Introduction
The electroencephalographic (EEG) signal represents bioelectric potentials generated by brain activity, recorded from the scalp using electrodes and specialized equipment. EEG activity reflects the temporal summation of synchronous firing from millions of spatially aligned cortical neurons. The measurement system captures weak electrical signals from the scalp; amplifies them; processes them, including digitization; and records the resulting data.[1]
Technique or Treatment
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Technique or Treatment
Analyzing and interpreting EEG tracings involves a combination of art and science. Normal EEGs exhibit high diversity and a broad range of physiological variability. Advanced processing techniques, such as Fourier analysis, joint time-frequency analysis, and specialized algorithms, expand the applications of EEG to general anesthesia and research areas, including neuroscience and cognitive psychology.
A systematic approach to interpreting EEG waveforms is essential. Potential confounding factors, including the patient's age, state of consciousness, physical and mental activity, and the influence of biological, environmental, and pharmacological factors on the waveforms, must be considered before analysis begins.
Clinical Significance
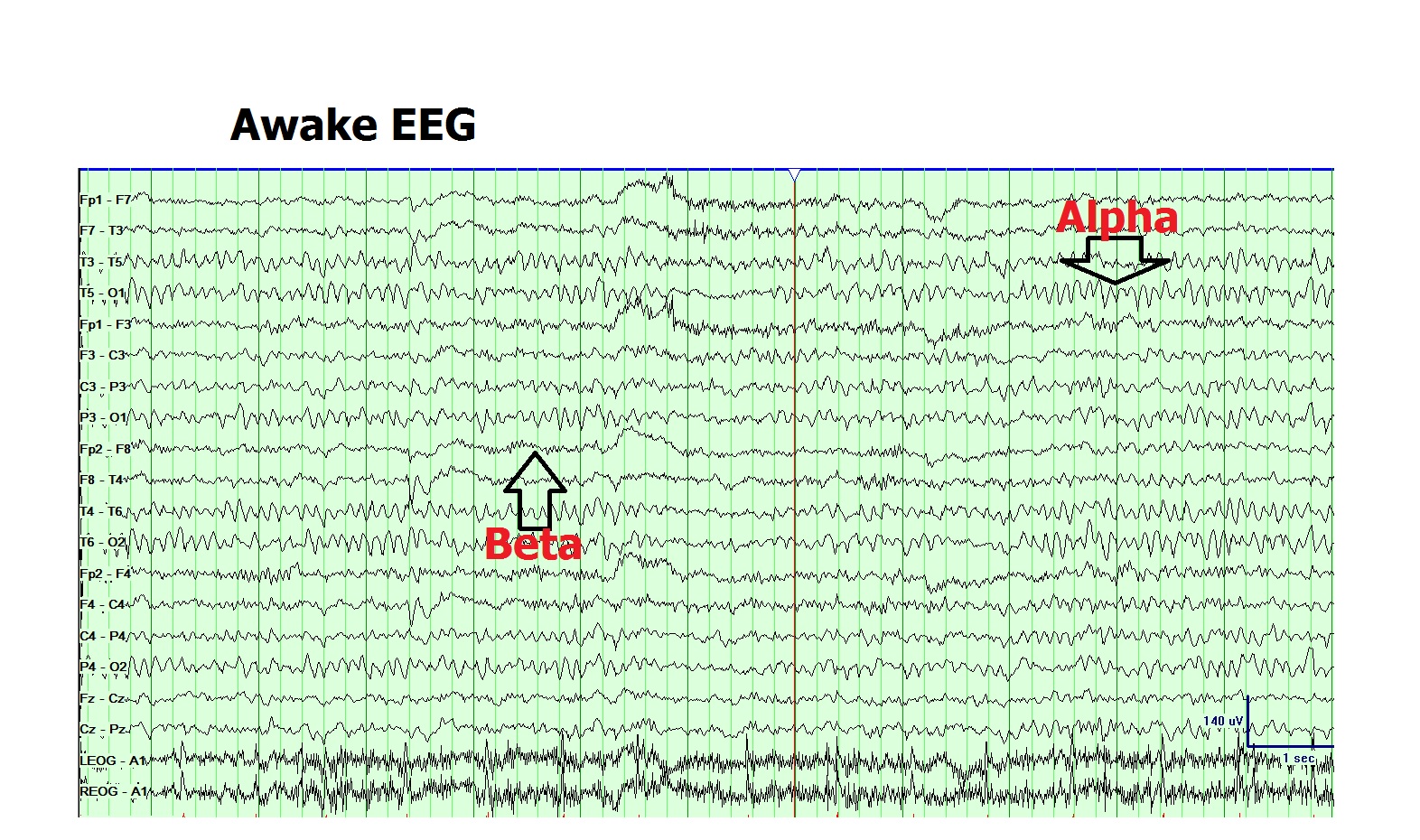
Differentiating normal waveforms from abnormal patterns is essential for EEG to achieve high specificity and diagnostic value. EEG waveforms can be characterized by location, amplitude, frequency, morphology, continuity (rhythmic, intermittent, or continuous), synchrony, symmetry, and reactivity (see Image. Normal Awake Electroencephalogram).
Frequency remains the most commonly used method to classify EEG waveforms, with waves named according to their frequency range using Greek letters. Key waveforms include δ (0.5-4 Hz), θ (4-7 Hz), α (8-12 Hz), σ (12-16 Hz), and β (13-30 Hz).
Normal rhythms are categorized into awake and sleep patterns.
- Awake patterns include the α rhythm and its variants, μ rhythm, λ waves, posterior slow waves of youth, hyperventilation-induced slowing, photic driving, and photomyogenic response.
- Sleep patterns include positive occipital sharp transients of sleep, vertex waves, spindles, K complexes, sleep-related hypersynchrony, and frontal arousal rhythm.
Breach rhythms can affect both awake and sleep patterns.
Additional waveforms exist outside the conventional clinical EEG bandwidth, but they have gained importance with the advent of digital signal processing. These patterns include infraslow oscillations (<0.5 Hz), γ waves (30-80 Hz), and high-frequency oscillations (>80 Hz).
Several patterns or variants of uncertain clinical significance previously considered abnormal in early EEG history are now classified as normal. These patterns include:
- Wicket spikes and wicket rhythms: The most commonly overread normal pattern for epileptiform activity
- Small sharp spikes: Also known as benign epileptiform transients of sleep
- Rhythmic midtemporal θ of drowsiness: Also called psychomotor variant
- Cigánek rhythm (midline θ)
- 6-Hz phantom spike-wave
- 14- and 6-Hz positive spikes
- Subclinical rhythmic epileptiform discharges of adults
- Slow-fused transients
- Occipital spikes associated with blindness
- Temporal slowing in older adults [2]
Frequency
Mapping the full frequency bandwidth of brain electrophysiological signals is crucial for understanding both physiological and pathological states. Conventional clinical EEG typically focuses on waveforms ranging from 0.5 to 70 Hz, analyzed using bandpass filtering techniques. However, broader EEG bandwidths have been examined by clinical neurophysiologists and researchers and shown to have clinical relevance in specific contexts.
Simultaneous recording of brain direct current shifts, infraslow oscillations (<0.1 Hz), typical local field potentials (0.1-80 Hz), and higher frequencies (80-600 Hz) from the same site holds promise for preclinical epilepsy research and may offer clinical biomarkers for more precise delineation of seizure onset zones.[3] Excluding infraslow or ultrafast frequency bands from routine EEG omits physiologically and pathologically significant features of brain activity.
Full-bandwidth EEG enables analysis of all physiologically and clinically meaningful waveforms without sacrificing a frequency band for another.[4] Despite its potential, routine clinical use of full-bandwidth EEG remains limited, as capturing extremely high-frequency signals requires specialized equipment capable of higher sampling rates and expanded data storage. Based on full-bandwidth EEG recordings, EEG waveforms can be characterized into several types, discussed below.
Infraslow Oscillations
In preterm neonates, infraslow oscillations dominate the EEG at frequencies below 0.5 Hz, typically ranging from 0.01 to 0.1 Hz. These patterns, also referred to as spontaneous activity transients, reflect endogenously driven, spontaneous activity that plays a critical role in shaping neuronal connectivity during early development when sensory input is minimal or absent.[5] Infraslow oscillations within a broader range (0.02-0.2 Hz) also occur during non-rapid eye movement sleep—a phase that coincides with higher-frequency EEG activity.[6]
Research on low-frequency EEG has largely focused on cognitive states and tasks, including contingent stimulation, such as contingent negative variation; voluntary motor activity, such as Bereitschafts potential; and orienting responses.[7][8] These slow scalp-recorded potentials often persist for several seconds and typically exhibit amplitudes of only a few microvolts. Accurate capture of these signals requires full-bandwidth EEG recordings and electrodes with true direct current properties, along with stable skin-electrode interfaces.
Both invasive and noninvasive EEG recordings in animal models and human studies have demonstrated that seizures are associated with very slow EEG responses and variable low-frequency fluctuations at the seizure focus.[9] Recent noninvasive recordings of ictal direct current activity have demonstrated that focal-onset seizures often coincide with prolonged and relatively high-amplitude direct current shifts.[10] Additional evidence indicates that infraslow activity (<0.2 Hz) may support long-range spatial coupling in the brain. A study demonstrated the potential to examine infraslow activity through its modulation of higher-frequency signals, even without direct current–coupled recordings, and emphasized the clinical significance of infraslow dynamics in evaluating seizure risk.[11]
δ Rhythm
The δ rhythm (0.5-4 Hz) appears physiologically during deep sleep and predominates in the frontocentral regions. Pathological δ activity arises during wakefulness in cases of generalized encephalopathy or focal cerebral dysfunction. Frontal intermittent rhythmic δ activity occurs in adults, whereas occipital intermittent rhythmic δ activity presents in children.[12][13] Temporal intermittent rhythmic δ activity is frequently observed in individuals with temporal lobe epilepsy.[14] Recent studies have identified stepping movements as δ-rhythmic (1-4 Hz), modulated by sensory input. Stepping-related δ-rhythmic neural activity, along with β (10-30 Hz) frequency dynamics, also emerges in sensorimotor circuits.[15]
θ Rhythm
The θ rhythm (4-7 Hz) appears during drowsiness and early sleep stages, such as N1 and N2. This pattern is most prominent in the frontocentral head regions and slowly migrates backward, replacing the α rhythm as drowsiness begins. Heightened emotional states can also enhance frontal rhythmic θ activity in children and young adults.
Focal θ activity during wakefulness suggests focal cerebral dysfunction. A recent study showed that patients with obstructive sleep apnea exhibit increased θ phase and β amplitude in the frontal and occipital regions during N1 sleep and wakefulness. This finding implies that cortical coupling is prevalent and displays sleep-stage–specific patterns in obstructive sleep apnea. θ-β Phase-amplitude coupling during N1 and wakefulness exhibited a positive correlation with hypoxia-related indices, suggesting a potential relationship between these neural oscillations and the severity of obstructive sleep apnea.[16]
Recent studies have shown that oscillatory activity in frontal brain regions in the θ range (4-8 Hz) correlates with cognitive processing and typically appears as increased θ activity, especially in post-response (late) periods. This oscillatory activity may be modulated by neurofeedback and has proven effective in upregulating frontal midline θ. Neural oscillatory activity in the θ band (approximately 3-8 Hz), which is linked to cognitive control in nonlinguistic tasks, serves as a real-time index of cognitive control during real-time language comprehension. Increases in θ activity may reflect greater conflict within a sentence. These effects emerge as early as 300 ms after the onset of the initiating event, indicating the rapid recruitment of cognitive control during sentence processing in response to conflicting representations.[17][18][19]
α Rhythm
The posterior dominant α rhythm (8-12 Hz) is characteristically present in normal awake EEG recordings in the occipital head region. This waveform defines the normal background rhythm of the adult EEG. The posterior rhythm reaches the α range of 8 Hz by age 3 and remains stable even into the 9th decade of life in healthy individuals. Fast variants of background α rhythm occur in the normal population. Slowing of the background α rhythm indicates generalized cerebral dysfunction.[20]
The amplitude of the α rhythm varies among individuals and over time in the same individual. Reactivity of the α rhythm is characteristic and aids in its identification. This pattern is most pronounced when eyes are closed and during mental relaxation, and is typically attenuated by eye opening and mental effort. In diffuse encephalopathy, patients may exhibit generalized α activity that is nonreactive to internal or external stimuli, a pattern known as α coma.
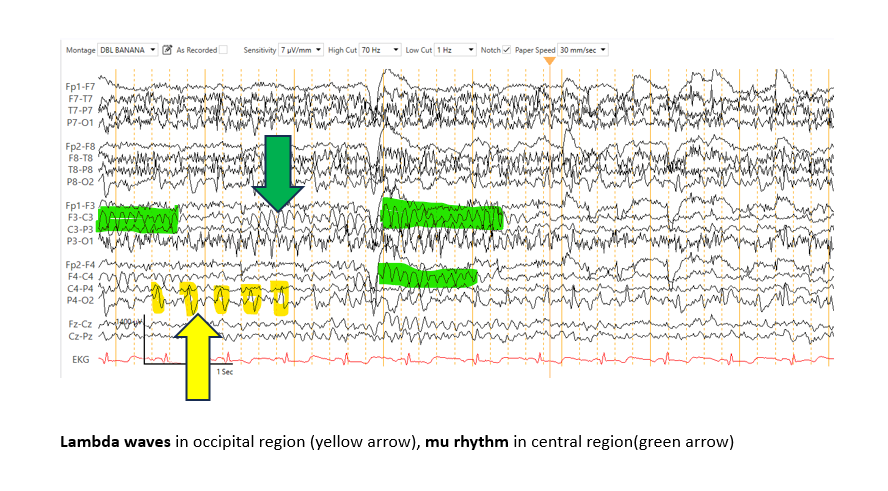
The μ rhythm, another type of α rhythm, appears in the central head regions and displays an arch-like morphology. This pattern disappears with motor activity of the contralateral limbs or with thoughts of initiating motor activity.[21] However, the μ rhythm remains relatively unchanged with eye-opening. This waveform is frequently observed in young adults and is less common in children and older individuals.
Attenuating factors of the μ rhythm include fatigue, somatosensory stimulation, and mental arithmetic. μ rhythms are often asymmetric and asynchronous on both sides. Desynchronization of the μ rhythm (8-13 Hz) over centroparietal areas reliably reflects activation of the mirror neuron system, which is associated with social skills. A recent study reported significant μ suppression during hand movement observation across mirror neuron system-attributed frontoparietal areas following neurofeedback training.[22]
Brain oscillatory activity in the α band has been associated with perception, memory, decision-making, and overall cognitive functioning. Recent literature has linked a higher peak α frequency to better visual temporal processing skills, with α oscillations playing a crucial role in temporal binding within and across different senses. Peak α frequency has been revealed to be an electrophysiological index of visual information processing speed, which is also linked to cognitive ability. These findings suggest that peak α frequency deficits are characteristic of schizophrenia but not of bipolar disorder and that individual differences in peak α frequency are due to abnormalities in visual information processing and cognition in schizophrenia.[23][24][25]
Individual α frequency, conventionally ranging from approximately 7 to 13 Hz, with faster α oscillations, is associated with greater temporal resolution and more refined perceptual experience. Research suggests that interindividual differences in α frequency contribute to performance variability in low-level perceptual tasks. These findings support the hypothesis that individual α frequency underlies a fundamental temporal sampling mechanism that shapes visual objective performance, with higher frequencies promoting enhanced sensory evidence per time unit.
In contrast, the α wave has been associated with behavioral inhibition and has been studied in relation to the default mode network, which is predominantly active during resting states. Studies have shown that individuals with low α activity exhibit greater behavioral inhibition and display distinct functional connectivity patterns within the default mode network, suggesting a significant role for low α oscillations in inhibitory control.[26][27]
σ Waves
σ Waves (12-16 Hz), commonly known as sleep spindles, are physiologically observed in N2 sleep. These waveforms serve as markers of sleep mechanisms, including brain development, sensory information processing, and consolidation, as they reflect thalamocortical circuit activity. A recent study involving 8-month-old infants found local differences in the non-rapid eye movement sleep quality that are mostly attributable to the topical phase of brain maturation associated with psychomotor development. Furthermore, sleep spindles in stage N2 sleep are highly heritable, whereas occipital σ activity during non-rapid eye movement sleep is significantly correlated with performance gains of visual perception learning.[28][29][30]
σ Waves may be slow (12-14 Hz) or fast (14-16 Hz) and are most prominently observed in the frontocentral head regions.[31] The fast spindles oscillate in the high-σ band and are related to sensorimotor processing, which is affected by deprived vision. A recent study revealed that children with blindness or severe visual impairment have no developmental spindles within the central area. In contrast, young ones presented low central high σ and high β (25-30 Hz) event-related spectral perturbations and showed no signs of maturational decrease. High σ and high β activity among children with blindness or severe visual impairment is associated with clinical indices that predict perceptual and motor disorders, suggesting that fast spindles are pivotal biomarkers for identifying early developmental deviations in children with blindness or severe visual impairment.[32]
Other studies also identified σ frequency activity as a hallmark of non-rapid eye movement sleep EEG and a key indicator of neural plasticity, central nervous system development, and cognition. Research has shown that sleep EEG spectral power densities within the σ frequency activity range in infants are more often linked to seasonal variation, likely due to changes in seasonal light exposure. Moreover, σ frequency activity was lower in the last part of the night sleep, suggesting different regulation of spindle activity in infants and toddlers compared to adults.[33] In adolescents, the decrease in σ activity is believed to reflect depressed sleep spindle activity, highlighting the impact of sleep restriction on cognitive performance during wakefulness.[34]
Pathological spindle rhythm, also known as spindle coma, is observed in generalized encephalopathy. Current literature suggests that reduced EEG power density in the σ range may represent early signs of overnight withdrawal from the continuous presence of daytime stimulants. Studies have shown that EEG power density in the σ frequencies (12-16 Hz) during non-rapid eye movement sleep is reduced in both caffeine and withdrawal conditions, indicating that daily caffeine intake in the morning and afternoon does not significantly affect nighttime sleep structure or subjective sleep quality in healthy individuals who regularly consume caffeine.[35]
Other findings have shown lower σ wave power in N2 sleep spindle activity and N3 waves in the last sleep cycle during daytime sleep in both the normal group and patients with Down syndrome. Cortical thickness in the σ band during non-rapid eye movement sleep is observed in individuals with Alzheimer disease.[36][37][38][39]
β Rhythm
The β rhythm (13-30 Hz) is the most frequently observed pattern in normal adults and children. This waveform is most prominent in the frontal and central head regions and attenuates as it goes posteriorly. The amplitude of β activity is typically 10 to 20 µV, rarely exceeding 30 µV.
The β rhythm often rises in amplitude during drowsiness and N1 sleep, and subsequently decreases in N2 and N3 sleep. Most sedative medications, such as barbiturates, chloral hydrate, and benzodiazepines, increase the amplitude and quantity of β activity in individuals who use them.[40] Focal, regional, or hemispheric attenuation of β can occur with a cortical injury, malformations, subdural, epidural, or subgaleal fluid collections. Recent case reports indicate an increase in β-band activity in the sensory thalamus associated with severe facial pain, suggesting the potential for closed-loop deep brain stimulation in facial pain management.[41]
Recent studies have shown that low- and high-frequency β rhythms in the motor cortex are correlated with band-specific roles in both movement control and spatiotemporal attention. Findings show that the low β band (<20 Hz) dominant in the primary motor cortex was linked positively to visual cue onset and negatively to uninstructed hand postural micro-movements. Meanwhile, a high β band (>20 Hz) dominant in the dorsal premotor cortex has been shown to exhibit temporal task prediction, with selective modulations before and during cues, which are enhanced in moments of increased focal attention when the gaze is directed at the work area.[42]
Another study investigated the influence of β-frequency sensory stimulation on stepping and dorsal striatal regulation of stepping. Although stimulation at 10- and 145-Hz frequencies produced locomotion and desynchronized striatal network, only 10-Hz stimulation enhanced the δ rhythmicity of stepping and strengthened the coupling between stepping and striatal local field potential δ and β oscillations. Findings revealed that an increase in the frequency of sensory stimulation can modulate lower-frequency striatal neural dynamics and enhance stepping rhythmicity, highlighting the translational potential of noninvasive β-frequency sensory stimulation for improving gait.
Another study suggested that excessive oscillatory activity across basal ganglia nuclei in the β frequency range (12-30 Hz) may be a hallmark of Parkinson disease. Findings revealed that pathophysiological strengthening of striatal and pallidal synapses following dopamine depletion can result in the emergence of synchronized oscillatory activity in the mid-β range, with spike-phase association between the neuronal populations of the basal ganglia. Furthermore, external pallidum inhibition, contrary to the subthalamic nucleus, eliminates oscillations.[43]
γ Waves
γ waves (30-80 Hz) were initially recorded in the visual cortex of monkeys and are now known to be widespread across various brain regions, including the premotor, parietal, temporal, and frontal cortices. γ oscillations are commonly observed in the cerebral cortex during different stages of the wake-sleep cycle and are associated with sensory processing and cognition.[44] The cortico-basal-ganglia-thalamo-cortical loop appears to communicate using this rhythm and is extensively involved in patients with Parkinson disease undergoing deep brain stimulation. An increase in β-γ phase-amplitude coupling over the sensorimotor cortex is consistently observed in patients with Parkinson disease, reflecting neural entrainment in the basal ganglia.[45]
Recent studies have identified narrowband γ oscillations as potential biomarkers, noting their decline with healthy aging and the onset of Alzheimer disease. Visual gratings induce narrowband γ oscillations accompanied by suppression in the α band (8-12 Hz), whereas auditory ripples elicit strong broadband high γ responses alongside suppression in the β band (14-26 Hz).[46]
In addition to its role in sensory perception and the integration of different brain regions, the γ rhythm is also linked to cognitive functions, including working memory and attention. Current literature describes θ-γ coupling as the modulation of γ oscillations by θ-phasic activity, which plays a key role in signaling information during working memory performance. Mental arithmetic, involving calculations with numbers, serves as an essential tool for evaluating sensory processing and working memory management. A decrease in θ-γ coupling and θ power has been observed in patients with panic disorder.[47]
High-Frequency Oscillations
High-frequency oscillations (>80 Hz) are further classified into ripples (80-200 Hz) and fast ripples (200-500 Hz). The γ rhythm is associated with sensory perception and the integration of different brain areas. Recent studies have analyzed spikes and high-frequency oscillations in epileptic EEG patterns, identifying them as potential biomarkers for surgical outcomes in patients with epilepsy, including pediatric patients with frequent seizures.
Spontaneous fast ripples are always pathological, while ripples may be physiological or pathological. Research shows that morphologically defined putative pathological high-frequency oscillations strongly correlate with expert-defined spikes and predominantly localize within the seizure onset zone. Novel pathological features identified include high power in the γ (30-80 Hz) and ripple (>80 Hz) bands centered on the event.
Differentiating pathological from physiological ripples can improve the prediction of surgical outcomes.[48][49][50][51][52] Furthermore, intracranial depth recordings from epileptic hippocampi in animal and human models report ultrafast frequency bursts (fast ripples), which likely correlate with the local epileptogenicity of brain tissue.[53] Subdural recordings during presurgical epilepsy evaluation also show that activity bursts in a relatively lower frequency range (60-100 Hz) may indicate the location of an epileptic focus.
Ultrafast EEG activity correlates with cognitive states and event-related potentials. The significance of γ rhythms in numerous cognitive functions has been well established.[54][55] Brainstem evoked potentials represent a well-established and routinely measured category of ultrafast EEG signals. Reports indicate that high-frequency oscillations greater than 200 Hz are associated with somatosensory stimulation or motor movements. The sensitivity of these waveforms to vigilance states, motor interference, and pharmacological manipulations, such as anesthetics or sedatives, offers new possibilities for brain monitoring and diagnostics.[56][57] These signals may also aid in the early detection of demyelination and other disorders affecting cortical integrity.
Morphology
EEG transients are isolated waveforms or complexes that stand out from the background activity. Several EEG transients observed in normal individuals are benign and must be distinguished from those that are pathological. Correct identification of these nonepileptic waveforms requires training and experience.
A recent cohort study examined the prevalence and characteristics of normal EEG variants. The distribution of these normal variants was reported as follows:
- Sharp transients, including wicket spikes: 19.21%
- Rhythmic temporal θ of drowsiness: 6.03%
- Temporal slowing in older adults: 2.89%
- Slowly fused transients: 2.59%
- 14- and 6-Hz bursts: 1.83%
- Breach rhythm: 1.25%
- Small sharp spikes: 1.05%
- 6-Hz spike-and-slow-wave complexes: 0.69%
- Subclinical rhythmic epileptiform discharges of adults: 0.03%
EEG readers must be familiar with these variants to avoid misinterpreting nonepileptiform transients, which could lead to overdiagnosis of epilepsy, unnecessary long-term antiepileptic treatment, and serious medicolegal consequences.[58]
Identification of Nonepileptiform Transients
Nonepileptiform transients are waveforms that resemble epileptiform activity but are not associated with epileptic seizures.[59] These patterns often have a sharp contour and may appear as isolated, arrhythmic bursts. Most commonly, these transients occur during drowsiness or light sleep.[60] Some of the frequently observed nonepileptiform transients are discussed below.
λ Waves: λ Waves are positive sharp transients observed in the occipital region during wakefulness, with maximal amplitude over the bilateral occipital areas and concurrent wicket spikes in the ipsilateral temporal lobe (see Image. λ Waves and μ Rhythms).[61] These transients appear most prominently during visual exploration and typically disappear with eye closure.[62]
Positive occipital sharp transients of sleep: Positive occipital sharp transients of sleep are sharply contoured waveforms resembling λ waves and are observed in approximately 50% to 80% of healthy individuals during non-rapid eye movement sleep. Like λ waves, positive occipital sharp transients of sleep show maximal amplitude over the bilateral occipital regions and may coincide with wicket spikes in the ipsilateral temporal lobe. These waveforms are considered physiological patterns of non-rapid eye movement sleep, most frequently observed in adolescents and young adults, with the highest prevalence during the early stages of drowsiness rather than deeper phases of non-rapid eye movement sleep.[63][64]
6-Hz Spike-and-wave complexes: 6-Hz spike-and-wave complexes, also known as phantom spike-and-wave, are low-amplitude, infrequently encountered, and poorly defined spikes occurring within a repeating spike-and-slow-wave pattern.[65] The frequency of these waveforms typically ranges from 5 to 6 Hz. The complexes have amplitudes below 40 μV and exhibit spike durations shorter than 30 ms.[66] Frontal or occipital predominance may be observed. These complexes are most commonly observed in adolescents and young adults.
14- and 6-Hz positive spikes: 14- and 6-Hz positive spikes, also known as ctenoids, are unilateral, bisynchronous or asynchronous waveforms with regular repetitions and arciform morphology. These waveforms are centered in the posterior temporal region but may have a broad distribution. These spikes are frequently observed during drowsiness and light sleep.[67]
Vertex sharp transients: Vertex sharp transients appear as monophasic, diphasic, or triphasic waves and surface-negative sharp waves with phase reversal at or near the vertex. These transients typically occur during drowsiness and non-rapid eye movement sleep. The duration of vertex sharp transients is typically around 100 ms.
K Complex: K complexes are sharp, well-delineated, high-voltage polyphasic waves lasting longer than 0.5 s. These patterns are less sharply contoured than epileptiform spikes and are often followed by sleep spindles.[68] The K complex is considered the most significant event in a healthy human EEG, characterized by a short positive voltage peak at approximately 200 ms, followed by a large negative complex around 550 ms, and a prolonged positive peak around 900 ms.
K complexes may occur in the following ways:
- Spontaneously, referred to as spontaneous K complexes.
- In response to internal stimuli, such as respiratory interruptions.
- In response to external stimuli, such as a tactile sensation. These K complexes are referred to as evoked K complexes.
These waveforms are most prominent over the frontal and superior frontal cortices. Recent studies indicate that K complexes can be generated across a wide range of cortical areas. Notably, the motor cortex exhibits awake-like EEG activity before the onset of a K complex, followed by microarousals. Although K complexes are a hallmark of N2 sleep, they may also play dual roles in region-specific sleep regulation and arousal responses.[69][70]
Benign epileptiform transients of sleep: Benign epileptiform transients of sleep, also known as small sharp spikes or benign sporadic sleep spikes, are the most common normal EEG variants. These waveforms appear as low-amplitude, sharply contoured monophasic or posteriorly propagating, inferiorly rotating diphasic patterns, most frequent during N1 and N2 sleep.[71] Benign epileptiform transients of sleep typically occur in adults aged 30 to 60. These patterns have amplitudes under 90 µV and durations longer than 90 ms. Benign epileptiform transients of sleep most often appear in the midtemporal region with a broad field extending into the adjacent frontal area.
Recent studies describe benign epileptiform transients of sleep as traveling, rotating hippocampal spikes, where the diphasic waveform propagates back and forth along the anteroposterior axis of the hippocampus. This finding suggests a sleep-related functional role for these hippocampal spikes.[72]
Wicket waves: Wicket waves, also known as wicket rhythms, are common EEG transients characterized by monophasic, sharply contoured waves with symmetric upward and downward phases. These patterns blend into ongoing background activity and do not cause disruption.[73] These physiological rhythms occur during non-rapid eye movement sleep and relaxed wakefulness facilitated by drowsiness. Wicket waves are most commonly observed in middle-to-late adulthood.
Rhythmic midtemporal θ of drowsiness: Previously referred to as the psychomotor variant, rhythmic midtemporal θ of drowsiness consists of trains of θ activity observed during non-rapid eye movement and rapid eye movement sleep, sleep-wake transitions, or triggered by hyperventilation, according to recent case reports.[74] Rhythmic midtemporal θ of drowsiness centers on the midtemporal region but may spread to anterior and posterior temporal areas, as well as the posterior parietal region. Rhythmic midtemporal θ of drowsiness displays a monomorphic pattern with a distinctly sharp or notched contour.[75]
Subclinical rhythmic electroencephalographic discharges of adults: Subclinical rhythmic epileptiform discharges of adults is a pattern of unclear clinical significance but is often misdiagnosed as epileptiform activity. This pattern consists of sharply contoured rhythmic θ activity that is bilateral, synchronous, and symmetric, ranging from slower δ to faster θ frequencies. The pattern typically peaks over the parietal and posterior temporal regions.[76] These paroxysms occur without objective or subjective clinical symptoms, commonly during wakefulness or light sleep, and may be induced by hyperventilation. Duration ranges from 10 s to 5 min, averaging 40 to 80 s, resolving either abruptly or gradually. Although subclinical rhythmic epileptiform discharges of adults is most commonly observed in older adults, recent reports have documented its occurrence in young adults and children as well.[77][78]
Enhancing Healthcare Team Outcomes
EEG tracings are essential for identifying and characterizing epileptiform and other pathological patterns associated with various neurological conditions. Clinicians must develop proficiency in recognizing normal EEG variations before interpreting abnormal findings. However, a single normal EEG does not rule out underlying pathology, as electrographic abnormalities may be transient. Overinterpreting benign variants of uncertain significance can lead to unnecessary concern, further testing, and possible misdiagnoses. Optimal patient outcomes depend on a collaborative approach involving clinicians, nurses, and advanced practice providers who are properly trained in EEG interpretation and subsequent management.
Interpreters must be equally skilled in identifying benign EEG variants and in detecting pathological patterns. Neurologists, in particular, should refine their ability to distinguish between a normal variant and true epileptiform activity to ensure diagnostic accuracy and guide appropriate treatment.[79]
Media
(Click Image to Enlarge)
(Click Image to Enlarge)
References
Cascella M, Bandyopadhyay S. Lambda Waves. StatPearls. 2025 Jan:(): [PubMed PMID: 31971728]
Amin U, Nascimento FA, Karakis I, Schomer D, Benbadis SR. Normal variants and artifacts: Importance in EEG interpretation. Epileptic disorders : international epilepsy journal with videotape. 2023 Oct:25(5):591-648. doi: 10.1002/epd2.20040. Epub 2023 Jul 27 [PubMed PMID: 36938895]
Bonaccini Calia A, Masvidal-Codina E, Smith TM, Schäfer N, Rathore D, Rodríguez-Lucas E, Illa X, De la Cruz JM, Del Corro E, Prats-Alfonso E, Viana D, Bousquet J, Hébert C, Martínez-Aguilar J, Sperling JR, Drummond M, Halder A, Dodd A, Barr K, Savage S, Fornell J, Sort J, Guger C, Villa R, Kostarelos K, Wykes RC, Guimerà-Brunet A, Garrido JA. Full-bandwidth electrophysiology of seizures and epileptiform activity enabled by flexible graphene microtransistor depth neural probes. Nature nanotechnology. 2022 Mar:17(3):301-309. doi: 10.1038/s41565-021-01041-9. Epub 2021 Dec 22 [PubMed PMID: 34937934]
Vanhatalo S, Voipio J, Kaila K. Full-band EEG (FbEEG): an emerging standard in electroencephalography. Clinical neurophysiology : official journal of the International Federation of Clinical Neurophysiology. 2005 Jan:116(1):1-8 [PubMed PMID: 15589176]
Vanhatalo S, Tallgren P, Andersson S, Sainio K, Voipio J, Kaila K. DC-EEG discloses prominent, very slow activity patterns during sleep in preterm infants. Clinical neurophysiology : official journal of the International Federation of Clinical Neurophysiology. 2002 Nov:113(11):1822-5 [PubMed PMID: 12417237]
Vanhatalo S, Palva JM, Holmes MD, Miller JW, Voipio J, Kaila K. Infraslow oscillations modulate excitability and interictal epileptic activity in the human cortex during sleep. Proceedings of the National Academy of Sciences of the United States of America. 2004 Apr 6:101(14):5053-7 [PubMed PMID: 15044698]
Birbaumer N, Elbert T, Canavan AG, Rockstroh B. Slow potentials of the cerebral cortex and behavior. Physiological reviews. 1990 Jan:70(1):1-41 [PubMed PMID: 2404287]
Level 3 (low-level) evidenceCui RQ, Huter D, Egkher A, Lang W, Lindinger G, Deecke L. High resolution DC-EEG mapping of the Bereitschaftspotential preceding simple or complex bimanual sequential finger movement. Experimental brain research. 2000 Sep:134(1):49-57 [PubMed PMID: 11026725]
Ikeda A, Terada K, Mikuni N, Burgess RC, Comair Y, Taki W, Hamano T, Kimura J, Lüders HO, Shibasaki H. Subdural recording of ictal DC shifts in neocortical seizures in humans. Epilepsia. 1996 Jul:37(7):662-74 [PubMed PMID: 8681899]
Level 3 (low-level) evidenceVoipio J, Tallgren P, Heinonen E, Vanhatalo S, Kaila K. Millivolt-scale DC shifts in the human scalp EEG: evidence for a nonneuronal generator. Journal of neurophysiology. 2003 Apr:89(4):2208-14 [PubMed PMID: 12612037]
Joshi RB, Duckrow RB, Goncharova II, Hirsch LJ, Spencer DD, Godwin DW, Zaveri HP. Stability of infraslow correlation structure in time-shifted intracranial EEG signals. Frontiers in network physiology. 2024:4():1441294. doi: 10.3389/fnetp.2024.1441294. Epub 2024 Aug 27 [PubMed PMID: 39258030]
CORDEAU JP. Monorhythmic frontal delta activity in the human electroencephalogram: a study of 100 cases. Electroencephalography and clinical neurophysiology. 1959 Nov:11():733-46 [PubMed PMID: 13811933]
Level 3 (low-level) evidenceDalby MA. Epilepsy and 3 per second spike and wave rhythms. A clinical, electroencephalographic and prognostic analysis of 346 patients. Acta neurologica Scandinavica. 1969:():Suppl 40:3+ [PubMed PMID: 4979890]
Reiher J, Beaudry M, Leduc CP. Temporal intermittent rhythmic delta activity (TIRDA) in the diagnosis of complex partial epilepsy: sensitivity, specificity and predictive value. The Canadian journal of neurological sciences. Le journal canadien des sciences neurologiques. 1989 Nov:16(4):398-401 [PubMed PMID: 2804800]
Sridhar S, Lowet E, Gritton HJ, Freire J, Zhou C, Liang F, Han X. Beta-frequency sensory stimulation enhances gait rhythmicity through strengthened coupling between striatal networks and stepping movement. Nature communications. 2024 Sep 27:15(1):8336. doi: 10.1038/s41467-024-52664-0. Epub 2024 Sep 27 [PubMed PMID: 39333151]
Zhang C, Wang Y, Li M, Niu P, Li S, Hu Z, Shi C, Li Y. Phase-Amplitude Coupling in Theta and Beta Bands: A Potential Electrophysiological Marker for Obstructive Sleep Apnea. Nature and science of sleep. 2024:16():1469-1482. doi: 10.2147/NSS.S470617. Epub 2024 Sep 21 [PubMed PMID: 39323903]
Brilliant, Yaar-Soffer Y, Herrmann CS, Henkin Y, Kral A. Theta and alpha oscillatory signatures of auditory sensory and cognitive loads during complex listening. NeuroImage. 2024 Apr 1:289():120546. doi: 10.1016/j.neuroimage.2024.120546. Epub 2024 Feb 21 [PubMed PMID: 38387743]
Ness T, Langlois VJ, Novick JM, Kim AE. Theta-band neural oscillations reflect cognitive control during language processing. Journal of experimental psychology. General. 2024 Sep:153(9):2279-2298. doi: 10.1037/xge0001621. Epub [PubMed PMID: 39235889]
Pfeiffer M, Kübler A, Hilger K. Modulation of human frontal midline theta by neurofeedback: A systematic review and quantitative meta-analysis. Neuroscience and biobehavioral reviews. 2024 Jul:162():105696. doi: 10.1016/j.neubiorev.2024.105696. Epub 2024 May 7 [PubMed PMID: 38723734]
Level 1 (high-level) evidenceAIRD RB, GASTAUT Y. Occipital and posterior electroencephalographic rhythms. Electroencephalography and clinical neurophysiology. 1959 Nov:11():637-56 [PubMed PMID: 13792196]
CHATRIAN GE, PETERSEN MC, LAZARTE JA. The blocking of the rolandic wicket rhythm and some central changes related to movement. Electroencephalography and clinical neurophysiology. 1959 Aug:11(3):497-510 [PubMed PMID: 13663823]
Dastgheib SS, Wang W, Kaufmann JM, Moratti S, Schweinberger SR. Mu-Suppression Neurofeedback Training Targeting the Mirror Neuron System: A Pilot Study. Applied psychophysiology and biofeedback. 2024 Sep:49(3):457-471. doi: 10.1007/s10484-024-09643-4. Epub [PubMed PMID: 38739182]
Level 3 (low-level) evidenceArioli M, Mattersberger M, Hoehl S, Brzozowska A. Peak alpha frequency is linked to visual temporal attention in 6-month-olds. Scientific reports. 2024 Nov 15:14(1):28173. doi: 10.1038/s41598-024-79129-0. Epub 2024 Nov 15 [PubMed PMID: 39548193]
Kawashima T, Nakayama R, Amano K. Theoretical and Technical Issues Concerning the Measurement of Alpha Frequency and the Application of Signal Detection Theory: Comment on Buergers and Noppeney (2022). Journal of cognitive neuroscience. 2024 Apr 1:36(4):691-699. doi: 10.1162/jocn_a_02010. Epub [PubMed PMID: 37255466]
Level 3 (low-level) evidenceCatalano LT, Reavis EA, Wynn JK, Green MF. Peak Alpha Frequency in Schizophrenia, Bipolar Disorder, and Healthy Volunteers: Associations With Visual Information Processing and Cognition. Biological psychiatry. Cognitive neuroscience and neuroimaging. 2024 Nov:9(11):1132-1140. doi: 10.1016/j.bpsc.2024.06.004. Epub 2024 Jun 21 [PubMed PMID: 38909899]
Kim YW, Kim S, Jin MJ, Im CH, Lee SH. The Importance of Low-frequency Alpha (8-10 Hz) Waves and Default Mode Network in Behavioral Inhibition. Clinical psychopharmacology and neuroscience : the official scientific journal of the Korean College of Neuropsychopharmacology. 2024 Feb 29:22(1):53-66. doi: 10.9758/cpn.22.1035. Epub 2023 May 30 [PubMed PMID: 38247412]
Tarasi L, Romei V. Individual Alpha Frequency Contributes to the Precision of Human Visual Processing. Journal of cognitive neuroscience. 2024 Apr 1:36(4):602-613. doi: 10.1162/jocn_a_02026. Epub [PubMed PMID: 37382485]
Satomaa AL, Mäkelä T, Saarenpää-Heikkilä O, Kylliäinen A, Huupponen E, Himanen SL. Slow-wave activity and sigma activities are associated with psychomotor development at 8 months of age. Sleep. 2020 Sep 14:43(9):. pii: zsaa061. doi: 10.1093/sleep/zsaa061. Epub [PubMed PMID: 32227230]
Goldschmied JR, Lacourse K, Maislin G, Delfrate J, Gehrman P, Pack FM, Staley B, Pack AI, Younes M, Kuna ST, Warby SC. Spindles are highly heritable as identified by different spindle detectors. Sleep. 2021 Apr 9:44(4):. doi: 10.1093/sleep/zsaa230. Epub [PubMed PMID: 33165618]
Tamaki M, Sasaki Y. Sleep-Dependent Facilitation of Visual Perceptual Learning Is Consistent with a Learning-Dependent Model. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2022 Mar 2:42(9):1777-1790. doi: 10.1523/JNEUROSCI.0982-21.2021. Epub 2022 Jan 12 [PubMed PMID: 35022218]
Gondeck AR, Smith JR. Dynamics of human sleep sigma spindles. Electroencephalography and clinical neurophysiology. 1974 Sep:37(3):293-7 [PubMed PMID: 4136646]
Vitali H, Campus C, Signorini S, De Giorgis V, Morelli F, Varesio C, Pasca L, Sammartano A, Gori M. Blindness affects the developmental trajectory of the sleeping brain. NeuroImage. 2024 Feb 1:286():120508. doi: 10.1016/j.neuroimage.2024.120508. Epub 2024 Jan 4 [PubMed PMID: 38181867]
Kärki A, Satomaa AL, Huhtala H, Saastamoinen A, Saarenpää-Heikkilä O, Paavonen EJ, Himanen SL. Season is related to the slow wave and sigma activity of infants and toddlers. Sleep medicine. 2022 Dec:100():364-377. doi: 10.1016/j.sleep.2022.09.006. Epub 2022 Sep 16 [PubMed PMID: 36201888]
Campbell IG, Cruz-Basilio A, Darchia N, Zhang ZY, Feinberg I. Effects of sleep restriction on the sleep electroencephalogram of adolescents. Sleep. 2021 Jun 11:44(6):. doi: 10.1093/sleep/zsaa280. Epub [PubMed PMID: 33507305]
Weibel J, Lin YS, Landolt HP, Kistler J, Rehm S, Rentsch KM, Slawik H, Borgwardt S, Cajochen C, Reichert CF. The impact of daily caffeine intake on nighttime sleep in young adult men. Scientific reports. 2021 Feb 25:11(1):4668. doi: 10.1038/s41598-021-84088-x. Epub 2021 Feb 25 [PubMed PMID: 33633278]
Yook S, Choi SJ, Zang C, Joo EY, Kim H. Are there effects of light exposure on daytime sleep for rotating shift nurses after night shift?: an EEG power analysis. Frontiers in neuroscience. 2024:18():1306070. doi: 10.3389/fnins.2024.1306070. Epub 2024 Mar 27 [PubMed PMID: 38601092]
Talukder A, Yeung D, Li Y, Anandanadarajah N, Umbach DM, Fan Z, Li L. Comparison of power spectra from overnight electroencephalography between patients with Down syndrome and matched control subjects. Journal of sleep research. 2024 Oct:33(5):e14187. doi: 10.1111/jsr.14187. Epub 2024 Feb 27 [PubMed PMID: 38410055]
D'Atri A, Gorgoni M, Scarpelli S, Cordone S, Alfonsi V, Marra C, Ferrara M, Rossini PM, De Gennaro L. Relationship between Cortical Thickness and EEG Alterations during Sleep in the Alzheimer's Disease. Brain sciences. 2021 Sep 4:11(9):. doi: 10.3390/brainsci11091174. Epub 2021 Sep 4 [PubMed PMID: 34573195]
Palepu K, Sadeghi K, Kleinschmidt DF, Donoghue J, Chapman S, Arslan AR, Westover MB, Cash SS, Pathmanathan J. An examination of sleep spindle metrics in the Sleep Heart Health Study: superiority of automated spindle detection over total sigma power in assessing age-related spindle decline. BMC neurology. 2023 Oct 6:23(1):359. doi: 10.1186/s12883-023-03376-3. Epub 2023 Oct 6 [PubMed PMID: 37803266]
Frost JD Jr, Carrie JR, Borda RP, Kellaway P. The effects of dalmane (flurazepam hydrochloride) on human EEG characteristics. Electroencephalography and clinical neurophysiology. 1973 Feb:34(2):171-5 [PubMed PMID: 4119530]
Lopez Ramos CG, Rockhill AP, Shahin MN, Gragg A, Tan H, Yamamoto EA, Fecker AL, Ismail M, Cleary DR, Raslan AM. Beta Oscillations in the Sensory Thalamus During Severe Facial Neuropathic Pain Using Novel Sensing Deep Brain Stimulation. Neuromodulation : journal of the International Neuromodulation Society. 2024 Dec:27(8):1419-1427. doi: 10.1016/j.neurom.2024.05.003. Epub 2024 Jun 15 [PubMed PMID: 38878055]
Nougaret S, López-Galdo L, Caytan E, Poitreau J, Barthélemy FV, Kilavik BE. Low and high beta rhythms have different motor cortical sources and distinct roles in movement control and spatiotemporal attention. PLoS biology. 2024 Jun:22(6):e3002670. doi: 10.1371/journal.pbio.3002670. Epub 2024 Jun 25 [PubMed PMID: 38917200]
Azizpour Lindi S, Mallet NP, Leblois A. Synaptic Changes in Pallidostriatal Circuits Observed in the Parkinsonian Model Triggers Abnormal Beta Synchrony with Accurate Spatio-temporal Properties across the Basal Ganglia. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2024 Feb 28:44(9):. doi: 10.1523/JNEUROSCI.0419-23.2023. Epub 2024 Feb 28 [PubMed PMID: 38123981]
Tahvili F, Destexhe A. A mean-field model of gamma-frequency oscillations in networks of excitatory and inhibitory neurons. Journal of computational neuroscience. 2024 May:52(2):165-181. doi: 10.1007/s10827-024-00867-1. Epub 2024 Mar 21 [PubMed PMID: 38512693]
Hodnik T, Roytman S, Bohnen NI, Marusic U. Beta-Gamma Phase-Amplitude Coupling as a Non-Invasive Biomarker for Parkinson's Disease: Insights from Electroencephalography Studies. Life (Basel, Switzerland). 2024 Mar 15:14(3):. doi: 10.3390/life14030391. Epub 2024 Mar 15 [PubMed PMID: 38541715]
Gulati D, Ray S. Auditory and Visual Gratings Elicit Distinct Gamma Responses. eNeuro. 2024 Apr:11(4):. pii: ENEURO.0116-24.2024. doi: 10.1523/ENEURO.0116-24.2024. Epub 2024 Apr 25 [PubMed PMID: 38604776]
Ahn JS, Hong HJ, Lee JH, Park JY. Theta power reduction and theta-gamma coupling desynchronization are associated with working memory interference and anxiety symptoms in panic disorder: a retrospective study. BMC psychiatry. 2024 Dec 3:24(1):875. doi: 10.1186/s12888-024-06272-3. Epub 2024 Dec 3 [PubMed PMID: 39623333]
Level 2 (mid-level) evidenceSindhu KR, Pinto-Orellana MA, Ombao HC, Riba A, Phillips D, Olaya J, Shrey DW, Lopour BA. Electrode Surface Area Impacts Measurement of High Frequency Oscillations in Human Intracranial EEG. IEEE transactions on bio-medical engineering. 2024 Nov:71(11):3283-3292. doi: 10.1109/TBME.2024.3416440. Epub 2024 Oct 25 [PubMed PMID: 38896508]
Chaibi S, Mahjoub C, Ayadi W, Kachouri A. Epileptic EEG patterns recognition through machine learning techniques and relevant time-frequency features. Biomedizinische Technik. Biomedical engineering. 2024 Apr 25:69(2):111-123. doi: 10.1515/bmt-2023-0332. Epub 2023 Oct 30 [PubMed PMID: 37899292]
Ye H, Chen C, Weiss SA, Wang S. Pathological and Physiological High-frequency Oscillations on Electroencephalography in Patients with Epilepsy. Neuroscience bulletin. 2024 May:40(5):609-620. doi: 10.1007/s12264-023-01150-6. Epub 2023 Nov 24 [PubMed PMID: 37999861]
Maeda K, Hosoda N, Fukumoto J, Kawai S, Hayafuji M, Tsuboi H, Fujita S, Ichino N, Osakabe K, Sugimoto K, Ishihara N. Association of Scalp High-Frequency Oscillation Detection and Characteristics With Disease Activity in Pediatric Epilepsy. Journal of clinical neurophysiology : official publication of the American Electroencephalographic Society. 2025 Jan 1:42(1):28-35. doi: 10.1097/WNP.0000000000001052. Epub 2023 Oct 30 [PubMed PMID: 37934062]
Zhang Y, Daida A, Liu L, Kuroda N, Ding Y, Oana S, Kanai S, Monsoor T, Duan C, Hussain SA, Qiao JX, Salamon N, Fallah A, Sim MS, Sankar R, Staba RJ, Engel J Jr, Asano E, Roychowdhury V, Nariai H. Self-Supervised Data-Driven Approach Defines Pathological High-Frequency Oscillations in Human. medRxiv : the preprint server for health sciences. 2024 Nov 5:():. pii: 2024.07.10.24310189. doi: 10.1101/2024.07.10.24310189. Epub 2024 Nov 5 [PubMed PMID: 39040207]
Level 2 (mid-level) evidenceWorrell G, Gotman J. High-frequency oscillations and other electrophysiological biomarkers of epilepsy: clinical studies. Biomarkers in medicine. 2011 Oct:5(5):557-66. doi: 10.2217/bmm.11.74. Epub [PubMed PMID: 22003904]
Ward LM. Synchronous neural oscillations and cognitive processes. Trends in cognitive sciences. 2003 Dec:7(12):553-9 [PubMed PMID: 14643372]
Tallon-Baudry C. Oscillatory synchrony and human visual cognition. Journal of physiology, Paris. 2003 Mar-May:97(2-3):355-63 [PubMed PMID: 14766151]
Level 3 (low-level) evidenceHaueisen J, Heuer T, Nowak H, Liepert J, Weiller C, Okada Y, Curio G. The influence of lorazepam on somatosensory-evoked fast frequency (600 Hz) activity in MEG. Brain research. 2000 Aug 18:874(1):10-4 [PubMed PMID: 10936218]
Klostermann F, Gobbele R, Buchner H, Siedenberg R, Curio G. Differential gating of slow postsynaptic and high-frequency spike-like components in human somatosensory evoked potentials under isometric motor interference. Brain research. 2001 Dec 13:922(1):95-103 [PubMed PMID: 11730706]
Wüstenhagen S, Terney D, Gardella E, Meritam Larsen P, Rømer C, Aurlien H, Beniczky S. EEG normal variants: A prospective study using the SCORE system. Clinical neurophysiology practice. 2022:7():183-200. doi: 10.1016/j.cnp.2022.06.001. Epub 2022 Jun 30 [PubMed PMID: 35865124]
Klass DW, Westmoreland BF. Nonepileptogenic epileptiform electroencephalographic activity. Annals of neurology. 1985 Dec:18(6):627-35 [PubMed PMID: 4083847]
Westmoreland BF, Klass DW. Unusual EEG patterns. Journal of clinical neurophysiology : official publication of the American Electroencephalographic Society. 1990 Apr:7(2):209-28 [PubMed PMID: 2187021]
Rahman S, Burch M, Parikh P, Zafar M. Source Localization of Normal Variants Seen on EEG. Journal of clinical neurophysiology : official publication of the American Electroencephalographic Society. 2024 Feb 1:41(2):155-160. doi: 10.1097/WNP.0000000000000948. Epub 2022 Sep 28 [PubMed PMID: 38306223]
Scott DF, Bickford RG. Electrophysiologic studies during scanning and passive eye movements in humans. Science (New York, N.Y.). 1967 Jan 6:155(3758):101-2 [PubMed PMID: 6015559]
Egawa I, Yoshino K, Hishikawa Y. Positive occipital sharp transients in the human sleep EEG. Folia psychiatrica et neurologica japonica. 1983:37(1):57-65 [PubMed PMID: 6884913]
Macorig G, Crespel A, Nilo A, Tang NPL, Valente M, Gigli GL, Gélisse P. Benign EEG variants in the sleep-wake cycle: A prospective observational study using the 10-20 system and additional electrodes. Neurophysiologie clinique = Clinical neurophysiology. 2021 Jun:51(3):233-242. doi: 10.1016/j.neucli.2021.03.006. Epub 2021 Apr 16 [PubMed PMID: 33875321]
Level 2 (mid-level) evidenceRathore C, Prakash S, Rana K, Makwana P. Prevalence of benign epileptiform variants from an EEG laboratory in India and frequency of their misinterpretation. Epilepsy research. 2021 Feb:170():106539. doi: 10.1016/j.eplepsyres.2020.106539. Epub 2021 Jan 5 [PubMed PMID: 33461042]
Hughes JR. Two forms of the 6/sec spike and wave complex. Electroencephalography and clinical neurophysiology. 1980 May:48(5):535-50 [PubMed PMID: 6153962]
MILLEN FJ, WHITE B. Fourteen and six per second positive spike activity in children. Neurology. 1954 Jul:4(7):541-9 [PubMed PMID: 13176673]
PAMPIGLIONE C. The phenomenon of adaptation in human E.E.G.; a study of K complexes. Revue neurologique. 1952:87(2):197-8 [PubMed PMID: 13014782]
Gandhi MH, Emmady PD. Physiology, K Complex. StatPearls. 2025 Jan:(): [PubMed PMID: 32491401]
Latreille V, von Ellenrieder N, Peter-Derex L, Dubeau F, Gotman J, Frauscher B. The human K-complex: Insights from combined scalp-intracranial EEG recordings. NeuroImage. 2020 Jun:213():116748. doi: 10.1016/j.neuroimage.2020.116748. Epub 2020 Mar 17 [PubMed PMID: 32194281]
A ST, Asranna A, Kenchaiah R, Mundlamuri RC, Lg V, Sinha S. Benign epileptiform variants in EEG: A comprehensive study of 3000 patients. Seizure. 2024 Aug:120():157-164. doi: 10.1016/j.seizure.2024.07.004. Epub 2024 Jul 3 [PubMed PMID: 39003934]
Wennberg R, Tarazi A, Zumsteg D, Garcia Dominguez L. Electromagnetic evidence that benign epileptiform transients of sleep are traveling, rotating hippocampal spikes. Clinical neurophysiology : official journal of the International Federation of Clinical Neurophysiology. 2020 Dec:131(12):2915-2925. doi: 10.1016/j.clinph.2020.07.023. Epub 2020 Aug 28 [PubMed PMID: 32988727]
Reiher J, Lebel M. Wicket spikes: clinical correlates of a previously undescribed EEG pattern. The Canadian journal of neurological sciences. Le journal canadien des sciences neurologiques. 1977 Feb:4(1):39-47 [PubMed PMID: 837263]
Asemota B, Dohmeier JM, Singh N, Gienapp AJ, Rivas-Coppola M, Chourasia N. Rhythmic mid-Temporal Theta of Drowsiness Activated by Hyperventilation- Uncommon Trigger of a Rare Benign EEG Variant in Pediatrics. An Educational Review. Child neurology open. 2023 Jan-Dec:10():2329048X231153506. doi: 10.1177/2329048X231153506. Epub 2023 Jan 26 [PubMed PMID: 36726798]
Lipman IJ, Hughes JR. Rhythmic mid-temporal discharges. An electro-clinical study. Electroencephalography and clinical neurophysiology. 1969 Jul:27(1):43-7 [PubMed PMID: 4182889]
Brigo F, Ausserer H, Nardone R, Tezzon F, Manganotti P, Bongiovanni LG. Subclinical rhythmic electroencephalogram discharge of adults occurring during sleep: a diagnostic challenge. Clinical EEG and neuroscience. 2013 Jul:44(3):227-31. doi: 10.1177/1550059412466549. Epub 2013 Mar 26 [PubMed PMID: 23536379]
Level 3 (low-level) evidenceAzman Iste F, Tezer Filik FI, Saygi S. SREDA: A Rare but Confusing Benign EEG Variant. Journal of clinical neurophysiology : official publication of the American Electroencephalographic Society. 2020 May:37(3):225-230. doi: 10.1097/WNP.0000000000000623. Epub [PubMed PMID: 31393275]
Bosisio L, Mancardi MM, Boeri S, Nobili L, Nobile G, Siri L, Prato G, Canale E. Subclinical rhythmic EEG discharge of adults (SREDA) in pediatric population: A case series with systematic review of the literature. Epileptic disorders : international epilepsy journal with videotape. 2025 Feb:27(1):71-81. doi: 10.1002/epd2.20294. Epub 2024 Oct 16 [PubMed PMID: 39412218]
Level 1 (high-level) evidenceSheng S,Nalleballe K,Yadala S, EEG Benign Variants 2020 Jan; [PubMed PMID: 32310359]