Introduction
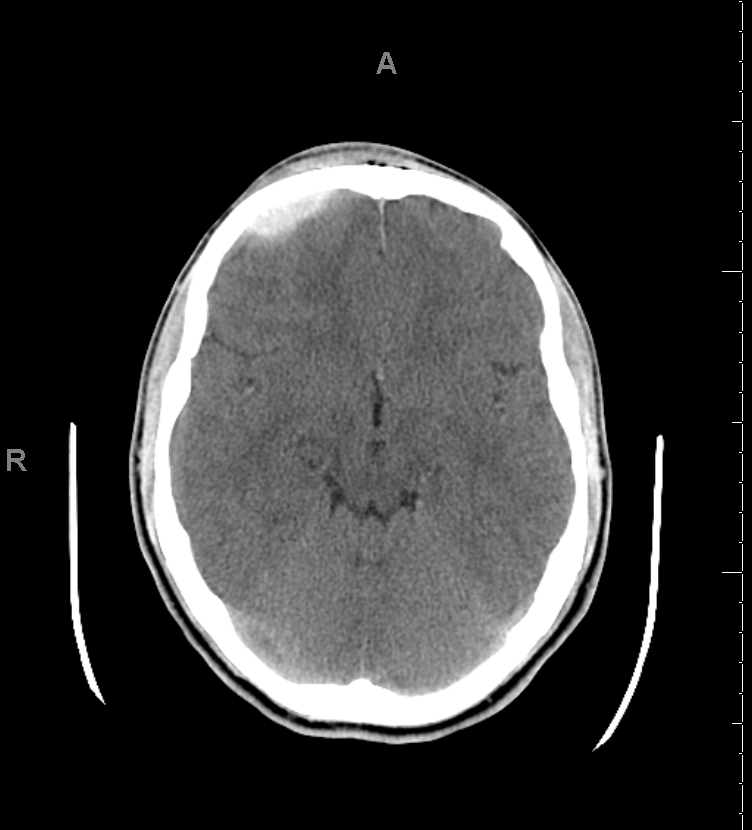
An epidural hematoma (EDH) is an extra-axial collection of blood within the potential space between the outer layer of the dura mater and the inner table of the skull. The hematoma is typically confined by the lateral sutures, particularly the coronal sutures, where the dura inserts. This condition is life-threatening and may require immediate intervention due to the risk of significant morbidity and mortality if left untreated (see Image. Epidural Hematoma on Computed Tomography). Rapid diagnosis and surgical evacuation are critical for favorable outcomes.[1][2][3]
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
EDH occurs in approximately 10% of traumatic brain injuries (TBIs) requiring hospitalization (see Image. Epidural Hematoma After Trauma, Computed Tomography). Although most cases result from trauma—typically motor vehicle collisions, physical assaults, or accidental falls—nontraumatic mechanisms have also been described.[4][5] Spontaneous EDH may rarely complicate acute craniofacial infections, such as frontal sinusitis, likely due to dural vessel erosion from osteomyelitis or venous thrombosis.[6] Additional nontraumatic causes include coagulopathy, hemorrhagic tumors, and vascular malformations.
Epidemiology
EDH occurs in 2% of all head injuries and up to 15% of fatal head traumas. Males are more frequently affected than females, with a higher incidence among adolescents and young adults. The mean age of affected patients ranges from 20 to 30 years, and EDH is rare in those older than 50. With advancing age, the dura mater becomes increasingly adherent to the inner table of the skull, reducing the likelihood of blood accumulating in the potential epidural space.[7]
A 2022 meta-analysis estimated EDH in 8% of TBIs worldwide. After adjustment for study bias, the pooled operative rate was 56%, 74% of patients were male, and nearly half of the cases were attributed to road traffic collisions. The authors estimated that approximately 3.1 million people globally meet surgical indications for traumatic EDH annually, with most cases occurring in working-age individuals.[8]
In the 65-center Collaborative European NeuroTrauma Effectiveness Research in Traumatic Brain Injury (CENTER-TBI) study, EDH was present on initial computed tomography (CT) in 11% of all TBI cases and 16% of severe TBI. Only 29% of EDHs were isolated, with most patients exhibiting concurrent subdural or intraparenchymal hemorrhages. The median patient age was 41 years.[9] In an Australian study, half of EDH cases occurred in individuals aged 0 to 24 years. Falls predominated among children younger than 10, while road-traffic crashes were the leading cause in adolescents. Skull fractures accompanied 75% of EDH and were associated with larger hematoma volumes and higher operative rates.[10]
Pathophysiology
Most EDHs result from arterial bleeding, typically from a branch of the middle meningeal artery. Arterial hemorrhage strips the dura from the inner table of the skull, producing a lentiform hematoma, most often in the temporoparietal region. The anterior meningeal artery or a dural arteriovenous fistula at the vertex may also be involved, though less commonly.[11][12]
Angiographic evaluation in 35 surgical cases identified meningeal arterial extravasation with epidural arteriovenous shunting in 60% of patients. Individuals with shunting experienced frequent clinical deterioration, suggesting that ongoing hemorrhage contributes to hematoma expansion and herniation risk.[13] Shunting may also increase the likelihood of a lucid interval. By diverting blood flow, the arteriovenous shunt can delay hematoma formation and the rise in intracranial pressure (ICP), postponing symptom onset.[14]
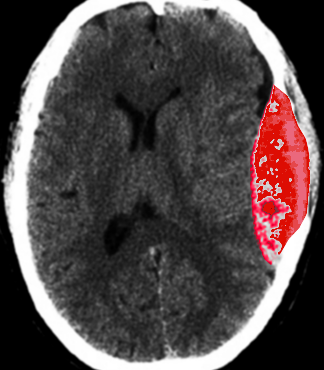
Up to 10% of EDH cases result from venous bleeding, typically due to laceration of a dural venous sinus or hemorrhage from diploic veins associated with skull fractures (see Image. Epidural Hematoma of Sinus Origin). In adults, approximately 75% of EDHs occur in the temporal region. In contrast, pediatric cases demonstrate a more even distribution, with similar frequencies observed in the temporal, occipital, frontal, and posterior fossa regions. A skull fracture is present in most patients with EDH and commonly overlies the squamous portion of the temporal bone. Spinal EDH refers to the occurrence of this condition within the spinal canal.
Radiographically, EDH may be classified by stage of evolution. Type I, or acute EDH, occurs on the first day and is characterized by a swirl sign indicating uncoagulated blood. Type II, or subacute EDH, emerges between days 2 and 4 and typically appears solid. Type III, or chronic EDH, develops between days 7 and 20 and exhibits mixed or lucent characteristics with contrast enhancement (see Image. Calcified Parietooccipital Epidural Hematoma).
History and Physical
The typical presentation of EDH involves an initial loss of consciousness after trauma, followed by a transient, complete recovery, commonly referred to as a "lucid interval," and subsequent rapid neurologic deterioration. This sequence occurs in approximately 14% to 21% of patients with EDH. However, presentations vary widely. Some patients may be unconscious from the outset, regain consciousness after a brief coma, or experience no loss of consciousness at all. Presentations range from transient unconsciousness to persistent coma. Importantly, the lucid interval is not pathognomonic for EDH and may be seen in other expanding intracranial mass lesions.
The classic lucid interval is typically associated with large, pure EDHs and is often accompanied by active bleeding visible on CT. Symptom onset depends on the rate of hematoma expansion within the cranial vault. Small EDHs may remain asymptomatic, although this presentation is uncommon. In some cases, EDH may develop in a delayed fashion. Additional historical features include progressively worsening headaches, nausea or vomiting, and, less frequently, focal or generalized seizures.
An enlarging EDH leads to progressive ICP elevation, which may be detected clinically by ipsilateral pupillary dilation due to uncal herniation, oculomotor nerve compression, elevated blood pressure, bradycardia, and irregular respirations. This triad, known as the Cushing reflex, signals impending brainstem compression and may necessitate immediate intracranial intervention to prevent central nervous system depression and death. On physical examination, ipsilateral pupillary dilation is frequently accompanied by contralateral hemiparesis, hyperreflexia, and a positive Babinski sign. In some cases, false localizing signs such as ipsilateral motor weakness may occur due to cerebral peduncle compression, a phenomenon known as the Kernohan notch or phenomenon.
Posterior fossa EDH is rare and accounts for approximately 5% of posttraumatic intracranial mass lesions. Patients may remain conscious until late in the hematoma’s evolution, at which point they may experience abrupt loss of consciousness, apnea, and death. These lesions often extend into the supratentorial compartment by stripping the dura from the transverse sinus, resulting in substantial intracranial hemorrhage.
A focused history must establish the mechanism and timing of head trauma, including details such as impact energy, fall height, motor vehicle involvement, and any witnessed loss of consciousness or lucid interval. The history should also assess the risk for concomitant injuries, including cervical spine fractures. Inquiry must include preceding symptoms such as headache, nausea, vomiting, focal seizures, anticoagulant or antiplatelet use, and coagulopathy risk factors. Past medical history should document prior neurologic disease, bleeding disorders, and baseline functional status.
Physical examination follows the Advanced Trauma Life Support paradigm. Airway patency should be secured with cervical spine control. Adequate ventilation and oxygenation should be confirmed. Hemodynamic status should be assessed by evaluating pulse, blood pressure, and peripheral perfusion. A rapid neurologic assessment should then be conducted, including the Glasgow Coma Scale scoring, pupillary size, and reactivity evaluation, with attention given to any ipsilateral dilation.
Motor functions should be examined for hemiparesis, hyperreflexia, or a positive plantar response. False localizing signs, such as ipsilateral weakness due to the Kernohan phenomenon, should be recognized. The patient should be fully exposed so scalp lacerations, skull deformities, or other injuries, such as cervical and limb fractures, can be inspected (see Image. Carotid Cavernous Fistula, Symptoms and Imaging). Continuous monitoring of vital signs may reveal bradycardia, hypertension, or respiratory irregularities suggestive of elevated ICP.
Evaluation
Laboratory Studies
Assessment should include prothrombin time, activated partial thromboplastin time, platelet count, and thromboelastography, where available. Blood typing and crossmatching are recommended to support perioperative management and guide the reversal of antithrombotic agents.
Plain Radiography
Skull radiographs have limited diagnostic utility. Up to 40% of EDH cases occur without a detectable fracture on plain imaging.
Computed Tomography
Head CT is the primary diagnostic modality for EDH due to its widespread availability and rapid image acquisition in emergency settings. Most EDHs are readily identifiable on noncontrast (CT computed tomography), typically as a biconvex or lens-shaped (lentiform) hyperdense mass. This shape reflects the limited blood expansion due to the dura's firm attachment at cranial sutures. EDH rarely crosses suture lines but may cross dural reflections such as the falx or tentorium, distinguishing it from subdural hematoma (SDH).
Although the lentiform appearance is characteristic of EDH, lentiform SDH can mimic this pattern. Four CT features favor SDH over EDH: a crescentic tail, obtuse inner margins, a visible dural line, and continuity with adjacent intraparenchymal hemorrhage.[15] Bone windows should be evaluated for associated skull fractures, which may aid localization and mechanism assessment.
Radiologists often estimate EDH volume using the standard formula, ABC/2, where A is the maximum hemorrhage diameter on the CT slice with the largest area of hemorrhage; B is the maximum diameter perpendicular to A on the same slice; and C is the number of CT slices with visible hemorrhage multiplied by slice thickness in centimeters. Additional CT features may influence both diagnosis and prognosis. A low-density region within the hematoma, known as the swirl sign, suggests active bleeding and is associated with larger hematoma volume, lower admission Glasgow Coma Score (GCS), abnormal pupillary findings, and temporoparietal location. This sign correlates with a 29% rate of unfavorable 3-month outcomes, compared with 8% when absent.[16]
Several factors may reduce CT sensitivity for EDH. A hematoma adjacent to contused or hemorrhagic brain tissue may be isodense and escape detection. Severe anemia can produce low-density blood, mimicking cerebrospinal fluid. Arterial extravasation may be minimal in the setting of hypotension. Early imaging after trauma may fail to detect smaller collections that have not yet accumulated sufficient volume. Venous-source EDHs, which accumulate more slowly, may also produce nondiagnostic scans.
CT also aids in detecting concurrent hematomas, evaluating midline shift, and informing surgical decisions. In addition to hematoma volume and the swirl sign, factors associated with an increased risk of EDH expansion include temporal or frontal location and younger patient age. In contrast, intrahematoma air, a contralateral hematoma, or parietal or occipital location is associated with a decreased risk of expansion.[17]
Magnetic Resonance Imaging
Brain magnetic resonance imaging (MRI) is more sensitive than CT, particularly for detecting EDH in the vertex region. MRI is typically reserved for delayed or clinically incongruent presentations when CT findings do not explain the patient's neurologic status. Fluid-attenuated inversion recovery and susceptibility-weighted sequences are especially useful for detecting subacute blood products not visible on CT. In suspected spinal EDH, spinal MRI is the preferred imaging modality due to its superior soft tissue resolution compared to spinal CT.
Angiography
For EDHs near the vertex, angiographic evaluation may be necessary to assess for an underlying dural arteriovenous fistula, often involving branches of the middle meningeal artery. Angiography can confirm the presence and vascular anatomy of such lesions when suspected based on imaging or clinical findings.
Emerging Modalities
A 2025 meta-analysis reported that near-infrared spectroscopy demonstrated a sensitivity of 0.86 and a specificity of 0.83 for detecting intracranial hematomas. These findings support its potential utility in prehospital triage settings.[18]
Treatment / Management
Initial Management and Surgical Consultation
EDH is a neurosurgical emergency that often requires urgent surgical evacuation to prevent irreversible neurologic injury and death from hematoma expansion and herniation. Neurosurgical consultation should be obtained without delay, particularly in patients who meet the criteria for operative intervention. Outcomes worsen progressively with increasing time from the first recorded decline in consciousness to surgical decompression, with especially poor prognosis when this interval exceeds 2 hours.[19] Initial management should prioritize patient stabilization, addressing airway, breathing, and circulation as a matter of urgency.(B2)
Reversal of Coagulopathy
Effective hemorrhage control requires prompt correction of coagulopathy. In the presence of ongoing bleeding, a massive transfusion protocol should be initiated immediately, typically involving packed red blood cells, fresh frozen plasma, and platelets. Reversal strategies must be tailored to the anticoagulant or antiplatelet agent involved. Patients receiving aspirin, clopidogrel, or ticagrelor benefit from platelet transfusion, often supplemented with desmopressin to enhance platelet function. Protamine is indicated for individuals on unfractionated heparin if the activated partial thromboplastin time remains elevated. Low-molecular-weight heparin is also reversed with protamine. Patients on warfarin should receive intravenous vitamin K and either 4-factor prothrombin complex concentrate or fresh frozen plasma. All interventions should be coordinated closely with the blood bank, anesthesiology, and critical care teams. Coagulation parameters must be rechecked to ensure that therapeutic targets are achieved.
Nonoperative Management
According to established guidelines, a nonoperative approach may be appropriate for patients with acute EDH who meet all of the following criteria:
- EDH volume less than 30 mL
- Clot thickness less than 15 mm
- Midline shift less than 5 mm
- GCS score greater than 8
- Absence of focal neurologic deficits
Patients selected for nonoperative management require close clinical observation with serial neurologic examinations and continuous radiographic surveillance. Follow-up head CT is typically recommended 6 hours after the initial scan to monitor for hematoma expansion or clinical deterioration.
Emerging evidence published after the guideline release suggests that select patients with EDH volumes greater than 30 mL may still be managed conservatively. A GCS score of 14 or higher and older age may favor this approach.[20] Conversely, surgical evacuation may be necessary even in patients with EDH volumes under 15 mL, particularly when the hematoma is located in the temporal region, which has been associated with an increased risk of deterioration.
Operative Management
Surgical evacuation is recommended for patients with acute EDH and a hematoma volume greater than 30 mL, regardless of GCS score (see Image. Right Temporal Epidural Hematoma with Skull Fracture and Bilateral Contusions). Prompt surgical intervention is critical in cases where this parameter is below 9 and accompanied by anisocoria.[21] The standard procedure for symptomatic acute EDH is craniotomy with hematoma evacuation. This operation is considered the most cost-effective neurosurgical procedure.[22] Trephination, or burr hole evacuation, may serve as a temporizing measure in settings where definitive neurosurgical care is unavailable. However, burr hole techniques rarely provide adequate hemorrhage control or complete hematoma evacuation.
Posterior fossa epidural hematoma
A meta-analysis of posterior fossa EDH found that surgical individuals frequently exhibited ventriculomegaly, ventricular compression, hydrocephalus, or associated brain contusions. These hematomas tended to be thicker and occupied a larger volume of the already restricted posterior fossa. Overall, 74% of patients underwent surgical intervention, while 26% were managed conservatively.[23](A1)
Other intracranial epidural hematomas
To minimize secondary brain injury, systolic blood pressure should be maintained at or above 100 mm Hg in patients aged 50 to 69, and at or above 110 mm Hg in younger or older patients. Sustained ICP elevations above 22 mm Hg require prompt treatment, and cerebral perfusion pressure should be maintained between 60 and 70 mm Hg, avoiding aggressive increases above this range. Seizure prophylaxis is recommended for 7 days following severe TBI.[24] Levetiracetam is often preferred due to its favorable dosing profile and lower incidence of adverse events.[25] In a global survey, 52% of clinicians reported using seizure prophylaxis for patients with EDH, even in the setting of mild TBI.[26](B3)
Spinal epidural hematoma
Surgical decompression of spinal EDH resulted in functional recovery in 87% of patients. Outcomes were most favorable when surgery was performed within 12 hours of symptom onset.[27]
Operative technique for typical supratentorial epidural hematoma
The patient should be supine on the operating table with a roll under the ipsilateral shoulder. The head must be turned nearly 90° to the contralateral side and secured in a horseshoe head holder. All pressure points should be padded, and the eyes should be protected. For a typical temporal EDH, a question mark–shaped scalp incision should be marked, beginning 1 cm anterior to the tragus, extending superiorly over the temporal region, curving posteriorly toward the parietal eminence, and ending near the frontal midline behind the hairline.
The musculocutaneous flap, including the temporalis fascia and muscle, must be elevated en bloc using periosteal elevators or electrocautery, and the tissues should be retracted with appropriate hooks. Burr holes should be made at the margins of the planned frontotemporal bone flap, ensuring that a sufficient bony margin is preserved for fixation hardware. A clear line of sight along the middle fossa floor to the foramen spinosum must be achieved when the hematoma extends to the floor of the temporal fossa. The root of the zygoma should be fully exposed for optimal access.
The burr holes must be connected circumferentially using a cutting bit with a footplate attachment. The bone flap should then be gently separated from the underlying dura using a Penfield 3 dissector. The additional bone should be removed using a Leksel rongeur to access the floor of the middle cranial fossa.
After removing the bone flap, the extradural space should be irrigated and suctioned to evacuate the hematoma. The source of bleeding, most commonly the middle meningeal artery or its branches, should be identified and coagulated using bipolar electrocautery. In venous bleeding from diploic veins or fracture margins, bone wax or fibrin-soaked hemostatic agents should be applied with gentle pressure. Bleeding from the foramen spinosum must be controlled using bone wax. The dura should be carefully inspected for signs of subdural collections, and a limited dural opening may be created to assess for an underlying SDH if clinically indicated.
Tack-up sutures should be placed between the dura and the inner table at approximately 2 to 3-centimeter intervals using 3-0 nonabsorbable sutures, typically Nurolon or silk, to eliminate potential epidural space. The bone flap must be repositioned and secured using a cranial plating system. If the flap is large, a central tack-up suture should be placed through a predrilled hole in the bone flap. A subgaleal drain should be inserted through a small stab incision and connected to bulb suction.
The temporalis fascia must be reapproximated with a 0 absorbable suture. The galea should be closed using inverted 2-0 absorbable sutures, and the scalp should be closed with either staples or nonabsorbable nylon sutures. A nonadherent gauze dressing should be applied, followed by a compressive head wrap.
Operative technique for typical infratentorial epidural hematoma
For ICP management, an external ventricular drain may be placed at the start of the procedure or deferred until after postoperative imaging, depending on the surgical plan. If selected as the initial step, the external ventricular drain should be inserted via a frontal burr hole while the patient is in the supine position or through an occipital approach once the patient has been positioned prone.
Patients must be positioned prone with 3-point Mayfield fixation unless contraindicated by the presence of a skull fracture, in which case a horseshoe head holder should be used. The neck should be flexed gently to expose the suboccipital region without obstructing venous return from the cervical vasculature. A midline linear incision should be made, or a hockey stick–shaped incision may be used if the hematoma extends laterally. In cases of bilateral extension, a T-shaped incision may be selected.
Posterior fossa EDHs frequently extend supratentorially. To facilitate the later placement of tack-up sutures and tamponade over the transverse sinus, a bridge of bone must be preserved in this region. Burr holes should be created both above and below the transverse sinus and connected with a craniotome to form a 2-piece supraoccipital and suboccipital bone flap.
After removing the bone flaps, the extradural clot must be evacuated gently. Hemostasis should be achieved with dural tack-up sutures and hemostatic agents. Direct sinus repair or ligation may be performed only in uncontrollable bleeding and should be reserved for nondominant sinuses.
Following hemostasis, nonabsorbable tack-up sutures must be placed at 2 to 3-centimeter intervals around the bone flap margin. Additional sutures should be placed along the bone bridge overlying the sinus to eliminate the epidural space. Replacement of the bone flap may be performed at the discretion of the surgical team. A subgaleal drain should be placed prior to layered closure of the fascia and skin.
Differential Diagnosis
Acute SDH can resemble EDH on initial imaging but typically appears crescentic and conforms to the brain surface, often crossing suture lines. Subdural bleeding usually originates from torn bridging veins and presents with a more gradual neurologic decline, in contrast to the rapid deterioration and classic lucid interval seen in EDH. Subdural hygromas and chronic SDHs appear hypodense or of mixed density on CT and typically present subacutely or chronically, differing from the uniformly hyperdense appearance of acute EDH. Intracerebral hemorrhage presents as a focal, intraparenchymal hyperdensity with surrounding edema on CT. EDH, by contrast, is strictly extra-axial.
Traumatic contusions are characterized by patchy, mixed-density lesions within the cortex or subcortical white matter that evolve over time. EDH, however, presents acutely as a well-demarcated collection between the skull and dura, with mass effect developing over minutes to hours. Hemorrhagic transformation of an ischemic stroke is suggested by a history of focal deficits and vascular risk factors, with bleeding localized to an infarct territory on CT. Intracranial abscess presents with headache, fever, and focal neurologic signs. Imaging typically shows a ring-enhancing lesion with surrounding edema, not the lentiform extra-axial blood of EDH. Dura-based primary or metastatic tumors can mimic EDH radiographically, but these masses enhance heterogeneously, progress slowly, and present with subacute symptoms, unlike the rapid evolution typical of EDH.
Prognosis
Patients with pure EDH typically have an excellent functional prognosis when the hematoma is promptly recognized and surgically evacuated. Delays in diagnosis and treatment are associated with increased morbidity and mortality. EDH caused by arterial injury tends to progress rapidly, whereas venous bleeding from a dural sinus tear may result in slower hematoma formation, leading to delayed symptom onset and recognition. Neurologic outcomes worsen significantly when hematoma volume exceeds 50 cm³ before evacuation.
Several factors negatively affect prognosis, including advanced age, prolonged time from injury to intervention, immediate coma, pupillary abnormalities, and low GCS scores before surgery. In a prospective series, no deaths occurred when preoperative GCS was 8 or higher.[28] A longer interval between neurologic deterioration and surgical evacuation also worsens outcomes. Imaging predictors of poor prognosis include hematoma volumes exceeding 30 to 150 mL, midline shift greater than 10 mm, the presence of a “swirl sign” suggesting active bleeding, and associated intracranial injuries such as contusions, intracerebral hemorrhage, subarachnoid hemorrhage, or diffuse cerebral edema. Persistently elevated ICP in the postoperative period increases the risk for poor outcomes.
In a national trauma database analysis of conservatively treated, low-severity EDH, independent predictors of mortality included advanced age, congestive heart failure, renal failure, cirrhosis, and preinjury anticoagulant use. The overall mortality rate was 1.9%.[29] Results from a separate study of surgically treated EDH reported a postoperative mortality rate of 6.8%. Unfavorable outcomes were associated with midline shift, concurrent injuries, elevated Injury Severity Score (ISS), and alcohol intoxication. Admission GCS score and anticoagulant use did not independently predict survival.[30]
Frailty significantly influences EDH prognosis. The 5-component modified Frailty Index (mFI-5) assigns 1 point each for chronic obstructive pulmonary disease, congestive heart failure, diabetes mellitus, functional dependency, and hypertension. The total score, divided by 5, yields the mFI-5. A value greater than 0.2 doubles the mortality risk in TBI and correlates with lower home discharge rates and increased facility placement. The mFI-5 predicts mortality with an area under the receiver operating characteristic curve of 0.71.[31]
In a large database cohort of patients with acute traumatic EDH, in-hospital mortality was 1.9%. Nonhome discharge occurred in 21.7%, prolonged hospitalization beyond 7 days in 17.6%, and intensive care unit stays longer than 4 days in 16.2%. Major complications developed in 3.2%. Machine learning models accurately predicted in-hospital mortality (area under the curve, 0.956), identifying verbal GCS score and age as the most important predictors.[32]
Complications
EDH may cause increased ICP, uncal herniation, and rapid neurological deterioration. Delayed recognition elevates the risk for permanent motor deficits, cognitive impairment, and death. Cortical irritation from blood in the epidural space increases the likelihood of seizures. Postoperative complications include surgical site infection and hematoma recurrence.
Medical complications following acute traumatic EDH include ventilator-associated pneumonia, delirium, cardiac arrest requiring resuscitation, acute kidney injury, and deep vein thrombosis. Less common events include pulmonary embolism, acute respiratory distress syndrome, severe sepsis, stroke, and additional surgical site infections. Rare complications involve myocardial infarction, catheter-associated bloodstream infection, osteomyelitis, and pressure ulcers. Despite a timely and thorough evacuation, some patients may experience persistent headaches or residual neurologic deficits.
Postoperative and Rehabilitation Care
Postoperative monitoring involves frequent neurologic assessments to detect early signs of deterioration. Serial imaging is used to evaluate for residual or recurrent hemorrhage. Blood pressure control is essential to minimize the risk of rebleeding and support hematoma resolution. Rehabilitation includes physical therapy to improve mobility and core strength, occupational therapy to support independence in daily activities, and speech therapy to assess and manage communication or swallowing dysfunction when indicated. Neuropsychological evaluation may reveal cognitive deficits that benefit from targeted rehabilitation strategies. Discharge planning should incorporate scheduled follow-up visits and referrals for community-based physical, occupational, or speech therapy services.
Consultations
Trauma surgery and neurosurgery should be consulted immediately, as outcomes depend on timely intervention. Immediate input from both teams supports efficient triage, monitoring, and treatment.
Deterrence and Patient Education
Primary prevention of EDH focuses on education and risk mitigation. Patients should receive individualized instruction on head injury prevention, including consistent use of protective headgear during contact sports or high-risk occupations. Home safety should be optimized by removing trip hazards and installing grab bars to reduce fall risk. Anticoagulant and antiplatelet use must be reviewed, with emphasis on strict adherence and regular monitoring.
Secondary prevention involves early recognition of complications and structured surveillance. Patients should be instructed to report any new or worsening headache, focal neurological deficits, or changes in consciousness promptly. Training in safe transfer and mobility techniques is essential to prevent recurrent trauma. Caregivers should be educated to recognize subtle cognitive or behavioral changes that may indicate delayed complications. Routine follow-up visits and imaging studies are necessary to detect hematoma recurrence at the earliest stage.
Pearls and Other Issues
EDH requires prompt recognition and intervention to prevent irreversible neurologic injury. The following clinical pearls highlight key diagnostic and management considerations.
- EDH is a neurosurgical emergency that requires rapid diagnosis and intervention.
- The condition should be suspected when a history of head trauma is followed by a period of loss of consciousness.
- Small EDHs may present without symptoms.
- A skull fracture is generally present, though EDH may occur in its absence.
- EDH is confined by cranial sutures and does not cross suture lines.
- Although a lucid interval is frequently described, it should not be considered pathognomonic, as it may also occur with other expanding intracranial lesions.
- Consciousness may be absent from the outset, briefly regained after trauma, or never lost in patients with EDH.
- An excellent functional outcome can be achieved if EDH is detected and evacuated promptly.
Understanding these key features of EDH can support more confident decision-making in acute care settings. Early neurosurgical consultation and intervention are the cornerstones of effective treatment.
Enhancing Healthcare Team Outcomes
EDH is a relatively common presentation of TBI in the emergency department and, if undiagnosed, carries a high risk of mortality. Optimal management requires coordination by an interprofessional team comprising the emergency clinician, neurosurgeon, trauma team, radiologist, intensivist, and critical care nursing staff. This condition can be life-threatening, and the initial level of consciousness is a key prognostic indicator. Early diagnosis and prompt surgical intervention in patients who meet operative criteria are critical to improving outcomes. Primary prevention is the most effective strategy. Public education regarding the use of protective headgear during sports and occupational activities should be emphasized to reduce the incidence of head trauma.[33][34]
Media
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
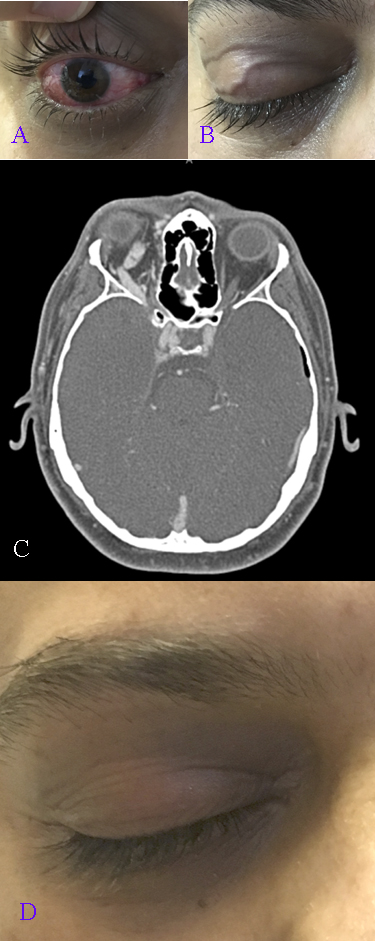
Carotid Cavernous Fistula, Symptoms and Imaging. A 33-year-old man with epidural hematomas and skull base fractures presents with right-sided ptosis, proptosis, pulsatile tinnitus, and limited extraocular movements in all directions (A). Chemosis with tortuous conjunctival vessels and elevated intraocular pressure is observed (B), along with dilated transverse eyelid veins (C). Computed tomography angiography confirms a right-sided Barrow type A carotid cavernous fistula with an engorged superior ophthalmic vein (D). Symptoms and signs resolve following embolization of the right cavernous sinus, and the previously dilated eyelid veins are no longer visible.
Contributed by BCK Patel, MD, FRCS
(Click Image to Enlarge)
Calcified Parietooccipital Epidural Hematoma. This noncontrast head computed tomography scan shows a chronic epidural hematoma with peripheral calcification along the right parietooccipital convexity. The dense, curvilinear rim suggests longstanding blood accumulation with organization and ossification.
Contributed by Sunil Munakomi, MD
(Click Image to Enlarge)
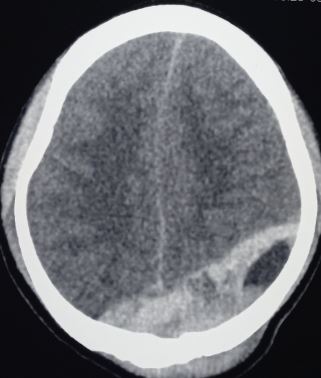
Epidural Hematoma of Sinus Origin. Computed tomography scan demonstrates a left parietooccipital epidural hematoma arising from injury to a dural venous sinus. This location is less common and often associated with slower progression due to venous rather than arterial bleeding.
Contributed by Sunil Munakomi,MD
(Click Image to Enlarge)
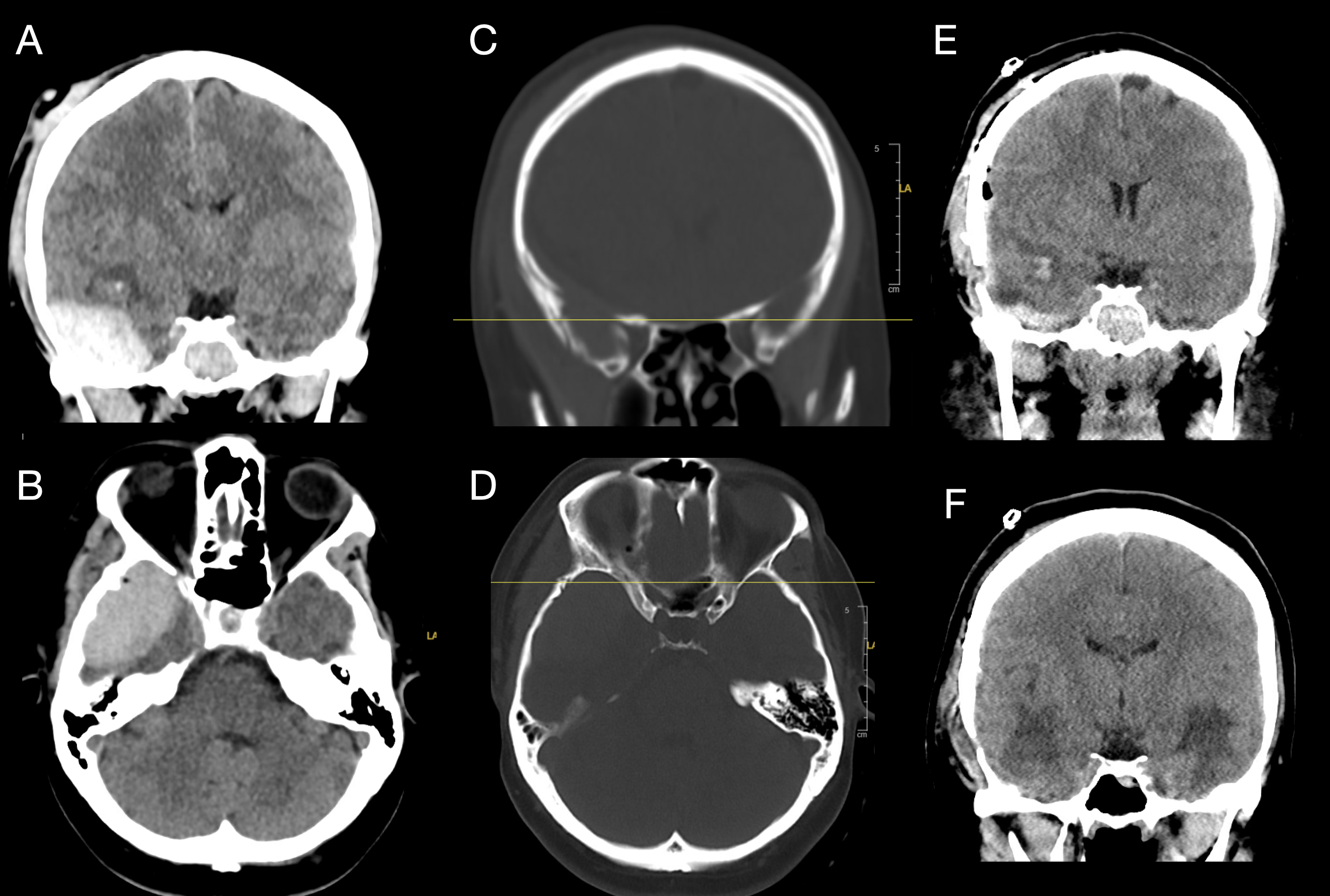
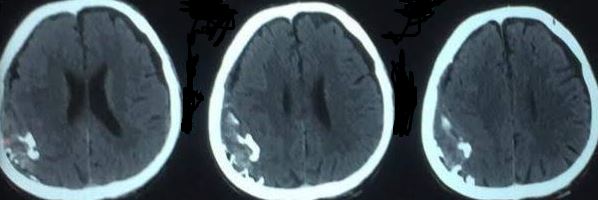
Right Temporal Epidural Hematoma with Skull Fracture and Bilateral Contusions. A coronal computed tomography scan (A) shows a right acute epidural hematoma with midline shift to the left and early right-sided uncal herniation. An axial view (B) further demonstrates the mass effect. Bone window images (C and D) reveal an acute traumatic fracture through the anterior aspect of the squamous portion of the right temporal bone. Immediate postoperative computed tomography (E) shows right temporal lobe contusions, with similar contralateral contusions not captured on this slice. Two-week postoperative imaging (F) demonstrates evolving hemorrhagic contusions in the right and left inferior temporal lobes, consistent with coup-contrecoup injury.
Contributed by Konstantinos Margetis MD, PhD
References
Rosenthal AA, Solomon RJ, Eyerly-Webb SA, Sanchez R, Lee SK, Kiffin C, Davare DL, Hranjec T, Carrillo EH. Traumatic Epidural Hematoma: Patient Characteristics and Management. The American surgeon. 2017 Nov 1:83(11):e438-e440 [PubMed PMID: 30401085]
Babu JM, Patel SA, Palumbo MA, Daniels AH. Spinal Emergencies in Primary Care Practice. The American journal of medicine. 2019 Mar:132(3):300-306. doi: 10.1016/j.amjmed.2018.09.022. Epub 2018 Oct 3 [PubMed PMID: 30291829]
Kanematsu R, Hanakita J, Takahashi T, Park S, Minami M. Radiologic Features and Clinical Course of Chronic Spinal Epidural Hematoma: Report of 4 Cases and Literature Review. World neurosurgery. 2018 Dec:120():82-89. doi: 10.1016/j.wneu.2018.08.058. Epub 2018 Aug 23 [PubMed PMID: 30145384]
Level 3 (low-level) evidenceTamburrelli FC, Meluzio MC, Masci G, Perna A, Burrofato A, Proietti L. Etiopathogenesis of Traumatic Spinal Epidural Hematoma. Neurospine. 2018 Mar:15(1):101-107. doi: 10.14245/ns.1834938.469. Epub 2018 Mar 28 [PubMed PMID: 29656630]
Fernández-Abinader JA, González-Colón K, Feliciano CE, Mosquera-Soler AM. Traumatic Brain Injury Profile of an Elderly Population in Puerto Rico. Puerto Rico health sciences journal. 2017 Dec:36(4):237-239 [PubMed PMID: 29220069]
Scafa AK, Jiang T, Pescatori L, Corsini M, Piccirilli M. Association between spontaneous intracranial epidural hematoma and craniofacial infections: A systematic literature review. Surgical neurology international. 2023:14():57. doi: 10.25259/SNI_1068_2022. Epub 2023 Feb 17 [PubMed PMID: 36895255]
Level 1 (high-level) evidenceChicote Álvarez E, González Castro A, Ortiz Lasa M, Jiménez Alfonso A, Escudero Acha P, Rodríguez Borregán JC, Peñasco Martín Y, Dierssen Sotos T. Epidemiology of traumatic brain injury in the elderly over a 25 year period. Revista espanola de anestesiologia y reanimacion... 2018 Dec:65(10):546-551. doi: 10.1016/j.redar.2018.06.003. Epub 2018 Jul 25 [PubMed PMID: 30054092]
Rahimi A, Corley JA, Ammar A, Shlobin NA, Rolle M, Mekary RA, Park KB. The unmet global burden of cranial epidural hematomas: A systematic review and meta-analysis. Clinical neurology and neurosurgery. 2022 Aug:219():107313. doi: 10.1016/j.clineuro.2022.107313. Epub 2022 Jun 6 [PubMed PMID: 35688003]
Level 1 (high-level) evidencePisică D, Volovici V, Yue JK, van Essen TA, den Boogert HF, Vande Vyvere T, Haitsma I, Nieboer D, Markowitz AJ, Yuh EL, Steyerberg EW, Peul WC, Dirven CMF, Menon DK, Manley GT, Maas AIR, Lingsma HF, CENTER-TBI Participants and Investigators. Clinical and Imaging Characteristics, Care Pathways, and Outcomes of Traumatic Epidural Hematomas: A Collaborative European NeuroTrauma Effectiveness Research in Traumatic Brain Injury Study. Neurosurgery. 2024 Nov 1:95(5):986-999. doi: 10.1227/neu.0000000000002982. Epub 2024 May 21 [PubMed PMID: 38771081]
Irie F, Le Brocque R, Kenardy J, Bellamy N, Tetsworth K, Pollard C. Epidemiology of traumatic epidural hematoma in young age. The Journal of trauma. 2011 Oct:71(4):847-53. doi: 10.1097/TA.0b013e3182032c9a. Epub [PubMed PMID: 21336188]
Burjorjee JE, Rooney R, Jaeger M. Epidural Hematoma Following Cessation of a Direct Oral Anticoagulant: A Case Report. Regional anesthesia and pain medicine. 2018 Apr:43(3):313-316. doi: 10.1097/AAP.0000000000000738. Epub [PubMed PMID: 29369958]
Level 3 (low-level) evidenceBonow RH, Barber J, Temkin NR, Videtta W, Rondina C, Petroni G, Lujan S, Alanis V, La Fuente G, Lavadenz A, Merida R, Jibaja M, Gonzáles L, Falcao A, Romero R, Dikmen S, Pridgeon J, Chesnut RM, Global Neurotrauma Research Group. The Outcome of Severe Traumatic Brain Injury in Latin America. World neurosurgery. 2018 Mar:111():e82-e90. doi: 10.1016/j.wneu.2017.11.171. Epub 2017 Dec 9 [PubMed PMID: 29229352]
Habash AH, Sortland O, Zwetnow NN. Epidural haematoma: pathophysiological significance of extravasation and arteriovenous shunting. An analysis of 35 patients. Acta neurochirurgica. 1982:60(1-2):7-27 [PubMed PMID: 7058702]
Ganz JC. The lucid interval associated with epidural bleeding: evolving understanding. Journal of neurosurgery. 2013 Apr:118(4):739-45. doi: 10.3171/2012.12.JNS121264. Epub 2013 Jan 18 [PubMed PMID: 23330993]
Level 3 (low-level) evidenceSu IC, Wang KC, Huang SH, Li CH, Kuo LT, Lee JE, Tseng HM, Tu YK. Differential CT features of acute lentiform subdural hematoma and epidural hematoma. Clinical neurology and neurosurgery. 2010 Sep:112(7):552-6. doi: 10.1016/j.clineuro.2010.03.001. Epub 2010 May 18 [PubMed PMID: 20483531]
Wang X, Ge R, Yuan J, Xu S, Fang X, Dai Y, Jiang X. Risk Factors and Prognostic Value of Swirl Sign in Traumatic Acute Epidural Hematoma. Frontiers in neurology. 2020:11():543536. doi: 10.3389/fneur.2020.543536. Epub 2020 Nov 9 [PubMed PMID: 33240193]
Hasanpour M, Elyassirad D, Gheiji B, Vatanparast M, Keykhosravi E, Shafiei M, Daneshkhah S, Fayyazi A, Faghani S. Predicting Epidural Hematoma Expansion in Traumatic Brain Injury: A Machine Learning Approach. The neuroradiology journal. 2025 Apr:38(2):200-206. doi: 10.1177/19714009241303052. Epub 2024 Nov 24 [PubMed PMID: 39582207]
Zarei H, Zarrin A, Janmohamadi M, Saadatipour N, Yarahmadi M, Moeini M, Shams Ardekani S, Safdarian A, Vazirizadeh-Mahabadi M, Babaei M, Bagheri N, Gholipour A, Azadi M, Parvari S, Azimi A. Near Infrared Spectroscopy as a Diagnostic Tool for Screening of Intracranial Hematomas; A Systematic Review and Meta-Analysis. Archives of academic emergency medicine. 2025:13(1):e9. doi: 10.22037/aaem.v13i1.2411. Epub 2024 Sep 13 [PubMed PMID: 39465060]
Level 1 (high-level) evidenceMendelow AD, Karmi MZ, Paul KS, Fuller GA, Gillingham FJ. Extradural haematoma: effect of delayed treatment. British medical journal. 1979 May 12:1(6173):1240-2 [PubMed PMID: 455011]
Level 2 (mid-level) evidenceSoon WC,Marcus H,Wilson M, Traumatic acute extradural haematoma - Indications for surgery revisited. British journal of neurosurgery. 2016; [PubMed PMID: 26742836]
Bullock MR, Chesnut R, Ghajar J, Gordon D, Hartl R, Newell DW, Servadei F, Walters BC, Wilberger JE, Surgical Management of Traumatic Brain Injury Author Group. Surgical management of acute epidural hematomas. Neurosurgery. 2006 Mar:58(3 Suppl):S7-15; discussion Si-iv [PubMed PMID: 16710967]
Pickard JD, Bailey S, Sanderson H, Rees M, Garfield JS. Steps towards cost-benefit analysis of regional neurosurgical care. BMJ (Clinical research ed.). 1990 Sep 29:301(6753):629-35 [PubMed PMID: 2121302]
Daoud SS, Jamous MA, Al Barbarawi MM, Jarrar S, Jaradat A, Aljabali AS, Altal MK, Hulliel AF, Hazaimeh EA, Jbarah OF, Alsharman MA, Abdallah A. Operative versus non-operative management of posterior fossa epidural hematoma: A systematic review and meta-analysis. Neuro-Chirurgie. 2024 Sep:70(5):101578. doi: 10.1016/j.neuchi.2024.101578. Epub 2024 Jun 28 [PubMed PMID: 38943702]
Level 1 (high-level) evidenceCarney N, Totten AM, O'Reilly C, Ullman JS, Hawryluk GW, Bell MJ, Bratton SL, Chesnut R, Harris OA, Kissoon N, Rubiano AM, Shutter L, Tasker RC, Vavilala MS, Wilberger J, Wright DW, Ghajar J. Guidelines for the Management of Severe Traumatic Brain Injury, Fourth Edition. Neurosurgery. 2017 Jan 1:80(1):6-15. doi: 10.1227/NEU.0000000000001432. Epub [PubMed PMID: 27654000]
Chartrain AG, Yaeger K, Feng R, Themistocleous MS, Dangayach NS, Margetis K, Hickman ZL. Antiepileptics for Post-Traumatic Seizure Prophylaxis after Traumatic Brain Injury. Current pharmaceutical design. 2017:23(42):6428-6441. doi: 10.2174/1381612823666171031100139. Epub [PubMed PMID: 29086674]
Hickman ZL, Spielman LA, Barthélemy EJ, Choudhri TF, Engelman B, Giwa AO, Greisman JD, Margetis K, Race M, Rahman J, Todor DR, Tsetsou S, Ullman JS, Unadkat P, Dams-O'Connor K. International Survey of Antiseizure Medication Use in Patients with Complicated Mild Traumatic Brain Injury: A New York Neurotrauma Consortium Study. World neurosurgery. 2022 Dec:168():e286-e296. doi: 10.1016/j.wneu.2022.09.110. Epub 2022 Oct 1 [PubMed PMID: 36191888]
Level 3 (low-level) evidenceLawton MT, Porter RW, Heiserman JE, Jacobowitz R, Sonntag VK, Dickman CA. Surgical management of spinal epidural hematoma: relationship between surgical timing and neurological outcome. Journal of neurosurgery. 1995 Jul:83(1):1-7 [PubMed PMID: 7782824]
Bricolo AP, Pasut LM. Extradural hematoma: toward zero mortality. A prospective study. Neurosurgery. 1984 Jan:14(1):8-12 [PubMed PMID: 6694798]
Erukulla N, Adachi K, Sureshkumar H, Hukamdad M, Jiang SH, Chiu RG, Mehta AI. Clinical risk factors for mortality in low severity acute epidural hematoma. Clinical neurology and neurosurgery. 2025 Feb:249():108764. doi: 10.1016/j.clineuro.2025.108764. Epub 2025 Jan 27 [PubMed PMID: 39879744]
Gutowski P, Meier U, Rohde V, Lemcke J, von der Brelie C. Clinical Outcome of Epidural Hematoma Treated Surgically in the Era of Modern Resuscitation and Trauma Care. World neurosurgery. 2018 Oct:118():e166-e174. doi: 10.1016/j.wneu.2018.06.147. Epub 2018 Jun 26 [PubMed PMID: 29959068]
Level 2 (mid-level) evidenceMaragkos GA, Matsoukas S, Cho LD, Legome EL, Wedderburn RV, Margetis K. Comparison of Frailty Indices and the Charlson Comorbidity Index in Traumatic Brain Injury. The Journal of head trauma rehabilitation. 2023 May-Jun 01:38(3):E177-E185. doi: 10.1097/HTR.0000000000000832. Epub 2022 Oct 14 [PubMed PMID: 36730992]
Karabacak M, Margetis K. Prognosis at Your Fingertips: A Machine Learning-Based Web Application for Outcome Prediction in Acute Traumatic Epidural Hematoma. Journal of neurotrauma. 2024 Jan:41(1-2):147-160. doi: 10.1089/neu.2023.0122. Epub 2023 Jul 18 [PubMed PMID: 37261977]
Aguilar MI, Brott TG. Update in intracerebral hemorrhage. The Neurohospitalist. 2011 Jul:1(3):148-59. doi: 10.1177/1941875211409050. Epub [PubMed PMID: 23983850]
Jeong YH, Oh JW, Cho S, Korean Trauma Data Bank System Committee. Clinical Outcome of Acute Epidural Hematoma in Korea: Preliminary Report of 285 Cases Registered in the Korean Trauma Data Bank System. Korean journal of neurotrauma. 2016 Oct:12(2):47-54 [PubMed PMID: 27857907]
Level 2 (mid-level) evidence