Introduction
Vision is the most dominant and highly elaborated sense in humans. More than 50% of the body’s sensory receptors are located in the eyes, and a considerable part of the cerebral cortex is devoted to visual processing. The eyes detect visible light with wavelengths of 400 to 700 nm. Objects appear colored according to the wavelengths of light they reflect. Materials that reflect the full spectrum appear white, and those that absorb all wavelengths appear black. The ciliary muscle is a critical component of the eye that enables clear visualization of objects at varying distances (see Image. Schematic of Eye Anatomy).[1][2][3][4]
Impaired function of the ciliary muscle contributes to accommodative disorders, such as presbyopia. In surgery, the ciliary muscle is important during cataract extraction and intraocular lens implantation, where preserving accommodative ability and preventing damage are critical. Knowledge of the anatomy and functions of this structure helps clinicians recognize abnormalities, plan procedures, and guide treatment to improve visual outcomes.
Structure and Function
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Structure and Function
The middle layer of the eyeball, the vascular tunic, consists of the choroid, ciliary body, and iris. In the anterior region, the choroid transitions into the ciliary body, which extends from the ora serrata—a serrated anterior margin of the retina—to a point just posterior to the corneoscleral junction. The ciliary body forms a circumferential band posterior to the limbus and contains the ciliary muscle and ciliary processes. The anterior portion, the pars plicata, includes the ciliary processes, whereas the posterior portion, the pars plana, is a relatively avascular zone that serves as an optimal site for posterior segment interventions.
A potential supraciliary space, continuous with the suprachoroidal space, separates the ciliary body from the sclera and can act as a pathway for fluid outflow. The dark brown coloration of the ciliary body results from its high melanocyte content. The ciliary processes are inward projections of the ciliary body that contain capillaries responsible for aqueous humor secretion. Zonular fibers extend from the ciliary processes to the lens, functioning as suspensory ligaments that transmit tension generated by the ciliary muscle.
The ciliary muscle is a circular band of smooth muscle with longitudinal, radial, and circular fibers. The longitudinal fibers insert into the scleral spur adjacent to the trabecular meshwork and the Schlemm canal. Through contraction and relaxation, the ciliary muscle modulates zonular fiber tension, thereby altering lens curvature and enabling accommodation for near or distance vision (see Image. Lens Shape Changes in Distant and Near Vision).[5][6][7][8]
Embryology
Eye formation begins at approximately the 3rd week of embryonic development and continues through the 10th week. Both ectodermal and mesodermal tissues contribute to this process. The eye develops from the neuroepithelium, surface ectoderm, and extracellular mesenchyme derived from the neural crest and mesoderm. The ciliary epithelium arises from the neuroepithelium of the optic cup, whereas the ciliary stroma and ciliary muscle are derived from neural crest and mesodermal mesenchyme.
Blood Supply and Lymphatics
Branches of the ophthalmic artery supply the eye, including the central artery of the retina, the short and long posterior ciliary arteries, and the anterior ciliary arteries. Six to 12 short posterior ciliary arteries arise from the ophthalmic artery as it crosses the optic nerve and supply the ciliary processes. Two long posterior ciliary arteries penetrate the posterior sclera near the optic nerve, providing blood to the choroid and ciliary muscle. Seven anterior ciliary arteries supply the conjunctiva, sclera, and rectus muscles. The 6 extrinsic eye muscles—superior rectus, inferior rectus, medial rectus, lateral rectus, superior oblique, and inferior oblique—coordinate ocular movements to align images on the fovea for optimal resolution.
The ciliary muscle also influences aqueous humor outflow. Contraction and relaxation of the muscle's longitudinal fibers alter the pore size of the trabecular meshwork, which regulates drainage into the Schlemm canal. These changes can either facilitate or impede aqueous humor flow.
Nerves
The ciliary muscle is primarily innervated by the parasympathetic division of the autonomic nervous system. Preganglionic parasympathetic neurons arise in the Edinger-Westphal nucleus of the midbrain, and their axons travel in the inferior division of the oculomotor nerve (cranial nerve III). These fibers synapse in the ciliary ganglion, located posterior to the globe between the optic nerve and the lateral rectus muscle. Postganglionic fibers then reach the eye through the short ciliary nerves.
Acetylcholine serves as the principal neurotransmitter at the ciliary muscle, acting on muscarinic receptors, particularly the M3 subtype, to induce smooth muscle contraction. Contraction reduces the diameter of the ciliary body ring, relaxing the zonular fibers (zonules of Zinn). The lens adopts a more spherical shape as zonular tension diminishes, increasing refractive power and permitting accommodation for near vision. This mechanism is essential for tasks such as reading, although accommodative capacity progressively declines with presbyopia.[9][10]
In addition to parasympathetic input, the ciliary muscle receives a minor sympathetic contribution. Postganglionic sympathetic fibers originate in the superior cervical ganglion, travel via the internal carotid plexus, and reach the orbit through the superior orbital fissure alongside the nasociliary branch of the ophthalmic nerve (cranial nerve V1). These fibers pass through the ciliary ganglion without synapsing and enter the globe via both the long and short ciliary nerves (see Images. Right Eye Muscles and Nerves at the Superior Orbital Fissure; Oculomotor Nerve Pathway). Sympathetic neurotransmission is mediated by norepinephrine acting at β2-adrenergic receptors in the ciliary muscle. This input serves a modulatory role, exerting minimal influence on muscle tone and aqueous humor outflow compared with parasympathetic control of accommodation.
Disruption of parasympathetic innervation, such as in oculomotor nerve palsy or treatment with muscarinic receptor antagonists like atropine and cyclopentolate, results in loss of accommodation (cycloplegia). Parasympathomimetic agents, such as pilocarpine, produce ciliary muscle contraction, thereby increasing accommodation and enhancing trabecular outflow, a mechanism used in glaucoma management.
Muscles
The ciliary muscle is composed of 3 distinct groups of smooth muscle fibers: longitudinal, radial, and circular. Parasympathetic innervation via the oculomotor nerve stimulates contraction of all 3 groups, enabling accommodation and enhancing trabecular outflow.
The longitudinal fibers, also termed "meridional fibers" or "Brücke muscle," run parallel to the scleral surface. These fibers extend posteriorly from the scleral spur and corneoscleral junction to insert into the anterior choroid and peripapillary tissue. Contraction of these fibers pulls the ciliary body and choroid anteriorly, exerting traction on the scleral spur. This mechanical action widens the trabecular meshwork and the Schlemm canal, thereby facilitating trabecular outflow. The longitudinal fibers form the largest portion of the ciliary muscle.
The radial fibers, or oblique fibers, lie between the longitudinal and circular layers, running obliquely from the scleral spur toward the lens equator. These fibers function as a transitional layer that coordinates the activity of the outer and inner muscle groups, although their direct contribution to accommodation and aqueous humor regulation remains uncertain. The circular fibers, also known as annular fibers or Müller muscle, are arranged circumferentially around the lens equator in a sphincter-like fashion. Contraction of these fibers decreases the inner diameter of the ciliary body, reducing zonular tension on the lens. The lens then assumes a more convex shape, increasing refractive power for near focus. Anatomical alterations of this component can therefore impair accommodation.[11][12]
Physiologic Variants
The ciliary muscle exhibits variability across populations, age groups, and refractive states, with important implications for accommodation disorders and surgical planning. These variations reflect its adaptive capacity and emphasize the need for individualized approaches to ciliary body-related interventions.
Age-associated changes are the most extensively studied. With advancing age, the ciliary muscle becomes shorter and shifts anteriorly and inward, producing a more compact configuration. Some studies also describe an increase in maximum width, although findings are inconsistent. The longitudinal and reticular portions decrease in area, while the circular portion enlarges.
Histologically, increased extracellular matrix deposition and progressive hyalinization occur between muscle bundles. These changes likely contribute to reduced uveoscleral outflow and to age-related limitations in accommodation. Despite structural remodeling, contractile function is largely preserved. In vivo imaging demonstrates an accommodation-induced ciliary muscle area increase of approximately 30% in individuals who are younger or have no presbyopia, compared with about 25% in people with presbyopia.[13][14][15]
Variations associated with refractive error reveal distinct morphological patterns, particularly in myopic eyes. In vivo imaging demonstrates that myopic eyes typically exhibit a longer ciliary muscle, with regional differences in thickness. The posterior portion is generally thicker in myopes, whereas the apical or anterior portion is similar to or thinner than in emmetropic eyes. Hyperopic eyes tend to display the greatest apical thickness. These differences correlate positively with axial length for both overall muscle length and posterior thickness. Although some studies suggest that morphological alterations and modified contractile behavior of the ciliary muscle may contribute to progressive myopia, no large-scale study has confirmed this association.[16][17][18]
Comparative studies report no consistent, clinically significant ethnic differences in ciliary muscle thickness after adjustment for confounding factors, although at least 1 multicenter imaging study observed a thinner ciliary body in Chinese versus Caucasian eyes. Large in vivo optical coherence tomography datasets also indicate no significant sex-linked differences in ciliary muscle dimensions after multivariable analysis.[19][20]
Surgical Considerations
Iatrogenic injury during anterior segment procedures can disinsert the longitudinal ciliary muscle fibers from the scleral spur, producing a cyclodialysis cleft that forms a direct pathway for aqueous flow from the anterior chamber to the suprachoroidal space. Patients typically present with hypotony, a shallow anterior chamber, and, often, choroidal effusions. Diagnosis is established through gonioscopy and may be supported by ultrasound biomicroscopy or anterior segment OCT. Management strategies range from cycloplegia to laser photocoagulation or surgical closure, depending on the cleft’s size and chronicity.
Entry through the pars plana avoids injury to the ciliary muscle and processes during scleral passes or sclerotomies, such as for vitrectomy ports or sclera-fixated intraocular lens sutures. Ports should generally be placed approximately 3.5 to 4.0 mm posterior to the limbus in phakic eyes and 3.0 to 3.5 mm posterior in pseudophakic or aphakic eyes, adjusted for axial length. Anterior placement increases the risk of ciliary muscle injury, bleeding, ciliary spasm, and possible cyclodialysis, whereas more posterior placement raises the risk of retinal tears.[21][22][23]
The ciliary body is a frequent target in glaucoma surgery for the reduction of IOP. Transscleral cyclophotocoagulation delivers laser energy through the sclera to partially ablate the ciliary processes, thereby decreasing aqueous humor production. Collateral injury to the adjacent ciliary muscle may occur. Surgeons should carefully titrate energy to avoid overtreatment, as excessive destruction can result in hypotony. Micropulse transscleral cyclophotocoagulation delivers laser in intermittent bursts to limit heat diffusion and collateral damage while maintaining the same therapeutic objective.
Two critical danger zones are the 3 and 9 o’clock meridians, which contain the long ciliary nerves and arteries. These regions are generally avoided to reduce the risk of neuropathy, bleeding, and postoperative pain. Special caution should be exercised in areas of thin or compromised scleral tissue and existing filtration blebs, where collateral injury and hypotony are more likely.[24][25]
Another glaucoma-related intervention is endoscopic cycloplasty, indicated for plateau iris or refractory angle-closure mechanisms. An endoscopic diode laser is introduced, often in combination with cataract surgery, to treat the ciliary processes under direct visualization. Confluent laser application induces shrinkage and posterior rotation of the ciliary processes, displacing the peripheral iris away from the trabecular meshwork and widening the anterior chamber angle. This anatomical adjustment can relieve angle crowding and assist in controlling IOP in selected primary angle-closure cases, particularly plateau iris syndrome. Preservation of ciliary muscle integrity is critical throughout these procedures. The surgical goal is to reduce aqueous humor production while maintaining sufficient ciliary tissue to support physiologic function and long-term IOP stability.[26]
Clinical Significance
The eyeball is a complex organ composed of multiple specialized structures essential for vision and ocular movement. Injury to any component can result in discomfort, visual impairment, or, in severe cases, blindness. The eye is also susceptible to infection and systemic disease manifestations. All health practitioners should be able to identify and describe the eye's anatomical features accurately. The ciliary muscle is a key structure that regulates lens shape, enabling accommodation for near and distance vision.[27][28]
Glaucoma is an optic neuropathy characterized by progressive damage to the optic nerve, potentially leading to irreversible vision loss. Ocular hypertension is a common, but not universal, risk factor for optic nerve damage. Aqueous humor continuously flows from the anterior chamber and drains at the iridocorneal angle to maintain IOP. In open-angle glaucoma, the drainage angle remains anatomically open, but resistance within the trabecular meshwork impedes outflow, resulting in increased pressure.
Increased IOP does not invariably cause glaucoma, nor is it required for disease development. Systemic vascular factors, including high blood pressure, can also influence optic nerve perfusion and contribute to glaucoma pathogenesis. The population at greatest risk for glaucoma includes African Americans older than 40, all adults older than 60, and people with a family history of the disease.
In angle-closure glaucoma, the iris apposes the trabecular meshwork, obstructing aqueous humor outflow from the anterior chamber and causing a rapid IOP rise. Both open-angle and angle-closure glaucoma may be treated with muscarinic receptor agonists, such as pilocarpine, which induce ciliary muscle contraction and widen the trabecular meshwork. Once the meshwork pores open, aqueous humor drains through the Schlemm canal, resulting in IOP reduction. Contraction of the ciliary muscle provides therapeutic benefit in both open-angle and angle-closure glaucoma, making it a key pharmacologic target.
Media
(Click Image to Enlarge)
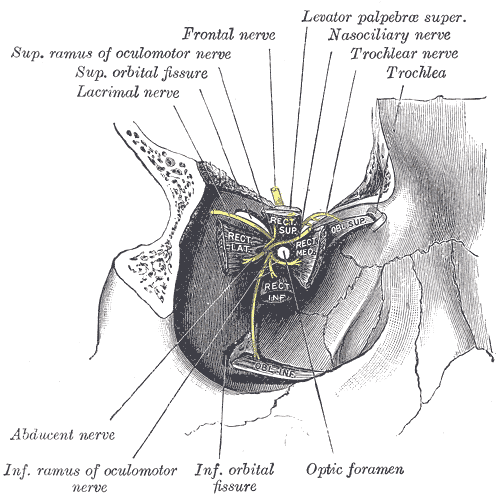
Right Eye Muscles and Nerves at the Superior Orbital Fissure. Dissection illustrating the origins of the right eye's extraocular muscles and the cranial nerves entering the orbit through the superior orbital fissure, including the superior and inferior rami of the oculomotor nerve, lacrimal nerve, frontal nerve, nasociliary nerve, trochlear nerve, abducens nerve, and related anatomical structures such as the optic foramen and inferior orbital fissure.
Henry Vandyke Carter, Public Domain, via Wikimedia Commons
(Click Image to Enlarge)
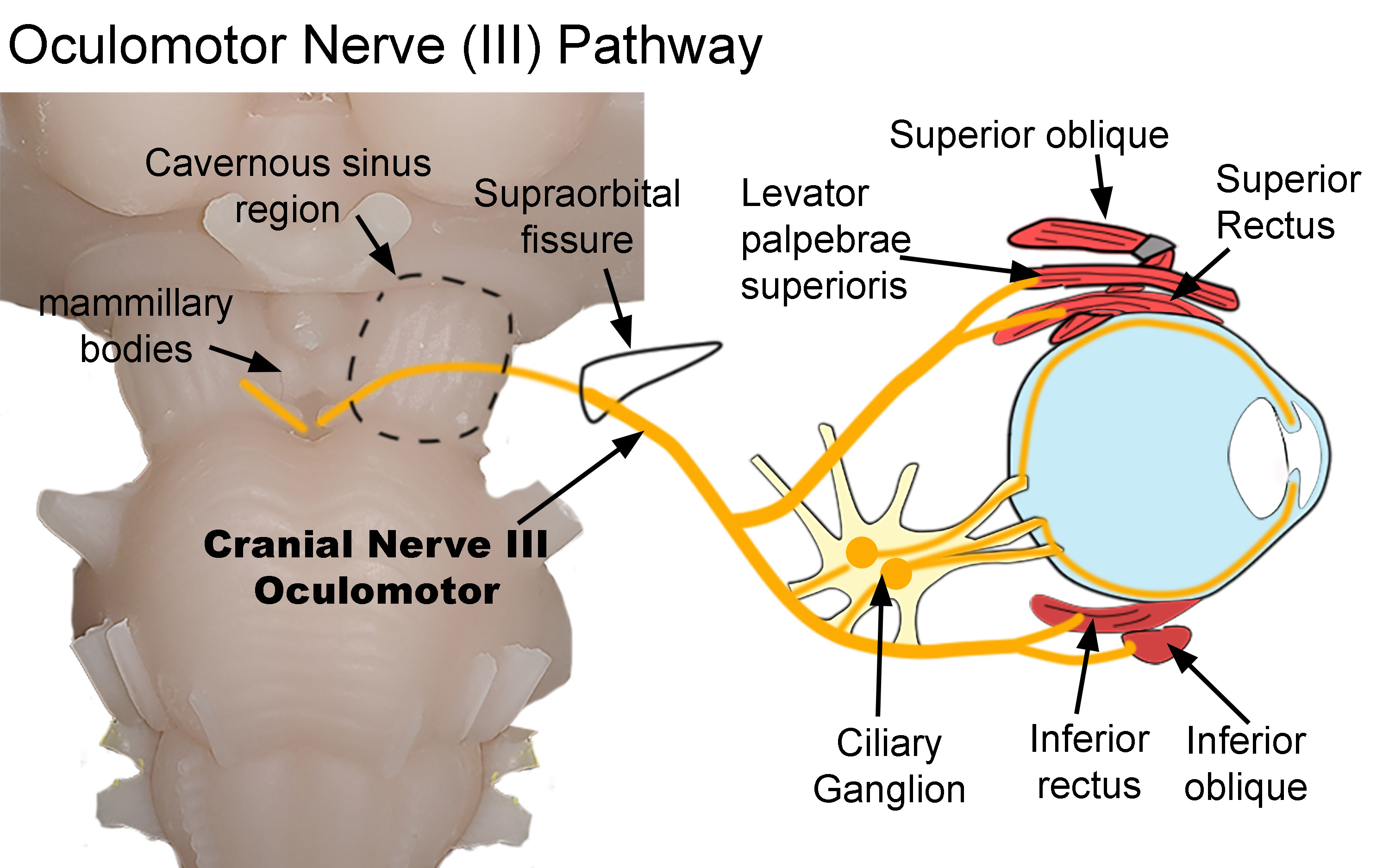
Oculomotor Nerve Pathway. The diagram shows the pathway of the oculomotor nerve as it exits the brainstem and terminates within the orbit. After exiting the brainstem, the nerve traverses both the cavernous sinus (dotted line) and supraorbital fissure (black line) before entering the orbit. Parasympathetic nerves synapse within the ciliary ganglion. Postganglionic nerves then innervate the sphincter papillae and ciliary muscles. Somatic nerves innervate the superior oblique, levator palpebrae superioris, superior and inferior recti, and inferior oblique.
Created by Diana Peterson, Ph.D. for use with StatPearls.
(Click Image to Enlarge)
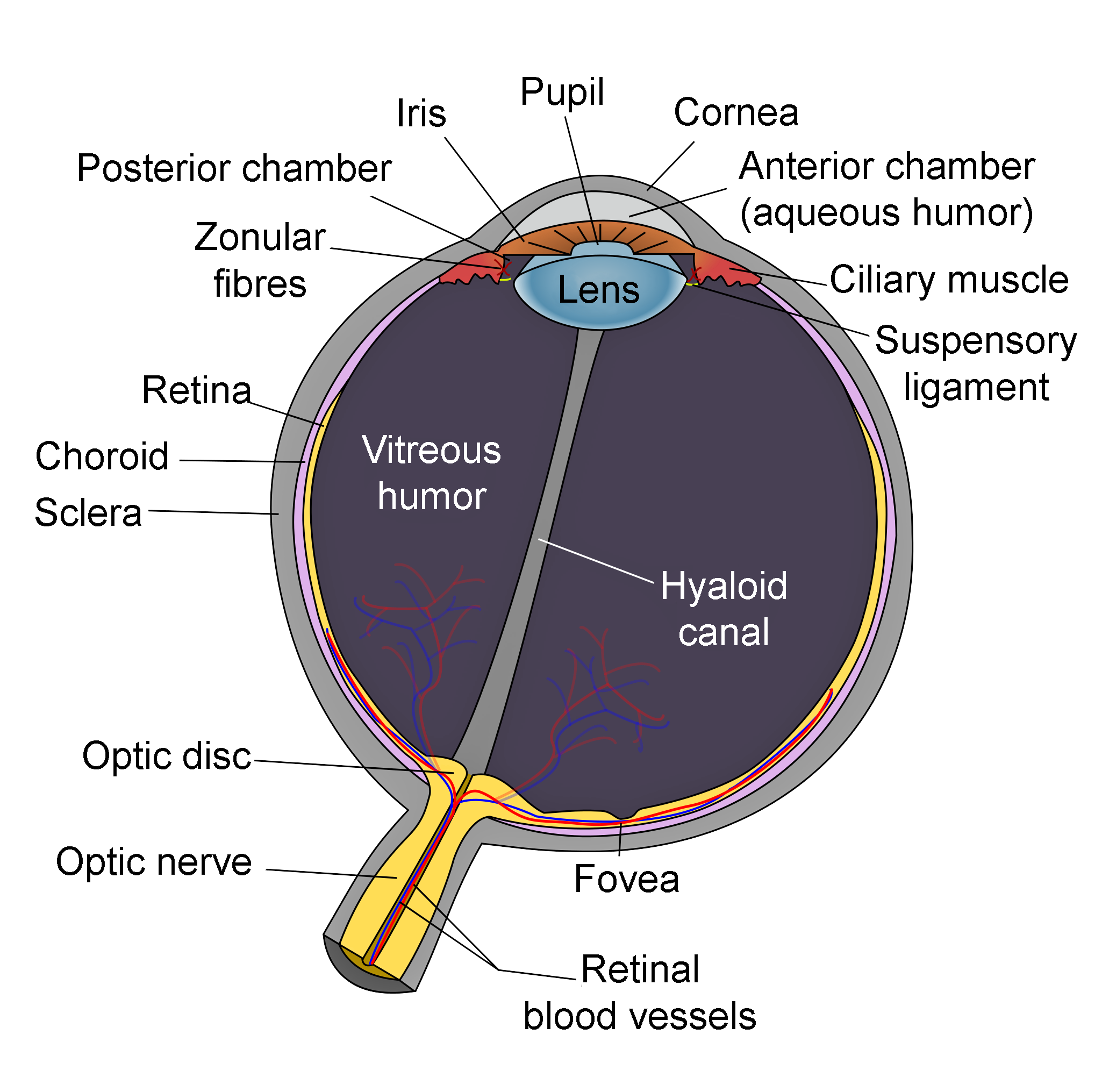
Schematic of Eye Anatomy. This image illustrates the anatomic relationships between the optic disc, optic nerve, fovea, sclera, choroid, vitreous humor, hyaloid canal, retina, retinal blood vessels, zonular fibers, iris, pupil, cornea, anterior chamber (aqueous humor), lens, posterior chamber, ciliary muscle, and suspensory ligament.
Contributed by R.H. Castilhos and Jordi March i Nogué (CC by SA-3.0 https://creativecommons.org/licenses/by-sa/3.0/deed.en)
(Click Image to Enlarge)
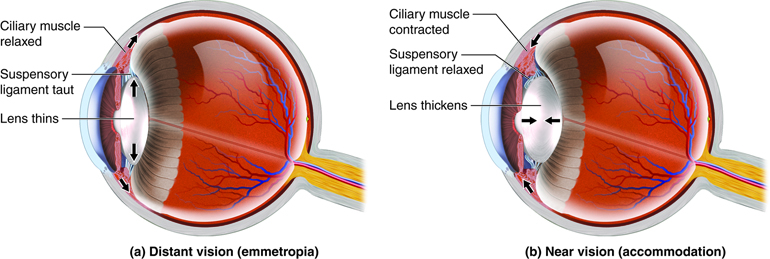
Lens Shape Changes in Distant and Near Vision. Illustration showing how the lens alters its shape through ciliary muscle and suspensory ligament tension changes during distant vision (emmetropia) with a relaxed ciliary muscle and taut ligaments, and near vision (accommodation) with a contracted ciliary muscle and relaxed ligaments, allowing the lens to thicken for focusing on close objects.
References
Shumway CL, Motlagh M, Wade M. Anatomy, Head and Neck, Orbit Bones. StatPearls. 2025 Jan:(): [PubMed PMID: 30285385]
Bird B, Stawicki SP. Anatomy, Head and Neck, Ophthalmic Arteries. StatPearls. 2025 Jan:(): [PubMed PMID: 29493942]
Modi P, Arsiwalla T. Cranial Nerve III Palsy. StatPearls. 2025 Jan:(): [PubMed PMID: 30252368]
Mao Y, Bai HX, Li B, Xu XL, Gao F, Zhang ZB, Jonas JB. Dimensions of the ciliary muscles of Brücke, Müller and Iwanoff and their associations with axial length and glaucoma. Graefe's archive for clinical and experimental ophthalmology = Albrecht von Graefes Archiv fur klinische und experimentelle Ophthalmologie. 2018 Nov:256(11):2165-2171. doi: 10.1007/s00417-018-4085-7. Epub 2018 Aug 15 [PubMed PMID: 30112579]
Liang YL, Jia SB. Clinical application of accommodating intraocular lens. International journal of ophthalmology. 2018:11(6):1028-1037. doi: 10.18240/ijo.2018.06.22. Epub 2018 Jun 18 [PubMed PMID: 29977819]
Vargas D, Castro C. Pupillometry in Chagas disease. Arquivos brasileiros de oftalmologia. 2018 Jun:81(3):195-201. doi: 10.5935/0004-2749.20180041. Epub [PubMed PMID: 29924199]
Khan Z, Bollu PC. Horner Syndrome. StatPearls. 2025 Jan:(): [PubMed PMID: 29763176]
Mohamed Farouk M, Naito T, Shinomiya K, Mitamura Y. Observation of Ciliary Body Changes during Accommodation Using Anterior OCT. The journal of medical investigation : JMI. 2018:65(1.2):60-63. doi: 10.2152/jmi.65.60. Epub [PubMed PMID: 29593195]
Bhardwaj N, Joshi A. Neuroanatomy, Ciliary Ganglion. StatPearls. 2025 Jan:(): [PubMed PMID: 31985975]
McDougal DH, Gamlin PD. Autonomic control of the eye. Comprehensive Physiology. 2015 Jan:5(1):439-73. doi: 10.1002/cphy.c140014. Epub [PubMed PMID: 25589275]
Level 3 (low-level) evidenceTamm ER, Lütjen-Drecoll E. Ciliary body. Microscopy research and technique. 1996 Apr 1:33(5):390-439 [PubMed PMID: 8695897]
Zuo H, Cheng H, Lin M, Gao X, Xiang Y, Zhang T, Gao N, Du M, Chen Y, Zheng S, Huang R, Wan W, Hu K. The effect of aging on the ciliary muscle and its potential relationship with presbyopia: a literature review. PeerJ. 2024:12():e18437. doi: 10.7717/peerj.18437. Epub 2024 Dec 24 [PubMed PMID: 39735562]
Tamm S, Tamm E, Rohen JW. Age-related changes of the human ciliary muscle. A quantitative morphometric study. Mechanisms of ageing and development. 1992 Feb:62(2):209-21 [PubMed PMID: 1569790]
Sheppard AL, Davies LN. The effect of ageing on in vivo human ciliary muscle morphology and contractility. Investigative ophthalmology & visual science. 2011 Mar 28:52(3):1809-16. doi: 10.1167/iovs.10-6447. Epub 2011 Mar 28 [PubMed PMID: 21071738]
Domínguez-Vicent A, Monsálvez-Romín D, Esteve-Taboada JJ, Montés-Micó R, Ferrer-Blasco T. Effect of age in the ciliary muscle during accommodation: Sectorial analysis. Journal of optometry. 2019 Jan-Mar:12(1):14-21. doi: 10.1016/j.optom.2018.01.001. Epub 2018 Apr 4 [PubMed PMID: 29627301]
Wagner S, Zrenner E, Strasser T. Emmetropes and myopes differ little in their accommodation dynamics but strongly in their ciliary muscle morphology. Vision research. 2019 Oct:163():42-51. doi: 10.1016/j.visres.2019.08.002. Epub 2019 Aug 28 [PubMed PMID: 31401218]
Kwok HY, Kwok HY, Ng TN, Leung TW, Kang BS, Kee CS. Characteristics of ciliary muscle profile in high myopes measured by swept-source anterior segment optical coherence tomography. PloS one. 2023:18(11):e0289135. doi: 10.1371/journal.pone.0289135. Epub 2023 Nov 30 [PubMed PMID: 38033137]
Kaphle D, Schmid KL, Davies LN, Suheimat M, Atchison DA. Ciliary Muscle Dimension Changes With Accommodation Vary in Myopia and Emmetropia. Investigative ophthalmology & visual science. 2022 Jun 1:63(6):24. doi: 10.1167/iovs.63.6.24. Epub [PubMed PMID: 35749128]
He N, Wu L, Qi M, He M, Lin S, Wang X, Yang F, Fan X. Comparison of Ciliary Body Anatomy between American Caucasians and Ethnic Chinese Using Ultrasound Biomicroscopy. Current eye research. 2016 Apr:41(4):485-91. doi: 10.3109/02713683.2015.1024869. Epub 2015 May 5 [PubMed PMID: 25942335]
Level 2 (mid-level) evidenceFernández-Vigo JI, Shi H, Kudsieh B, Arriola-Villalobos P, De-Pablo Gómez-de-Liaño L, García-Feijóo J, Fernández-Vigo JÁ. Ciliary muscle dimensions by swept-source optical coherence tomography and correlation study in a large population. Acta ophthalmologica. 2020 Jun:98(4):e487-e494. doi: 10.1111/aos.14304. Epub 2019 Nov 26 [PubMed PMID: 31773907]
Ioannidis AS,Barton K, Cyclodialysis cleft: causes and repair. Current opinion in ophthalmology. 2010 Mar; [PubMed PMID: 20051856]
Level 3 (low-level) evidenceGonzález-Martín-Moro J, Contreras-Martín I, Muñoz-Negrete FJ, Gómez-Sanz F, Zarallo-Gallardo J. Cyclodialysis: an update. International ophthalmology. 2017 Apr:37(2):441-457. doi: 10.1007/s10792-016-0282-8. Epub 2016 Jul 8 [PubMed PMID: 27392912]
Dogramaci M, Lee EJ, Williamson TH. The incidence and the risk factors for iatrogenic retinal breaks during pars plana vitrectomy. Eye (London, England). 2012 May:26(5):718-22. doi: 10.1038/eye.2012.18. Epub 2012 Feb 17 [PubMed PMID: 22344186]
Bloom PA,Dharmaraj S, Endoscopic and transscleral cyclophotocoagulation. The British journal of ophthalmology. 2006 Jun; [PubMed PMID: 16714260]
Dastiridou AI, Katsanos A, Denis P, Francis BA, Mikropoulos DG, Teus MA, Konstas AG. Cyclodestructive Procedures in Glaucoma: A Review of Current and Emerging Options. Advances in therapy. 2018 Dec:35(12):2103-2127. doi: 10.1007/s12325-018-0837-3. Epub 2018 Nov 17 [PubMed PMID: 30448885]
Level 3 (low-level) evidenceFrancis BA,Pouw A,Jenkins D,Babic K,Vakili G,Tan J,Chopra V,Green RL, Endoscopic Cycloplasty (ECPL) and Lens Extraction in the Treatment of Severe Plateau Iris Syndrome. Journal of glaucoma. 2016 Mar; [PubMed PMID: 25794042]
Park HK, Rha HK, Lee KJ, Chough CK, Joo W. Microsurgical Anatomy of the Oculomotor Nerve. Clinical anatomy (New York, N.Y.). 2017 Jan:30(1):21-31. doi: 10.1002/ca.22811. Epub [PubMed PMID: 27859787]
Kaser-Eichberger A, Schrödl F, Trost A, Strohmaier C, Bogner B, Runge C, Motloch K, Bruckner D, Laimer M, Schlereth SL, Heindl LM, Reitsamer HA. Topography of Lymphatic Markers in Human Iris and Ciliary Body. Investigative ophthalmology & visual science. 2015 Jul:56(8):4943-53. doi: 10.1167/iovs.15-16573. Epub [PubMed PMID: 26225635]