Congenital Cytomegalovirus Infection
Congenital Cytomegalovirus Infection
Introduction
Cytomegalovirus (CMV) infection is the most common congenital infection worldwide and the leading cause of nonhereditary sensorineural hearing loss in children.[1][2] Congenital CMV infection (cCMV) is asymptomatic in 85% to 90% of newborns at birth.[3][4] Symptomatic congenital disease occurs most frequently after primary maternal infection in pregnancy. Although much less common, symptomatic cCMV carries a mortality risk of up to 7% to 12% in the early neonatal period.
The risk of severe morbidity is increased due to central nervous system damage, leading to neurodevelopmental delays, hearing loss, and vision impairment. Asymptomatic disease is not entirely benign, as 10% to 15% go on to develop long-term morbidities.[4][5] Despite these risks, clinician awareness of cCMV among women of reproductive age is much more limited than for congenital toxoplasmosis or Down syndrome, which occur at a lower incidence.[6][7][8]
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
CMV is the largest member of the Herpesviridae, a neurotropic virus family that favors persistence and immune evasion. Humans are the only reservoir. Primary infection of the host occurs when a previously uninfected individual acquires the virus for the first time through any contact with bodily fluids, such as saliva, urine, semen, or blood, except through the air. Infection is lifelong, and reactivation of the latent infection is a common occurrence.
Infection with a different strain of the virus is also possible. Reactivation and reinfection are referred to as a nonprimary infection.[9] Hormonal changes associated with pregnancy and lactation may stimulate reactivation of CMV.[2][10] A significant risk factor for primary infection before and during pregnancy is close contact with young children.[11] CMV-infected children younger than 2 can secrete the virus in their urine and saliva for approximately 24 months, meaning they can continue to secrete it for extended periods, even after recovery.[12]
Epidemiology
Seroprevalence rates in women of reproductive age are high and differ based on socioeconomic status. In developed countries, the seroprevalence rate ranges from 40% to 83% among women,[13] and in developing countries, it approaches nearly 100%.[14] However, in women of lower socioeconomic status in developed countries, the seroprevalence rate approaches that seen in developing countries. The acquisition of CMV in industrialized nations is typically due to frequent contact with small children. At the same time, in the developing world, transmission occurs early in life through breastfeeding and crowded living conditions.[15]
Since only about 10% of infected newborns are symptomatic at birth, the disease is difficult to detect at birth. Nevertheless, the live birth prevalence rate in developed countries is 0.6% to 0.7% or more than 1 in 200, resulting in about 40,000 cases annually in the United States.[16] The incidence of cCMV is higher in developing countries, ranging from 1% to 5%.
The risk of long-term neurological consequences is high in symptomatic newborns, with 40% to 58% developing permanent sequelae, including sensorineural hearing loss, ophthalmological deficits, and neurodevelopmental delays.[17] The most common complication of cCMV infection is hearing loss in 35% of symptomatic newborns and 7% to 10% of newborns asymptomatic at birth.[15][18]
Pathophysiology
The fetus is infected after maternal viremia and placental infection. The greatest risk of transplacental transmission (30% to 35%) is associated with maternal primary infection, compared to only 1.4% for nonprimary infections, where viremia is transient.[2][10][14] As pregnancy progresses, shedding of CMV in urine and cervicovaginal secretions increases and transmission to the fetus is more likely, with 58% to 78% in the third trimester compared to 30% to 45% in the first trimester.[19][20]
When CMV infects the placenta, it impairs trophoblast function, leading to placental insufficiency and hypoxia, which can cause intrauterine growth restriction.[21] CMV infects neural progenitor cells in the developing fetal brain, especially in the ventricular and subventricular zones. This disrupts their growth, differentiation, and migration, leading to neuronal cell loss, impaired cortical development, and the development of structural brain abnormalities, including cortical atrophy, ventriculomegaly, and microcephaly.[22] Hearing loss, the most common complication, results from direct viral injury to cochlear structures, immune-mediated inflammation and neuronal loss, and correlates with the extent of early viral load and inflammation, rather than viral presence at later stages.[23]
If the fetus is infected later in pregnancy, long-term sequelae are much less likely; 24% to 26% of those infected in the first trimester, versus 2.5% to 6% of those infected after 20 weeks, have long-term sequelae. In contrast, only 0.1% of those infected in the second trimester and 0% of those infected in the third trimester experience long-term sequelae.[24][25][26] The risk of transmission of CMV in human immunodeficiency virus-positive mothers on antiretroviral therapy is not different from that of uninfected women, even in resource-limited settings.[27]
History and Physical
Maternal CMV infection often remains asymptomatic. When symptoms do occur, primary infection may manifest with fever, malaise, headache, pharyngitis, lymphadenopathy, hepatosplenomegaly, arthralgias, and rash—features that closely resemble Epstein-Barr virus infection and offer no clear clinical distinction. Antenatal ultrasound may reveal fetal abnormalities, including microcephaly, intracranial calcifications, ventriculomegaly, lenticulostriate vasculopathy, occipital horn anomalies, echogenic bowel, intrauterine growth restriction, hepatomegaly, pericardial effusion, ascites, placental inflammation, or fetal death (see Image. Congenital Cytomegalovirus [CMV]).[2][28]
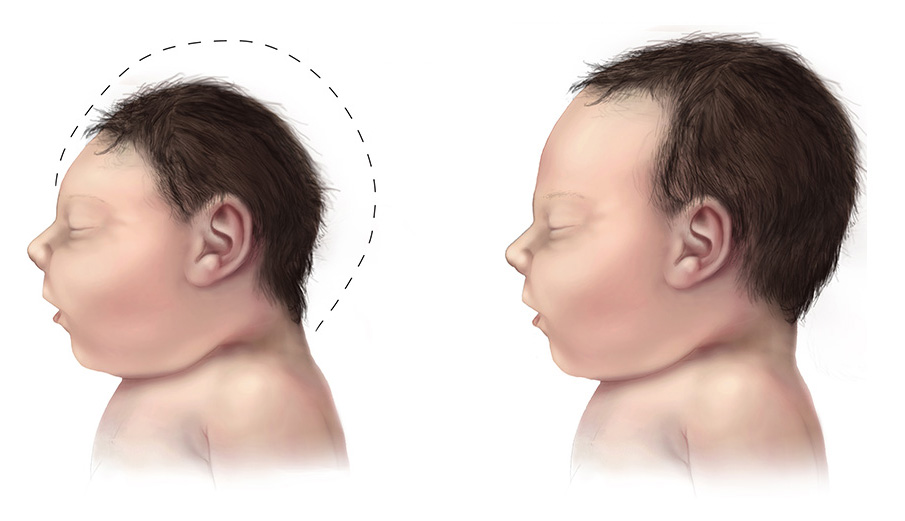
In affected newborns, jaundice, petechiae, hepatosplenomegaly, and microcephaly represent the most common clinical signs (see Image. Fetal Microcephaly).[4][5][16][29][30] Prematurity or growth restriction may also be present. Ophthalmologic manifestations include chorioretinitis, with or without optic atrophy, and cataracts. Hearing loss—a hallmark of cCMV infection—may be present at birth or develop later, underscoring the importance of regular audiologic monitoring in affected children. Laboratory findings may include conjugated hyperbilirubinemia, thrombocytopenia, elevated aspartate aminotransferase levels, and increased cerebrospinal fluid protein.[29] Cranial imaging findings frequently reveal periventricular calcifications, ventricular dilatation, ventricular cysts, and lenticulostriate vasculopathy.[16][18]
Evaluation
Although cCMV can be diagnosed prenatally, this condition is most often diagnosed after birth. Prenatal diagnosis involves maternal and fetal diagnostic studies.
Maternal Cytomegalovirus Testing
Testing should be pursued in cases of mononucleosis-like illness during pregnancy, known exposure to an individual with CMV infection, occupational exposure in healthcare or childcare settings, or when fetal ultrasound reveals findings suggestive of cCMV infection, including ventriculomegaly, hyperechogenic bowel, intracranial calcifications, or hydrops. Universal screening during pregnancy remains unrecommended due to the absence of tests with sufficient sensitivity and specificity, combined with limited available interventions.[31] In suspected primary CMV infection, both maternal and fetal testing becomes essential.
Maternal evaluation includes assessment of antibody status. A confirmed diagnosis of primary CMV infection requires documented seroconversion from a previously negative to a positive antibody status.[32] In Europe, guidelines recommend first-trimester seroscreening as early as possible, with follow-up testing every 4 weeks in seronegative women until 14 to 16 weeks of gestation.[26] In regions where routine screening is not part of standard prenatal care, seroconversion often goes undetected. The presence of immunoglobulin (Ig) M antibodies may indicate a primary infection, though these antibodies lack specificity, can persist for months, and frequently cross-react. IgG antibody avidity testing provides additional insight by determining the timing of infection—low avidity strongly suggests a recent primary infection, especially when paired with detectable IgM.[33] Standard serologic tests and polymerase chain reaction (PCR) cannot differentiate between viral reactivation and reinfection.
Fetal and Newborn Cytomegalovirus Evaluation
Fetal diagnosis is achieved through amniocentesis, which involves collecting amniotic fluid for PCR analysis, with or without viral culture. Replication of the virus in the fetal kidney, leading to shedding in the urine, occurs at least 5 to 7 weeks after infection. Thus, the optimal time for performing this test is after 21 weeks of gestation and 7 weeks after maternal infection.[9][31] The sensitivity of amniocentesis after 21 weeks is approximately 71%, and it is 30% if performed before 21 weeks.[32] A fetal sonogram is also recommended; however, the possible findings are not specific for CMV and are present in only around 15% of infected fetuses.[34] The presence of ultrasound abnormalities along with primary maternal infection strongly suggests fetal infection.[34] Amniocentesis is adequate for diagnosing fetal CMV infection, and cordocentesis for fetal IgM testing is not recommended due to the test's poor sensitivity and the associated risks.[35] The viral load of CMV in the amniotic fluid may be determined for prognostic value; a higher CMV deoxyribonucleic acid (DNA) load greater than 100,000 correlates with symptoms in the newborn.[36]
Testing becomes necessary in cases of confirmed maternal primary CMV infection and in symptomatic newborns. In cases of symmetric intrauterine growth restriction, where both weight and head circumference are affected, testing is suggested.[26] Without a high index of suspicion, cCMV frequently remains undiagnosed at birth due to the asymptomatic presentation in most infected newborns. Diagnosis in the neonatal period requires isolation of CMV through DNA PCR or viral culture from urine or saliva collected within the first 3 weeks of life. After this window, distinguishing congenital infection from intrapartum or postnatal acquisition via breast milk or blood transfusion becomes impossible. Notably, infections acquired during delivery or postnatally generally do not result in long-term complications.[10]
Because CMV may be present in breast milk, infant specimen collection for urine or saliva testing should occur at least 1 hour after breastfeeding to prevent contamination.[37] Recommended laboratory assessments include a complete blood count, liver enzyme levels, total and direct bilirubin measurements, and cranial imaging, starting with ultrasound and followed by magnetic resonance imaging if necessary.[30] Although a 2017 informal recommendation by the International Congenital Cytomegalovirus Recommendations Group supported the use of saliva as a diagnostic specimen, its lower specificity compared to urine increases the likelihood of false-positive PCR results.[38]
Treatment / Management
Prenatal Management
Fetal cCMV has no definitive treatment in utero. For fetuses with positive isolation of the virus, termination of the pregnancy can be offered to parents. This offer must be accompanied by thorough counseling to enable the parents to make an informed decision. If the parents opt to continue the pregnancy, close follow-up with regular ultrasound exams is essential. CMV hyperimmune globulin does not reduce maternal–fetal transmission but could reduce the number of symptomatic newborns with cCMV. However, routine use is not recommended.[8][26][39][40] European experts recommend oral valacyclovir at 8 g/day as soon as possible during the primary infection in the periconceptional period and the first trimester, until a negative amniocentesis result or 17 to 18 weeks postconception.[26] Valganciclovir is toxic to the bone marrow and a teratogen, and is not recommended for use prenatally, as it increases mutagenicity in the recipient.[8](A1)
Newborn Management
Newborns with symptomatic cCMV, other than isolated sensorineural hearing loss, should receive oral valganciclovir for 6 months. This therapy has been shown to preserve normal hearing or prevent the progression of hearing loss, and also correlates with improved long-term neurodevelopmental outcomes.[41] Oral valganciclovir is superior to intravenous ganciclovir, which is associated with bone marrow suppression manifesting as neutropenia and gonadal toxicity.[42] (A1)
Unfortunately, long-term follow-up revealed that most patients have developed hearing loss, regardless of the 6-week intravenous ganciclovir treatment, which was the prior standard of care. Therefore, more studies are needed to investigate the long-term effects of valganciclovir.[26] Newborns with confirmed cCMV should be closely monitored, both symptomatic and asymptomatic, to detect the development of long-term sequelae, particularly through regular hearing screens for at least 6 years.[26] Pediatric subspecialists involved in the care of congenitally infected neonates include infectious disease specialists, audiologists, otolaryngologists, ophthalmologists, neurologists, developmental and behavioral specialists, and physical and occupational therapists, as needed.[30] (B3)
Differential Diagnosis
Other congenital infections, commonly referred to by the acronym ToRCHeS, include toxoplasmosis, rubella, cytomegalovirus, herpes simplex virus, and syphilis; Zika virus is also recognized as a significant congenital infection, though not part of the traditional ToRCHeS group.[10][43] Toxoplasmosis can present with chorioretinitis, microphthalmia, hydrocephalus, scattered calcifications, and maculopapular rash. Newborns with congenital rubella syndrome may have cataracts and congenital heart disease. A vesicular rash usually distinguishes herpes simplex infection. Syphilis may be associated with rhinitis and osteochondritis. Acute neonatal viral, bacterial, or fungal infections can mimic cCMV, as well as inborn errors of metabolism (eg, galactosemia and tyrosinemia). A positive newborn metabolic screen would be the expectation with inborn errors of metabolism. History, physical examination, and bacterial and viral cultures help distinguish other infections.
Prognosis
Prognosis is variable. Symptomatic cCMV leads to long-term sequelae including sensorineural hearing loss, mental and developmental disabilities, and impaired vision. Around 10% of symptomatic newborns will perish in the neonatal period, a number that may be an underestimate. Asymptomatic babies are not spared long-term sequelae, with 10% to 12% developing hearing loss and a smaller percentage developing neuromotor disabilities and vision problems.[17] Infants of mothers who acquired primary CMV in pregnancy or who are symptomatic with microcephaly, intracranial calcifications, or chorioretinitis have the worst prognosis.[43]
Complications
Complications occur more commonly in symptomatic cCMV infection, including the following:
- Sensorineural hearing loss is the most common complication, which affects both symptomatic and asymptomatic neonates
- Neurodevelopmental delays, including motor deficits, eg, cerebral palsy, and cognitive deficits
- Ophthalmological deficits ranging from the clinical finding of chorioretinitis to optic atrophy that may be associated with vision loss (see Image. Cytomegalovirus Retinitis)
- Seizures
- Death
Deterrence and Patient Education
Primary prevention aims at preventing a mother from becoming infected or reinfected. As long as no vaccine is available, the only means of primary prevention is increasing awareness about CMV and cCMV in women pre- and periconceptionally.[8] Healthcare professionals play a crucial role in education, but they have also been shown to have knowledge gaps themselves.[8]
Currently, the only proven strategy for mothers to reduce their risk of infection, or horizontal transmission, involves hygienic measures, as exposure to the urine or saliva of young children poses the greatest risk for maternal primary or reinfection. These include thorough hand washing after handling potentially infected articles, such as soiled diapers and toys, and limiting intimate contact with children younger than 6 years, including kissing on the mouth or cheek, bed sharing, and wiping drool from their mouth. Pregnant women should not share utensils with their children or put a child’s pacifier in their mouth.[7][44][45] Childcare workers who plan to become pregnant may need to inform their employers, so that the risk can be decreased as much as possible. Regarding the reactivation of a latent infection, no clear risk factors are known.[26]
Secondary prevention aims to reduce the likelihood of vertical transmission from an infected mother to her fetus. For this, a maternal CMV infection diagnosis must be made, and prophylactic treatment may be considered in follow-up, with hyperimmune globulin or antiviral therapy as discussed above.[8] Others consider secondary prevention to include the diagnosis of fetal infection and follow-up of infected fetuses. Tertiary prevention aims to reduce symptomatic cCMV infections in newborns, for example, through transplacental administration of valaciclovir or valganciclovir.[8]
Enhancing Healthcare Team Outcomes
Optimal care of cCMV infection requires an integrated, interprofessional approach involving clinicians, nurses, pharmacists, and allied health professionals. Primary care clinicians and obstetricians play a central role in prevention by educating women of reproductive age about CMV transmission risks, particularly those working in high-risk environments such as childcare centers and healthcare settings. Patient-centered education should include practical hygiene measures and the importance of routine prenatal care. Effective communication between team members ensures that maternal serologic testing and ultrasound findings are appropriately interpreted, documented, and conveyed to specialists, such as infectious disease clinicians, when needed. Pharmacists contribute by advising on the safety, dosing, and monitoring of antivirals, especially considering the toxicities of valganciclovir. Nurses reinforce health education, monitor adherence to follow-up protocols, and assist in coordinating care transitions between maternal and pediatric teams.
When prenatal diagnosis of cCMV is confirmed or strongly suspected, timely communication with the neonatal care team is critical. Coordination ensures that newborns receive diagnostic testing within the first 3 weeks of life, the only period during which a definitive diagnosis of congenital infection can be made. Early identification enables the prompt initiation of antiviral therapy when indicated and facilitates timely referrals to audiology, neurology, ophthalmology, and early intervention services. Interdisciplinary care conferences, structured handoffs, and shared electronic health records enhance team performance and patient safety by reducing communication breakdowns and ensuring continuity of care. Collaborative follow-up by developmental specialists and physical and occupational therapists further improves long-term outcomes, supporting the child’s health and developmental potential.
Media
(Click Image to Enlarge)
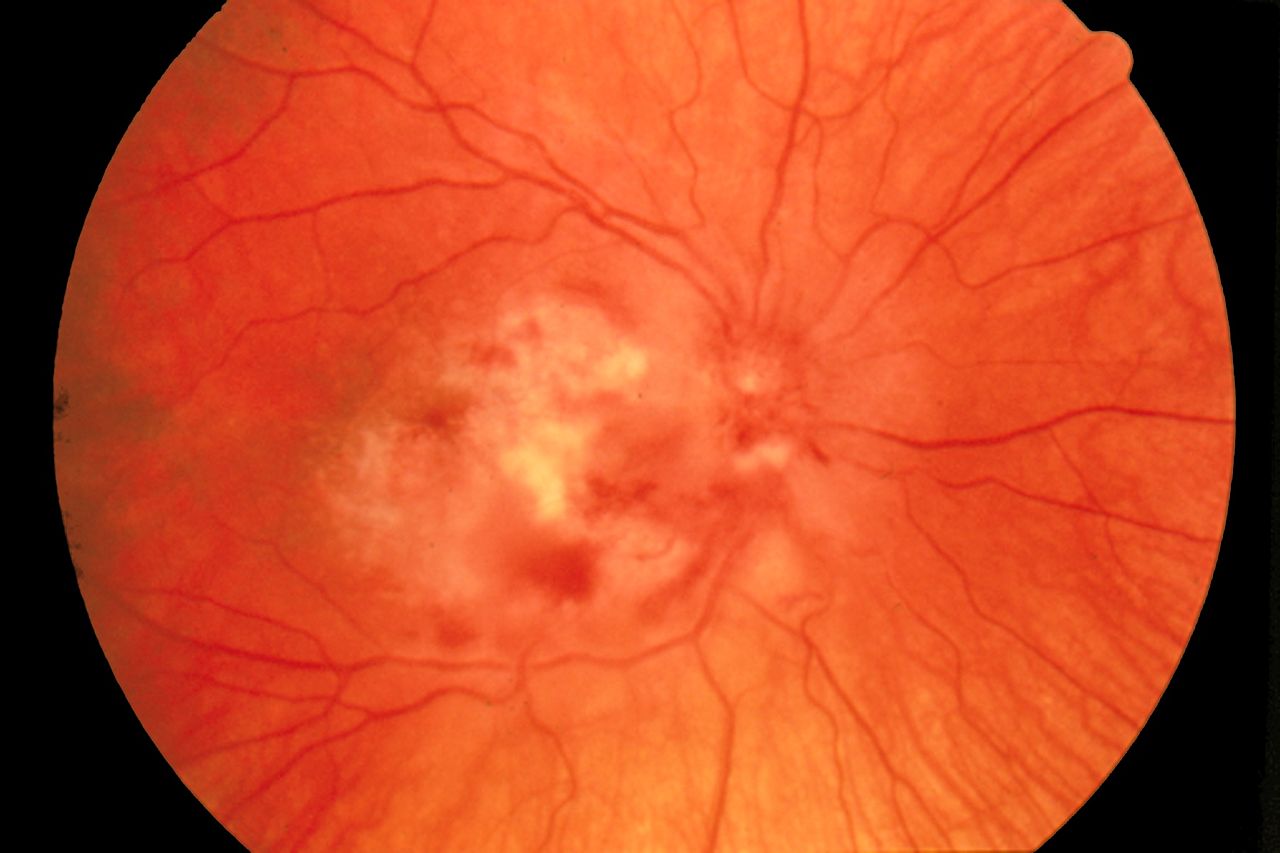
Cytomegalovirus Retinitis. The image depicts a view of a fundus affected by cytomegalovirus retinitis.
National Eye Institute, Public Domain, via Wikimedia Commons
(Click Image to Enlarge)
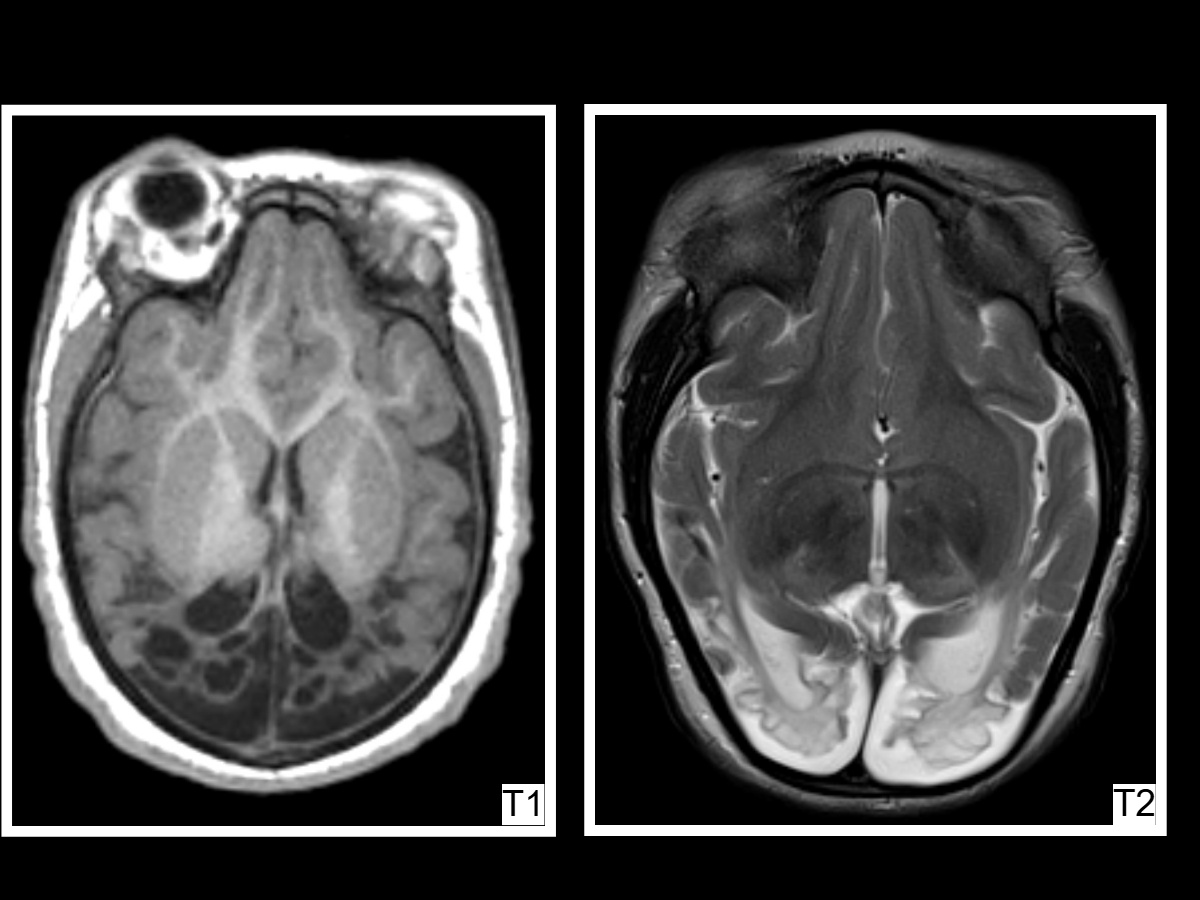
Congenital Cytomegalovirus (CMV). Axial T1- and T2-weighted magnetic resonance images of a 2-year-old with a history of congenital CMV demonstrate microcephaly with diffuse white matter volume loss, periventricular and subcortical cysts in the posterior parietal and occipital lobes, and ex vacuo dilatation of the occipital and temporal horns.
Contributed by A Thomas, MD
(Click Image to Enlarge)
References
Lazzarotto T, Guerra B, Gabrielli L, Lanari M, Landini MP. Update on the prevention, diagnosis and management of cytomegalovirus infection during pregnancy. Clinical microbiology and infection : the official publication of the European Society of Clinical Microbiology and Infectious Diseases. 2011 Sep:17(9):1285-93. doi: 10.1111/j.1469-0691.2011.03564.x. Epub 2011 Jun 1 [PubMed PMID: 21631642]
Picone O, Vauloup-Fellous C, Cordier AG, Guitton S, Senat MV, Fuchs F, Ayoubi JM, Grangeot Keros L, Benachi A. A series of 238 cytomegalovirus primary infections during pregnancy: description and outcome. Prenatal diagnosis. 2013 Aug:33(8):751-8. doi: 10.1002/pd.4118. Epub 2013 May 1 [PubMed PMID: 23553686]
Level 2 (mid-level) evidenceBoppana SB, Pass RF, Britt WJ, Stagno S, Alford CA. Symptomatic congenital cytomegalovirus infection: neonatal morbidity and mortality. The Pediatric infectious disease journal. 1992 Feb:11(2):93-9 [PubMed PMID: 1311066]
Kylat RI, Kelly EN, Ford-Jones EL. Clinical findings and adverse outcome in neonates with symptomatic congenital cytomegalovirus (SCCMV) infection. European journal of pediatrics. 2006 Nov:165(11):773-8 [PubMed PMID: 16835757]
Lanzieri TM, Leung J, Caviness AC, Chung W, Flores M, Blum P, Bialek SR, Miller JA, Vinson SS, Turcich MR, Voigt RG, Demmler-Harrison G. Long-term outcomes of children with symptomatic congenital cytomegalovirus disease. Journal of perinatology : official journal of the California Perinatal Association. 2017 Jul:37(7):875-880. doi: 10.1038/jp.2017.41. Epub 2017 Apr 6 [PubMed PMID: 28383538]
Jeon J, Victor M, Adler SP, Arwady A, Demmler G, Fowler K, Goldfarb J, Keyserling H, Massoudi M, Richards K, Staras SA, Cannon MJ. Knowledge and awareness of congenital cytomegalovirus among women. Infectious diseases in obstetrics and gynecology. 2006:2006():80383 [PubMed PMID: 17485810]
Cannon MJ, Westbrook K, Levis D, Schleiss MR, Thackeray R, Pass RF. Awareness of and behaviors related to child-to-mother transmission of cytomegalovirus. Preventive medicine. 2012 May:54(5):351-7. doi: 10.1016/j.ypmed.2012.03.009. Epub 2012 Mar 21 [PubMed PMID: 22465669]
Sartori P, Egloff C, Hcini N, Vauloup Fellous C, Périllaud-Dubois C, Picone O, Pomar L. Primary, Secondary, and Tertiary Prevention of Congenital Cytomegalovirus Infection. Viruses. 2023 Mar 23:15(4):. doi: 10.3390/v15040819. Epub 2023 Mar 23 [PubMed PMID: 37112800]
Revello MG, Gerna G. Diagnosis and management of human cytomegalovirus infection in the mother, fetus, and newborn infant. Clinical microbiology reviews. 2002 Oct:15(4):680-715 [PubMed PMID: 12364375]
Pass RF. Cytomegalovirus infection. Pediatrics in review. 2002 May:23(5):163-70 [PubMed PMID: 11986492]
Bristow BN, O'Keefe KA, Shafir SC, Sorvillo FJ. Congenital cytomegalovirus mortality in the United States, 1990-2006. PLoS neglected tropical diseases. 2011 Apr 26:5(4):e1140. doi: 10.1371/journal.pntd.0001140. Epub 2011 Apr 26 [PubMed PMID: 21541359]
Adler SP, Nigro G, Pereira L. Recent advances in the prevention and treatment of congenital cytomegalovirus infections. Seminars in perinatology. 2007 Feb:31(1):10-8 [PubMed PMID: 17317422]
Level 3 (low-level) evidenceYinon Y, Farine D, Yudin MH. Screening, diagnosis, and management of cytomegalovirus infection in pregnancy. Obstetrical & gynecological survey. 2010 Nov:65(11):736-43. doi: 10.1097/OGX.0b013e31821102b4. Epub [PubMed PMID: 21375790]
Kenneson A, Cannon MJ. Review and meta-analysis of the epidemiology of congenital cytomegalovirus (CMV) infection. Reviews in medical virology. 2007 Jul-Aug:17(4):253-76 [PubMed PMID: 17579921]
Level 2 (mid-level) evidenceManicklal S, Emery VC, Lazzarotto T, Boppana SB, Gupta RK. The "silent" global burden of congenital cytomegalovirus. Clinical microbiology reviews. 2013 Jan:26(1):86-102. doi: 10.1128/CMR.00062-12. Epub [PubMed PMID: 23297260]
de Vries LS, Gunardi H, Barth PG, Bok LA, Verboon-Maciolek MA, Groenendaal F. The spectrum of cranial ultrasound and magnetic resonance imaging abnormalities in congenital cytomegalovirus infection. Neuropediatrics. 2004 Apr:35(2):113-9 [PubMed PMID: 15127310]
Dollard SC, Grosse SD, Ross DS. New estimates of the prevalence of neurological and sensory sequelae and mortality associated with congenital cytomegalovirus infection. Reviews in medical virology. 2007 Sep-Oct:17(5):355-63 [PubMed PMID: 17542052]
Level 1 (high-level) evidenceBoppana SB, Ross SA, Fowler KB. Congenital cytomegalovirus infection: clinical outcome. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2013 Dec:57 Suppl 4(Suppl 4):S178-81. doi: 10.1093/cid/cit629. Epub [PubMed PMID: 24257422]
Level 2 (mid-level) evidenceBodéus M, Kabamba-Mukadi B, Zech F, Hubinont C, Bernard P, Goubau P. Human cytomegalovirus in utero transmission: follow-up of 524 maternal seroconversions. Journal of clinical virology : the official publication of the Pan American Society for Clinical Virology. 2010 Feb:47(2):201-2. doi: 10.1016/j.jcv.2009.11.009. Epub 2009 Dec 16 [PubMed PMID: 20006542]
Level 3 (low-level) evidenceEnders G, Daiminger A, Bäder U, Exler S, Enders M. Intrauterine transmission and clinical outcome of 248 pregnancies with primary cytomegalovirus infection in relation to gestational age. Journal of clinical virology : the official publication of the Pan American Society for Clinical Virology. 2011 Nov:52(3):244-6. doi: 10.1016/j.jcv.2011.07.005. Epub 2011 Aug 5 [PubMed PMID: 21820954]
Level 2 (mid-level) evidenceNjue A, Coyne C, Margulis AV, Wang D, Marks MA, Russell K, Das R, Sinha A. The Role of Congenital Cytomegalovirus Infection in Adverse Birth Outcomes: A Review of the Potential Mechanisms. Viruses. 2020 Dec 24:13(1):. doi: 10.3390/v13010020. Epub 2020 Dec 24 [PubMed PMID: 33374185]
Huang SN, Pan YT, Zhou YP, Wang XZ, Mei MJ, Yang B, Li D, Zeng WB, Cheng S, Sun JY, Cheng H, Zhao F, Luo MH. Human Cytomegalovirus IE1 Impairs Neuronal Migration by Downregulating Connexin 43. Journal of virology. 2023 May 31:97(5):e0031323. doi: 10.1128/jvi.00313-23. Epub 2023 Apr 25 [PubMed PMID: 37097169]
Smith MD, Seleme MC, Marquez-Lago T, Chen JW, Mach M, Britt WJ. Early control of cochlear viral load limits cochlear inflammation and prevents virus-induced sensorineural hearing loss. Journal of neuroinflammation. 2025 Mar 23:22(1):92. doi: 10.1186/s12974-025-03416-4. Epub 2025 Mar 23 [PubMed PMID: 40122833]
Pass RF, Fowler KB, Boppana SB, Britt WJ, Stagno S. Congenital cytomegalovirus infection following first trimester maternal infection: symptoms at birth and outcome. Journal of clinical virology : the official publication of the Pan American Society for Clinical Virology. 2006 Feb:35(2):216-20 [PubMed PMID: 16368262]
Revello MG, Zavattoni M, Furione M, Lilleri D, Gorini G, Gerna G. Diagnosis and outcome of preconceptional and periconceptional primary human cytomegalovirus infections. The Journal of infectious diseases. 2002 Aug 15:186(4):553-7 [PubMed PMID: 12195384]
Leruez-Ville M, Chatzakis C, Lilleri D, Blazquez-Gamero D, Alarcon A, Bourgon N, Foulon I, Fourgeaud J, Gonce A, Jones CE, Klapper P, Krom A, Lazzarotto T, Lyall H, Paixao P, Papaevangelou V, Puchhammer E, Sourvinos G, Vallely P, Ville Y, Vossen A. Consensus recommendation for prenatal, neonatal and postnatal management of congenital cytomegalovirus infection from the European congenital infection initiative (ECCI). The Lancet regional health. Europe. 2024 May:40():100892. doi: 10.1016/j.lanepe.2024.100892. Epub 2024 Apr 1 [PubMed PMID: 38590940]
Level 3 (low-level) evidenceMhandire D, Duri K, Kaba M, Mhandire K, Musarurwa C, Chimusa E, Munjoma P, Mazengera L, Stray-Pedersen B, Dandara C. Seroprevalence of Cytomegalovirus Infection Among HIV-Infected and HIV-Uninfected Pregnant Women Attending Antenatal Clinic in Harare, Zimbabwe. Viral immunology. 2019 Sep:32(7):289-295. doi: 10.1089/vim.2019.0024. Epub 2019 Jul 26 [PubMed PMID: 31347990]
La Torre R, Nigro G, Mazzocco M, Best AM, Adler SP. Placental enlargement in women with primary maternal cytomegalovirus infection is associated with fetal and neonatal disease. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2006 Oct 15:43(8):994-1000 [PubMed PMID: 16983610]
Dreher AM, Arora N, Fowler KB, Novak Z, Britt WJ, Boppana SB, Ross SA. Spectrum of disease and outcome in children with symptomatic congenital cytomegalovirus infection. The Journal of pediatrics. 2014 Apr:164(4):855-9. doi: 10.1016/j.jpeds.2013.12.007. Epub 2014 Jan 14 [PubMed PMID: 24433826]
Schleiss MR. Congenital cytomegalovirus: Impact on child health. Contemporary pediatrics. 2018 Jul:35(7):16-24 [PubMed PMID: 30740598]
Leber AL. Maternal and congenital human cytomegalovirus infection: laboratory testing for detection and diagnosis. Journal of clinical microbiology. 2024 Apr 10:62(4):e0031323. doi: 10.1128/jcm.00313-23. Epub 2024 Feb 23 [PubMed PMID: 38391188]
Liesnard C, Donner C, Brancart F, Gosselin F, Delforge ML, Rodesch F. Prenatal diagnosis of congenital cytomegalovirus infection: prospective study of 237 pregnancies at risk. Obstetrics and gynecology. 2000 Jun:95(6 Pt 1):881-8 [PubMed PMID: 10831985]
D'Alberti E, Rizzo G, Khalil A, Mappa I, Pietrolucci ME, Capannolo G, Alameddine S, Sorrenti S, Zullo F, Giancotti A, Di Mascio D, D'Antonio F. Counseling in fetal medicine: Congenital cytomegalovirus infection. European journal of obstetrics, gynecology, and reproductive biology. 2024 Apr:295():8-17. doi: 10.1016/j.ejogrb.2024.01.037. Epub 2024 Feb 1 [PubMed PMID: 38310675]
Guerra B, Simonazzi G, Puccetti C, Lanari M, Farina A, Lazzarotto T, Rizzo N. Ultrasound prediction of symptomatic congenital cytomegalovirus infection. American journal of obstetrics and gynecology. 2008 Apr:198(4):380.e1-7. doi: 10.1016/j.ajog.2007.09.052. Epub 2008 Jan 14 [PubMed PMID: 18191802]
Level 2 (mid-level) evidenceAzam AZ, Vial Y, Fawer CL, Zufferey J, Hohlfeld P. Prenatal diagnosis of congenital cytomegalovirus infection. Obstetrics and gynecology. 2001 Mar:97(3):443-8 [PubMed PMID: 11239654]
Guerra B, Lazzarotto T, Quarta S, Lanari M, Bovicelli L, Nicolosi A, Landini MP. Prenatal diagnosis of symptomatic congenital cytomegalovirus infection. American journal of obstetrics and gynecology. 2000 Aug:183(2):476-82 [PubMed PMID: 10942490]
Naing ZW, Scott GM, Shand A, Hamilton ST, van Zuylen WJ, Basha J, Hall B, Craig ME, Rawlinson WD. Congenital cytomegalovirus infection in pregnancy: a review of prevalence, clinical features, diagnosis and prevention. The Australian & New Zealand journal of obstetrics & gynaecology. 2016 Feb:56(1):9-18. doi: 10.1111/ajo.12408. Epub 2015 Sep 22 [PubMed PMID: 26391432]
Rawlinson WD, Boppana SB, Fowler KB, Kimberlin DW, Lazzarotto T, Alain S, Daly K, Doutré S, Gibson L, Giles ML, Greenlee J, Hamilton ST, Harrison GJ, Hui L, Jones CA, Palasanthiran P, Schleiss MR, Shand AW, van Zuylen WJ. Congenital cytomegalovirus infection in pregnancy and the neonate: consensus recommendations for prevention, diagnosis, and therapy. The Lancet. Infectious diseases. 2017 Jun:17(6):e177-e188. doi: 10.1016/S1473-3099(17)30143-3. Epub 2017 Mar 11 [PubMed PMID: 28291720]
Level 3 (low-level) evidenceNigro G, Adler SP, La Torre R, Best AM, Congenital Cytomegalovirus Collaborating Group. Passive immunization during pregnancy for congenital cytomegalovirus infection. The New England journal of medicine. 2005 Sep 29:353(13):1350-62 [PubMed PMID: 16192480]
Revello MG, Lazzarotto T, Guerra B, Spinillo A, Ferrazzi E, Kustermann A, Guaschino S, Vergani P, Todros T, Frusca T, Arossa A, Furione M, Rognoni V, Rizzo N, Gabrielli L, Klersy C, Gerna G, CHIP Study Group. A randomized trial of hyperimmune globulin to prevent congenital cytomegalovirus. The New England journal of medicine. 2014 Apr 3:370(14):1316-26. doi: 10.1056/NEJMoa1310214. Epub [PubMed PMID: 24693891]
Level 1 (high-level) evidenceRawlinson WD, Hamilton ST, van Zuylen WJ. Update on treatment of cytomegalovirus infection in pregnancy and of the newborn with congenital cytomegalovirus. Current opinion in infectious diseases. 2016 Dec:29(6):615-624 [PubMed PMID: 27607910]
Level 3 (low-level) evidenceLackner A, Acham A, Alborno T, Moser M, Engele H, Raggam RB, Halwachs-Baumann G, Kapitan M, Walch C. Effect on hearing of ganciclovir therapy for asymptomatic congenital cytomegalovirus infection: four to 10 year follow up. The Journal of laryngology and otology. 2009 Apr:123(4):391-6. doi: 10.1017/S0022215108003162. Epub 2008 Jun 30 [PubMed PMID: 18588736]
Level 1 (high-level) evidenceLeung AK, Sauve RS, Davies HD. Congenital cytomegalovirus infection. Journal of the National Medical Association. 2003 Mar:95(3):213-8 [PubMed PMID: 12749681]
Cannon MJ, Davis KF. Washing our hands of the congenital cytomegalovirus disease epidemic. BMC public health. 2005 Jun 20:5():70 [PubMed PMID: 15967030]
Adler SP, Finney JW, Manganello AM, Best AM. Prevention of child-to-mother transmission of cytomegalovirus among pregnant women. The Journal of pediatrics. 2004 Oct:145(4):485-91 [PubMed PMID: 15480372]
Level 1 (high-level) evidence