can confirm active infection and monitor the response to treatment. Ultrasound of subcutaneous nodules can diagnose onchocerciasis and dirofilariasis. Lymphoscintigraphy assesses lymphatic function and can detect early structural and functional damage to lymphatic vessels before clinical symptoms develop, helping guide disease staging and management.[37]
Table. Summary of Filarial Species: Characteristic Morphologic Features and Preferred Diagnostic Tests
| Species | Periodicity | Preferred Diagnostic Test | Length | Characteristic Morphologic Features on Blood Smear |
| Wuchereria bancrofti | Nocturnal | Antigen testing with blood smear as an alternative | 244–296 µm |
|
| Brugia malayi | Nocturnal | Blood smear | 177–230 µm |
|
| Brugia timori | Nocturnal | Blood smear | 310 µm |
|
| Loa Loa | Diurnal | Blood smear or identifying a migrating adult worm in the subcutaneous tissue or conjunctiva | 230–250 µm |
|
| Mansonella perstans | None | Blood smear or identifying adult worms in tissue specimens | 190–200 µm | Sheath is absent, with a round terminal nucleus at the tip of the tail |
| Mansonella ozzardi | None | Blood smear or skin snip | 170–240 µm | Sheath and terminal nuclei are absent |
| Mansonella streptocerca | None | Skin snip | 180–240 µm |
|
| Onchocerca volvulus | None | Skin snip | 304–315 µm |
|
| Dirofilaria immitis | Nocturnal | Tissue sample | 10–30 cm | Smooth, multilayered cuticle |
|
Dirofilaria repens |
Nocturnal | Tissue sample | 10–30 cm | Spiked, multilayered cuticle |
Treatment / Management
All patients with lymphatic filariasis should be treated, even if asymptomatic, because early therapy may reverse lymphatic damage.[38] Before starting treatment, patients from regions where onchocerciasis or loiasis are endemic should be tested for possible coinfection. Administering diethylcarbamazine (DEC) in individuals with onchocerciasis can trigger a severe inflammatory reaction, while both DEC and ivermectin can cause potentially fatal encephalopathy in patients with loiasis.[1][13][39](B3)
Onchocerciasis
Ivermectin 150 µg/kg as a single dose is the treatment of choice for onchocerciasis monoinfection.[40] Clinicians should instruct patients to take ivermectin on an empty stomach with a full glass of water, and to repeat the dose every 3 to 6 months until the patient is asymptomatic. Treatment may take 10 years or more. Common adverse effects of ivermectin are fever, rash, dizziness, pruritus, myalgia, arthralgia, and tender lymphadenopathy. These symptoms are due to the immune response triggered by the release of Wolbachia antigens, typically appearing around day 3 of treatment. Analgesics and antihistamines may improve these effects. Though not approved for human use, the veterinary medication closantel has shown some promise in treating onchocerciasis. Additional studies are necessary to evaluate its safety, given reports of potential ocular, liver, and central nervous system toxicity.[41] Please see StatPearls' companion topic, "Onchocerciasis," for additional treatment options.(A1)
Loiasis
All patients with L loa infection require treatment, though standardized guidelines are lacking. DEC, which kills adult worms and microfilariae, is not commercially available in the United States; however, it can be obtained through the Centers for Disease Control and Prevention (CDC). DEC can cause severe inflammatory reactions, including encephalitis and shock, in patients with high microfilarial loads. Therefore, the microfilarial count determines the appropriate treatment. The CDC recommends 8 to 10 mg/kg day in 3 divided doses for 21 days for patients with fewer than 8000 microfilariae/mL.[43] For counts greater than 8000 microfilariae/mL, clinicians initially use albendazole 200 mg twice daily for 21 days to reduce the burden, with apheresis for very high loads. Some experts suggest a lower DEC threshold of 2500 microfilariae/mL, with albendazole used for counts greater than 20,000 microfilariae/mL and ivermectin 150 mcg/kg for counts between 2500 and 20,000 microfilariae/mL, repeated until counts drop below 2500 microfilariae/mL. Ivermectin does not kill adult worms and is not a curative treatment for parasitic infections.
DEC is not appropriate in pregnancy. Albendazole is pregnancy category C but is generally considered safe in the second and third trimesters and during breastfeeding, per WHO guidelines. Coinfection with L loa and O volvulus presents a challenge, as DEC is contraindicated in patients with onchocerciasis due to the risk of a severe Mazzotti reaction. For patients with L loa coinfection and microfilariae counts below 20,000/mL, ivermectin plus albendazole is appropriate. If microfilariae counts exceed 20,000/mL, inpatient ivermectin followed by albendazole may be considered. Reevaluation should occur after 6 to 12 months, with retreatment as needed.
Dirofilariasis
Treatment consists of worm extraction or surgical excision. Systemic therapy is generally not necessary. However, clinicians may elect to administer ivermectin, and in cases of D repens infection, doxycycline is a viable option to target the bacterial endosymbiont Wolbachia.[42][43]
Treatment of Lymphatic Filariasis Monoinfection
Diethylcarbamazine
DEC, a microfilaricidal and macrofilaricidal agent, is the treatment of choice for patients with monoinfection caused by W bancrofti, B malayi, and B timori.[44] The dose is 6 mg/kg once daily or in 3 doses for 12 days. Patients with tropical pulmonary eosinophilia should complete a 14- to 21-day course of treatment.
Alternative regimens
Doxycycline is an alternative when DEC is unavailable or contraindicated. This antibiotic exhibits both microfilaricidal and macrofilaricidal activity by targeting the Wolbachia bacteria and is effective against W bancrofti and B malayi infections.[45] For patients who cannot take doxycycline or who have concomitant loiasis or onchocerciasis, albendazole is an additional alternative. The combination of albendazole with ivermectin enhances the suppression of microfilaremia.[46](A1)
Treatment of Lymphatic Filariasis Coinfection
Lymphatic filariasis with onchocerciasis
Doxycycline 200 mg orally once daily for 6 weeks plus ivermectin 150 µg/kg as a single dose is first-line therapy. Alternative options are ivermectin plus albendazole, moxidectin plus albendazole, or doxycycline.
Lymphatic filariasis with loiasis
Treatment of lymphatic filariasis coinfection with loiasis is the same as for loiasis monoinfection. Refer to "Loiasis" for more information on treating lymphatic filariasis coinfection with L loa.
Treatment of Mansonella species
- M sstreptocera: DEC 6 mg/kg/d orally for 14 days is effective against adult worms and microfilariae. Ivermectin 150 µg/kg is an alternative, but it only effectively reduces microfilariae.
- M ozzardi: Experts recommend ivermectin 200 µg/kg orally as a single dose.
- M perstans: Relatively resistant to most antifilarial medications, a combination of DEC and mebendazole for 21 days achieves a 37% clearance of microfilariae after 1 month.[47] Doxycycline 200 mg daily for 6 weeks is 100% effective for Wolbachia in M perstans in patients from Mali, Cameroon, and Ghana at 24 and 36 months. However, strains of M perstans in Uganda and Gabon lack Wolbachia, making the use of doxycycline in other geographic regions unclear.
Managing Complications
Adenolymphangitis and dermatolymphangioadenitis significantly contribute to the progression of lymphedema and elephantiasis, making skin hygiene and prompt treatment of secondary infections crucial. Clinicians should consider antibiotic prophylaxis in patients who experience recurrent infections despite appropriate preventative care.
The WHO's Global Programme to Eliminate Lymphatic Filariasis aims to eliminate lymphatic filariasis as a public health issue through mass drug administration programs and providing a recommended "essential package of care." The core components of the essential package of care are linking patients with local healthcare services, treating acute adenolymphangitis, providing patient education to reduce further disability, and performing surgery for a hydrocele. The core educational elements of the essential package of care are:
- Information to increase awareness about the availability and benefits of health services and self-management, encouraging patients to seek necessary care and overcome barriers such as fear or a lack of information.
- Proper skin care instructions include washing the affected limbs with pH-neutral soap and water twice daily. Patients should wash both legs, even if only 1 leg appears affected, as lymphatic dysfunction may still exist in the unaffected leg. Dry the skin thoroughly, focusing on skin folds and interdigital spaces, using a gentle dabbing motion.
- Clip nails regularly and keep nails clean.
- Immediately treat local wounds or infections with topical antibiotics and apply antifungal creams to areas between the toes and skin folds to prevent fungal infections.
- Wear properly fitting, adapted, and comfortable shoes.
- Keep the affected limb elevated overnight.
- Exercises such as deep breathing, walking, drawing the knees up to the chest, and lifting the lower legs up and down without touching the floor can improve lymphatic flow.
- Proper lymphatic massage techniques gently move excess lymphatic fluid away from congested areas and towards functional lymph nodes.
- Though expensive and often impractical in resource-poor settings, clinicians should offer compression therapy whenever possible. Additional studies are necessary to identify suitable and affordable alternatives.[13][14][48][49][50] (A1)
Hydrocele
Hydrocelectomy is an effective management strategy for patients affected by a hydrocele. However, barriers to surgical intervention, such as cost, lack of awareness, limited number of trained surgeons, social stigma, fear of surgery, mistrust in the healthcare system, and inadequate access to surgical facilities, limit the number of surgeries.[5][51][52] To enhance access, programs that support surgical training in endemic areas and aid countries in developing their own training and surgical programs are essential. Additionally, setting up mobile surgery camps with trained collaborative surgical teams helps bring services directly to isolated communities. Programs that provide financial assistance to cover the costs of transportation, lodging, and lost wages may help alleviate the financial burden associated with surgery.(B3)
Chyluria
Patients can lose a significant amount of fat and protein via the urine and are at risk for nutritional deficiencies and anemia. Patients affected by this condition should follow a low-fat, high-protein diet supplemented with medium-chain triglycerides such as coconut oil.
Prevention
- Endemic lymphatic filariasis without onchocerciasis or loiasis: A single dose of ivermectin, DEC, or one of the 2 in combination with albendazole once a year for 3 years [53][54]
- Coendemic lymphatic filariasis and onchocerciasis: Ivermectin plus albendazole once a year [55]
- Coendemic lymphatic filariasis and loiasis: Albendazole twice a year [56] (A1)
Lymphatic filariasis infection requires multiple mosquito bites over months to years. Short-term travelers to endemic areas do not require medication prophylaxis; however, they should wear protective clothing and use insect repellents to avoid bites from vectors. Long-term travelers should consult with a travel medicine clinician to discuss prophylaxis with DEC to prevent L loa and possibly lymphatic filariasis. No studies are available regarding chemoprophylaxis for Mansonella species. However, repeated mass drug administration of ivermectin reduces M perstans.[57] As with other filarial diseases, implementing protective measures to prevent bites from midges may provide some protection.(B2)
Surgical treatments
Surgical options for early-stage lymphedema (stages I–II) include lymphovenous bypass and vascularized lymph node transplant, which create alternative drainage pathways.[14] Advanced cases may require reductive procedures, such as excision, liposuction, or both. Severe lymphedema may require a combination of techniques. Continued conservative care, including compression therapy, is necessary following surgical interventions.[58] In onchocerciasis, nodulectomy may reduce the worm burden but is not curative due to the presence of deeper-seated worms. Dirofilariasis treatment consists of the worm's extraction or surgical excision in affected patients.[59] Topical agents, such as coumarin and flavonoids, have shown potential in reducing edema through macrophage activation; however, safety concerns limit their use.[14][60] Carbon dioxide laser therapy has also shown promise in nonfilarial elephantiasis by improving skin lesions and fistulas through dermal remodeling.[61][62](B2)
Differential Diagnosis
The differential diagnoses for filarial illnesses include:
- Acute poststreptococcal glomerulonephritis
- Allergic bronchopulmonary aspergillosis
- Bacterial lymphangitis
- Cutaneous larva migrans
- Damage to the lymphatic system secondary to surgery or radiation
- Dipetalonema infection (zoonotic)
- Gnathostoma infection (gnathostomiasis)
- Idiopathic hypereosinophilic syndrome
- Leprosy
- Lymphoma
- Nonfilarial hydrocele
- Pelvic malignancy
- Podoconiosis
- Primary lymphedema
- Recurrent cellulitis
- Loaina infection (zoonotic)
- Meningonema infection (zoonotic)
- Strongyloides infection (strongyloidiasis)
- Systemic vasculitis
- Testicular malignancy
- Tuberculosis [14][63]
Prognosis
The prognosis of filariasis depends on the type of infection, the stage at diagnosis, and the timing of treatment. Early or asymptomatic infections respond well to antiparasitic therapy, preventing complications. However, chronic conditions such as lymphedema, hydrocele, and elephantiasis may persist due to irreversible lymphatic damage, affecting quality of life, causing disability, and creating social stigma. Early detection, mass drug administration, and supportive care, including hygiene, physical therapy, and surgery, can improve outcomes.
Global public health initiatives have significantly reduced disease burden in endemic areas. Untreated onchocerciasis may lead to permanent complications such as blindness, severe skin disease, and disfigurement. In contrast, L loa infections generally have a favorable prognosis, with transient symptoms resolving without long-term effects, though repeated infections can cause chronic inflammation. Mansonella infections are usually mild or asymptomatic, with a good overall prognosis.
Complications
The complications of lymphatic filariasis are:
- Chyluria
- Economic hardship
- Elephantiasis
- Encephalitis
- Filarial arthritis
- Filarial breast abscess
- Immune complex glomerulonephritis
- Lymphedema of the arms, breasts, genitalia, and legs
- Mental illness
- Secondary bacterial infection of limbs affected by lymphedema
- Seizures
- Social stigma
- Tropical pulmonary eosinophilia [64][65][66][67]
Complications of loiasis are:
- Arthritis
- Cardiomyopathy
- Cataract
- Encephalopathy
- Endomyocardial fibrosis
- Entrapment neuropathy
- Glaucoma
- Glomerulonephritis
- Lymphangitis
- Meningitis
- Myelopathy
- Pleural effusion
- Uveitis [68]
Complications of onchocerciasis are:
- Blindness
- Cataract
- Chorioretinitis
- Developmental delay
- Epilepsy
- Glaucoma
- Growth restriction
- Iridocyclitis and uveitis
- Keratitis
- Optic atrophy
- Optic neuritis [69]
Deterrence and Patient Education
Healthcare professionals play a crucial role in preventing and managing filarial infections through education and proactive counseling. Clinicians should educate patients that lymphatic filariasis, onchocerciasis, and loiasis are transmitted by insect vectors, including mosquitoes, blackflies, and deerflies, respectively, and that multiple bites over time are typically required for infection. Emphasizing the importance of personal protective measures, such as wearing long sleeves, using insect repellent, and sleeping under insecticide-treated nets, is crucial for individuals living in or traveling to endemic areas. Participation in mass drug administration programs is key to reducing transmission and preventing long-term complications. Patients should also understand that early treatment improves outcomes and helps prevent irreversible damage such as lymphedema, hydrocele, or vision loss.
In cases with chronic complications, such as severe lymphedema or hydrocele, surgical intervention may provide additional benefit. Procedures such as hydrocelectomy, lymphovenous anastomosis, and excisional debulking can help relieve symptoms, restore function, and improve quality of life. Educating patients about the availability, risks, and goals of surgical treatment can help reduce social stigma and improve quality of life. Clinicians should reinforce adherence to antiparasitic therapy, promote ongoing hygiene and limb care, and coordinate with surgical and rehabilitation specialists as part of comprehensive, patient-centered care.
Pearls and Other Issues
The Global Programme to Eliminate Lymphatic Filariasis initially aimed to eliminate the disease as a public health problem by 2020. Although the COVID-19 pandemic caused delays, mass drug administration programs have led to a 74% reduction in infections, preventing an estimated $100.5 billion in economic loss. As of 2023, 21 countries have eliminated filariasis, while 14 others are undergoing surveillance for the disease. The WHO has since extended its goal to 2030, aiming for 80% of the 71 endemic countries to eliminate lymphatic filariasis as a public health concern.
Enhancing Healthcare Team Outcomes
Filariasis is a parasitic disease caused by thread-like nematodes transmitted through the bites of infected insects. Lymphatic filariasis, the most common form, is caused by W bancrofti, B malayi, or B timori. Other forms include L loa and O volvulus, the cause of river blindness. While lymphatic filarial infections may remain asymptomatic for years, they progressively damage the lymphatic system, kidneys, and immune function. Lymphatic filariasis can lead to chronic, disfiguring conditions such as lymphedema, hydrocele, and elephantiasis, contributing to disability, stigma, and economic hardship. Moreover, loiasis may progress to encephalopathy, cardiomyopathy, arthritis, and nephropathy, while onchocerciasis causes blindness, epilepsy, and skin disease. Diagnosis depends on clinical findings and laboratory tests such as antigen assays, blood smears, or skin snips, depending on the suspected organism. Treatment includes antiparasitic medications, hygiene-based care, and, in some cases, surgical intervention. Prevention relies on personal protective measures, vector control, and mass drug administration programs in endemic regions to interrupt transmission and reduce disease burden.
Effective filariasis management requires a coordinated, patient-centered interprofessional approach. Physicians and advanced practitioners play a central role in diagnosis, treatment planning, and long-term care, while nurses provide patient education, monitor for complications, and support lymphedema care. Pharmacists ensure the safe use of antiparasitic medication and promote adherence, especially in areas where multiple diseases co-occur. Community engagement, facilitated by trained volunteers, local leaders, religious leaders, and educators, is crucial for the success of mass drug administration efforts. Clear interprofessional communication supports treatment alignment, timely referrals, and attention to social determinants of health. Together, this collaboration improves outcomes, reduces stigma, and ensures culturally sensitive care.
Media
(Click Image to Enlarge)
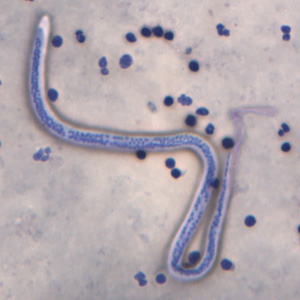
Brugia malayi. This threadlike nematode causes lymphatic filariasis, an infection transmitted to humans through the bites of infected insect vectors.
Lee Moore, MD, Public Health Image Library, Public Domain, Centers for Disease Control and Prevention
(Click Image to Enlarge)
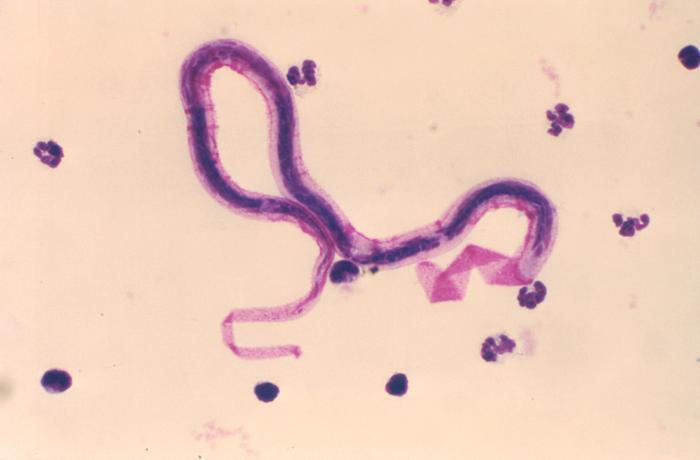
Wuchereria bancrofti.
Centers for Disease Control and Prevention, Public Domain, via Wikimedia Commons
(Click Image to Enlarge)
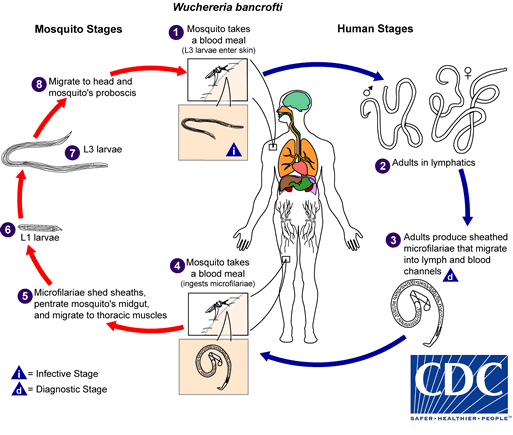
W Bancrofti Lifecycle.
Centers for Disease Control and Prevention, Public Domain, via Wikimedia Commons
(Click Image to Enlarge)
References
Rebollo MP, Bockarie MJ. Can Lymphatic Filariasis Be Eliminated by 2020? Trends in parasitology. 2017 Feb:33(2):83-92. doi: 10.1016/j.pt.2016.09.009. Epub 2016 Oct 17 [PubMed PMID: 27765440]
Maldjian C, Khanna V, Tandon B, Then M, Yassin M, Adam R, Klein MJ. Lymphatic filariasis disseminating to the upper extremity. Case reports in radiology. 2014:2014():985680. doi: 10.1155/2014/985680. Epub 2014 Feb 19 [PubMed PMID: 24707427]
Level 3 (low-level) evidenceShukla SK, Kusum A, Sharma S, Kandari D. Filariasis presenting as a solitary testicular mass. Tropical parasitology. 2019 Jul-Dec:9(2):124-126. doi: 10.4103/tp.TP_15_19. Epub 2019 Sep 18 [PubMed PMID: 31579667]
Saha BK, Bonnier A, Chong WH, Chieng H, Austin A, Hu K, Shkolnik B. Human Pulmonary Dirofilariasis: A Review for the Clinicians. The American journal of the medical sciences. 2022 Jan:363(1):11-17. doi: 10.1016/j.amjms.2021.07.017. Epub 2021 Oct 16 [PubMed PMID: 34666060]
Thomas G, Richards FO Jr, Eigege A, Dakum NK, Azzuwut MP, Sarki J, Gontor I, Abimiku J, Ogah G, Jindau MY, Jiya JY, Miri ES. A pilot program of mass surgery weeks for treatment of hydrocele due to lymphatic filariasis in central Nigeria. The American journal of tropical medicine and hygiene. 2009 Mar:80(3):447-51 [PubMed PMID: 19270297]
Level 3 (low-level) evidenceGardon J, Gardon-Wendel N, Demanga-Ngangue, Kamgno J, Chippaux JP, Boussinesq M. Serious reactions after mass treatment of onchocerciasis with ivermectin in an area endemic for Loa loa infection. Lancet (London, England). 1997 Jul 5:350(9070):18-22 [PubMed PMID: 9217715]
Level 3 (low-level) evidenceTwum-Danso NA. Loa loa encephalopathy temporally related to ivermectin administration reported from onchocerciasis mass treatment programs from 1989 to 2001: implications for the future. Filaria journal. 2003 Oct 24:2 Suppl 1(Suppl 1):S7 [PubMed PMID: 14975064]
Local Burden of Disease 2019 Neglected Tropical Diseases Collaborators. The global distribution of lymphatic filariasis, 2000-18: a geospatial analysis. The Lancet. Global health. 2020 Sep:8(9):e1186-e1194. doi: 10.1016/S2214-109X(20)30286-2. Epub [PubMed PMID: 32827480]
Jabir M, Rahi M. Global insights can accelerate India's journey towards the elimination of lymphatic filariasis as a public health problem. BMJ global health. 2025 Jul 10:10(7):. doi: 10.1136/bmjgh-2025-018851. Epub 2025 Jul 10 [PubMed PMID: 40639855]
Borlase A, Le Rutte EA, Castaño S, Blok DJ, Toor J, Giardina F, Davis EL, NTD Modelling Consortium. Evaluating and mitigating the potential indirect effect of COVID-19 on control programmes for seven neglected tropical diseases: a modelling study. The Lancet. Global health. 2022 Nov:10(11):e1600-e1611. doi: 10.1016/S2214-109X(22)00360-6. Epub [PubMed PMID: 36240827]
Lenk EJ, Redekop WK, Luyendijk M, Rijnsburger AJ, Severens JL. Productivity Loss Related to Neglected Tropical Diseases Eligible for Preventive Chemotherapy: A Systematic Literature Review. PLoS neglected tropical diseases. 2016 Feb:10(2):e0004397. doi: 10.1371/journal.pntd.0004397. Epub 2016 Feb 18 [PubMed PMID: 26890487]
Level 1 (high-level) evidenceWitt C, Ottesen EA. Lymphatic filariasis: an infection of childhood. Tropical medicine & international health : TM & IH. 2001 Aug:6(8):582-606 [PubMed PMID: 11555425]
Chandy A, Thakur AS, Singh MP, Manigauha A. A review of neglected tropical diseases: filariasis. Asian Pacific journal of tropical medicine. 2011 Jul:4(7):581-6. doi: 10.1016/S1995-7645(11)60150-8. Epub [PubMed PMID: 21803313]
Level 3 (low-level) evidenceShenoy RK. Clinical and pathological aspects of filarial lymphedema and its management. The Korean journal of parasitology. 2008 Sep:46(3):119-25. doi: 10.3347/kjp.2008.46.3.119. Epub [PubMed PMID: 18830049]
Hemilembolo MC, Niama AC, Campillo JT, Pion SD, Missamou F, Whittaker C, Kankou JM, Ndziessi G, Bileckot RR, Boussinesq M, Chesnais CB. Excess Mortality Associated With Loiasis: Confirmation by a New Retrospective Cohort Study Conducted in the Republic of Congo. Open forum infectious diseases. 2023 Mar:10(3):ofad103. doi: 10.1093/ofid/ofad103. Epub 2023 Feb 24 [PubMed PMID: 36968967]
Level 2 (mid-level) evidenceVeletzky L, Schlicker V, Hergeth J, Stelzl DR, Zoleko Manego R, Mombo-Ngoma G, Eberhardt KA, McCall MBB, Adegnika AA, Lell B, Mordmüller B, Adegnika S, Ramharter M, Budke C. Reported healthcare-seeking of loiasis patients and estimation of the associated monetary burden in Gabon: Data from a cross-sectional survey. PLoS neglected tropical diseases. 2024 Aug:18(8):e0012389. doi: 10.1371/journal.pntd.0012389. Epub 2024 Aug 19 [PubMed PMID: 39159280]
Level 2 (mid-level) evidenceChesnais CB, Takougang I, Paguélé M, Pion SD, Boussinesq M. Excess mortality associated with loiasis: a retrospective population-based cohort study. The Lancet. Infectious diseases. 2017 Jan:17(1):108-116. doi: 10.1016/S1473-3099(16)30405-4. Epub 2016 Oct 21 [PubMed PMID: 27777031]
Level 2 (mid-level) evidenceMshana MI, Silvestri V, Mushi V, Bonaventura WM, Tarimo D, Ngasala B, Gasarasi DB. Burden and factors associated with onchocerciasis transmission among school-aged children after more than 20 years of Community Directed Treatment with Ivermectin in Ulanga district, Tanzania: A school-based cross-sectional study. PLOS global public health. 2023:3(5):e0001919. doi: 10.1371/journal.pgph.0001919. Epub 2023 May 12 [PubMed PMID: 37172010]
Level 2 (mid-level) evidenceGBD 2017 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet (London, England). 2018 Nov 10:392(10159):1789-1858. doi: 10.1016/S0140-6736(18)32279-7. Epub 2018 Nov 8 [PubMed PMID: 30496104]
Level 1 (high-level) evidenceDahmer KJ, Palma-Cuero M, Ciuoderis K, Patiño C, Roitman S, Li Z, Sinha A, Hite JL, Cuellar OB, Hernandez-Ortiz JP, Osorio JE, Christensen BM, Carlow CKS, Zamanian M. Molecular Surveillance Detects High Prevalence of the Neglected Parasite Mansonella ozzardi in the Colombian Amazon. The Journal of infectious diseases. 2023 Nov 11:228(10):1441-1451. doi: 10.1093/infdis/jiad331. Epub [PubMed PMID: 37566913]
Kozek WJ, Palma G, Henao A, García H, Hoyos M. Filariasis in Colombia: prevalence and distribution of Mansonella ozzardi and Mansonella (=Dipetalonema) perstans infections in the Comisaría del Guainía. The American journal of tropical medicine and hygiene. 1983 Mar:32(2):379-84 [PubMed PMID: 6340542]
Jelinek T, Schulte-Hillen J, Löscher T. Human dirofilariasis. International journal of dermatology. 1996 Dec:35(12):872-5 [PubMed PMID: 8970844]
Blaizot R, Receveur MC, Millet P, Otranto D, Malvy DJM. Systemic Infection With Dirofilaria repens in Southwestern France. Annals of internal medicine. 2018 Feb 6:168(3):228-229. doi: 10.7326/L17-0426. Epub 2017 Oct 31 [PubMed PMID: 29086799]
Ehrens A, Hoerauf A, Hübner MP. Eosinophils in filarial infections: Inducers of protection or pathology? Frontiers in immunology. 2022:13():983812. doi: 10.3389/fimmu.2022.983812. Epub 2022 Oct 31 [PubMed PMID: 36389745]
Punkosdy GA, Addiss DG, Lammie PJ. Characterization of antibody responses to Wolbachia surface protein in humans with lymphatic filariasis. Infection and immunity. 2003 Sep:71(9):5104-14 [PubMed PMID: 12933853]
Lammie PJ, Cuenco KT, Punkosdy GA. The pathogenesis of filarial lymphedema: is it the worm or is it the host? Annals of the New York Academy of Sciences. 2002 Dec:979():131-42; discussion 188-96 [PubMed PMID: 12543723]
Level 3 (low-level) evidenceNutman TB. Insights into the pathogenesis of disease in human lymphatic filariasis. Lymphatic research and biology. 2013 Sep:11(3):144-8. doi: 10.1089/lrb.2013.0021. Epub [PubMed PMID: 24044755]
Level 3 (low-level) evidenceBabu S, Nutman TB. Immunopathogenesis of lymphatic filarial disease. Seminars in immunopathology. 2012 Nov:34(6):847-61. doi: 10.1007/s00281-012-0346-4. Epub 2012 Oct 3 [PubMed PMID: 23053393]
Level 3 (low-level) evidenceMishra R, Panda SK, Sahoo PK, Mishra S, Satapathy AK. Self-reactive IgG4 antibodies are associated with blocking of pathology in human lymphatic filariasis. Cellular immunology. 2019 Jul:341():103927. doi: 10.1016/j.cellimm.2019.103927. Epub 2019 May 21 [PubMed PMID: 31130239]
Chakraborty S, Gurusamy M, Zawieja DC, Muthuchamy M. Lymphatic filariasis: perspectives on lymphatic remodeling and contractile dysfunction in filarial disease pathogenesis. Microcirculation (New York, N.Y. : 1994). 2013 Jul:20(5):349-64. doi: 10.1111/micc.12031. Epub [PubMed PMID: 23237232]
Level 3 (low-level) evidencePani SP, Srividya A. Clinical manifestations of bancroftian filariasis with special reference to lymphoedema grading. The Indian journal of medical research. 1995 Sep:102():114-8 [PubMed PMID: 8543349]
Lammie PJ, Weil G, Noordin R, Kaliraj P, Steel C, Goodman D, Lakshmikanthan VB, Ottesen E. Recombinant antigen-based antibody assays for the diagnosis and surveillance of lymphatic filariasis - a multicenter trial. Filaria journal. 2004 Sep 3:3(1):9 [PubMed PMID: 15347425]
Level 1 (high-level) evidenceSteel C, Golden A, Stevens E, Yokobe L, Domingo GJ, de los Santos T, Nutman TB. Rapid Point-of-Contact Tool for Mapping and Integrated Surveillance of Wuchereria bancrofti and Onchocerca volvulus Infection. Clinical and vaccine immunology : CVI. 2015 Aug:22(8):896-901. doi: 10.1128/CVI.00227-15. Epub 2015 May 27 [PubMed PMID: 26018537]
Burbelo PD, Ramanathan R, Klion AD, Iadarola MJ, Nutman TB. Rapid, novel, specific, high-throughput assay for diagnosis of Loa loa infection. Journal of clinical microbiology. 2008 Jul:46(7):2298-304. doi: 10.1128/JCM.00490-08. Epub 2008 May 28 [PubMed PMID: 18508942]
Pion SD, Montavon C, Chesnais CB, Kamgno J, Wanji S, Klion AD, Nutman TB, Boussinesq M. Positivity of Antigen Tests Used for Diagnosis of Lymphatic Filariasis in Individuals Without Wuchereria bancrofti Infection But with High Loa loa Microfilaremia. The American journal of tropical medicine and hygiene. 2016 Dec 7:95(6):1417-1423. doi: 10.4269/ajtmh.16-0547. Epub 2016 Oct 10 [PubMed PMID: 27729568]
Gurung S, Karki S, Kharal K, Thapa S, Thapa S, Baral S. Filariasis diagnosed by real-time ultrasound scanning as filarial dance sign - A case report. IDCases. 2022:30():e01621. doi: 10.1016/j.idcr.2022.e01621. Epub 2022 Sep 29 [PubMed PMID: 36210858]
Level 3 (low-level) evidenceKelly-Hope LA, Karim MJ, Sultan Mahmood A, Al Kawsar A, Khair A, Betts H, Douglass J, Forrer A, Taylor MJ. Infrared Thermal Imaging as a Novel Non-Invasive Point-of-Care Tool to Assess Filarial Lymphoedema. Journal of clinical medicine. 2021 May 25:10(11):. doi: 10.3390/jcm10112301. Epub 2021 May 25 [PubMed PMID: 34070599]
Moore TA, Reynolds JC, Kenney RT, Johnston W, Nutman TB. Diethylcarbamazine-induced reversal of early lymphatic dysfunction in a patient with bancroftian filariasis: assessment with use of lymphoscintigraphy. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 1996 Nov:23(5):1007-11 [PubMed PMID: 8922794]
Kamgno J, Pion SD, Chesnais CB, Bakalar MH, D'Ambrosio MV, Mackenzie CD, Nana-Djeunga HC, Gounoue-Kamkumo R, Njitchouang GR, Nwane P, Tchatchueng-Mbouga JB, Wanji S, Stolk WA, Fletcher DA, Klion AD, Nutman TB, Boussinesq M. A Test-and-Not-Treat Strategy for Onchocerciasis in Loa loa-Endemic Areas. The New England journal of medicine. 2017 Nov 23:377(21):2044-2052. doi: 10.1056/NEJMoa1705026. Epub 2017 Nov 8 [PubMed PMID: 29116890]
Gardon J, Boussinesq M, Kamgno J, Gardon-Wendel N, Demanga-Ngangue, Duke BO. Effects of standard and high doses of ivermectin on adult worms of Onchocerca volvulus: a randomised controlled trial. Lancet (London, England). 2002 Jul 20:360(9328):203-10 [PubMed PMID: 12133654]
Level 1 (high-level) evidenceVenkatesh R, Pereira A, Aseem A, Yadav NK. Commentary: Closantel - A lesser-known evil. Indian journal of ophthalmology. 2019 Oct:67(10):1771-1772. doi: 10.4103/ijo.IJO_1150_19. Epub [PubMed PMID: 31546561]
Level 3 (low-level) evidenceHuebl L, Tappe D, Giese M, Mempel S, Tannich E, Kreuels B, Ramharter M, Veletzky L, Jochum J. Recurrent Swelling and Microfilaremia Caused by Dirofilaria repens Infection after Travel to India. Emerging infectious diseases. 2021 Jun:27(6):1701-1704. doi: 10.3201/eid2706.210592. Epub [PubMed PMID: 34013860]
Frenzen FS, Loewe I, Müller G, Schoenlebe J, Tappe D, Teichmann D. Dirofilaria repens infection of the eye with concomitant microfilaremia in a traveller. Journal of travel medicine. 2021 Jan 6:28(1):. pii: taaa119. doi: 10.1093/jtm/taaa119. Epub [PubMed PMID: 32701137]
Sankari T, Subramanian S, Hoti SL, Pani SP, Jambulingam P, Das PK. Heterogeneous response of Wuchereria bancrofti-infected persons to diethylcarbamazine (DEC) and its implications for the Global Programme to Eliminate Lymphatic Filariasis (GPELF). Parasitology research. 2021 Jan:120(1):311-319. doi: 10.1007/s00436-020-06950-7. Epub 2020 Nov 4 [PubMed PMID: 33146778]
Taylor MJ. Wolbachia bacteria of filarial nematodes in the pathogenesis of disease and as a target for control. Transactions of the Royal Society of Tropical Medicine and Hygiene. 2000 Nov-Dec:94(6):596-8 [PubMed PMID: 11198638]
Albadrani MS, Molla A, Elbadawy HM, Eltahir HM, Sriram S, Abouzied MM, Elsayed EMS. Antifilarial treatment strategies: a systematic review and network meta-analysis. BMC infectious diseases. 2025 May 16:25(1):712. doi: 10.1186/s12879-025-11105-z. Epub 2025 May 16 [PubMed PMID: 40380307]
Level 1 (high-level) evidenceBregani ER, Rovellini A, Mbaïdoum N, Magnini MG. Comparison of different anthelminthic drug regimens against Mansonella perstans filariasis. Transactions of the Royal Society of Tropical Medicine and Hygiene. 2006 May:100(5):458-63 [PubMed PMID: 16257021]
Mackenzie CD, Kapa DR, Krishnasastry S, Douglass J, Hoerauf A, Ottesen EA. Managing Lymphedema Induced by Lymphatic Filariasis: Implementing and Improving Care at the Individual and Programmatic Levels. The American journal of tropical medicine and hygiene. 2024 Oct 1:111(4_Suppl):3-21. doi: 10.4269/ajtmh.23-0905. Epub 2024 Jul 30 [PubMed PMID: 39084208]
Stocks ME, Freeman MC, Addiss DG. The Effect of Hygiene-Based Lymphedema Management in Lymphatic Filariasis-Endemic Areas: A Systematic Review and Meta-analysis. PLoS neglected tropical diseases. 2015 Oct:9(10):e0004171. doi: 10.1371/journal.pntd.0004171. Epub 2015 Oct 23 [PubMed PMID: 26496129]
Level 1 (high-level) evidenceHall JM, De Silva NL, Ruben J, Thilakarathne SS, Yahathugoda TC, Budge PJ. Efficacy and Feasibility of Short-Stretch Compression Therapy for Filarial Lymphedema in Sri Lanka. The American journal of tropical medicine and hygiene. 2024 May 1:110(5):936-942. doi: 10.4269/ajtmh.23-0496. Epub 2024 Mar 26 [PubMed PMID: 38531106]
Level 2 (mid-level) evidence. Corrigendum to: The development and roll-out of a new hydrocoele surgery facility assessment tool for the elimination of lymphatic filariasis. International health. 2023 Mar 1:15(2):233. doi: 10.1093/inthealth/ihac086. Epub [PubMed PMID: 36538769]
Lama Yonzon C, Padmawati RS, Subedi RK, Paudel S, Ghimire A, Murhandarwati EH. Exploring determinants of hydrocele surgery coverage related to Lymphatic Filariasis in Nepal: An implementation research study. PloS one. 2021:16(2):e0244664. doi: 10.1371/journal.pone.0244664. Epub 2021 Feb 26 [PubMed PMID: 33635870]
King CL, Suamani J, Sanuku N, Cheng YC, Satofan S, Mancuso B, Goss CW, Robinson LJ, Siba PM, Weil GJ, Kazura JW. A Trial of a Triple-Drug Treatment for Lymphatic Filariasis. The New England journal of medicine. 2018 Nov 8:379(19):1801-1810. doi: 10.1056/NEJMoa1706854. Epub [PubMed PMID: 30403937]
Hast MA, Tufa A, Brant TA, Suiaunoa-Scanlan L, Camacho J, Vaifanua-Leo J, Robinson K, Dodd E, Sili B, Lees LS, Won KY, Utu F. Notes from the Field: Impact of a Mass Drug Administration Campaign Using a Novel Three-Drug Regimen on Lymphatic Filariasis Antigenemia - American Samoa, 2019. MMWR. Morbidity and mortality weekly report. 2020 May 29:69(21):656-657. doi: 10.15585/mmwr.mm6921a3. Epub 2020 May 29 [PubMed PMID: 32463808]
Thomsen EK, Sanuku N, Baea M, Satofan S, Maki E, Lombore B, Schmidt MS, Siba PM, Weil GJ, Kazura JW, Fleckenstein LL, King CL. Efficacy, Safety, and Pharmacokinetics of Coadministered Diethylcarbamazine, Albendazole, and Ivermectin for Treatment of Bancroftian Filariasis. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2016 Feb 1:62(3):334-341. doi: 10.1093/cid/civ882. Epub 2015 Oct 20 [PubMed PMID: 26486704]
Ouattara AF, Bjerum CM, Aboulaye M, Kouadio O, Marius VK, Andersen B, Lew D, Goss CW, Weil GJ, Koudou BG, King CL. Semiannual Treatment of Albendazole Alone is Efficacious for Treatment of Lymphatic Filariasis: A Randomized Open-label Trial in Cote d'Ivoire. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2022 Jul 6:74(12):2200-2208. doi: 10.1093/cid/ciab194. Epub [PubMed PMID: 33674871]
Level 1 (high-level) evidenceWanji S, Tayong DB, Layland LE, Datchoua Poutcheu FR, Ndongmo WP, Kengne-Ouafo JA, Ritter M, Amvongo-Adjia N, Fombad FF, Njeshi CN, Nkwescheu AS, Enyong PA, Hoerauf A. Update on the distribution of Mansonella perstans in the southern part of Cameroon: influence of ecological factors and mass drug administration with ivermectin. Parasites & vectors. 2016 May 31:9(1):311. doi: 10.1186/s13071-016-1595-1. Epub 2016 May 31 [PubMed PMID: 27245442]
Level 2 (mid-level) evidenceVictor J, Stephen T, Guin D, Victor J. The Nodovenous Shunt and Reduction Surgery for Post-Filarial Lymphedema-Surgical Technique and Clinical Outcomes. Indian journal of plastic surgery : official publication of the Association of Plastic Surgeons of India. 2021 Jan:54(1):75-81. doi: 10.1055/s-0041-1723908. Epub 2021 Mar 2 [PubMed PMID: 33814746]
Level 2 (mid-level) evidenceKhurana S, Singh G, Bhatti HS, Malla N. Human subcutaneous dirofilariasis in India: a report of three cases with brief review of literature. Indian journal of medical microbiology. 2010 Oct-Dec:28(4):394-6. doi: 10.4103/0255-0857.71836. Epub [PubMed PMID: 20966580]
Level 3 (low-level) evidenceSharifi-Rad J, Cruz-Martins N, López-Jornet P, Lopez EP, Harun N, Yeskaliyeva B, Beyatli A, Sytar O, Shaheen S, Sharopov F, Taheri Y, Docea AO, Calina D, Cho WC. Natural Coumarins: Exploring the Pharmacological Complexity and Underlying Molecular Mechanisms. Oxidative medicine and cellular longevity. 2021:2021():6492346. doi: 10.1155/2021/6492346. Epub 2021 Aug 23 [PubMed PMID: 34531939]
Robinson CG, Lee KR, Thomas VD. The Successful Treatment of Elephantiasis Nostras Verrucosa With Ablative Carbon Dioxide Laser. Journal of cutaneous medicine and surgery. 2018 Nov/Dec:22(6):611-613. doi: 10.1177/1203475418763548. Epub 2018 Mar 8 [PubMed PMID: 29519146]
Menzer C, Aleisa A, Wilson BN, Musthaq S, Rossi A. Efficacy of laser CO(2) treatment for refractory lymphedema secondary to cancer treatments. Lasers in surgery and medicine. 2022 Mar:54(3):337-341. doi: 10.1002/lsm.23498. Epub 2021 Nov 27 [PubMed PMID: 34837392]
Orihel TC, Eberhard ML. Zoonotic filariasis. Clinical microbiology reviews. 1998 Apr:11(2):366-81 [PubMed PMID: 9564568]
Level 3 (low-level) evidenceBhalla D, Dumas M, Preux PM. Neurological manifestations of filarial infections. Handbook of clinical neurology. 2013:114():235-42. doi: 10.1016/B978-0-444-53490-3.00018-2. Epub [PubMed PMID: 23829914]
Shrivastava A, Arora P, Khare A, Goel G, Kapoor N. Central nervous system filariasis masquerading as a glioma: case report. Journal of neurosurgery. 2017 Sep:127(3):691-693. doi: 10.3171/2016.9.JNS161092. Epub 2016 Dec 23 [PubMed PMID: 28009239]
Level 3 (low-level) evidenceKumar RR, Balakrishnan A, Suman SK, Dasgupta S, Gupta N, Vallonthaiel AG, Arava S, Mirdha BR, Kumar U. Filariasis: a vasculitis mimic. Rheumatology advances in practice. 2019:3(2):rkz045. doi: 10.1093/rap/rkz045. Epub 2019 Dec 17 [PubMed PMID: 31858075]
Level 3 (low-level) evidencevan Velthuysen ML, Florquin S. Glomerulopathy associated with parasitic infections. Clinical microbiology reviews. 2000 Jan:13(1):55-66, table of contents [PubMed PMID: 10627491]
Pallara E, Cotugno S, Guido G, De Vita E, Ricciardi A, Totaro V, Camporeale M, Frallonardo L, Novara R, Panico GG, Puzo P, Alessio G, Sablone S, Mariani M, De Iaco G, Milano E, Bavaro DF, Lattanzio R, Patti G, Papagni R, Pellegrino C, Saracino A, Di Gennaro F. Loa loa in the Vitreous Cavity of the Eye: A Case Report and State of Art. The American journal of tropical medicine and hygiene. 2022 Aug 1:107(3):504-16. doi: 10.4269/ajtmh.22-0274. Epub 2022 Aug 1 [PubMed PMID: 35914685]
Level 3 (low-level) evidenceNewland HS, White AT, Greene BM, Murphy RP, Taylor HR. Ocular manifestations of onchocerciasis in a rain forest area of west Africa. The British journal of ophthalmology. 1991 Mar:75(3):163-9 [PubMed PMID: 2012784]
Level 3 (low-level) evidence