Introduction
Pediatric brain tumors are the most common type of solid childhood cancer and are only second to leukemia as a cause of pediatric malignancies. These tumors can be classified further based on the age at diagnosis. Tumors are often referred to as congenital brain tumors if diagnosed antenatally or within the first 60 days of life [1], or tumors of infancy if diagnosed younger than 1 year. Brain tumors in this population of patients often present in the supratentorial compartment, as compared to tumors in older children, which are most often located infratentorially.[2][3] The prognosis of brain tumors in these young children depends primarily on tumor histology and extent of resection.
Brain tumors in neonates and infants pose difficult treatment challenges due to the small size of patients and limited blood volume, as well as limited adjuvant therapies due to the risk of treatment-related morbidities. With the advances in imaging techniques, molecular biology, and genetics, neonatal and infant brain tumors are increasingly being diagnosed early in the disease course, subgrouped, and treated with more targeted strategies.[4] Despite these advances, the prognosis remains poor in a large subset of these patients.
The most common neonatal histological brain tumor subtypes include teratoma, choroid plexus tumors, desmoplastic infantile tumors (DIA/DIG), glioblastoma multiforme (GBM), and atypical teratoid/rhabdoid tumors (ATRT). Please see StatPearls' companion resources, "Medulloblastoma" and "Ependymoma", for further information on these conditions.
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
Risk factors for developing brain tumors have been well recognized, including exposure to ionizing radiation, several birth defects, maternal consumption of N-nitroso compounds, and the cancer predisposition syndromes.[5][6] One well-known example is Li-Fraumeni syndrome, which is associated with an increased risk of choroid plexus carcinomas. Some other predisposition syndromes include tuberous sclerosis, neurofibromatosis, and rhabdoid predisposition syndrome.[7] The parental age at birth may also play a role, with studies suggesting advanced paternal age may be a risk factor for congenital cancers [8][9][10], although this is not definitive. Congenital anomalies, eg, oral clefts, have been associated with the development of childhood brain tumors, including medulloblastoma and germ cell tumors.[11][12]
Epidemiology
Neonatal brain tumors are rare and account for only 0.5% to 1.9% of all pediatric brain tumors.[13][14] The incidence ranges from 1.15 to 5.14 cases per 100,000 children, with the highest rates reported in the United States.[8] The incidence of congenital brain tumors ranges from approximately 0.3 to 2.9 cases per 100,000 live births worldwide.[15][16] Their prognosis and survival rates depend on multiple factors, including the histological subtype and location. For the neonatal cohort, the most common type is teratoma (26.6% to 48%), followed by astrocytoma (7.4% to 28.8%), choroid plexus papilloma (3.7% to 13.2%), embryonal tumor (3% to 13%), craniopharyngioma (5.6% to 6.8%), and ependymoma (4.4%).[17]
Histopathology
The histopathology and molecular basis of neonatal brain tumors vary tremendously depending on the subtype.
Teratomas
Teratomas contain tissue derived from all 3 germ layers.[18] They can be classified as mature or immature. Mature teratomas contain well-differentiated cells, while immature teratomas contain tissue that is poorly differentiated, resembling fetal tissue.[19] In both subtypes, ectodermal components are commonly seen, including skin and teeth. Mesodermal components may include cartilage, bone, and fat. Immature teratomas may also contain malignant cells, often in the form of an undifferentiated sarcoma.[20]
Gliomas and Mixed Neuroglial Tumors
Gliomas arise from glial precursor cells of the brain and spinal cord. Astrocytic tumors comprise a large group of neoplasms with various clinicopathologic and histological subtypes and grades, ranging from WHO grades 1 to 4.[21] Congenital GBM is a very rare brain tumor (WHO grade 4). This lesion shows clinical and molecular characteristics that are different from its pediatric or adult counterparts and has a better prognosis than the pediatric and adult GBMs.[22]
Mutations in several genes have been identified and are unique to infantile gliomas, including MET, ALK, and NTRK.[23] Desmoplastic infantile astrocytoma and ganglioglioma (DIA/DIG) are WHO grade I tumors.[24] DIG demonstrates neuronal elements, whereas DIAs only have astrocytic components.[12] Both of these tumors exhibit desmoplastic features, characterized by a stroma rich in collagen and reticulin fibers.[25][26]
Embryonal Tumors
Atypical teratoid/rhabdoid tumors (ATRT) are embryonal tumors characterized by sheets of cells with round, eccentric nuclei, necrosis, and frequent mitoses. Areas composed of small, round, blue cells may also be noted.[27] These tumors are characterized by inactivation of the SMARCB1 and SMARCA4 genes. They have been subtyped into 3 subgroups: ATRT-MYC, ATRT-SHH, and ATRT-TYR. Among these, the ATRT-TYR group has superior overall survival, whereas the ATRT-SHH group has a higher likelihood of metastatic disease and poorer outcomes.[28]
Choroid Plexus Tumors
Choroid plexus tumors are classified into 3 types, ranging from WHO grade 1 to 3. Choroid plexus papillomas are WHO grade 1 tumors that are composed of a fibrovascular core surrounded by cuboidal and columnar epithelial cells, similar to the choroid plexus. Atypical choroid plexus papillomas are WHO grade 2 tumors that demonstrate foci of hypercellular epithelium with varying degrees of atypia and pleomorphism. There are often areas of focal necrosis; however, no direct invasion of the surrounding brain occurs. Lastly, choroid plexus carcinomas are WHO grade 3 tumors that exhibit hypercellularity, increased mitotic activity, pleomorphic nuclei, and direct invasion of the surrounding brain parenchyma.[29]
History and Physical
When diagnosed prenatally, the age of gestation may help predict tumor pathology. If detected before 22 weeks, tumors may be teratomas or hamartomas, and if detected between 22 and 32 weeks gestation, germinal tumors are more likely. Astrocytic tumors are more likely to be detected after 32 weeks gestation.[12][30] Polyhydramnios is a common finding when diagnosed prenatally due to the dysfunction of the hypothalamus as well as fetal distress, and complications related to craniopelvic disproportion.[31][32] Stillborn births are seen in roughly a third of patients with congenital tumors, or patients may succumb after only minutes.[33][34][19][1]
Neonates and infants often present with macrocephaly due to the size of the tumor itself, as in cases of desmoplastic infantile gangliogliomas, or from coexisting hydrocephalus.[7][35] Due to large tumor sizes, intra-tumoral hemorrhage can occur, leading to further increases in intracranial pressure.[36] Common signs associated with increased intracranial pressure in this age group include bulging or tense fontanelles, sutural diastasis, irritability, vomiting, and a preference for downgaze. Seizures can also be a presenting feature.[37] More rare findings include ophthalmological manifestations such as proptosis and exophthalmos, as well as cranium rupture due to excessive pressure.[38][39]
Evaluation
Congenital brain tumors can occasionally be discovered during the second or third-trimester ultrasound. Antenatal ultrasound may show an intracranial mass, hydrocephalus, or polyhydramnios, among other signs.[31][40] Fetal MRI is increasingly used to confirm findings visualized on ultrasound.[41][35] The imaging characteristics of individual tumors vary depending on their histology.
Teratomas
Teratomas are typically visualized as a mixed echogenic and cystic intracranial mass on antenatal ultrasound during the second and third trimesters.[42] Magnetic resonance imaging (MRI) typically shows heterogeneous signal intensity on T1 and T2-weighted sequences, with the presence of a T1 hyperintense signal due to fat content is almost pathognomonic. Lesions are often contrast-enhancing and frequently contain cystic components.[13] Although computed tomography (CT) is generally avoided in patients this young, teratomas appear as a mixed-density lesion, with frequent coarse calcifications visible. Teratomas may grow significantly large and extend into extracranial compartments, eg, the orbit or oral cavity, and have even been reported to cause skull rupture.[43][39]
Choroid Plexus Tumors
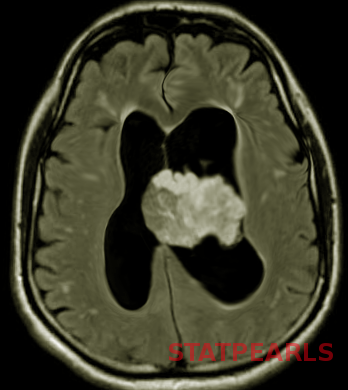
Choroid plexus tumors occur intraventricularly, primarily within the lateral ventricles, although they can occur within the third or fourth ventricles. Hydrocephalus is common with these tumors due to obstruction of the foramen of Monro, but also from cerebrospinal fluid (CSF) hypersecretion from the tumor itself. Choroid plexus tumors are most often isointense on T1-weighted sequences and hyperintense on T2-weighted sequences and are frequently described as having a “frond-like” appearance (see Image. Neonatal Brain Tumors). They are highly vascular tumors and therefore have avid contrast enhancement.[13]
Gliomas and Mixed Neuroglial Tumors
Neuronal and mixed neuronal-glial tumors, eg, DIG and DIA, are often very large at diagnosis and affect multiple lobes. These tumors have a large cystic component and usually involve the leptomeninges and dura.[37] Typical imaging characteristics include hypointense signal within the cystic components and isointense signal of the solid components on T1-weighted sequences. On T2, the cystic portions are hyperintense, and the solid portion is heterogeneous.[13] The solid portion of the tumor usually demonstrates substantial enhancement.[25]
Glioblastoma Multiforme
GBM is a high-grade astrocytoma, typically presenting as a large, ill-defined, intracranial mass occupying most of 1 hemisphere or spreading through the corpus callosum to the contralateral hemisphere. It appears as an echogenic heterogeneous mass on ultrasonography and commonly shows intralesional hemorrhage and necrosis.[44] Diffusion restriction in MRI is a key feature that reflects the high-grade nature of the lesion and can help distinguish GBMs from DIG/DIAs.[45][44]
Embryonal Tumors
Embryonal tumors in this age group include ATRT, medulloblastoma, and embryonal tumor with multilayered rosettes (ETMR), C19MC-altered. MRI characteristics of ATRT include a heterogeneous mass that may be partially cystic and demonstrate areas of necrosis, intralesional hemorrhage, or mineralization. On both T1- and T2-weighted images, they are typically isointense to hypointense with heterogeneous contrast enhancement. Due to the high cellularity, the solid portion of the tumor often shows restricted diffusion.[13] Because of the propensity for drop metastasis, diagnostic evaluation of the entire spinal axis should ideally occur preoperatively to help distinguish between postoperative blood products within the CSF.
Treatment / Management
If diagnosed prenatally, corticosteroid treatment to accelerate fetal lung maturation may be appropriate. Furthermore, transabdominal aspiration for hydrocephalus may be used in extreme circumstances.[30] The principal treatment for neonatal tumors is surgical resection with the goal of gross total resection; however, due to the patient’s size, tumor vascularity, and the concern for intraoperative blood loss, subtotal resections may be advised.[46](B2)
A retrospective cohort study assessing outcomes and overall survival suggested delaying surgery until patients are at least 1.3 months of age and have a body weight of at least 5.2 kg.[47] The extent of the congenital brain tumors and the general condition of the patient are important determinants of surgical outcome.[35] Adjuvant therapies are sometimes used in neonates and are primarily used on a case-by-case basis. Craniospinal radiotherapy can result in significant developmental impairments and is generally avoided during the first 3 years of life.[48] (B3)
Differential Diagnosis
Abscess
Neonatal brain abscess could occur as a complication of bacterial meningitis in 1.3% to 4.0% neonates.[49] Several congenital CNS infections like TORCH (toxoplasmosis, rubella, cytomegalovirus, and herpes simplex) infections may mimic metastatic disease from extracranial neoplasms in children.
Intracranial Hemorrhage
Variable stages of evolving hematoma may mimic tumors on imaging, especially in the subacute and chronic phases, which exhibit central clot retraction and peripheral enhancement, with or without surrounding edema, on CT and MRI. MRI sequences, eg, SWI for blood products, DWI, and magnetic resonance spectroscopy, could aid in differentiation, as well as provide follow-up images. If an underlying vascular malformation or vascular lesion is suspected, then a dedicated MRI angiography or CT angiogram can be performed.[50]
Ischemic Stroke
Ischemic arterial or venous stroke may mimic a tumor on imaging. A diagnostic dilemma typically arises when imaging reveals mass effects or irregular contrast enhancements; advanced MRI sequences, eg, DWI and perfusion MRI, can provide further insight. As a general rule, if the edema shows restricted diffusion, then tumoral edema is the culprit. However, clinical history and knowledge of arterial and venous territories are of paramount importance for an accurate diagnosis.
Neurocutaneous Syndromes
Neurofibromatosis (NF), tuberous sclerosis, Sturge-Weber syndrome, Von Hippel-Lindau (VHL) syndrome, and hereditary telangiectasia are well-known syndromes with central nervous system (CNS) manifestations that are non-neoplastic. These syndromes are known to predispose to specific types of brain tumors. For example, NF-1 is associated with low-grade gliomas. Tuberous sclerosis may be associated with SEGA (subependymal giant cell astrocytomas), and hemangioblastomas are seen in VHL. Physicians may find it challenging to differentiate between the non-neoplastic NF-related myelin vacuolation spots and low-grade glioma in settings of NF-1. Cortical and subcortical T2 hyperintense tubers in tuberous sclerosis may mimic gliomas.
Medical Oncology
While surgery is the mainstay of treatment for many of the neonatal brain tumors, immunotherapy-based approaches are emerging as alternatives to standard chemotherapies. An in-depth discussion of these therapies, utilizing vaccines and monoclonal antibodies, has been presented in various reviews.[51]
Prognosis
Congenital brain tumors have a poor prognosis, with an overall survival ranging from 30% to 78%.[52][3][53][54][55] Patients who survive are often left with numerous sequelae of the tumor itself and treatment-related morbidities, eg, neurological deficits, epilepsy, endocrinopathies, and cognitive deficits.[56][57][58][59][60] In a retrospective cohort of 70 patients, Isikay et al reported an overall survival rate of 78% and 63% at 5 and 10 years, respectively. They reported that 55.5% of survivors had neurological sequelae. Several factors, eg, tumor grade, preoperative hydrocephalus, larger tumor size, and ventriculoperitoneal shunt placement, were associated with lower survival.[52] Younger age and the presence of leptomeningeal spread at diagnosis are also associated with increased mortality.[47]
In a retrospective cohort study on patients who developed ATRT in the first year of life, patients younger than 6 months had worse outcomes even when receiving active therapies.[61] Other studies have found that patients younger than 1 year with the presence of leptomeningeal disease have worse outcomes.[62][63] A recent meta-analysis of patients with ATRT found significantly worse outcomes in patients with spinal ATRT, while the extent of resection and adjuvant therapies were associated with increased survival.[64]
A meta-analysis of patients with DIG/DIA reported that gross total resection was predictive of overall survival; however, the presence of leptomeningeal spread at diagnosis was associated with a higher mortality.[65] Another retrospective study evaluating outcomes of children younger than 3 years old reported several factors associated with lower survival rates, including embryonal tumors, leptomeningeal disease, subtotal resection, tumor recurrence, and age younger than 1 year.[55]
Complications
Complications of surgery in this age group include extensive blood loss, neurological deficits, hydrocephalus, endocrinopathies, wound breakdown, and seizures, among others.
Consultations
Interprofessional consultations in the management of neonatal brain tumors may include:
- Neurosurgery
- Medical oncology
- Radiation oncology
- Neuroradiology
- Neuropathology
- Genetics
- Physical medicine and rehabilitation
- Pediatricians
- Physical, occupational, and speech therapies
Deterrence and Patient Education
An interprofessional approach is instrumental in the successful management of brain tumors, especially those that occur in infants and neonates. Full and detailed education of families, as well as their involvement in the decision-making process, is vital. The treatment protocols are typically discussed at interprofessional meetings to explore the best available treatment options and the expected prognosis for the patient. Genetic testing and profiling are offered on certain occasions to help families with prognosis and future family planning.
Enhancing Healthcare Team Outcomes
The management of neonatal brain tumors demands a highly coordinated interprofessional approach, with each healthcare professional contributing specialized knowledge and skills to ensure optimal patient outcomes and safety. Physicians, particularly pediatric neurosurgeons, neurologists, neonatologists, and radiologists, must collaborate closely to achieve accurate diagnosis, staging, and surgical planning. Early detection of brain tumors and prompt referral to pediatric neurosurgery and pediatric neuro-oncology teams is vital. This usually starts with maternal-fetal medicine specialists, pediatricians, family physicians, or emergency physicians. The optimal care of patients with brain tumors occurs through an interprofessional collaboration that includes neurosurgery, oncology, diagnostic imaging, pathology, radiation oncology, nursing, rehabilitation, and social work.
Early and precise interpretation of imaging studies by radiologists, along with timely communication with the surgical team, is critical to guiding decisions about resectability and potential intraoperative risks. Neonatologists play a key role in stabilizing patients preoperatively and postoperatively, managing ventilation, intracranial pressure, and fluid balance, while also monitoring for signs of neurological decline. Advanced practitioners, such as pediatric nurse practitioners and physician assistants, support continuity of care by synthesizing complex clinical data, facilitating coordination between subspecialties, and helping families navigate the diagnostic and treatment process.
Nurses provide continuous bedside care and are essential in monitoring vital signs, recognizing early signs of neurologic deterioration, and supporting both the infant and family during hospitalization. Pharmacists contribute by evaluating drug dosing, managing perioperative medications, and ensuring the safe use of supportive therapies, particularly in contexts where neonatal pharmacologic data are limited. Interprofessional communication is crucial when discussing the timing and extent of surgical intervention, potential use of adjuvant therapy, and post-discharge care planning. Social workers, palliative care teams, and genetic counselors also contribute by addressing psychosocial needs, supporting informed decision-making, and identifying hereditary cancer syndromes. Through deliberate care coordination, shared decision-making, and unified communication strategies, the care team can reduce errors, enhance patient safety, and provide compassionate, individualized care for infants and their families navigating these life-threatening diagnoses.
Media
(Click Image to Enlarge)
References
Wakai S, Arai T, Nagai M. Congenital brain tumors. Surgical neurology. 1984 Jun:21(6):597-609 [PubMed PMID: 6372141]
Toescu SM, James G, Phipps K, Jeelani O, Thompson D, Hayward R, Aquilina K. Intracranial Neoplasms in the First Year of Life: Results of a Third Cohort of Patients From a Single Institution. Neurosurgery. 2019 Mar 1:84(3):636-646. doi: 10.1093/neuros/nyy081. Epub [PubMed PMID: 29617945]
Rivera-Luna R, Medina-Sanson A, Leal-Leal C, Pantoja-Guillen F, Zapata-Tarrés M, Cardenas-Cardos R, Barrera-Gómez R, Rueda-Franco F. Brain tumors in children under 1 year of age: emphasis on the relationship of prognostic factors. Child's nervous system : ChNS : official journal of the International Society for Pediatric Neurosurgery. 2003 Jun:19(5-6):311-4 [PubMed PMID: 12732940]
Ostrom QT, de Blank PM, Kruchko C, Petersen CM, Liao P, Finlay JL, Stearns DS, Wolff JE, Wolinsky Y, Letterio JJ, Barnholtz-Sloan JS. Alex's Lemonade Stand Foundation Infant and Childhood Primary Brain and Central Nervous System Tumors Diagnosed in the United States in 2007-2011. Neuro-oncology. 2015 Jan:16 Suppl 10(Suppl 10):x1-x36. doi: 10.1093/neuonc/nou327. Epub [PubMed PMID: 25542864]
Level 3 (low-level) evidenceAl Shoufy M, Kafa G, Ibrahim B, Ibrahem H, Dakour A, Haidar A, Alshehabi Z. Exploring neonatal brain tumors: a narrative review about epidemiology, classification, and management. Annals of medicine and surgery (2012). 2025 May:87(5):2838-2846. doi: 10.1097/MS9.0000000000003229. Epub 2025 Apr 2 [PubMed PMID: 40337379]
Level 3 (low-level) evidenceYang W, Cai Y, Chen J, Yang P, Ying Z, Liang Y, Ling M, Zhu K, Sun H, Ji Y, Peng X, Zhang N, Ma W, Ge M. Epidemiological characteristics, clinical presentations, and prognoses of pediatric brain tumors: Experiences of national center for children's health. Frontiers in oncology. 2023:13():1067858. doi: 10.3389/fonc.2023.1067858. Epub 2023 Jan 27 [PubMed PMID: 36776329]
Level 2 (mid-level) evidenceToniutti M, Sasso AL, Carai A, Colafati GS, Piccirilli E, Del Baldo G, Mastronuzzi A. Central nervous system tumours in neonates: what should the neonatologist know? European journal of pediatrics. 2024 Apr:183(4):1485-1497. doi: 10.1007/s00431-023-05404-3. Epub 2024 Jan 11 [PubMed PMID: 38206395]
Johnson KJ, Cullen J, Barnholtz-Sloan JS, Ostrom QT, Langer CE, Turner MC, McKean-Cowdin R, Fisher JL, Lupo PJ, Partap S, Schwartzbaum JA, Scheurer ME. Childhood brain tumor epidemiology: a brain tumor epidemiology consortium review. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2014 Dec:23(12):2716-36. doi: 10.1158/1055-9965.EPI-14-0207. Epub 2014 Sep 5 [PubMed PMID: 25192704]
Yip BH, Pawitan Y, Czene K. Parental age and risk of childhood cancers: a population-based cohort study from Sweden. International journal of epidemiology. 2006 Dec:35(6):1495-503 [PubMed PMID: 17008361]
Hemminki K, Kyyrönen P, Vaittinen P. Parental age as a risk factor of childhood leukemia and brain cancer in offspring. Epidemiology (Cambridge, Mass.). 1999 May:10(3):271-5 [PubMed PMID: 10230837]
Hoang TT, Schraw JM, Shumate C, Desrosiers TA, Nembhard WN, Yazdy M, Nestoridi E, Janitz AE, Kirby RS, Salemi JL, Tanner JP, Chambers TM, Taylor MD, Huff CD, Plon SE, Lupo PJ, Scheurer ME. Co-occurrence of congenital anomalies and childhood brain tumors in 22 million live births. Neuro-oncology. 2025 Sep 8:27(7):1910-1922. doi: 10.1093/neuonc/noaf087. Epub [PubMed PMID: 40138258]
Shahab S, Fangusaro J. Neonatal Central Nervous System Tumors. Clinics in perinatology. 2021 Mar:48(1):35-51. doi: 10.1016/j.clp.2020.11.003. Epub 2021 Jan 11 [PubMed PMID: 33583506]
Shekdar KV, Schwartz ES. Brain Tumors in the Neonate. Neuroimaging clinics of North America. 2017 Feb:27(1):69-83. doi: 10.1016/j.nic.2016.09.001. Epub [PubMed PMID: 27889024]
Orbach D, Sarnacki S, Brisse HJ, Gauthier-Villars M, Jarreau PH, Tsatsaris V, Baruchel A, Zerah M, Seigneur E, Peuchmaur M, Doz F. Neonatal cancer. The Lancet. Oncology. 2013 Dec:14(13):e609-20. doi: 10.1016/S1470-2045(13)70236-5. Epub [PubMed PMID: 24275134]
Oi S, Kokunai T, Matsumoto S. Congenital brain tumors in Japan (ISPN Cooperative Study): specific clinical features in neonates. Child's nervous system : ChNS : official journal of the International Society for Pediatric Neurosurgery. 1990 Mar:6(2):86-91 [PubMed PMID: 2340534]
Carstensen H, Juhler M, Bøgeskov L, Laursen H. A report of nine newborns with congenital brain tumours. Child's nervous system : ChNS : official journal of the International Society for Pediatric Neurosurgery. 2006 Nov:22(11):1427-31 [PubMed PMID: 16804715]
Level 2 (mid-level) evidenceAlamo L, Beck-Popovic M, Gudinchet F, Meuli R. Congenital tumors: imaging when life just begins. Insights into imaging. 2011 Jun:2(3):297-308 [PubMed PMID: 22347954]
Goyal N, Kakkar A, Singh PK, Sharma MC, Chandra PS, Mahapatra AK, Sharma BS. Intracranial teratomas in children: a clinicopathological study. Child's nervous system : ChNS : official journal of the International Society for Pediatric Neurosurgery. 2013 Nov:29(11):2035-42. doi: 10.1007/s00381-013-2091-y. Epub 2013 Apr 9 [PubMed PMID: 23568500]
Level 2 (mid-level) evidenceSimone V, Rizzo D, Cocciolo A, Caroleo AM, Carai A, Mastronuzzi A, Tornesello A. Infantile Brain Tumors: A Review of Literature and Future Perspectives. Diagnostics (Basel, Switzerland). 2021 Apr 8:11(4):. doi: 10.3390/diagnostics11040670. Epub 2021 Apr 8 [PubMed PMID: 33917833]
Level 3 (low-level) evidenceDiengdoh JV, Buxton PH, Foy PM. Intracranial malignant teratoma. Neuropathology and applied neurobiology. 1985 May-Jun:11(3):245-50 [PubMed PMID: 4033875]
Louis DN, Perry A, Reifenberger G, von Deimling A, Figarella-Branger D, Cavenee WK, Ohgaki H, Wiestler OD, Kleihues P, Ellison DW. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: a summary. Acta neuropathologica. 2016 Jun:131(6):803-20. doi: 10.1007/s00401-016-1545-1. Epub 2016 May 9 [PubMed PMID: 27157931]
Arslanca SB, Söylemez F, Koç A. Congenital glioblastoma multiforme presented with intracranial bleeding: a case report. Journal of obstetrics and gynaecology : the journal of the Institute of Obstetrics and Gynaecology. 2019 Apr:39(3):427-428. doi: 10.1080/01443615.2018.1475470. Epub 2018 Sep 18 [PubMed PMID: 30226408]
Level 3 (low-level) evidenceViaene AN, Pu C, Perry A, Li MM, Luo M, Santi M. Congenital tumors of the central nervous system: an institutional review of 64 cases with emphasis on tumors with unique histologic and molecular characteristics. Brain pathology (Zurich, Switzerland). 2021 Jan:31(1):45-60. doi: 10.1111/bpa.12885. Epub 2020 Aug 4 [PubMed PMID: 32681571]
Level 3 (low-level) evidenceBale TA, Rosenblum MK. The 2021 WHO Classification of Tumors of the Central Nervous System: An update on pediatric low-grade gliomas and glioneuronal tumors. Brain pathology (Zurich, Switzerland). 2022 Jul:32(4):e13060. doi: 10.1111/bpa.13060. Epub 2022 Feb 25 [PubMed PMID: 35218102]
Bianchi F, Tamburrini G, Massimi L, Caldarelli M. Supratentorial tumors typical of the infantile age: desmoplastic infantile ganglioglioma (DIG) and astrocytoma (DIA). A review. Child's nervous system : ChNS : official journal of the International Society for Pediatric Neurosurgery. 2016 Oct:32(10):1833-8. doi: 10.1007/s00381-016-3149-4. Epub 2016 Sep 20 [PubMed PMID: 27659826]
Rout P, Santosh V, Mahadevan A, Kolluri VR, Yasha TC, Shankar SK. Desmoplastic infantile ganglioglioma--clinicopathological and immunohistochemical study of four cases. Child's nervous system : ChNS : official journal of the International Society for Pediatric Neurosurgery. 2002 Oct:18(9-10):463-7 [PubMed PMID: 12382166]
Level 3 (low-level) evidenceBlessing MM, Alexandrescu S. Embryonal Tumors of the Central Nervous System: An Update. Surgical pathology clinics. 2020 Jun:13(2):235-247. doi: 10.1016/j.path.2020.01.003. Epub 2020 Apr 7 [PubMed PMID: 32389264]
Upadhyaya SA, Robinson GW, Onar-Thomas A, Orr BA, Johann P, Wu G, Billups CA, Tatevossian RG, Dhanda SK, Srinivasan A, Broniscer A, Qaddoumi I, Vinitsky A, Armstrong GT, Bendel AE, Hassall T, Partap S, Fisher PG, Crawford JR, Chintagumpala M, Bouffet E, Gururangan S, Mostafavi R, Sanders RP, Klimo P Jr, Patay Z, Indelicato DJ, Nichols KE, Boop FA, Merchant TE, Kool M, Ellison DW, Gajjar A. Relevance of Molecular Groups in Children with Newly Diagnosed Atypical Teratoid Rhabdoid Tumor: Results from Prospective St. Jude Multi-institutional Trials. Clinical cancer research : an official journal of the American Association for Cancer Research. 2021 May 15:27(10):2879-2889. doi: 10.1158/1078-0432.CCR-20-4731. Epub 2021 Mar 18 [PubMed PMID: 33737307]
Crawford JR, Isaacs H Jr. Perinatal (fetal and neonatal) choroid plexus tumors: a review. Child's nervous system : ChNS : official journal of the International Society for Pediatric Neurosurgery. 2019 Jun:35(6):937-944. doi: 10.1007/s00381-019-04135-x. Epub 2019 Apr 5 [PubMed PMID: 30953158]
Sugimoto M, Kurishima C, Masutani S, Tamura M, Senzaki H. Congenital Brain Tumor within the First 2 Months of Life. Pediatrics and neonatology. 2015 Dec:56(6):369-75. doi: 10.1016/j.pedneo.2015.04.004. Epub 2015 Apr 30 [PubMed PMID: 26044848]
Bedei IA, Huisman TAGM, Whitehead W, Axt-Fliedner R, Belfort M, Sanz Cortes M. Fetal Brain Tumors, a Challenge in Prenatal Diagnosis, Counselling, and Therapy. Journal of clinical medicine. 2022 Dec 21:12(1):. doi: 10.3390/jcm12010058. Epub 2022 Dec 21 [PubMed PMID: 36614855]
Soares FA, Fischer SE, Reis MA, Soares EG. Massive intracranial immature teratoma. Report of a case with polyhidramnios and intense pelvic pain. Arquivos de neuro-psiquiatria. 1996 Jun:54(2):309-12 [PubMed PMID: 8984992]
Level 3 (low-level) evidenceVrionis A, Hegert J, Matsumoto L, Hayes L, Kucera JN. The prenatal imaging of a rare congenital intracranial teratoma. Radiology case reports. 2024 Oct:19(10):4213-4218. doi: 10.1016/j.radcr.2024.06.073. Epub 2024 Jul 20 [PubMed PMID: 39101018]
Level 3 (low-level) evidenceIsaacs H. Fetal brain tumors: a review of 154 cases. American journal of perinatology. 2009 Jun:26(6):453-66. doi: 10.1055/s-0029-1214245. Epub 2009 Apr 24 [PubMed PMID: 19396744]
Level 3 (low-level) evidenceIsaacs H Jr. II. Perinatal brain tumors: a review of 250 cases. Pediatric neurology. 2002 Nov:27(5):333-42 [PubMed PMID: 12504200]
Level 3 (low-level) evidenceSeverino M, Schwartz ES, Thurnher MM, Rydland J, Nikas I, Rossi A. Congenital tumors of the central nervous system. Neuroradiology. 2010 Jun:52(6):531-48. doi: 10.1007/s00234-010-0699-0. Epub 2010 Apr 29 [PubMed PMID: 20428859]
Pai V, Laughlin S, Ertl-Wagner B. Imaging of pediatric glioneuronal and neuronal tumors. Child's nervous system : ChNS : official journal of the International Society for Pediatric Neurosurgery. 2024 Oct:40(10):3007-3026. doi: 10.1007/s00381-024-06502-9. Epub 2024 Jul 3 [PubMed PMID: 38960918]
Saleem A, Alqallaf A, Alduwailah M, Zulfiqar N, Saleh S, Alrabea A, Mijalcic R, Alsheikh T. Congenital intracranial immature teratoma in a preterm infant: illustrative case. Child's nervous system : ChNS : official journal of the International Society for Pediatric Neurosurgery. 2024 Nov 27:41(1):4. doi: 10.1007/s00381-024-06666-4. Epub 2024 Nov 27 [PubMed PMID: 39601929]
Level 3 (low-level) evidenceBolat F, Kayaselcuk F, Tarim E, Kilicdag E, Bal N. Congenital intracranial teratoma with massive macrocephaly and skull rupture. Fetal diagnosis and therapy. 2008:23(1):1-4 [PubMed PMID: 17934288]
Woodward PJ, Sohaey R, Kennedy A, Koeller KK. From the archives of the AFIP: a comprehensive review of fetal tumors with pathologic correlation. Radiographics : a review publication of the Radiological Society of North America, Inc. 2005 Jan-Feb:25(1):215-42 [PubMed PMID: 15653597]
Gkasdaris G, Chourmouzi D. Congenital intracranial mature teratoma: the role of fetal MRI over ultrasound in the prenatal diagnosis and the perinatal management. BMJ case reports. 2019 May 6:12(5):. doi: 10.1136/bcr-2019-229774. Epub 2019 May 6 [PubMed PMID: 31064792]
Level 3 (low-level) evidenceSherer DM, Onyeije CI. Prenatal ultrasonographic diagnosis of fetal intracranial tumors: a review. American journal of perinatology. 1998 May:15(5):319-28 [PubMed PMID: 9643639]
Parmar HA, Pruthi S, Ibrahim M, Gandhi D. Imaging of congenital brain tumors. Seminars in ultrasound, CT, and MR. 2011 Dec:32(6):578-89. doi: 10.1053/j.sult.2011.07.001. Epub [PubMed PMID: 22108220]
Lee DY, Kim YM, Yoo SJ, Cho BK, Chi JG, Kim IO, Wang KC. Congenital glioblastoma diagnosed by fetal sonography. Child's nervous system : ChNS : official journal of the International Society for Pediatric Neurosurgery. 1999 Apr:15(4):197-201 [PubMed PMID: 10361971]
Level 3 (low-level) evidenceBader A, Heran M, Dunham C, Steinbok P. Radiological features of infantile glioblastoma and desmoplastic infantile tumors: British Columbia's Children's Hospital experience. Journal of neurosurgery. Pediatrics. 2015 Aug:16(2):119-25. doi: 10.3171/2014.10.PEDS13634. Epub 2015 May 8 [PubMed PMID: 25955808]
Lang SS, Beslow LA, Gabel B, Judkins AR, Fisher MJ, Sutton LN, Storm PB, Heuer GG. Surgical treatment of brain tumors in infants younger than six months of age and review of the literature. World neurosurgery. 2012 Jul:78(1-2):137-44. doi: 10.1016/j.wneu.2011.09.012. Epub 2011 Nov 7 [PubMed PMID: 22120270]
Level 2 (mid-level) evidenceLee JS, Lee JY, Kim KH, Park SH, Koh EJ, Kim SK, Phi JH. The Role of Early and Delayed Surgery for Infants with Congenital Brain Tumors. Cancer research and treatment. 2024 Jul:56(3):909-919. doi: 10.4143/crt.2023.1174. Epub 2023 Dec 28 [PubMed PMID: 38186242]
Shin HJ, Kwon YJ, Park HJ, Park BK, Shin SH, Kim JY, Lee SH, Kim HS, Kim DW. An infant with prenatally diagnosed congenital anaplastic astrocytoma who remains disease-free after proton therapy. Journal of Korean medical science. 2013 Sep:28(9):1394-8. doi: 10.3346/jkms.2013.28.9.1394. Epub 2013 Aug 28 [PubMed PMID: 24015049]
Level 3 (low-level) evidenceFeferbaum R, Diniz EM, Valente M, Giolo CR, Vieira RA, Galvani AL, Ceccon ME, Araujo MC, Krebs VL, Vaz FA. Brain abscess by citrobacter diversus in infancy: case report. Arquivos de neuro-psiquiatria. 2000 Sep:58(3A):736-40 [PubMed PMID: 10973119]
Level 3 (low-level) evidenceHakimi R, Garg A. Imaging of Hemorrhagic Stroke. Continuum (Minneapolis, Minn.). 2016 Oct:22(5, Neuroimaging):1424-1450 [PubMed PMID: 27740983]
Tran S, Plant-Fox AS, Chi SN, Narendran A. Current advances in immunotherapy for atypical teratoid rhabdoid tumor (ATRT). Neuro-oncology practice. 2023 Aug:10(4):322-334. doi: 10.1093/nop/npad005. Epub 2023 Jan 28 [PubMed PMID: 37457224]
Level 3 (low-level) evidenceIsikay AI, Gurses ME, Gecici NN, Baylarov B, Cekic E, Narin F, Karakaya D, Hanalioglu S, Bilginer B. Congenital Brain Tumors: Surgical Outcomes and Long-Term Prognostic Factors. World neurosurgery. 2024 Nov:191():e664-e673. doi: 10.1016/j.wneu.2024.09.021. Epub 2024 Sep 10 [PubMed PMID: 39265942]
Lundar T, Due-Tønnessen BJ, Frič R, Brandal P, Stensvold E, Due-Tønnessen P. Adult outcome after neurosurgical treatment of brain tumours in the first year of life: long-term follow-up of a single consecutive institutional series of 34 patients. Acta neurochirurgica. 2019 Sep:161(9):1793-1798. doi: 10.1007/s00701-019-04014-z. Epub 2019 Jul 15 [PubMed PMID: 31309304]
Jurkiewicz E, Brożyna A, Grajkowska W, Bekiesińska-Figatowska M, Daszkiewicz P, Nowak K, Malczyk K, Walecki J, Perek D, Syczewska M. Congenital brain tumors in a series of 56 patients. Child's nervous system : ChNS : official journal of the International Society for Pediatric Neurosurgery. 2012 Aug:28(8):1193-201. doi: 10.1007/s00381-012-1798-5. Epub 2012 May 31 [PubMed PMID: 22648076]
Eker N, Tokuç G, Sarısaltık A, Dağçınar A, Gül D, Atasoy BM, Yılmaz B, Taş BT. Clinical factors, management, and outcomes of children under 3 years old with central nervous system tumors: single-center experience. Child's nervous system : ChNS : official journal of the International Society for Pediatric Neurosurgery. 2024 Aug:40(8):2311-2320. doi: 10.1007/s00381-024-06386-9. Epub 2024 Apr 15 [PubMed PMID: 38619586]
Nomura Y, Yasumoto S, Yanai F, Akiyoshi H, Inoue T, Nibu K, Tsugu H, Fukushima T, Hirose S. Survival and late effects on development of patients with infantile brain tumor. Pediatrics international : official journal of the Japan Pediatric Society. 2009 Jun:51(3):337-41. doi: 10.1111/j.1442-200X.2008.02760.x. Epub 2008 Dec 29 [PubMed PMID: 19400825]
Gerber NU, Zehnder D, Zuzak TJ, Poretti A, Boltshauser E, Grotzer MA. Outcome in children with brain tumours diagnosed in the first year of life: long-term complications and quality of life. Archives of disease in childhood. 2008 Jul:93(7):582-9 [PubMed PMID: 17634182]
Level 2 (mid-level) evidenceYoung HK, Johnston H. Intracranial tumors in infants. Journal of child neurology. 2004 Jun:19(6):424-30 [PubMed PMID: 15446390]
Gurney JG, Ness KK, Stovall M, Wolden S, Punyko JA, Neglia JP, Mertens AC, Packer RJ, Robison LL, Sklar CA. Final height and body mass index among adult survivors of childhood brain cancer: childhood cancer survivor study. The Journal of clinical endocrinology and metabolism. 2003 Oct:88(10):4731-9 [PubMed PMID: 14557448]
Pillai S, Metrie M, Dunham C, Sargent M, Hukin J, Steinbok P. Intracranial tumors in infants: long-term functional outcome, survival, and its predictors. Child's nervous system : ChNS : official journal of the International Society for Pediatric Neurosurgery. 2012 Apr:28(4):547-55. doi: 10.1007/s00381-012-1707-y. Epub 2012 Feb 4 [PubMed PMID: 22307825]
Fossey M, Li H, Afzal S, Carret AS, Eisenstat DD, Fleming A, Hukin J, Hawkins C, Jabado N, Johnston D, Brown T, Larouche V, Scheinemann K, Strother D, Wilson B, Zelcer S, Huang A, Bouffet E, Lafay-Cousin L. Atypical teratoid rhabdoid tumor in the first year of life: the Canadian ATRT registry experience and review of the literature. Journal of neuro-oncology. 2017 Mar:132(1):155-162. doi: 10.1007/s11060-016-2353-0. Epub 2017 Jan 19 [PubMed PMID: 28102486]
Silva AHD, Habermann S, Craven CL, Bhagawati D, O'Hare P, Jorgensen M, Dahl C, Mankad K, Thompson DNP, Hargrave D, Jeelani NUO, Aquilina K. Atypical teratoid rhabdoid tumours (ATRTs)-a 21-year institutional experience. Child's nervous system : ChNS : official journal of the International Society for Pediatric Neurosurgery. 2023 Jun:39(6):1509-1518. doi: 10.1007/s00381-023-05828-0. Epub 2023 Feb 15 [PubMed PMID: 36790496]
Bachu VS, Shah P, Jimenez AE, Khalafallah AM, Tailor J, Mukherjee D, Cohen AR. Clinical predictors of survival for patients with atypical teratoid/rhabdoid tumors. Child's nervous system : ChNS : official journal of the International Society for Pediatric Neurosurgery. 2022 Jul:38(7):1297-1306. doi: 10.1007/s00381-022-05511-w. Epub 2022 Apr 1 [PubMed PMID: 35362829]
Egiz A, Kannan S, Asl SF. The Impact of Surgical Resection and Adjuvant Therapy on Survival in Pediatric Patients with Atypical Teratoid/Rhabdoid Tumor: Systematic Review and Pooled Survival Analysis. World neurosurgery. 2022 Aug:164():216-227. doi: 10.1016/j.wneu.2022.04.073. Epub 2022 Apr 22 [PubMed PMID: 35470083]
Level 1 (high-level) evidenceWang S, Sun MZ, Abecassis IJ, Weil AG, Ibrahim GM, Fallah A, Ene C, Leary SES, Cole BL, Lockwood CM, Olson JM, Geyer JR, Ellenbogen RG, Ojemann JG, Wang AC. Predictors of mortality and tumor recurrence in desmoplastic infantile ganglioglioma and astrocytoma-and individual participant data meta-analysis (IPDMA). Journal of neuro-oncology. 2021 Nov:155(2):155-163. doi: 10.1007/s11060-021-03860-1. Epub 2021 Oct 6 [PubMed PMID: 34613581]
Level 1 (high-level) evidence