Introduction
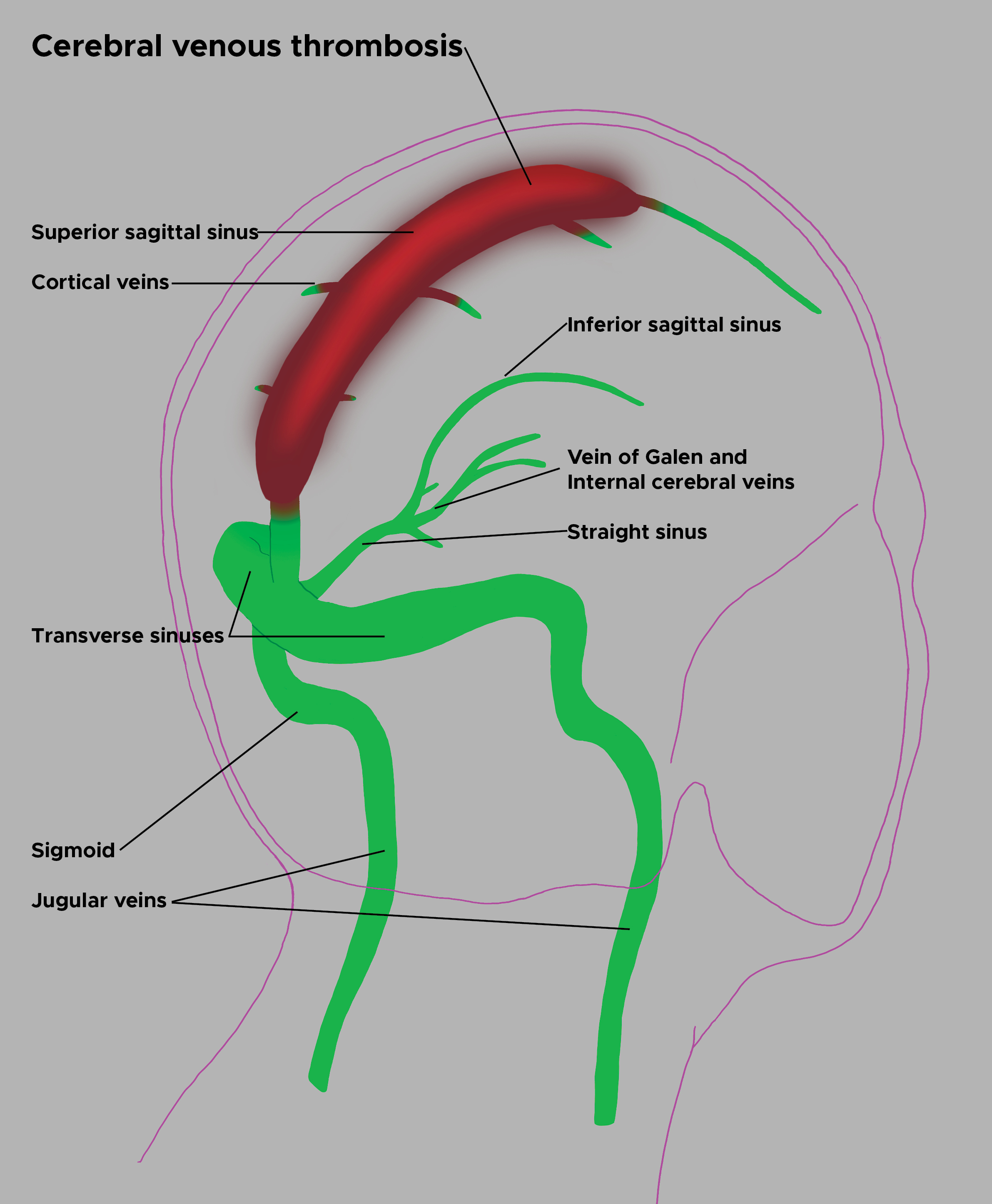
Cerebral venous sinus thrombosis (CVST) is a rare condition associated with substantial morbidity and mortality (see Image. Cerebral Venous Sinus Thrombosis Illustration). This disorder may present with a wide range of signs and symptoms that resemble acute stroke, subarachnoid hemorrhage (SAH), meningoencephalitis, or benign intracranial hypertension.[1][2] The broad spectrum of risk factors and clinical manifestations complicates the diagnostic process. Delays are common, with median intervals of 4 days from symptom onset to hospital admission and 7 days to diagnosis. Multiple studies have documented a high prevalence of residual symptoms, which can hinder a return to baseline function.[3][4] Maintaining a high index of suspicion remains critical for ensuring timely diagnosis and appropriate treatment.[5][6]
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
Multiple risk factors contribute to the development of CVST. At least 1 risk factor was identified in over 85% of patients, and more than 50% had multiple risk factors.[7] The condition often arises in the context of prothrombotic conditions, such as pregnancy, the postpartum period, or oral contraceptive pill (OCP) use. Nearly 60% of pregnancy-related strokes are attributed to CVST.[8] More recently, COVID-19 has also been linked to cerebral venous thrombosis (CVT), with 1 study identifying COVID-19 in 7.6% of all CVST admissions.
The International Study on Cerebral Vein and Dural Sinus Thrombosis (ISCVT) reported genetic and acquired thrombophilia in 34% of patients with CVST. Inherited thrombophilias include deficiencies in protein C, protein S, and antithrombin, as well as the factor V Leiden mutation, the prothrombin gene mutation 20210, and hyperhomocysteinemia.[9][10][11]
Acquired thrombophilia should be considered in patients with nephrotic syndrome, where antithrombin loss increases thrombotic risk, or in those with antiphospholipid antibodies. Chronic inflammatory conditions, including systemic lupus erythematosus and inflammatory bowel disease, have also been associated with CVST, along with malignancy and vasculitides such as Wegener granulomatosis. Local infections like otitis media and mastoiditis may lead to thrombosis in the adjacent sigmoid and transverse sinuses. Additional risk arises following head trauma, certain neurosurgical interventions, direct injury to the venous sinuses or jugular veins (eg, catheterization), and even after lumbar puncture.[12][13]
Salient risk factors for CVST can be categorized as follows:
-
Estrogen-related: Obesity, OCP use, pregnancy and puerperium, and estrogen-containing hormone replacement therapy (associated with an 8-fold increase in risk)
-
Acquired thrombophilia: Antiphospholipid antibody syndrome, JAK2 mutations, myeloproliferative disorders, and autoimmune diseases
-
Genetic thrombophilia: Protein C and protein S deficiencies, Factor V Leiden mutation, and prothrombin G20210A polymorphism
-
Other provoking triggers: COVID-19 infection, otitis media, mastoiditis, dehydration, head injuries, compressive lesions affecting venous sinuses, and vaccine-induced thrombocytopenia.[14]
Recognizing these diverse risk factors is essential for early identification and prevention of CVST, especially in high-risk populations.
Epidemiology
CVST is a rare disorder, with an estimated annual incidence of 3 to 4 cases per million. Among pregnant women, the frequency of peripartum and postpartum CVST reaches approximately 12 cases per 100,000 deliveries, only slightly lower than that of peripartum and postpartum arterial stroke. Women experience CVST 3 times more often than men, likely due to sex-specific risk factors such as OCP use, pregnancy, the puerperium, and hormone replacement therapy. Recent studies have highlighted a marked female predominance among young adults, with 70% to 80% of cases occurring in women of childbearing age.
Pathophysiology
Two distinct pathophysiologic mechanisms contribute to the clinical manifestations of CVST. First, thrombosis in the cerebral veins raises venous and capillary pressure, reducing cerebral perfusion. The resulting ischemia produces cytotoxic edema, which impairs energy-dependent membrane pumps and causes intracellular swelling. Disruption of the blood-brain barrier follows, leading to vasogenic edema and interstitial fluid accumulation. Elevated venous pressure can culminate in intraparenchymal hemorrhage.
Second, obstruction of the cerebral sinuses, especially when thrombi persist, interferes with cerebrospinal fluid absorption. Cerebrospinal fluid normally flows from the cerebral ventricles through the subarachnoid space to the arachnoid granulations, where it enters the venous sinuses. Sinus thrombosis blocks this absorption, causing elevated intracranial pressure (ICP), which in turn promotes both cytotoxic and vasogenic edema and may result in parenchymal hemorrhage.
The superior sagittal sinus (SSS) is most frequently affected in CVST, followed by the transverse sinus. Increased length and narrower diameter heighten the risk of thrombosis in the SSS. The left transverse sinus, often hypoplastic relative to the right, typically demonstrates lower flow velocity, further predisposing it to thrombosis.[15]
History and Physical
Physicians should maintain a high index of suspicion for CVST due to its variable presentation and low incidence. The International Study on Cerebral Vein and Dural Sinus Thrombosis categorized symptom onset into acute (<48 hours), subacute (48 hours-4 weeks), and chronic (>4 weeks).[16] A subacute presentation occurred in nearly 60% of cases, while acute and chronic patterns were observed in 37% and 7%, respectively.
Headache occurs in 80% to 90% of patients. These headaches may be diffuse or generalized, often resembling migraines, but typically worsen gradually over several days and are not relieved by sleep. In some patients, the headache presents as a thunderclap, reaching peak intensity at onset and mimicking SAH. Exacerbation with the Valsalva maneuver or coughing suggests elevated ICP. Papilledema and visual disturbances, such as diplopia from a 6th cranial nerve palsy, may accompany the headache when ICP rises significantly. Funduscopic examination reveals papilledema, which can result in visual loss or permanent blindness if unrecognized. An isolated headache, without focal neurologic deficits or papilledema, occurs in up to 25% of CVST cases and may further obscure diagnosis.
Focal neurologic signs occur in up to 44% of patients. Motor weakness, particularly hemiparesis, represents the most frequent focal deficit. Unlike arterial strokes, these deficits do not typically localize to a single vascular territory. Hemispheric symptoms such as aphasia and hemiparesis, though characteristic, remain relatively uncommon.
Seizures affect approximately 40% of patients, with focal seizures being the most prevalent type. Among those who develop seizures, 50% experience focal onset, which may progress to generalized seizures or status epilepticus. Any presentation involving a headache with either a focal neurologic deficit or new-onset seizure should prompt consideration of CVST. Involvement of the straight sinus or severe venous infarction with hemorrhagic transformation can compress the diencephalon and brainstem, leading to coma and potentially death due to cerebral herniation.[17]
A subacute clinical picture often includes signs of raised ICP, including headaches (90% of cases), papilledema, diplopia, seizures (20%-40%), focal neurologic deficits (20%-50%), and encephalopathy (20%). An acute presentation may involve a thunderclap headache resembling SAH, often accompanied by focal neurologic deficits.
Evaluation
Clinical and Laboratory Assessments
The diagnosis of CVST is primarily clinical, with neuroimaging serving to confirm the condition. Due to its varied presentation and wide range of symptoms, a high index of suspicion is essential for accurate diagnosis. CVST should be considered in young and middle-aged patients, particularly those with risk factors such as postpartum status, genetic or acquired thrombophilia, and focal neurological findings. The condition must also be suspected in the presence of the following risk factors or manifestations:
- Age younger than 50
- Atypical headaches or persistent ones requiring repeated evaluations
- Focal neurological deficits
- Stroke-like symptoms, especially in the absence of typical vascular contributors, such as carotid atherosclerosis
- Seizures, whether focal, generalized, or in status epilepticus
- Signs of intracranial hypertension, such as papilledema observed on funduscopic examination
- Computed tomography (CT) evidence of hemorrhagic infarcts, particularly if multiple and occurring outside a single vascular territory
Salient clinical clues to the diagnosis include slow progression, bilateral involvement, and concurrent seizures. Neuroimaging (see below) plays a crucial role in confirming the diagnosis once clinical suspicion is raised.
Laboratory evaluation for CVST should include a complete blood count, coagulation panel, chemistry panel, and inflammatory markers such as sedimentation rate and C-reactive protein to assess for proinflammatory states. An ideal screening test would effectively rule out CVST without unnecessary neuroimaging, which would greatly benefit clinical practice. The D-dimer assay has been evaluated for this purpose, but one study found an unacceptable false-negative rate of up to 26%. This low sensitivity contrasts with the test's utility in ruling out deep vein thrombosis (DVT), likely due to the lower thrombotic burden of CVST compared to this condition.[18][19]
Recent guidelines from the American Heart Association and the American Stroke Association (AHA/ASA) state that a negative D-dimer test does not reliably exclude CVST. A negative result should not prevent neuroimaging if CVST is suspected clinically.[20][21] However, incorporating D-dimer (≥500 μg/L) into the clinical CVST score, which includes variables such as seizure, known thrombophilia, OCP use, symptom duration of more than 6 days, a description as the "worst headache ever," and focal neurological deficits, has been shown to enhance predictive value.[22]
Neuroimaging
Neuroimaging plays a crucial role in diagnosing CVST, with key objectives including visualization of the thrombus, identification of venous filling defects, and detection of sequelae such as infarction, hemorrhagic transformation, and raised ICP. Noncontrast CT should be the first imaging modality used in patients presenting with atypical headaches, focal neurologic deficits, seizures, altered mental status, or coma, given its speed and accessibility.
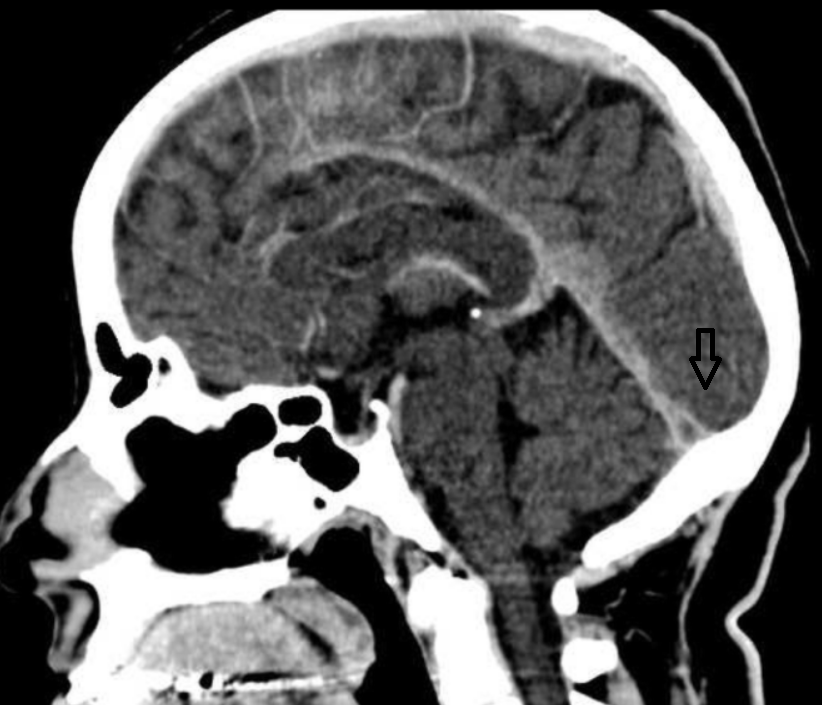
Direct signs of CVST on CT include the cord sign, a curvilinear hyperdensity within a cortical vein that can be seen up to 2 weeks after thrombus formation, and the dense triangle sign, a triangular hyperdensity in the SSS (see Image. Cord Sign). Intraparenchymal hemorrhages or infarcts may appear on noncontrast-enhanced CT and often cross vascular boundaries, with bilateral involvement in some cases. A multicentric study reported brain infarction in 36.4%, hemorrhagic transformation in 17.3%, and intraparenchymal hemorrhage in 3.8% of CVST cases.
Hyperdensity within a cortical vein or dural sinus is seen in only 1/3 of CVST cases on noncontrast-enhanced CT scans. Increased sensitivity in diagnosis has been noted when hyperdensity exceeds 50 Hounsfield units and when the target sinus-lowest attenuation sinus ratio exceeds 1.3. Additionally, deep machine learning models have been applied to distinguish intracranial hemorrhage following CVST in noncontrast-enhanced CT images appropriately.[23]
CT venography (CTV) remains a cornerstone in the diagnosis of CVST. Although magnetic resonance imaging (MRI) offers superior sensitivity and specificity compared to conventional CT, confirmatory venography is essential for excluding CVT. The advent of helical CT scanners has improved the resolution and accuracy of CTV, with emerging evidence showing it may surpass magnetic resonance venography (MRV) in delineating cerebral veins.
Studies also indicate that CTV and MRV perform comparably in diagnosing CVT. The ability to perform CTV immediately after a noncontrast-enhanced head CT, while the patient remains in the scanner, makes it particularly valuable in emergency settings where MRI access may be constrained. Contrast-enhanced CT can demonstrate the empty delta sign, which occurs when contrast outlines the thrombosed SSS, producing a triangular central hypodensity surrounded by enhancement from collateral venous flow (see Image. Empty Delta Sign).
MRI and MRV remain the gold standard for diagnosing CVT due to their superior sensitivity compared to CT. MRI is especially effective in evaluating parenchymal edema resulting from CVST. Imaging findings vary depending on the age of the thrombus, as signal characteristics evolve over time. Thrombi formed within the first 7 days are more challenging to visualize, but by the 2nd week, T1- and T2-weighted sequences typically demonstrate hyperintense signals. The combination of abnormal venous sinus signals on MRI and absent flow on MRV confirms CVT. A 2-dimensional time-of-flight (TOF) sequence revealing the absence of a flow void in the dural sinus is considered the most sensitive modality. MRV is preferred when assessing for low-flow states or hypoplastic sinuses, and both TOF and phase-contrast techniques are used when contrast is contraindicated.
However, these sequences may produce false positives or miss acute thrombi due to limitations such as complex flow artifacts, operator dependence, and prolonged acquisition time. Validation with susceptibility-weighted imaging, gradient-recalled echo (GRE), or MRV is often necessary.[24][25] Advanced MRI methods, such as T1-based black-blood imaging, where the signal from flowing blood is suppressed, show considerable promise. Multiscale entropy analysis of hemoglobin degradation products within thrombi also provides strong diagnostic value.[26]
A diffusion-weighted imaging (DWI) abnormality within affected sinuses or veins correlates with a lower likelihood of recanalization. Gradient-recalled echo, susceptibility-weighted imaging (SWI) with blooming artifacts, T1 black-blood imaging, and contrast-enhanced MRV can reach sensitivities approaching 100%. In contrast, CT and conventional MRI yield a sensitivity of 0.80 and a specificity of 0.90 in diagnosing CVST.
Cerebral angiography is reserved for situations in which the diagnosis remains uncertain after MRI and MRV. Intraarterial angiography provides superior visualization of the cerebral venous system and helps identify anatomical variants that may mimic CVT. This modality is especially valuable in rare instances of isolated cortical vein thrombosis without concurrent sinus involvement. Intraarterial angiography may reveal indirect findings, such as dilated and tortuous collateral veins resembling a corkscrew pattern, suggesting upstream venous obstruction. Angiography also plays a role when considering endovascular therapy (EVT) as part of the management strategy.
Several diagnostic pitfalls may complicate the interpretation of imaging studies across modalities. On plain CT, hyperdense blood within a patent dural sinus, acute subdural hematomas, or retained contrast material may falsely suggest CVT. On contrast-enhanced CT, intrasinus septa or split or fenestrated dural sinuses can appear as filling defects. MRI pitfalls include slow venous flow that leads to loss of the normal flow void, and anatomical variants such as intrasinus septa or fenestrated sinuses. Additionally, acute and early subacute hemorrhages can obscure or mimic thrombus. On contrast-enhanced MRI, a thrombus containing methemoglobin may resemble a patent sinus, particularly on T1-weighted sequences. Across all imaging modalities, hypoplastic or aplastic dural sinuses, intrasinus arachnoid granulations, or compression and invasion of dural sinuses by tumors may lead to diagnostic confusion.[27]
Treatment / Management
Initial Measures
Management begins with identifying and treating life-threatening complications of CVST, such as increased ICP, seizures, and coma. Patients who present with seizures and have a hemorrhage or infarction on neuroimaging require specific anticonvulsant therapy along with seizure prophylaxis.
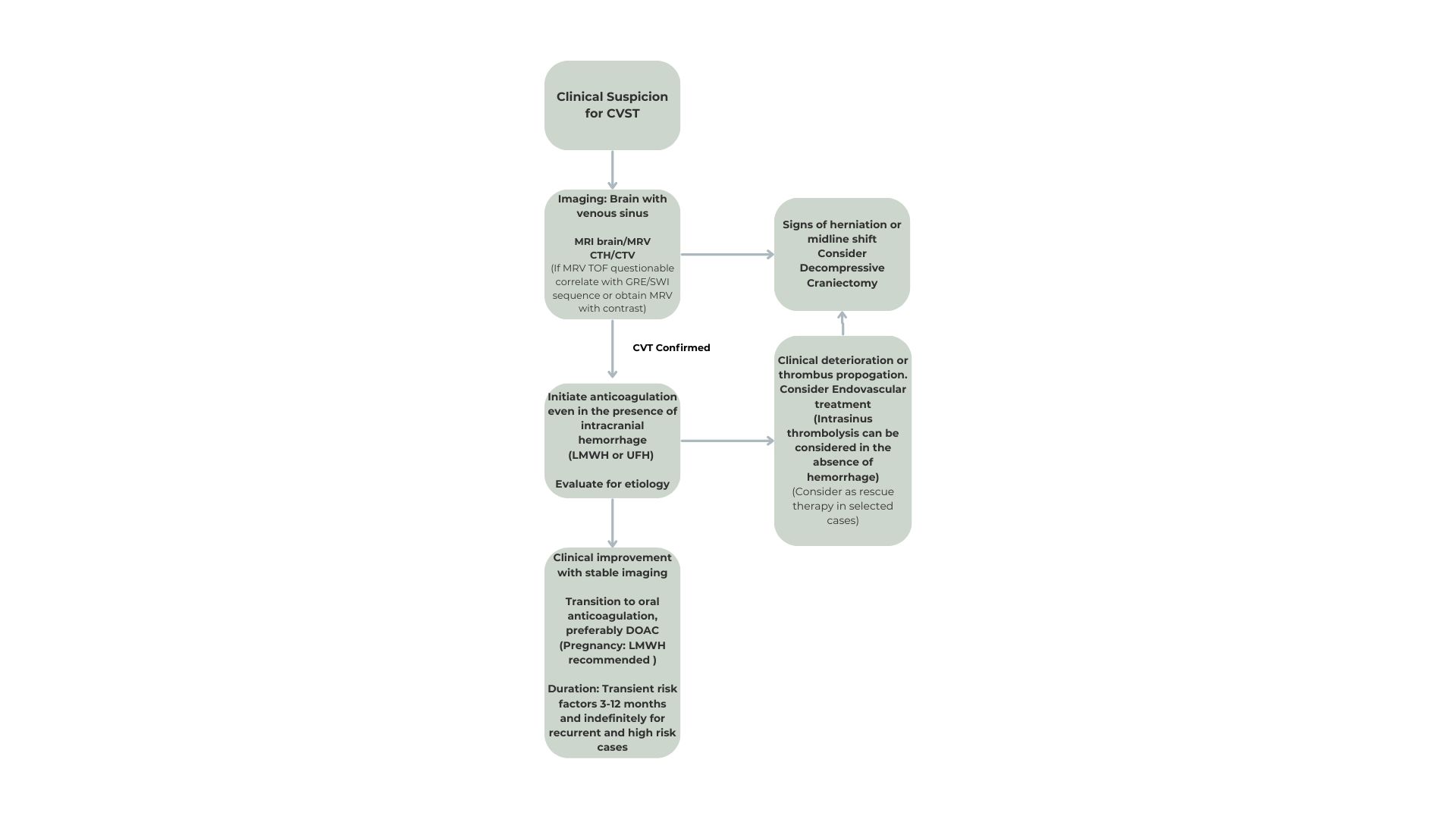
For increased ICP, elevating the head of the bed and administering osmotherapy are immediate interventions. Admission to the intensive care or stroke unit enables close monitoring of ICP, and neurosurgical consultation is warranted if the patient decompensates and surgical decompression becomes necessary. Once stabilized, focus shifts to definitive treatment, including anticoagulation and, in selected cases, catheter-directed fibrinolysis or surgical thrombectomy (see Image. Management and Treatment of Cerebral Venous Sinus Thrombosis).
Anticoagulation
Anticoagulation in CVST aims to prevent thrombus propagation, facilitate recanalization, and reduce the risk of recurrence. The approach has been debated due to concerns about hemorrhagic transformation of cerebral infarcts prior to anticoagulant administration. However, anticoagulation remains essential for limiting thrombus extension, reopening occluded cerebral veins, and minimizing complications such as DVT and pulmonary embolism in patients who already have a thrombotic predisposition. Results from 2 randomized controlled trials (RCTs) comparing anticoagulation to placebo, while not statistically significant, indicated more favorable outcomes with anticoagulation and demonstrated its safety, even in patients with cerebral hemorrhage.
Evidence from these RCTs, along with observational studies, supports anticoagulation as a safe and effective treatment for CVT. Therapy should begin as soon as the diagnosis is confirmed. Intravenous unfractionated heparin or subcutaneous low-molecular-weight heparin (LMWH) serves as a bridge to oral anticoagulation using a vitamin K antagonist (VKA), with a target international normalized ratio between 2.0 and 3.0. No difference in outcomes has been observed between unfractionated and LMWH. According to European Stroke Organization (ESO) guidelines, unfractionated heparin is preferred in patients with renal insufficiency or in situations where emergent reversal may be required.
Recent evidence suggests that direct oral anticoagulants (DOACs) are a suitable alternative to VKAs for treating CVT. RCTs such as RE-SPECT CVT and SECRET demonstrated that DOACs, including dabigatran and rivaroxaban, matched warfarin in efficacy, with no reported cases of recurrent venous thromboembolism (VTE) and comparable bleeding risks.[28][29](A1)
A large retrospective study, ACTION-CVT, also showed no difference in recurrent VTE rates between DOACs and VKAs, but it did identify a significantly lower risk of major hemorrhage, including intracerebral hemorrhage, in patients treated with DOACs.[30] A systematic review confirmed similar outcomes for recurrent VTE, major hemorrhage, and recanalization between the 2 drug classes.[31] DOACs offer a reasonable alternative following initial parenteral anticoagulation. However, all studies excluded pregnant patients, for whom LMWH remains the preferred treatment.(A1)
Treatment duration depends on the underlying cause. For provoked CVT, anticoagulation is generally continued for 3 to 6 months, while unprovoked CVT typically requires 6 to 12 months of therapy. Indefinite anticoagulation may be warranted in patients with recurrent CVT, DVT or pulmonary embolism alongside CVT, or CVT in the context of severe thrombophilia. LMWH remains the most commonly used agent, favored in 83% of cases.[32] The role of CTV or MRV in evaluating the extent of revascularization to guide the length of anticoagulation remains unclear.(B2)
Reperfusion Therapies
Although most patients experience clinical improvement with anticoagulation therapy, a small subset does not, and these individuals may clinically deteriorate despite receiving anticoagulation. EVT may be considered in such selected cases, provided that the medical staff are experienced in interventional radiology. Studies conducted over the last decade have demonstrated the effectiveness of various techniques, including mechanical thrombectomy (either balloon-assisted or through aspiration or vacuum aspiration systems), intrasinus thrombolysis, a combination of mechanical thrombectomy and intrasinus thrombolysis, intraarterial thrombolysis, and intrasinus stenting. However, the findings regarding safety and complication rates remain inconclusive.[33][34]
Catheter-directed thrombolysis may be considered in settings where the prognosis is poor, for example, in cases involving extremely large cerebral venous thrombi and clinical deterioration despite anticoagulation therapy. Fibrinolytics, however, carry an increased risk of intracranial hemorrhage, making their use preferable in individuals without evidence of ICH. A systematic review conducted in 2003, which examined 72 studies and 169 patients with CVT, suggested a potential clinical benefit of intrasinus fibrinolytics in patients with severe presentations. ICH occurred in 17% of patients treated with fibrinolytics and was associated with clinical deterioration in 5% of cases.[35](A1)
CVST can be categorized based on the age of the clot as follows: acute (<5 days postsymptom onset), subacute (5–15 days), and chronic (>15 days). The success of EVT is most pronounced in the acute and subacute phases. Despite this outcome, significant heterogeneity remains in the algorithm for EVT in CVT, with aspiration thrombectomy and stent retrievers being the most common modalities.
Surgical Intervention
In cases of large venous infarcts and hemorrhages causing a mass effect with a risk of herniation, decompressive surgery may improve clinical outcomes, particularly when performed early. However, the evidence supporting this intervention is level C. Decompressive surgery can be lifesaving, with favorable outcomes seen in more than 50% of patients, and a mortality rate of approximately 20%.
Supportive Care
Identifying the underlying contributing factors of CVST and developing a targeted treatment strategy is essential. Women on hormonal contraceptive therapy should consider nonestrogen-based contraception methods, such as levonorgestrel and copper intrauterine devices or progestin-only pills. Further testing to identify the etiology of all acquired and reversible thrombophilic states should be conducted and, when possible, corrected. In addition to clinical follow-up, the AHA/ASA recommend follow-up imaging 3 to 6 months after diagnosis to assess recanalization.
The risks for ICH following anticoagulation therapy range from zero to 5.4%. A systematic review has shown that the overall mortality rate was 9.4%, and dependency rates were 9.4% and 9.7%, respectively.[36][37] The ESO's 2017 guidelines for the diagnosis and treatment of CVST outline the quality of evidence and strength of recommendations as follows:(B2)
Table. Evidence and Recommendations for the Diagnosis and Treatment of Cerebral Venous Sinus Thrombosis
|
Recommendations |
Quality of evidence |
Strength of recommendations |
|
MRV and CTV as an alternative to digital subtraction angiography |
Very low |
Weak |
|
CTV as an alternative to MRV |
Very low |
Weak |
|
D-dimer before neuroimaging |
Low |
Weak |
|
Thrombophilia screening is not recommended |
Very low |
Weak |
|
Screening for occult malignancy is not recommended |
Very low |
Weak |
|
Treatment with heparin at a therapeutic dose, including those with ICH |
Moderate |
Strong |
|
Use of LMWH instead of UFH |
Low |
Weak |
|
No recommendation on thrombolysis |
Very low |
Uncertain |
|
Oral VKA for 3-12 months |
Very low |
Weak |
|
No recommendation for using novel oral anticoagulants |
Very low |
Weak |
|
No recommendation on therapeutic lumbar puncture |
Very low |
Uncertain |
|
Acetazolamide and steroids are not recommended |
Low |
Weak |
|
Shunting alone is not recommended |
Very low |
Uncertain |
|
Decompressive surgery in impending herniation |
Low |
Strong |
|
Antiepileptic drugs in supratentorial lesions and seizures |
Very low |
Uncertain |
|
LMWH in pregnant and puerperal patients |
Low |
Weak |
|
OCP use is not advised in women of fertile age and prior CVT |
Very low |
Weak |
|
LMWH during pregnancy or puerperium with a previous history of CVT |
Very low |
Weak |
The AHA/ASA and ESO guidelines recommend LMWH followed by oral VKAs for 3 to 12 months for transient risk factors or an indefinite period for significant risk factors for thrombosis or recurrent VTE. The duration of parenteral anticoagulation remains unspecified. DOACs are a reasonable alternative for oral anticoagulation in selected cohorts, as demonstrated by the RE-SPECT CVT, SECRET, and ACTION-CVT trials, showing no significant differences in VTE recurrence, bleeding events, or recanalization rates.
Antiplatelet therapy after discontinuation of oral anticoagulation is determined based on patient characteristics and through a shared decision-making process. EVT is recommended for patients who have progressive clinical deterioration or who have failed standard therapy or have contraindications to it. Decompressive craniectomy is warranted only as a life-saving measure in patients with impending herniation.
Differential Diagnosis
The differential diagnosis for CVST includes several conditions that present with similar symptoms. Such conditions include the following:
- Acute SAH
- Acute stroke
- Internal carotid artery dissection
- Cerebral vasculitis
- Meningoencephalitis
- Space-occupying lesion
- Preeclampsia or eclampsia
- Reversible vasoconstriction syndrome
- Pituitary apoplexy
- Benign intracranial hypertension
- Acute metabolic encephalopathies [38][39]
Besides these clinical mimics, filling defects within the dural sinuses must be considered in the differential. These defects can arise from artefactual causes, hypoplastic venous sinuses, or anatomical variations such as venous septations or arachnoid granulations.[40] Careful evaluation and consideration of these differential diagnoses are critical for accurate diagnosis and appropriate management of CVST. A comprehensive clinical and imaging assessment helps distinguish CVST from other conditions with similar presentations.
Prognosis
The natural history of CVST is highly variable, with some patients experiencing minimal symptoms and a full recovery, while others face a rapid, severe course leading to significant dependency or death. While almost 90% of individuals with CVST achieve clinical recovery with good functional independence, nearly 40% continue to experience persistent headache, fatigue, and cognitive or mood impairments even 6 months postevent, hindering a full return to their previous level of activity. Overall mortality is approximately 3%, with nearly half of those cases linked to hemorrhagic complications.[41]
Factors such as advanced age, altered consciousness at presentation, concurrent neurological deficits, intracerebral hemorrhage, high infarction burden, and lymphopenia are associated with worse outcomes.[42] A poor prognosis is also indicated by involvement of the posterior 2/3 of the SSS, confluence, straight sinus, or deep venous system, with an odds ratio of 6 for modified Rankin Scale (mRS) scores of at least 1.[43] Thrombolysis, particularly for CVST cases involving the deep venous system, has been shown to improve clinical and radiological outcomes significantly.[44]
Clinical scoring systems such as the Cerebral venOus thrombosis DEterioration (CODE) score are used to guide prognosis and risk assessment. A comprehensive classification integrating clinical, radiological, and prognostic data remains crucial for managing CVST and predicting patient outcomes.
Complications
Complications of CVST include seizures, occurring in 10% to 25% of cases, with an increased propensity following involvement of the SSS and Trolard vein.[45] ICH complicates nearly 40% of cases, with diverse patterns of involvement such as intraventricular hemorrhage, SAH, and subdural hematoma.[46] Other complications include intracranial hypertension, dural arteriovenous fistula (DAVF), and recurrent CVT, which occurs in 1% to 4% of cases annually. Mortality remains a significant concern, though the rate is relatively low.
Deterrence and Patient Education
CVST is a rare but potentially life-threatening condition requiring proactive prevention and patient education. Prevention strategies target managing risk factors such as coagulopathy, dehydration, and hormonal contraceptive use, alongside promoting lifestyle changes like regular exercise and hydration.
Patient education plays a critical role in empowering individuals to recognize early signs and symptoms, including headaches, visual disturbances, and neurological deficits. Early detection facilitates timely medical intervention, making it crucial to emphasize adherence to anticoagulation therapy, necessary lifestyle changes, and regular follow-up appointments. These efforts help reduce complications and improve outcomes.
Through increased awareness, healthcare providers can significantly enhance patient safety and lower CVST incidence in at-risk populations. Collaboration between providers and patients fosters prevention and ensures timely intervention in CVST cases.
Enhancing Healthcare Team Outcomes
The diagnosis and management of CVST require a collaborative approach from an interprofessional team, including neurologists, neurosurgeons, radiologists, hematologists, anesthesiologists, intensive care nurses, and intensivists. Additional team members, such as nursing staff, midlevel practitioners (eg, nurse practitioners and physician assistants), and pharmacists, also play essential roles.
Initial management focuses on addressing life-threatening complications like increased ICP, seizures, and coma. Attention shifts afterward to specific therapies, including anticoagulation and, when necessary, catheter-directed fibrinolysis or surgical thrombectomy. The natural history of CVST varies widely, with some patients experiencing minimal symptoms and a smooth recovery, while others may face a rapid decline, leading to dependency or death.[47][48]
Media
(Click Image to Enlarge)
(Click Image to Enlarge)
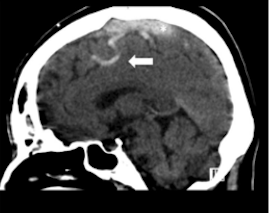
Cord Sign. A direct sign of cerebral venous thrombosis is the cord sign, a curvilinear hyperdensity within a cortical vein in the presence of thrombosis that can be seen for up to 2 weeks following thrombus formation.
Alshurafa S, Alfilfil W, Alshurafa A, Alhashim K. Cerebral venous sinus thrombosis in a young female misdiagnosed as migraine ending in a permanent vegetative state: a case report and review of the literature. J Med Case Rep. 2018;12:323. doi: 10.1186/s13256-018-1846-1.
(Click Image to Enlarge)
(Click Image to Enlarge)
References
Rawala MS, Noorani MM, Gulati R, Waqas S, Dave D. Elevated Factor VIII Level Associated with Transverse Cerebral Venous Sinus Thrombosis. The American journal of case reports. 2019 Mar 2:20():274-277. doi: 10.12659/AJCR.913917. Epub 2019 Mar 2 [PubMed PMID: 30824680]
Level 3 (low-level) evidenceFarooq S, Testai FD. Neurologic Complications of Sickle Cell Disease. Current neurology and neuroscience reports. 2019 Feb 28:19(4):17. doi: 10.1007/s11910-019-0932-0. Epub 2019 Feb 28 [PubMed PMID: 30820687]
Hiltunen S, Putaala J, Haapaniemi E, Tatlisumak T. Long-term outcome after cerebral venous thrombosis: analysis of functional and vocational outcome, residual symptoms, and adverse events in 161 patients. Journal of neurology. 2016 Mar:263(3):477-84. doi: 10.1007/s00415-015-7996-9. Epub 2016 Jan 2 [PubMed PMID: 26725090]
Preter M, Tzourio C, Ameri A, Bousser MG. Long-term prognosis in cerebral venous thrombosis. Follow-up of 77 patients. Stroke. 1996 Feb:27(2):243-6 [PubMed PMID: 8571417]
Zhu L, Cheng J, Gu P, Liu Y, Liu J, Wang J, Shen H. Therapeutic strategies of thromboembolic events in patients with inflammatory bowel diseases: Two case reports. Medicine. 2019 Mar:98(9):e14622. doi: 10.1097/MD.0000000000014622. Epub [PubMed PMID: 30817579]
Level 3 (low-level) evidenceTekesin A, Tunç A. Inflammatory markers are beneficial in the early stages of cerebral venous thrombosis. Arquivos de neuro-psiquiatria. 2019 Feb:77(2):101-105. doi: 10.1590/0004-282X20190001. Epub [PubMed PMID: 30810594]
Ulivi L, Squitieri M, Cohen H, Cowley P, Werring DJ. Cerebral venous thrombosis: a practical guide. Practical neurology. 2020 Oct:20(5):356-367. doi: 10.1136/practneurol-2019-002415. Epub [PubMed PMID: 32958591]
Pandit MV, Frishman WH. Cerebral Venous Sinus Thrombosis During Pregnancy and the Postpartum Period: A Review of Pathophysiological Mechanisms, Clinical Manifestations, and Treatment Approaches. Cardiology in review. 2025 Feb 12:():. doi: 10.1097/CRD.0000000000000853. Epub 2025 Feb 12 [PubMed PMID: 39928512]
Leite J, Ribeiro A, Gonçalves D, Sargento-Freitas J, Trindade L, Duque V. Cerebral Venous Thrombosis as Rare Presentation of Herpes Simplex Virus Encephalitis. Case reports in infectious diseases. 2019:2019():7835420. doi: 10.1155/2019/7835420. Epub 2019 Jan 17 [PubMed PMID: 30800483]
Level 3 (low-level) evidenceTaneda K, Adachi T, Watanabe Y, Hanajima R. Cerebral Venous Thrombosis due to Nontyphoidal Salmonella Bacteremia. Internal medicine (Tokyo, Japan). 2019 Jul 1:58(13):1943-1946. doi: 10.2169/internalmedicine.2266-18. Epub 2019 Feb 25 [PubMed PMID: 30799361]
Kasiviswanathan G, Sivashanmugam S, Bakthavatchalam R, Gaur A, Sugunakar Reddy K, Varatharajan S. Clinical Profile of Cerebral Sinus Venous Thrombosis: A Prospective Observational Study in South India. The Kurume medical journal. 2025 Mar 7:():. doi: 10.2739/kurumemedj.MS7112012. Epub 2025 Mar 7 [PubMed PMID: 40058869]
Level 2 (mid-level) evidenceMikulenka P, Peisker T, Vasko P, Stetkarova I. Diagnosis of cerebral venous thrombosis: a single centre experience. Neuro endocrinology letters. 2019 Jan:39(6):473-479 [PubMed PMID: 30796798]
Level 3 (low-level) evidenceMartín-Masot R, Ortiz Pérez P, Serrano Nieto J, Martínez León M, Pascual Martínez A, Blasco-Alonso J, Navas-López VM. Central Venous Sinus Thrombosis in a Boy With Acute Severe Ulcerative Colitis. Frontiers in pediatrics. 2019:7():19. doi: 10.3389/fped.2019.00019. Epub 2019 Feb 1 [PubMed PMID: 30775357]
Saposnik G, Bushnell C, Coutinho JM, Field TS, Furie KL, Galadanci N, Kam W, Kirkham FC, McNair ND, Singhal AB, Thijs V, Yang VXD, American Heart Association Stroke Council; Council on Cardiopulmonary, Critical Care, Perioperative and Resuscitation; Council on Cardiovascular and Stroke Nursing; and Council on Hypertension. Diagnosis and Management of Cerebral Venous Thrombosis: A Scientific Statement From the American Heart Association. Stroke. 2024 Mar:55(3):e77-e90. doi: 10.1161/STR.0000000000000456. Epub 2024 Jan 29 [PubMed PMID: 38284265]
Maghbooli M, Kermani M, Sany SNT, Arfaei M. Determining the Diagnostic Value of Venous Sinus Density Indices in Non-Contrast Brain CT Scan for Early Diagnosis of Cerebral Venous Sinus Thrombosis. Brain and behavior. 2025 Feb:15(2):e70324. doi: 10.1002/brb3.70324. Epub [PubMed PMID: 39935195]
Vojjala N, Peshin S, Jayakumar J, Kotla N, Dharia A, Balla M, Krishnamoorthy G. Re-optimizing the Time Frame for Classifying Cerebral Venous Sinus Thrombosis: An Unmet Need. Cureus. 2024 Dec:16(12):e75951. doi: 10.7759/cureus.75951. Epub 2024 Dec 18 [PubMed PMID: 39834963]
Aarju G, Birinder Singh P, Vipin K, Alisha S, Gunchan P. Neurological Predictors of Functional Outcome in Cortical Venous Sinus Thrombosis. Journal of neurosciences in rural practice. 2022 Apr:13(2):290-294. doi: 10.1055/s-0042-1744123. Epub 2022 Mar 10 [PubMed PMID: 35694057]
Weimar C, Holzhauer S, Knoflach M, Koennecke HC, Masuhr F, Mono ML, Niederstadt T, Nowak-Göttl U, Schellong SM, Kurth T. [Cerebral venous and sinus thrombosis : S2k guidelines]. Der Nervenarzt. 2019 Apr:90(4):379-387. doi: 10.1007/s00115-018-0654-6. Epub [PubMed PMID: 30758512]
Monagle P, Newall F. Management of thrombosis in children and neonates: practical use of anticoagulants in children. Hematology. American Society of Hematology. Education Program. 2018 Nov 30:2018(1):399-404. doi: 10.1182/asheducation-2018.1.399. Epub [PubMed PMID: 30504338]
Monagle P, Cuello CA, Augustine C, Bonduel M, Brandão LR, Capman T, Chan AKC, Hanson S, Male C, Meerpohl J, Newall F, O'Brien SH, Raffini L, van Ommen H, Wiernikowski J, Williams S, Bhatt M, Riva JJ, Roldan Y, Schwab N, Mustafa RA, Vesely SK. American Society of Hematology 2018 Guidelines for management of venous thromboembolism: treatment of pediatric venous thromboembolism. Blood advances. 2018 Nov 27:2(22):3292-3316. doi: 10.1182/bloodadvances.2018024786. Epub [PubMed PMID: 30482766]
Level 3 (low-level) evidenceWangqin R, Laskowitz DT, Wang Y, Li Z, Wang Y, Liu L, Liang L, Matsouaka RA, Saver JL, Fonarow GC, Bhatt DL, Smith EE, Schwamm LH, Prvu Bettger J, Hernandez AF, Peterson ED, Xian Y. International Comparison of Patient Characteristics and Quality of Care for Ischemic Stroke: Analysis of the China National Stroke Registry and the American Heart Association Get With The Guidelines--Stroke Program. Journal of the American Heart Association. 2018 Oct 16:7(20):e010623. doi: 10.1161/JAHA.118.010623. Epub [PubMed PMID: 30371291]
Level 2 (mid-level) evidenceHeldner MR, Zuurbier SM, Li B, Von Martial R, Meijers JCM, Zimmermann R, Volbers B, Jung S, El-Koussy M, Fischer U, Kohler HP, Schroeder V, Coutinho JM, Arnold M. Prediction of cerebral venous thrombosis with a new clinical score and D-dimer levels. Neurology. 2020 Aug 18:95(7):e898-e909. doi: 10.1212/WNL.0000000000009998. Epub 2020 Jun 23 [PubMed PMID: 32576633]
Yang KC, Xu Y, Lin Q, Tang LL, Zhong JW, An HN, Zeng YQ, Jia K, Jin Y, Yu G, Gao F, Zhao L, Tong LS. Explainable deep learning algorithm for identifying cerebral venous sinus thrombosis-related hemorrhage (CVST-ICH) from spontaneous intracerebral hemorrhage using computed tomography. EClinicalMedicine. 2025 Mar:81():103128. doi: 10.1016/j.eclinm.2025.103128. Epub 2025 Feb 26 [PubMed PMID: 40093990]
Dmytriw AA, Song JSA, Yu E, Poon CS. Cerebral venous thrombosis: state of the art diagnosis and management. Neuroradiology. 2018 Jul:60(7):669-685. doi: 10.1007/s00234-018-2032-2. Epub 2018 May 11 [PubMed PMID: 29752489]
van Dam LF, van Walderveen MAA, Kroft LJM, Kruyt ND, Wermer MJH, van Osch MJP, Huisman MV, Klok FA. Current imaging modalities for diagnosing cerebral vein thrombosis - A critical review. Thrombosis research. 2020 May:189():132-139. doi: 10.1016/j.thromres.2020.03.011. Epub 2020 Mar 19 [PubMed PMID: 32220779]
Boukobza M, Crassard I, Bousser MG, Chabriat H. MR imaging features of isolated cortical vein thrombosis: diagnosis and follow-up. AJNR. American journal of neuroradiology. 2009 Feb:30(2):344-8. doi: 10.3174/ajnr.A1332. Epub 2008 Dec 18 [PubMed PMID: 19095790]
Poon CS, Chang JK, Swarnkar A, Johnson MH, Wasenko J. Radiologic diagnosis of cerebral venous thrombosis: pictorial review. AJR. American journal of roentgenology. 2007 Dec:189(6 Suppl):S64-75 [PubMed PMID: 18029905]
Ferro JM, Coutinho JM, Dentali F, Kobayashi A, Alasheev A, Canhão P, Karpov D, Nagel S, Posthuma L, Roriz JM, Caria J, Frässdorf M, Huisman H, Reilly P, Diener HC, RE-SPECT CVT Study Group. Safety and Efficacy of Dabigatran Etexilate vs Dose-Adjusted Warfarin in Patients With Cerebral Venous Thrombosis: A Randomized Clinical Trial. JAMA neurology. 2019 Dec 1:76(12):1457-1465. doi: 10.1001/jamaneurol.2019.2764. Epub [PubMed PMID: 31479105]
Level 1 (high-level) evidenceKoike T, Tsuchiya T, Takenobu A, Teraoka A. Sensory Aphasia After Cerebral Venous Sinus Thrombosis: Quantitative Evaluation of the Recovery Process of Language Function Using Tractography. Cureus. 2025 Mar:17(3):e80628. doi: 10.7759/cureus.80628. Epub 2025 Mar 15 [PubMed PMID: 40230757]
Yaghi S, Shu L, Bakradze E, Salehi Omran S, Giles JA, Amar JY, Henninger N, Elnazeir M, Liberman AL, Moncrieffe K, Lu J, Sharma R, Cheng Y, Zubair AS, Simpkins AN, Li GT, Kung JC, Perez D, Heldner M, Scutelnic A, Seiffge D, Siepen B, Rothstein A, Khazaal O, Do D, Kasab SA, Rahman LA, Mistry EA, Kerrigan D, Lafever H, Nguyen TN, Klein P, Aparicio H, Frontera J, Kuohn L, Agarwal S, Stretz C, Kala N, El Jamal S, Chang A, Cutting S, Xiao H, de Havenon A, Muddasani V, Wu T, Wilson D, Nouh A, Asad SD, Qureshi A, Moore J, Khatri P, Aziz Y, Casteigne B, Khan M, Cheng Y, Mac Grory B, Weiss M, Ryan D, Vedovati MC, Paciaroni M, Siegler JE, Kamen S, Yu S, Leon Guerrero CR, Atallah E, De Marchis GM, Brehm A, Dittrich T, Psychogios M, Alvarado-Dyer R, Kass-Hout T, Prabhakaran S, Honda T, Liebeskind DS, Furie K. Direct Oral Anticoagulants Versus Warfarin in the Treatment of Cerebral Venous Thrombosis (ACTION-CVT): A Multicenter International Study. Stroke. 2022 Mar:53(3):728-738. doi: 10.1161/STROKEAHA.121.037541. Epub 2022 Feb 10 [PubMed PMID: 35143325]
Yaghi S, Saldanha IJ, Misquith C, Zaidat B, Shah A, Joudi K, Persaud B, Abdul Khalek F, Shu L, de Havenon A, Mistry EA, Bakradze E, Goldstein ED, Reagan J, Theodorou A, Palaiodimou L, Furie K, Field TS, Tsivgoulis G, Mac Grory B. Direct Oral Anticoagulants Versus Vitamin K Antagonists in Cerebral Venous Thrombosis: A Systematic Review and Meta-Analysis. Stroke. 2022 Oct:53(10):3014-3024. doi: 10.1161/STROKEAHA.122.039579. Epub 2022 Aug 8 [PubMed PMID: 35938419]
Level 1 (high-level) evidenceBrakel BA, Rebchuk AD, Ospel J, Chen Y, Heran MK, Goyal M, Hill MD, Miao Z, Huo X, Sacco S, Yaghi S, Mai TD, Thomalla G, Boulouis G, Yamagami H, Hu W, Nagel S, Puetz V, Kristoffersen ES, Demeestere J, Qiu Z, Abdalkader M, Al Kasab S, Siegler JE, Strbian D, Fischer U, Coutinho J, Munckhof A, Aguiar de Sousa D, Campbell BC, Raymond J, Ji X, Saposnik G, Nguyen TN, Field TS. International practice patterns and perspectives on endovascular therapy for the treatment of cerebral venous thrombosis. International journal of stroke : official journal of the International Stroke Society. 2025 Mar:20(3):319-327. doi: 10.1177/17474930241304206. Epub 2024 Dec 17 [PubMed PMID: 39569543]
Level 2 (mid-level) evidenceAguiar de Sousa D, Lucas Neto L, Arauz A, Sousa AL, Gabriel D, Correia M, Gil-Gouveia R, Penas S, Carvalho Dias M, Correia MA, Carvalho M, Canhão P, Ferro JM. Early Recanalization in Patients With Cerebral Venous Thrombosis Treated With Anticoagulation. Stroke. 2020 Apr:51(4):1174-1181. doi: 10.1161/STROKEAHA.119.028532. Epub 2020 Mar 2 [PubMed PMID: 32114929]
Siddiqui FM, Weber MW, Dandapat S, Scaife S, Buhnerkempe M, Ortega-Gutierrez S, Aksan N, Elias A, Coutinho JM. Endovascular Thrombolysis or Thrombectomy for Cerebral Venous Thrombosis: Study of Nationwide Inpatient Sample 2004-2014. Journal of stroke and cerebrovascular diseases : the official journal of National Stroke Association. 2019 Jun:28(6):1440-1447. doi: 10.1016/j.jstrokecerebrovasdis.2019.03.025. Epub 2019 Apr 2 [PubMed PMID: 30952531]
Canhão P, Falcão F, Ferro JM. Thrombolytics for cerebral sinus thrombosis: a systematic review. Cerebrovascular diseases (Basel, Switzerland). 2003:15(3):159-66 [PubMed PMID: 12646773]
Level 1 (high-level) evidenceFerro JM, Canhão P, Stam J, Bousser MG, Barinagarrementeria F, ISCVT Investigators. Prognosis of cerebral vein and dural sinus thrombosis: results of the International Study on Cerebral Vein and Dural Sinus Thrombosis (ISCVT). Stroke. 2004 Mar:35(3):664-70 [PubMed PMID: 14976332]
Level 2 (mid-level) evidenceSaposnik G, Barinagarrementeria F, Brown RD Jr, Bushnell CD, Cucchiara B, Cushman M, deVeber G, Ferro JM, Tsai FY, American Heart Association Stroke Council and the Council on Epidemiology and Prevention. Diagnosis and management of cerebral venous thrombosis: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2011 Apr:42(4):1158-92. doi: 10.1161/STR.0b013e31820a8364. Epub 2011 Feb 3 [PubMed PMID: 21293023]
Borhani-Haghighi A, Hooshmandi E. Cerebral venous thrombosis: a practical review. Postgraduate medical journal. 2024 Jan 21:100(1180):68-83. doi: 10.1093/postmj/qgad103. Epub [PubMed PMID: 37978050]
Moriles KE, Lockhart C. Isolated Cortical Venous Thrombosis. StatPearls. 2025 Jan:(): [PubMed PMID: 35593823]
Zedde M, Pascarella R. Non-Thrombotic Filling Defects in Cerebral Veins and Sinuses: When Normal Structures Mimic a Disease. Neurology international. 2025 Jan 17:17(1):. doi: 10.3390/neurolint17010009. Epub 2025 Jan 17 [PubMed PMID: 39852773]
Sitthilok P, Niprapan P, Tantiworawit A, Punnachet T, Hantrakun N, Piriyakhuntorn P, Rattanathammethee T, Hantrakool S, Rattarittamrong E, Norasetthada L, Chai-Adisaksopha C. Clinical course and neurological outcomes of cerebral venous sinus thrombosis: A single center retrospective observational study. PloS one. 2025:20(1):e0316849. doi: 10.1371/journal.pone.0316849. Epub 2025 Jan 13 [PubMed PMID: 39804849]
Level 2 (mid-level) evidenceLin L, Liu S, Wang W, He XK, Romli MH, Rajen Durai R. Key prognostic risk factors linked to poor functional outcomes in cerebral venous sinus thrombosis: a systematic review and meta-analysis. BMC neurology. 2025 Feb 6:25(1):52. doi: 10.1186/s12883-025-04059-x. Epub 2025 Feb 6 [PubMed PMID: 39915720]
Level 1 (high-level) evidenceFeng Y, Mo S, Li X, Jiang P, Wu J, Li J, Liu P, Wang S, Liu Q, Tong X. Development and validation of a score for clinical deterioration in patients with cerebral venous thrombosis. Neurosurgical review. 2025 Jan 16:48(1):56. doi: 10.1007/s10143-025-03224-7. Epub 2025 Jan 16 [PubMed PMID: 39815140]
Level 1 (high-level) evidenceMorel B, Hoffman J, Roark C, Folzenlogen Z, Seinfeld J, Case D. Endovascular treatment of cerebral venous thrombosis involving the deep venous system. Interventional neuroradiology : journal of peritherapeutic neuroradiology, surgical procedures and related neurosciences. 2025 Mar 28:():15910199251330723. doi: 10.1177/15910199251330723. Epub 2025 Mar 28 [PubMed PMID: 40152202]
Level 3 (low-level) evidenceAmini Harandi A, Jafari Khaljiri H, Jahangiri Zarkani N, Pakdaman H, Khalili N. Exploring Seizure Risks in Cerebral Venous Sinus Thrombosis Based on Thrombosis Site. The neurologist. 2025 Apr 7:():. doi: 10.1097/NRL.0000000000000625. Epub 2025 Apr 7 [PubMed PMID: 40191892]
Jha S, Kulanthaivelu K, Raja P, Kenchiah R, Ramakrishnan S, Kulkarni GB, Asranna A. Spectrum of Intracranial Hemorrhages in Cerebral Venous Thrombosis: A Pictorial Case Series and Review of Pathophysiology and Management. The neurologist. 2025 Jan 1:30(1):45-51. doi: 10.1097/NRL.0000000000000604. Epub 2025 Jan 1 [PubMed PMID: 39618313]
Level 2 (mid-level) evidenceXu Y, Meng R, Rajah GB, Ding Y, Wu Y, Wu Y, Ji K, Wu C, Zhao W, Ji X. Long-term Outcomes of Cerebral Venous Sinus Stenosis Corrected by Stenting. Current neurovascular research. 2019:16(1):77-81. doi: 10.2174/1567202616666190206185133. Epub [PubMed PMID: 30727893]
Lal D, Gujjar AR, Ramachandiran N, Obaidi A, Kumar S, El-Tigani M, Al-Azri F, Al-Asmi AR. Spectrum of Cerebral Venous Thrombosis in Oman. Sultan Qaboos University medical journal. 2018 Aug:18(3):e329-e337. doi: 10.18295/squmj.2018.18.03.011. Epub 2018 Dec 19 [PubMed PMID: 30607274]