Introduction
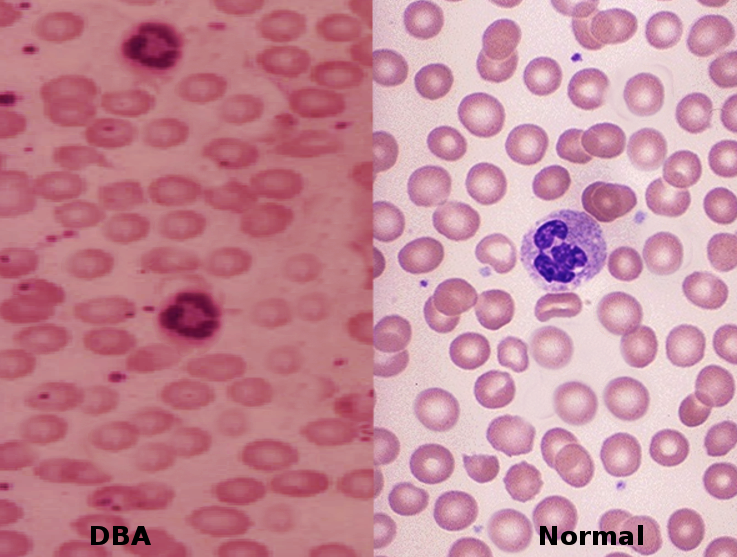
Anemia refers to a hemoglobin level below the normal range for a given age group. Classification typically falls into 3 categories based on the mean corpuscular volume (MCV) of red blood cells. Iron deficiency anemia remains the most prevalent type across all age groups worldwide.[1] In contrast, a rare anemia with a genetic origin—Diamond-Blackfan anemia is a congenital condition that features pure red cell aplasia and frequently accompanies congenital skeletal abnormalities. Diamond-Blackfan anemia presents as a chronic anemia, typically characterized by either macrocytic or normocytic features. In approximately 40% to 45% of cases, inheritance follows an autosomal dominant pattern, while 55% to 60% arise sporadically (see Image. Diamond-Blackfan Anemia).[2][3][4] A smaller subset of cases involves autosomal recessive inheritance. Although possible, the occurrence of hydrops remains exceedingly rare.[2]
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
Often, Diamond-Blackfan anemia results from a genetic mutation. The most common mutation involves a ribosomal protein on chromosome 11. However, newer studies have also noted mutations of the transcription factor GATA1.[2][3][5][4] Ribosomal gene mutations are more common and present in about 60% to 70% of Diamond-Blackfan anemia cases.[2][3][5][4] It involves the gene that codes for both small and large ribosomal protein units, including but not limited to RPL5, RPL11, RPS7, RPS17, RPS24, RPS10, RPS19, and RPS26. In some families, mutations in RPL3, RPL7, RPL14, RPL19, RPL26, RPL36, RPL23A, RPL35, RPS 15, RPS 8, RPS27A, RPL35, and RPL18 gene has occurred.[2][3][6][4] Despite a significant genetic component to Diamond-Blackfan anemia, 30% to 35% of cases remain genetically indeterminate.[2][7][4] Defective ribosomal protein biosynthesis initiates apoptosis of erythroid progenitor cells through the activation and stabilization of p53, leading to erythroid failure. This mechanism is known as "ribosomal stress".[2] Up to 50% of Diamond-Blackfan anemia cases have a heterozygous mutation in the ribosomal gene, resulting in a loss of function.[2]
Epidemiology
Diamond-Blackfan anemia is a rare disease with an incidence rate of 1 in 500,000 live births. The onset is generally within the first year of life. The incidence of Diamond-Blackfan anemia is similar among different ethnicities and genders.[2][7] In pregnant patients with Diamond-Blackfan anemia, relapse may occur, which is thought to be secondary to the hormonal stress of pregnancy.[8]
Pathophysiology
Diamond-Blackfan anemia is a chronic macrocytic-normochromic anemia characterized by low reticulocytes, normal platelets, and normal white blood cells (WBCs) in the bone marrow. In 25% of cases, a transient thrombocytopenia and neutropenia may be present.[9] Laboratory evaluation is significant for elevated MCV and erythrocyte adenosine deaminase (eADA). The eADA is elevated in 80% to 85% of patients.[8] Persistently elevated hemoglobin F is commonly seen in patients with Diamond-Blackfan anemia as well.[2][5][4]
Among all ribosomal protein genes, RPS19, which encodes a small subunit of the ribosome, represents the most frequently mutated gene in Diamond-Blackfan anemia, accounting for approximately 25% of cases.[2][4] More than 50% of mutations involving ribosomal protein genes affect RPS genes. These mutations include deletions, insertions, nonsense mutations, splice site alterations, and frameshift changes. Such alterations result in a deficiency of the RPS19 protein, a condition known as "haploinsufficiency."[2][4]
Up to 50% of Diamond-Blackfan anemia cases have a heterozygous mutation of the ribosomal gene, resulting in loss of function in a single copy of the gene.[2] RPS19 plays a crucial role in 18S rRNA maturation and 40S ribosome subunit synthesis. RPS19 mutation causes a decrease in the production of RPS19 and 40S ribosomal subunits by reducing translation initiation. This reduction disrupts the differentiation and production of normal primary hematopoietic progenitor cells and increases apoptosis.[2][4] The overexpression of exogenous RPS19 can compensate for the deficiency of RPS19, which helps explain the haploinsufficiency phenomenon and suggests that Diamond-Blackfan anemia might be treatable by augmenting the RPS gene.[2][4]
According to the "ribosomal stress" mechanism, ribosomal deficiency activates and stabilizes p53, subsequently initiating apoptosis to cause bone marrow failure by terminating cell lines. Murine double minute (MDM2) is a ringer figure ubiquitin ligase that controls the level and activity of p53. When interacting with the normal ribosomal protein, MDM2 enhances p53 degradation.[2] In Diamond-Blackfan anemia, ribosomal stress causes many free ribosomal proteins (RPS) to accumulate due to disrupted ribosomal biosynthesis. These free RPS, such as RPL5, RPL11, RPL23, RPS27, and RPS7, bind with MDM2 to stabilize p53, leading to apoptosis, cell cycle arrest, and eventually erythroid hypoplasia.[2] Diamond-Blackfan anemia also results from non-Rp gene mutations, eg, GATA1. GATA1 encodes for transcription factors.[2][5][4] It plays a crucial role in erythroid differentiation. GATA1 mutation of G to C transversion on the X chromosome leads to substituting leucine for valine. This mutation affects the splicing process of GATA1, leading to the termination of GATA1, which plays a crucial role in the differentiation of erythroid cells. GATA1 mutation ultimately acts by a global translation blockade.[2][5][4]
History and Physical
Diamond-Blackfan Anemia Clinical Manifestations
In approximately 90% of patients, Diamond-Blackfan anemia typically begins before the age of 12 months. It commonly presents with congenital bony malformations (in 50% of cases) and growth retardation (in 30% of cases).[3] The median age of presentation and diagnosis is 2 months.[2][3] Children typically present with lethargy and pallor first. Patients usually present with severe macrocytic anemia and normochromic anemia, along with erythroid aplasia due to congenital bone marrow failure. Typically, platelet and leukocyte counts are within the normal range; however, some patients have been found to have low leukocyte and high platelet counts. Patients can have significantly low reticulocyte counts.[2] Diamond-Blackfan anemia is also associated with elevated fetal hemoglobin levels, erythropoietin, and eADA activities.[2][7][5][4]
Diamond-Blackfan anemia presents a broad spectrum of phenotypes, from mild to severe. Physical abnormalities are present in 50% of cases.[2][3][9] The most common congenital physical abnormalities are thumb and upper extremity malformations, craniofacial anomalies (eg, microtia), and short stature. RPL26 favors variable radial ray anomalies.[10] The patient may also have a snub nose and wide-spaced eyes. They may manifest congenital glaucoma or cataracts, and strabismus. The characteristic Diamond-Blackfan anemia anomalies include a distinct facial appearance along with triphalangeal thumbs.[2][3][4] The PRL5 mutation is associated with cleft lip or soft palate, while RPL11 largely correlates with thumb abnormalities but is also observed in cases of cleft lip or palate.[2][3][4] Other physical anomalies include urogenital (eg, absence of or horseshoe kidney), atrial septal, and ventricular septal defects as well as aortic coarctation.[2][3][7] Due to cervical spine anomalies, patients may manifest Klippel-Feil deformity.
Diagnostic Criteria for Classical Diamond-Blackfan Anemia
The following criteria identify classic Diamond-Blackfan anemia
- Age of onset less than 12 months
- Macrocytic anemia without other significant cytopenias
- Reticulocytopenia
- Bone marrow with normal cellularity with a lack of erythroid precursors [3][7][11]
Major supporting criteria include:
- Gene mutation described in Diamond-Blackfan anemia
- Positive family history
Minor supporting criteria include:
- Elevated ADA activity
- Congenital anomalies described in classical Diamond-Blackfan anemia
- Elevated HbF
- There is no evidence of another inherited bone marrow failure syndrome
Evaluation
After meeting the clinical criteria for diagnosis, laboratory tests help physicians make a correct diagnosis. Erythropoietin (EPO) levels are elevated in Diamond-Blackfan anemia due to a lack of EPO receptors in the setting of erythroid aplasia. Other biological tests may include immune phenotyping and titers of IgG and IgA agglutinins.[12] For molecular diagnosis, the first step is to characterize the phenotype with a bone marrow evaluation. Molecular tests are performed to identify a heterozygous pathogenic variant in genes commonly associated with Diamond-Blackfan anemia.
The types of molecular testing include serial single-gene testing and multigene panels. Parvovirus B19 is a common cause of bone marrow failure, making it mandatory to have parvovirus B19 serology or blood parvovirus B19 PCR done in patients where Diamond-Blackfan anemia is suspected.[12] A common hematological workup includes a complete blood count with differential, hemoglobin F, eADA, erythropoietin level, reticulocyte count, and a peripheral blood smear.
Treatment / Management
Corticosteroid Therapy
Corticosteroids are the first-line treatment of Diamond-Blackfan anemia. However, despite the efficacy of corticosteroids, patients with Diamond-Blackfan anemia often require chronic blood transfusions (90% of patients before the age of 1 year) and concurrent iron chelation therapy.[2][3][5] A patient who is responsive to steroid therapy but with intolerable adverse effects requires chronic blood transfusions with a hemoglobin goal of 8 g/dL and requires a blood transfusion usually every 35 weeks.[2] Approximately 40% of patients became steroid dependent, while steroid resistance occurred in 35%.[9][8] Frequent monitoring of serum ferritin levels helps determine if iron chelation therapy is necessary.(B3)
Clinicians should bear in mind that ferritin is an "acute-phase reactant" (ie, phase reactant protein) and can be elevated in conditions of inflammation, infarction, ischemia, and infection, thereby giving a false impression of the iron level. Generally, iron chelation is started after 12 to 15 units of blood transfusions if serum ferritin concentration increases to 1000 to 1500 µg/L or if hepatic iron concentration increases to 6 to 7 mg of the dry weight of liver tissue.[2][3][5] When iron chelation is required, deferasirox and desferrioxamine are the therapeutic choices. Deferiprone is not a recommendation due to its adverse effect of neutropenia.
The mechanism of action of corticosteroids remains obscure, but it seems to have a nonspecific anti-apoptotic effect on erythroid progenitors.[2] Metoclopramide can be used as a supplement to steroid therapy to decrease steroid dose and, therefore, the adverse effects.[4] Clinical trials have also suggested leucine as a supplemental therapy to steroids.[4] For patients on chronic steroids, vitamin D supplementation and regular bone density checks are recommended.[8](B3)
Hematopoietic Stem Cell Transplantation
Diamond-Blackfan anemia may also receive treatment with hematopoietic stem cell transplantation (HSCT). This approach is the only treatment that cures the hematological manifestation of Diamond-Blackfan anemia; the procedure proves to be risky if a matched sibling donor is not available.[3][7][6][4] HSCT has a high success rate in patients younger than 10 treated with an HLA-identical donor. HSCT is indicated as an alternative to chronic blood transfusions if the patient becomes nonresponsive to chronic blood transfusions or develops adverse effects of iron overload.[2][3][8] However, HSCT also has adverse effects to consider, including infection and graft-versus-host disease.[2][6] The indications for HSCT are resistance to chronic steroids, transfusion dependency, and steroid toxicity.[8] Stem cells from the bone marrow are preferred over those from the peripheral blood. Total body irradiation is not recommended as a preconditioning component, as Diamond-Blackfan anemia patients are already predisposed to cancer.(B3)
Gene Therapy
According to recent literature, gene therapy and gene editing may be potential future treatments for Diamond-Blackfan anemia.[6][13] The primary difference between gene therapy and allogeneic HSCT lies in the source of stem cells. In gene therapy, the source of stem cells is the patient's normal HSCs; then, a normal copy of the mutated gene can be inserted into the patient's cells. In allogeneic HSCT, normal stem cells are obtained from a donor.[13] Since the RPS19 gene is present in 25% of Diamond-Blackfan anemia cases, gene therapy may benefit patients with the mutation, which involves replacing the normal copy of the RPS19 gene to alleviate symptoms of Diamond-Blackfan anemia in the patient.[2][13][4] The viral vector system is the most common method for gene therapy, achieving a high success rate and safety profile.[6][13] Approximately 20% to 25% of Diamond-Blackfan anemia patients experience spontaneous remission.[5]
Differential Diagnosis
Diamond-Blackfan anemia has a unique quantitative defect in erythropoiesis rather than hematopoiesis, as seen in other bone marrow failure syndromes (BMF).[12] Various BMF syndromes include:
- Transient erythroblastopenia of childhood is a type of acquired anemia of unknown etiology, which typically (over 80%) presents at 1 year of age. In contrast, Diamond-Blackfan anemia usually (90%) presents before the age of 1. Another distinguishing feature of transient erythroblastopenia of childhood is that only 10% of patients have elevated eADA, and anemia is normocytic.[14]
- Fanconi anemia is a bone marrow failure syndrome characterized by pancytopenia and physical abnormalities, typically presenting within the first decade of life.[15]
- Shwachman-Diamond syndrome is a clinical syndrome characterized by exocrine pancreatic dysfunction with malabsorption, single or multi-lineage cytopenia, growth failure, bone abnormalities, and susceptibility to myelodysplastic syndrome (MDS) and acute myeloid leukemia (AML).[16][17]
- Pearson syndrome is a maternally inherited mDNA mutation characterized by sideroblastic anemia of childhood, exocrine pancreatic failure, liver failure, renal tubular defects, and pancytopenia. Death generally occurs in infancy due to progressive liver failure and severe metabolic acidosis.[18][19]
- Dyskeratosis congenita is a genetically inherited disorder with a wide spectrum of severity. Dyskeratosis congenita is a telomere biology disorder characterized by the classic triad of lacy reticular pigmentation on the upper chest and neck, dysplastic nails, and oral leukoplakia. These patients have an increased risk of MDS, BMF, or AML.[20][21]
- Cartilage-hair dysplasia is an autosomal recessive inherited disorder with a high incidence in the Amish and Finnish populations. This condition is characterized by anemia, macrocytosis, defective T cell-mediated immune response, short tubular bones, and fine, sparse blond hair.[22][23]
- Thrombocytopenia absent radii syndrome presents in the neonatal period or infancy with thrombocytopenia, absent radii, and the presence of bilateral thumbs. Other physical findings include rib, cervical, ulnar, and humeral deformities, cardiac septal defects, gastrointestinal abnormalities, and genitourinary abnormalities. In rare cases, aplastic anemia, multilineage cytopenia, or leukemia have been clinical features.[24][25]
- Congenital amegakaryocytic thrombocytopenia usually presents at birth or in the neonatal period with severe thrombocytopenia, petechiae, and intracranial or intestinal mucosal bleeding. No congenital physical findings are associated with this disease. In childhood, these patients may develop pancytopenia, MDS, or leukemia.[3][5][26][27]
Acquired conditions with BMF include viral infections (eg, parvovirus B19, viral hepatitis and mononucleosis, and human T-cell lymphotropic virus type 1, HIV-associated pure red cell aplasia), immune-mediated diseases (myasthenia gravis, thymoma, multiple endocrinopathy, and systemic lupus erythematosus), and drugs (eg, antiepileptic drugs, sulfonamide, isoniazid, chloramphenicol, procainamide, azathioprine, and thiamphenicol).[3][4]
Prognosis
The prognosis is relatively good; however, complications related to treatment may impact the patient's quality of life.[2] Severe complications as a result of treatment or the development of cancer may reduce life expectancy.[2] Disease severity is determined by the quality of life and response to treatment.[2]
Complications
Diamond-Blackfan anemia patients are at a high risk of developing hematological complications in the first year of life. Diamond-Blackfan anemia has a high risk of developing AML, MDS, and solid tumors. Other complications like growth failure, organ failure, and infection are related to iron overload due to blood transfusion, chronic steroid use, and HSCT.
Deterrence and Patient Education
Patients with Diamond-Blackfan anemia should have their complete blood counts monitored frequently.[3] Periodic bone marrow biopsy or aspiration to evaluate cellularity and morphology helps diagnose any new cytopenia or bone marrow failure.[3] Patients who are steroid-dependent or transfusion-dependent should be monitored for blood pressure and growth as they are at high risk of organ failure; often, an endocrinology consult is necessary.[3] Healthy Diamond-Blackfan anemia patients must follow up every 4 to 6 months with history, physical examination, and complete blood count for cancer surveillance.[3] The rapid decline of any cell line indicates bone marrow failure, and bone marrow biopsy with cytogenetic studies is required to diagnose chromosomal abnormalities associated with cancer.[3]
Relatives of individuals with Diamond-Blackfan anemia are at risk of developing the condition, as approximately 40% to 45% of autosomal dominant Diamond-Blackfan anemia patients have inherited a pathognomonic variant from 1 parent. Additionally, X-linked inheritance has been noted.[3] Offering genetic testing if a pathognomonic variant is known is appropriate. The patient may receive an offer for other hematological tests for early diagnosis of Diamond-Blackfan anemia and cancer.[3] Approximately 55% to 60% of cases of Diamond-Blackfan anemia are found to have a de-novo pathognomonic variant; this may be a result of alternate paternity or maternity, including assisted reproduction or adoption.[3]
An international consensus advocates for a maximum prednisone dose of 0.3 mg/kg/d, increasing the pretransfusion threshold to a hemoglobin level of 9 to 10 g/dL (regardless of age), early chelation, and a greater focus on stem cell transplantation, as well as early cancer detection.[28] Young adults with Diamond-Blackfan anemia are at an increased risk of early cancers, especially colorectal and osteogenic sarcoma.
Enhancing Healthcare Team Outcomes
Effective management of Diamond-Blackfan anemia demands a cohesive, interprofessional strategy that prioritizes patient-centered care, safety, and long-term outcomes. Given the chronic nature and complexity of Diamond-Blackfan anemia, physicians, advanced practitioners, nurses, pharmacists, and other health professionals must collaborate closely to implement evidence-based treatment strategies. Physicians and hematology specialists bear primary responsibility for diagnosis, initiating corticosteroid therapy, coordinating blood transfusion schedules, and considering hematopoietic stem cell transplantation when necessary. Pharmacists contribute to patient care by educating patients about corticosteroid therapy, monitoring for adverse effects, ensuring accurate dosing, and performing comprehensive medication reconciliation to prevent harmful drug interactions. Nurses play a pivotal role in administering transfusions, monitoring clinical responses, and educating patients about iron overload, a common complication of repeated transfusions.[29][30][31] Obstetricians and labor and delivery nurses must provide high-risk counseling, as pregnancy can exacerbate or trigger relapses in Diamond-Blackfan anemia, requiring specialized prenatal and genetic care.
In addition to clinical management, supporting patients holistically requires integration of mental health, genetic counseling, and social services. Mental health nurses should offer counseling to address the psychological burden associated with chronic treatment and poor quality of life. Social workers assist patients in navigating financial challenges and accessing necessary care resources. Genetic counselors play a critical role in prenatal screening and education, particularly given the autosomal dominant inheritance pattern of Diamond-Blackfan anemia, which carries a 50% transmission risk.[3] Coordination of imaging studies and cardiac evaluations by radiology and cardiology teams enables early detection of renal, hepatic, or cardiac complications. Open, consistent communication among all team members enhances care continuity, reduces medical errors, and improves patient satisfaction. Through shared responsibilities and mutual respect across disciplines, the interprofessional team can optimize treatment efficacy, ensure patient safety, and promote long-term wellness in individuals living with Diamond-Blackfan anemia.
Media
References
Wang M. Iron Deficiency and Other Types of Anemia in Infants and Children. American family physician. 2016 Feb 15:93(4):270-8 [PubMed PMID: 26926814]
Engidaye G, Melku M, Enawgaw B. Diamond Blackfan Anemia: Genetics, Pathogenesis, Diagnosis and Treatment. EJIFCC. 2019 Mar:30(1):67-81 [PubMed PMID: 30881276]
Adam MP, Feldman J, Mirzaa GM, Pagon RA, Wallace SE, Amemiya A, Sieff C. Diamond-Blackfan Anemia. GeneReviews(®). 1993:(): [PubMed PMID: 20301769]
Da Costa L, O'Donohue MF, van Dooijeweert B, Albrecht K, Unal S, Ramenghi U, Leblanc T, Dianzani I, Tamary H, Bartels M, Gleizes PE, Wlodarski M, MacInnes AW. Molecular approaches to diagnose Diamond-Blackfan anemia: The EuroDBA experience. European journal of medical genetics. 2018 Nov:61(11):664-673. doi: 10.1016/j.ejmg.2017.10.017. Epub 2017 Oct 26 [PubMed PMID: 29081386]
Khincha PP, Savage SA. Neonatal manifestations of inherited bone marrow failure syndromes. Seminars in fetal & neonatal medicine. 2016 Feb:21(1):57-65. doi: 10.1016/j.siny.2015.12.003. Epub 2015 Dec 24 [PubMed PMID: 26724991]
Aspesi A, Borsotti C, Follenzi A. Emerging Therapeutic Approaches for Diamond Blackfan Anemia. Current gene therapy. 2018:18(6):327-335. doi: 10.2174/1566523218666181109124538. Epub [PubMed PMID: 30411682]
van Dooijeweert B, van Ommen CH, Smiers FJ, Tamminga RYJ, Te Loo MW, Donker AE, Peters M, Granzen B, Gille HJJP, Bierings MB, MacInnes AW, Bartels M. Pediatric Diamond-Blackfan anemia in the Netherlands: An overview of clinical characteristics and underlying molecular defects. European journal of haematology. 2018 Feb:100(2):163-170. doi: 10.1111/ejh.12995. Epub 2017 Dec 1 [PubMed PMID: 29114930]
Level 3 (low-level) evidenceLiu Y, Karlsson S. Perspectives of current understanding and therapeutics of Diamond-Blackfan anemia. Leukemia. 2024 Jan:38(1):1-9. doi: 10.1038/s41375-023-02082-w. Epub 2023 Nov 16 [PubMed PMID: 37973818]
Level 3 (low-level) evidenceDa Costa L, Leblanc T, Mohandas N. Diamond-Blackfan anemia. Blood. 2020 Sep 10:136(11):1262-1273. doi: 10.1182/blood.2019000947. Epub [PubMed PMID: 32702755]
Vanlerberghe C, Frénois F, Smol T, Jourdain AS, Escande F, Aït-Yahya E, Aldeeri AA, Yu TW, Cormier-Daire V, Ghoumid J, Jacob M, Newbury-Ecob R, Manouvrier S, Platon J, Sailer S, Brunelle P, Da Costa L, Petit F. RPL26 variants: A rare cause of Diamond-Blackfan anemia syndrome with multiple congenital anomalies at the forefront. Genetics in medicine : official journal of the American College of Medical Genetics. 2024 Dec:26(12):101266. doi: 10.1016/j.gim.2024.101266. Epub 2024 Sep 10 [PubMed PMID: 39268718]
Khurana M, Edwards D, Rescorla F, Miller C, He Y, Sierra Potchanant E, Nalepa G. Whole-exome sequencing enables correct diagnosis and surgical management of rare inherited childhood anemia. Cold Spring Harbor molecular case studies. 2018 Oct:4(5):. doi: 10.1101/mcs.a003152. Epub 2018 Oct 1 [PubMed PMID: 30275003]
Level 3 (low-level) evidenceDa Costa L, Narla A, Mohandas N. An update on the pathogenesis and diagnosis of Diamond-Blackfan anemia. F1000Research. 2018:7():. pii: F1000 Faculty Rev-1350. doi: 10.12688/f1000research.15542.1. Epub 2018 Aug 29 [PubMed PMID: 30228860]
Li H, Lodish HF, Sieff CA. Critical Issues in Diamond-Blackfan Anemia and Prospects for Novel Treatment. Hematology/oncology clinics of North America. 2018 Aug:32(4):701-712. doi: 10.1016/j.hoc.2018.04.005. Epub 2018 Jun 5 [PubMed PMID: 30047421]
Burns RA, Woodward GA. Transient Erythroblastopenia of Childhood: A Review for the Pediatric Emergency Medicine Physician. Pediatric emergency care. 2019 Mar:35(3):237-240. doi: 10.1097/PEC.0000000000001760. Epub [PubMed PMID: 30817707]
Sharma P, Sharma N, Sharma D. A Narrative Review on Fanconi Anemia: Genetic and Diagnostic Considerations. Global medical genetics. 2022 Sep:9(3):237-241. doi: 10.1055/s-0042-1751303. Epub 2022 Sep 5 [PubMed PMID: 36071913]
Level 3 (low-level) evidenceNelson AS, Myers KC. Diagnosis, Treatment, and Molecular Pathology of Shwachman-Diamond Syndrome. Hematology/oncology clinics of North America. 2018 Aug:32(4):687-700. doi: 10.1016/j.hoc.2018.04.006. Epub 2018 Jun 5 [PubMed PMID: 30047420]
Reilly CR, Shimamura A. Predisposition to myeloid malignancies in Shwachman-Diamond syndrome: biological insights and clinical advances. Blood. 2023 Mar 30:141(13):1513-1523. doi: 10.1182/blood.2022017739. Epub [PubMed PMID: 36542827]
Level 3 (low-level) evidenceYoshimi A, Ishikawa K, Niemeyer C, Grünert SC. Pearson syndrome: a multisystem mitochondrial disease with bone marrow failure. Orphanet journal of rare diseases. 2022 Oct 17:17(1):379. doi: 10.1186/s13023-022-02538-9. Epub 2022 Oct 17 [PubMed PMID: 36253820]
Alter BP. Pearson syndrome in a Diamond-Blackfan anemia cohort. Blood. 2014 Jul 17:124(3):312-3. doi: 10.1182/blood-2014-04-571687. Epub [PubMed PMID: 25035146]
Savage SA, Alter BP. Dyskeratosis congenita. Hematology/oncology clinics of North America. 2009 Apr:23(2):215-31. doi: 10.1016/j.hoc.2009.01.003. Epub [PubMed PMID: 19327580]
Gitto L, Stoppacher R, Richardson TE, Serinelli S. Dyskeratosis congenita. Autopsy & case reports. 2020 Sep 2:10(3):e2020203. doi: 10.4322/acr.2020.203. Epub 2020 Sep 2 [PubMed PMID: 33344307]
Level 3 (low-level) evidenceVakkilainen S, Taskinen M, Mäkitie O. Immunodeficiency in cartilage-hair hypoplasia: Pathogenesis, clinical course and management. Scandinavian journal of immunology. 2020 Oct:92(4):e12913. doi: 10.1111/sji.12913. Epub 2020 Jun 22 [PubMed PMID: 32506568]
Vakkilainen S, Taskinen M, Klemetti P, Pukkala E, Mäkitie O. A 30-Year Prospective Follow-Up Study Reveals Risk Factors for Early Death in Cartilage-Hair Hypoplasia. Frontiers in immunology. 2019:10():1581. doi: 10.3389/fimmu.2019.01581. Epub 2019 Jul 16 [PubMed PMID: 31379817]
Monteiro C, Gonçalves A, Oliveira J, Salvado R, Tomaz J, Morais S, Lima M, Santos R. Thrombocytopenia-Absent Radius Syndrome: Descriptions of Three New Cases and a Novel Splicing Variant in RBM8A That Expands the Spectrum of Null Alleles. International journal of molecular sciences. 2022 Aug 25:23(17):. doi: 10.3390/ijms23179621. Epub 2022 Aug 25 [PubMed PMID: 36077017]
Level 3 (low-level) evidenceStrauss G, Mott K, Klopocki E, Schulze H. Thrombocytopenia Absent Radius (TAR)-Syndrome: From Current Genetics to Patient Self-Empowerment. Hamostaseologie. 2023 Aug:43(4):252-260. doi: 10.1055/a-2088-1801. Epub 2023 Aug 23 [PubMed PMID: 37611607]
Germeshausen M, Ballmaier M. Congenital amegakaryocytic thrombocytopenia - Not a single disease. Best practice & research. Clinical haematology. 2021 Jun:34(2):101286. doi: 10.1016/j.beha.2021.101286. Epub 2021 Jul 14 [PubMed PMID: 34404532]
Balduini CL. The name counts: the case of 'congenital amegakaryocytic thrombocytopenia'. Haematologica. 2023 May 1:108(5):1216-1219. doi: 10.3324/haematol.2022.282024. Epub 2023 May 1 [PubMed PMID: 36226496]
Level 3 (low-level) evidenceWlodarski MW, Vlachos A, Farrar JE, Da Costa LM, Kattamis A, Dianzani I, Belendez C, Unal S, Tamary H, Pasauliene R, Pospisilova D, de la Fuente J, Iskander D, Wolfe L, Liu JM, Shimamura A, Albrecht K, Lausen B, Bechensteen AG, Tedgard U, Puzik A, Quarello P, Ramenghi U, Bartels M, Hengartner H, Farah RA, Al Saleh M, Hamidieh AA, Yang W, Ito E, Kook H, Ovsyannikova G, Kager L, Gleizes PE, Dalle JH, Strahm B, Niemeyer CM, Lipton JM, Leblanc TM, international Diamond-Blackfan anaemia syndrome guideline panel. Diagnosis, treatment, and surveillance of Diamond-Blackfan anaemia syndrome: international consensus statement. The Lancet. Haematology. 2024 May:11(5):e368-e382. doi: 10.1016/S2352-3026(24)00063-2. Epub [PubMed PMID: 38697731]
Level 3 (low-level) evidenceVan Hook JW, Gill P, Cyr D, Kapur RP. Diamond-Blackfan anemia as an unusual cause of nonimmune hydrops fetalis: a case report. The Journal of reproductive medicine. 1995 Dec:40(12):850-4 [PubMed PMID: 8926615]
Level 3 (low-level) evidenceCancado R, Watman NP, Lobo C, Chona Z, Manzur F, Traina F, Park M, Drelichman G, Zarate JP, Marfil L. Assessment of liver and cardiac iron overload using MRI in patients with chronic anemias in Latin American countries: results from ASIMILA study. Hematology (Amsterdam, Netherlands). 2018 Oct:23(9):676-682. doi: 10.1080/10245332.2018.1461292. Epub 2018 Apr 17 [PubMed PMID: 29663858]
Gomes RF, Munerato MC. The Stomatological Complications of Diamond-Blackfan Anemia: A Case Report. Clinical medicine & research. 2016 Jun:14(2):97-102. doi: 10.3121/cmr.2015.1305. Epub 2016 Feb 10 [PubMed PMID: 26864506]
Level 3 (low-level) evidence