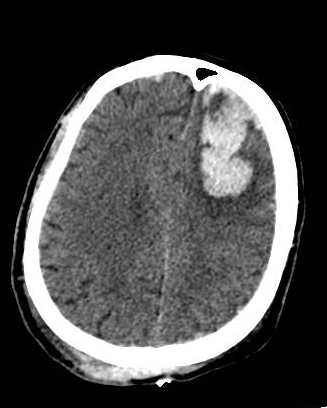
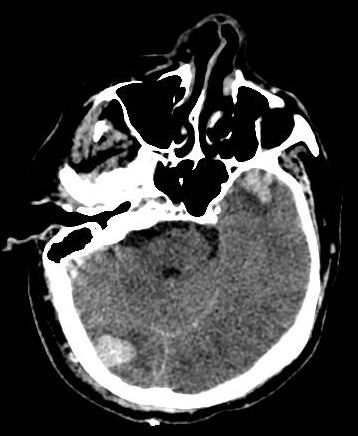
Introduction
Traumatic brain injury (TBI) is considered the most disabling of traumatic phenomena, almost always encompassing lifelong emotional, behavioral, and permanent physical impairment.[1][2] Nearly 50% of hospitalized survivors of TBI experience long-term disabilities. TBI encompasses several types of brain injuries, with hemorrhagic cerebral contusion being one of the most severe damage mechanisms. Cerebral contusions in patients with TBI are associated with an increased risk of disability and mortality.
Cerebral contusions cause permanent damage to the tissues of the brain. The severity of the damage is related to the primary injury initiated by the kinetic energy absorbed during the collision and the cascade of secondary injury responses that exacerbate the primary damage. The hemorrhagic lesion is produced in the immediate moments after the head impact.[3] These contusions extend to variable depths within the cortical gyri and invariably into subcortical regions, ranging from tiny punctate lesions to large lesions causing brain herniation.
Contusions can progress and expand, and, in many cases, other hemorrhagic contusions are present. Hemorrhagic contusions overlie brain parenchyma, which can invariably cause loss of associated function. Because blood is highly toxic to healthy brain tissue, cerebral contusions represent one of the most damaging forms of secondary injury in TBI. Brain contusions have been associated with bleeding from the continuous flow of injured microvessels during the initial traumatic episode. This concept suggests that the formation of a contusion may be due to an underlying or overt coagulopathy.
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
The etiology of cerebral contusion is trauma to the head. Most of the time, it is a closed head injury, although open injuries can also result in contusions. Various mechanisms can cause such trauma, including:
- Road traffic accidents (motor vehicle, motorcycle, and pedestrian)
- Falls
- Physical aasault
- Recreation-related head injuries
- Sports injury
- Domestic violence
- Child abuse
- Blast injury
- Penetrating head injuries (missiles and non-missiles)
Epidemiology
TBI is a significant global cause of disability, death, and economic cost. In the United States, approximately 2.8 million TBIs occur annually, with one-third affecting children.[1][2] Over 250,000 patients are hospitalized for nonfatal TBIs, including 10% hospitalizations in children. Over 2.5 million patients are treated in the emergency department, with children comprising 25% of these cases. More than 50,000 patients die from TBI, with 4.5% of deaths related to children.[1] Fatalities in children are more common with falls and road traffic accidents.[4] The mortality rate has steadily declined over the past decades due to improvements in patient management.[5]
The most common sports-related injuries resulting in emergency department visits are superficial injuries and cerebral contusions.[6] One year after TBI hospitalization, 43% of Americans have a residual disability, including physical, cognitive, behavioral, and psychosocial impairments; the prevalence of residents living with disabilities is 3.2 million.[1][2] The prevalence of self-reported head injury among adults aged 40 or older is 15.7% in the United States.[7] The rate is higher in men (20.0%) compared to women (12.0%), with non-Hispanic White respondents showing the highest (18.0%) compared to non-Hispanic Black respondents (8.9%).[7]
TBI leads to 1 million hospitalizations per year in the European Union, accounting for the most deaths (50,000) following road traffic accidents.[8][9] Unfortunately, three-fourths of these victims are young people. A Chinese head trauma data bank has shown important information regarding cerebral contusions. The mortality rates of patients with no cerebral contusions, single cerebral contusions, and multiple cerebral contusions are 3.9%, 7.8%, and 14.8%, respectively. The mortality rate of patients with and without traumatic subarachnoid hemorrhage was 9.5% and 5.4%, respectively. The mortality rates for patients with no intracranial hematomas, single intracranial hematomas, and multiple intracranial hematomas were 5.8%, 8.4%, and 20.6%, respectively.[10] Approximately 51.6% of the patients had a cerebral contusion, and 49.9% had a traumatic subarachnoid hemorrhage.[10]
TBI related to recreation most commonly involved cerebral contusion in 29% of the cases, followed by a traumatic subarachnoid hemorrhage in 26%, subdural hematoma in 25%, and epidural hematoma in 23%.[11] In the Netherlands, road traffic accidents resulted in a contusion incidence of 46.6% among cyclists and 74.2% among motorcyclists.[12] A cerebral contusion occurs in over 50% of patients with severe TBI.[12] In adults older than 60 with TBI, cerebral contusions arise in approximately 18% of them.[13] Pediatric TBI frequently shows brain edema (72.9%), skull fracture (69.5%), and brain contusion (55.9%).[14]
The risk of a male patient having a TBI is double that of a female patient. However, after an injury is sustained, there is no statistically significant difference in mortality rates between male (7.5%) and female (7.2%) patients with TBI.[10]
Pathophysiology
Cerebral contusions are typically observed in the temporal and frontal lobes, although other sites can be affected through coup (beneath the impact) and contrecoup (opposite to the impact) mechanisms. The relative motion between the brain and skull produces compressive strains and skull deformation, most pronounced in the orbital roof, the temporal squama, and the ala major of the sphenoid bone.[15]
Different Forms of Cerebral Contusions
- Coup contusions: These contusions result from cavitation that arises due to the elastic movement of the skull following an impact. The negative pressures at the impact site may lead to the formation of gas bubbles, which then collapse rapidly, causing damage to the brain tissue.
- Contrecoup contusions: The distribution of contusions in this category does not align with the anticipated areas of negative pressure as proposed by cavitation theory. Ommaya has attributed these contusions to shear strains and deformation of the skull that act upon bony protuberances.
- Fracture contusions: These contusions occur as a result of intruding bone fragments and associated cavitation that follows blunt trauma.
- Gliding contusions: Frequently observed in the parasagittal regions and associated with diffuse axonal injury.
Theories Explaining the Formation of Cerebral Contusions
- Vibration theory: According to this theory, an impact generates vibratory waves that exert significant force on the contralateral side of the skull.
- Transmitted wave theory: This theory posits that a wave originating at the impact point can create a vacuum, leading to the disruption of blood vessels and resulting damage to the brain parenchyma.
- Brain displacement theory: This theory advocates that the momentum generated by acceleration-deceleration dynamics can cause the brain to detach from its supporting attachments.
- Skull deformation theory: This theory examines the alterations in skull structure that occur during impact events.
- Pressure gradient theory: This theory suggests that movement between the brain and the skull generates intracranial cavitation as a consequence of a localized, nonuniform pressure gradient.
- Rotational shear force theory: This theory illustrates that rapid rotational movements of the head may contribute to the occurrence of contusive injuries.
Brain contusions are lesions with a hemorrhagic character that typically begin in the brain's cortical area and, more commonly, at the crests of the gyri of the cerebrum. These lesions can progress to the subcortical white matter in the more severe forms of injury. Despite the primary cortical area's preference, they can also develop at the white-gray border with expansion into the overlying gray matter. The occurrence of hemorrhage within the contusion can lead to ischemia and edema in the local area, which progresses to tissue destruction, neuronal necrosis, and cavitation, accompanied by overlying reactive gliosis.
There are many instances where the progression and expansion of the hemorrhagic components in the areas surrounding the initial contusion have been observed. Contusion progression occurred with a frequency of 63% to 70%.[16][17] Progression typically occurs within the first 12 hours but may also develop within 3 to 4 days after TBI.[18] Historically, it was postulated that the hemorrhagic brain contusion progression occurred secondary to a coagulopathic event associated with TBI.[18][19][20]
Endothelial injury and ruptured microvessels have been implicated in this process. More recently, the concept of traumatic penumbra has been postulated.[15]
In TBI, the contused brain is a major source of tissue factor release. Recent studies have proposed that the cerebrum's vasculature is mechanosensitive, activating endothelial cells in the penumbra that were not initially damaged by the contusion core. In this situation, the contusion penumbra experiences activation of 2 transcription factors (endothelial mechanosensitive mechanisms), which are the specificity protein 1 (transcription factor 1) and nuclear factor kappa B.[21] The importance of these factors in controlling sulfonylurea receptor 1-transient receptor potential melastatin 4, which forms the regulatory subunit of the NCCa-ATP channel, is of interest in the context of the damage cascade.[22][23][24][25]
The nuclear factor kappa B leads to apoptosis, whereas the specificity factor 1 causes vascular fragmentation. Both factors are associated with dysfunction or damage to vascular entities, resulting in microvascular failure and endothelial necrosis.[18][26] Contusion progression risk factors independently associated with the hematoma growth included an initial volume, cisternal compression, decompressive craniectomy, age, falls as the mechanism of trauma, multiple hematomas, and hypoxia.[16]
The initial trauma from the brain injury can lead to immediate cell death through necrosis. The cell lyses and releases harmful substances, including inflammatory chemokines and cytokines, reactive oxygen species, and proteases. The release of inflammatory mediators, including pro-inflammatory cytokines, as well as the breakdown of the blood-brain barrier, leads to the development of cerebral edema. Secondary injury from excitatory amino acids, including glutamate and aspartate, increased intercellular cytosolic calcium concentration, acidosis, and free radical production. Glutamate excitotoxicity can cause persistent membrane depolarization, which activates ion channels, particularly N-methyl-D-aspartate channels. Secondary to the permanent opening of N-methyl-D-aspartate channels, sodium and calcium ions enter cells, whereas potassium ions are extruded into the extracellular space.[27] A shift of potassium into the extracellular space results in rapid swelling of astrocytes, which absorb potassium to preserve ionic homeostasis.[28]
Free radicals cause damage to genetic material, leading to the downregulation of genes and mitochondrial disruption. This disruption results in ion dyshomeostasis and consequent neuronal and astrocytic swelling, ultimately leading to cell death. Once the autoregulatory mechanisms have been abolished, cerebral blood flow passively follows changes in arterial blood pressure and impaired cerebral pressure. In the central core area, cerebral blood flow is 4.7 mL/100 g/min, whereas in the peripheral zone, it ranges from 16 to 18 mL/100 g/min.[28] Irreversible neuronal death occurs when cerebral blood flow is below 8 cc/100g/min, whereas neurons can recover if they have a cerebral blood flow of 9 to 18 cc/100g/min.[29]
Brain edema formation begins with vasogenic edema, resulting from the breakdown of the blood-brain barrier and the extravasation of fluid into the extracellular space, which typically occurs within 12 to 24 hours after injury. Then, a secondary injury caused by a cascade of mechanisms initiated at the moment of injury sets in after 24 to 72 hours and progresses for 7 to 10 days. A third phase occurs with the lysis of red blood cells in the intracerebral clot.
Histopathology
Edema is a rapid response that typically begins within minutes of an injury, peaks over several days, and resolves within 6 days. In contrast, hemorrhage initiates and expands within hours, particularly around perivascular areas, reaching maximum accumulation within just 24 hours. Notably, red blood cells retain their viability for up to 5 to 6 months after the injury. Polymorphonuclear leukocytes act quickly, initiating their response within hours of the injury and remaining visible for up to a month. Within just 2 hours, degenerated neurons, including red neurons, become apparent, signaling the immediate impact of injury. Macrophages and scavenger cells emerge within 24 hours, reach their peak between 7 and 14 days, and then gradually decline, illustrating the body's dynamic response to the healing process. Hemosiderin and siderophages can be detected as early as 5 days post injury and can persist for years as indicators of the body's healing trajectory. Hematoidin pigment appears 10 to 12 days after the injury and persists for years within hemorrhagic lesions. Angiogenesis, the formation of new blood vessels, begins 5 to 7 days post injury and reaches its peak by 3 weeks, illustrating the body's remarkable capacity for regeneration. The astrocytic reaction involves initial protoplasmic astroglial proliferation starting 4 to 5 days post injury, followed by fibrillary astroglial proliferation from 7 to 10 days, culminating in the formation of a glial scar that is crucial for restoring the blood-brain barrier. Additionally, fibroblasts appear within 1 week of the injury and can persist for months or even years, playing a vital role in collagen production and the development of fibrosis. This complex interplay of cellular responses highlights the body's remarkable ability to respond to injury and facilitate healing.
History and Physical
An initial trauma assessment is performed. Rapid imaging studies, with identification of possible surgical injuries, are performed after adequate resuscitation. Proper immobilization should be maintained until the stability of the entire spine has been confirmed. The mechanism of trauma is obtained from the patient, relatives, or paramedics.
A detailed neurologic examination is performed. If sedation was given, it should be stopped for an adequate neurological assessment. The patient is evaluated for response to stimuli in eye, motor, and verbal forms. The Glasgow Coma Scale (GCS) was established by Teasdale and Jennett in 1974.[30] This tool helps obtain the best response from a patient and presents the status to the healthcare team. Additionally, GCS helps in comparing future evaluations. The pupillary response is critical in the initial assessment.
Evaluation
X-rays should routinely be performed for a trauma workup. Imaging of the entire spine, chest, and pelvis should be obtained along with any other evident bones involved. Laboratories should include a complete blood count, a comprehensive metabolic panel, an international normalized ratio, and toxicology testing. Arterial blood gases are required for intubated patients and those with respiratory distress.
A head computed tomography (CT) scan should be performed for patients with impaired consciousness or history of loss of consciousness, focal neurologic findings, persistent vomiting, seizures, and an abnormal GCS score.[31] Initially, the CT scan may show isodense or hypodense areas, sometimes mixed-density lesions, commonly surrounded by perilesional hypodense regions. However, as time progresses, more surrounding edema develops. Multiple focal contusions may have a salt-and-pepper appearance. The bone window is used for evaluating skull fractures. A minor contusion near the skull base may be missed due to partial volume averaging. Models such as Brain Lesion Analysis and Segmentation Tool for Computed Tomography (BLAST-CT) help better categorize and quantify the cerebral contusions.[32]
Brain magnetic resonance imaging (MRI) is not indicated in the acute management of TBI.[31] If acutely performed, there are isointense to hyperintense areas on T1-weighted images and hyperintense on T2-weighted images. Fluid-attenuated inversion recovery images demonstrate petechial hemorrhages that are isointense to the brain. The MRI can detect ischemic areas and changes associated with diffuse axonal injury. The MRI can also be used later for cognitive problems. The logistic barriers to acquiring and analyzing CT data are lower compared to MRI-based approaches in the acute phases of the cerebral contusions.[32]
Treatment / Management
A dictum is a stringent neurological assessment used to determine the level of consciousness. Assessment of pupillary size and GCS score is the most straightforward and effective approach for monitoring patients.[32] Preventing hypotension or hypoxia according to the Advanced Trauma Life Support guidelines is crucial. Optimising cerebral perfusion and monitoring intracranial pressure (ICP) is pivotal.[15] Tranexamic acid helps stabilise the contusions.
After the head CT scan is obtained, management is decided. In patients with no immediate neurosurgical lesions, the priority is managing ICP. Monitoring ICP is performed if the GCS is below 8. An external ventricular drain has the advantage of cerebrospinal fluid (CSF) drainage. Intraparenchymal devices can also be used. In patients with cerebral contusions, seizure prophylaxis is started for 7 days to prevent posttraumatic seizures. Cerebral perfusion pressure (CPP) is defined as the difference between the mean arterial pressure (MAP) and the ICP. CPP = MAP − ICP. The treatment goals are to maintain an ICP of under 20 mm Hg and a CPP of above 60 mm Hg. Patients with increased ICP are treated using sedation, diuresis, and hypertonic saline.
Some patients need immediate craniotomy for evacuation of the contusion; however, there is still debate about the value of removing intraparenchymal lesions. Indications for surgical removal include progressive neurological deterioration, signs of mass effect on brain CT, unresponsive increased ICP, midline deviation of more than 5 mm, cistern compression evidenced on brain CT, and temporal contusions of greater than 20 cc of volume.[33] In extreme cases, unresponsive to the usual mechanisms to reduce the ICP, decompressive craniectomy can be performed, resulting in lower mortality but also with more patients in a vegetative state.[34]
Hemorrhage/contusion progression in up to 87% of the cases is a potential complication of decompressive craniectomy.[17][35] Corticosteroids are not recommended for the management of increased ICP. Early enteral nutritional support should be started within 72 hours. The Brain Trauma Foundation has specific guideline recommendations, along with the level of evidence for each one, that can be used to develop treatment protocols.[36][37](B2)
The American College of Surgeons published a three-tiered protocol in 2015 for managing increased ICP.
Tier 1
- The head of the bed is elevated at a 30° angle.
- Sedation and analgesia using short-acting agents for intubated patients.
- External ventricular drain with intermittent drainage.
- Repeat head CT imaging if no improvement.
- If ICP remains ≥20 to 25 mm Hg, proceed to Tier 2.
Tier 2
- If using a parenchymal ICP device, change to an external ventricular drain.
- Hyperosmolar therapy (mannitol or hypertonic saline) is given intermittently.
- Mannitol is administered in intermittent boluses (0.25 to 1 g/kg body weight). Check the serum sodium and osmolality frequently; additional doses are held if the serum osmolality exceeds 320 mOsm/L.
- Hypertonic saline, a 3% sodium chloride solution, is administered in intermittent boluses of 250 mL over 0.5 hours (2-5 mL/kg over 10-20 minutes). Serum sodium and osmolality are monitored every 6 hours. Additional doses are held if the serum sodium level exceeds 160 mEq/L. A bolus of up to 23.4% sodium chloride solution can be given for refractory increased ICP.
- CPP no less than 50 mm Hg, with a target range of 60 to 70 mm Hg.
- PaCO2 should be maintained between 30 and 35 mmHg.
- Repeat head CT imaging if no improvement.
- Administer a test bolus of neuromuscular paralytic agent if indicated.
- If ICP remains ≥20 to 25 mm Hg, proceed to the next tier.
Tier 3
- Decompressive hemicraniectomy or bilateral craniectomy.
- Neuromuscular paralysis through continuous infusion (titrated to maintain at least 2 twitches out of a train-of-four). Adequate sedation must be utilized.
- Barbiturate or propofol coma. Hypotension is a common adverse effect. Continuous EEG is used to achieve burst suppression.
- Hypothermia below 36 °C is not indicated as an initial TBI treatment. Use only as salvage therapy after all previous Tier 3 treatments have failed.
Evolution in the Management Strategies for Cerebral Contusions
In the early 20th century, techniques such as gentle dehydration using magnesium sulfate and sodium chloride administered rectally, along with the removal of CSF, were commonly practiced.[15] Later, in the 1950s, hyperosmolar compounds such as urea were introduced, followed by mannitol and hypertonic saline in the 1960s. In the same decade, Nils Lundberg pioneered the use of a ventricular catheter for monitoring ICP. In 1981, Galbraith and Teasdale established ICP-based management guidelines. Harvey Cushing performed a subtemporal decompressive craniectomy in 1908, and Polin recommended performing decompressive craniectomy within 48 hours if ICP exceeded 40 mm Hg.[15] Multimodal monitoring emerged in the early 2000s, and currently, algorithm-based ICP management is the recommended approach.
According to the Brain Trauma Foundation guidelines and expert opinion from the Scandinavian Neurotrauma Committee, surgery is indicated under the following conditions:
- Contusion volume greater than 50 cm³
- GCS score of 6 to 8 with frontal or temporal contusions greater than 20 cm³, a midline shift greater than 5 mm, and cisternal compression
- Neurological deterioration and refractory high ICP [15]
Surgical strategies include focal lesionectomy and decompressive craniectomy.
Venous Thromboembolism Prophylaxis in Cerebral Contusion
Patients with TBI, particularly those with cerebral contusions, face a substantially elevated risk of venous thromboembolism with reported incidence rates approaching 25% in moderate-to-severe cases and exceeding 30% in specific high-risk cohorts.[36] This predisposition stems from a hypercoagulable state triggered by the release of tissue factor, endothelial injury, and prolonged immobilization, which collectively amplify thrombogenic mechanisms.[38][39] Current guidelines strongly advocate early initiation of pharmacological prophylaxis, barring contraindications, such as active intracranial hemorrhage or coagulopathy. Low-molecular-weight heparin has demonstrated superiority over unfractionated heparin for venous thromboembolism prevention in trauma populations, including those with TBI, due to its more predictable pharmacokinetics, reduced bleeding complications, and lower failure rates.[36] The optimal timing for chemoprophylaxis initiation remains nuanced, although contemporary evidence supports administration within 24 to 72 hours post injury, provided follow-up neuroimaging confirms hematoma stability. Mechanical prophylaxis, including intermittent pneumatic compression, should be employed during periods when pharmacological agents are contraindicated; however, its efficacy as monotherapy remains inferior to that of anticoagulant-based approaches. Geriatric patients and those with obesity or polytrauma warrant heightened vigilance given their compounded thrombotic risks. In contrast, paediatric protocols now preferentially recommend direct oral anticoagulants, such as dabigatran or rivaroxaban, over traditional agents based on 2025 guideline updates.[38] Critically underutilization persists with nearly 50% of eligible patients receiving delayed or suboptimal prophylaxis, contributing to preventable morbidity, including fatal pulmonary embolism, which frequently manifests within 5 to 7 days post injury.[40](B2)
Differential Diagnosis
After a traumatic event, cerebral contusions are typically distinguishable from other conditions. However, in rare instances, a patient may first have a nontraumatic hemorrhage, which then provokes a traumatic event, including:
- Traumatic intracerebral hemorrhage
- Hypertensive hemorrhagic stroke
- Lobar bleed from amyloid angiopathy
- Cerebral venous thrombosis with hemorrhagic transformation
- Intraparenchymal hemorrhage from a ruptured aneurysm
- Reperfusion injury in strokes
- Diffuse axonal injury
- Cerebral cavernoma
- Acute intratumoral bleed
- Hemorrhagic metastasis
Prognosis
Mortality increases as the GCS decreases. The most critical factors in the outcome are the patient's age and mechanism of injury.[41] A prolonged duration of coma is a strong predictor of long-term disability. For moderate head injuries, approximately 60% of the patients make a positive recovery, but 25% have a residual moderate degree of disability. In severe head injury cases, only 25% to 33% have positive outcomes. Patients may experience impaired concentration, attention, and memory. Cognitive deficits and personality changes may persist. A brain contusion is an essential factor in the development of posttraumatic seizures.[42]
The number of non-reactive pupils (0-2) has recently been used as a prognostic indicator in TBI.[43] A commonly used formula subtracts the number of non-reactive pupils from GCS. In patients with a GCS of 3 and no reactive pupils, the mortality rate 6 months after injury is 74%, and the unfavorable outcome is 90%. If one pupil is reactive, the mortality rate 6 months after injury is 47%, and the unfavorable outcome is 70%. If both pupils are reactive, the mortality rate 6 months after injury is 28%, and the unfavorable outcome is 50%. Very similar results are found in patients with a GCS of 4. For patients with a GCS of 5 and no reactive pupil, the mortality rate 6 months after injury is 54%, and the unfavorable outcome rate is 82%.
Increasing age is a significant factor for mortality and unfavorable recovery 6 months after TBI.[44] The same study found that the outcomes based on single CT findings, such as hematoma, cisterns, or subarachnoid hemorrhage, are very similar among patients. However, when the 3 findings are combined, a progression toward an unfavorable outcome is observed, based on the number of abnormalities present in the study.
The Glasgow Outcome Scale is used for outcome assessment, employing a 5-point scale.[45]
- Score 5—Good recovery: Minor disabilities may be present, but the individual can resume everyday daily life
- Score 4—Moderate disability: More significant disabilities are present, but the individual is still able to live independently. Individuals can use public transportation and work in an assisted situation.
- Score 3—Severe disability: The individual is conscious but dependent on others for daily care, often requiring institutional support.
- Score 2—Persistent vegetative state: The individual is not conscious, though eyes may be open and may track movement
- Score 1—Death
Classifications to predict outcomes on TBI have been developed and published. The Marshall CT classification evaluates midline shift, cistern patency, hematoma size, and whether surgery was performed.[46] The Rotterdam CT score assesses midline shift, cistern patency, presence of an epidural mass, and the presence of intraventricular or subarachnoid blood.[47][48] The Rotterdam CT score has been validated recently in the literature.[49]
The largest temporal contusions impose higher odds of dependency and mortality compared to similarly sized frontal contusions, due to the early risk of central descending transtentorial herniation.[32] Machine learning algorithms have also been devised to prognosticate clinical outcomes.[50] The outcome of surgery in cerebral contusions is derived from the Surgical Trial in Traumatic Intracerebral Hemorrhage (STITCH) randomized controlled trial and the CENTER-TBI study.[15] DECRA and RESCUEicp trials have shown increased dependency, though improved survival rates among cohorts undergoing decompressive surgery.[15]
Complications
Hemorrhagic Progression of Contusion
The reported prevalence of hemorrhagic progression of contusion (HPC) ranges from 16% to 75%, with up to 20% of the same requiring surgery, conferring high rates of morbidity and mortality.[51] This discrepancy may be due to the lack of a standardized definition of HPC, which ranges from a 5% to 50% increase in diameter to a 1 cm increase, and a 12.5 mL increase in volume. Various methods are employed for assessing contusion volume, including the ABC/2 formula, manual segmentation, and automated image segmentation. Additionally, some authors refer to this phenomenon as progressive hemorrhagic injury, encompassing any hemorrhagic lesions, such as subarachnoid hemorrhage and subdural hematoma, in conjunction with contusions.[52] HPC typically occurs within the first 24 hours and may last for up to 1 week.[50][52]
Pathophysiology of progression: Initially, it was believed that the progression of contusions resulted from fractured microvessels, which can be exacerbated by coagulopathy. Recently, the concept of traumatic penumbra surrounding the contusion core has been postulated, involving specificity protein 1 and nuclear factor kappa B.[52]
Risk variables and predictors for progression:
- Advanced age: Due to microvasculature fragility and reduced resting cerebral blood flow
- Low GCS at presentation
- Male sex: Estrogen and progesterone in females confer membrane stability (inhibiting lipid peroxidation) and decrease apoptosis (through Bcl-2 upregulation)
- Hypertension: Increased blood-brain barrier permeability. Chronic hypertension increases the lower limit of autoregulation through a rightward shift of the cerebral autoregulatory curve
- Smoking increases vessel fragility and reduces cerebral blood flow through vasospasm
- Alcohol impairs platelet function and minimizes vascular tone
- Hypoxia
- Coagulopathy: The most determining variables are the international normalized ratio and platelet count
- Low triglyceride levels (<150 mg/dL): Cholesterol is an essential constituent of the cell membrane
- Timing of the initial CT
- Baseline contusion characteristics:
- Volume ≤4 mL is unlikely to progress
- Location: Frontal contusions have a high predilection for progression
- Bilateral or multiple contusions can coalesce
- Coexisting lesions: Subdural hematoma, subarachnoid hemorrhage, or skull fractures involving cortical veins, bridging veins, and venous sinuses
- A Rotterdam score of 5 or higher
- Basal cistern compression, midline shift ≥5 mm
- Leakage sign in CTA
- Decompressive craniectomy: Loss of the tamponade effect
- Multihematoma fuzzy sign
- Low monocyte-to-lymphocyte ratio in CSF
- Low glucose-to-lactate ratio in CSF
- Use of antiplatelets [15][50][53][54]
Dual-energy CT (190 keV) is ideal for assessing the HPC.[52][55] HPC increases the length of surgical intervention, intensive care unit stay, ventilatory support, hospital stay, nosocomial infections, unfavorable clinical outcomes, and short- and long-term mortality.[52]
Other Complications
- Seizures: Early seizures are due to bleeding. The involved regions are later replaced with cavitated regions of gliosis and retracted plaque of hemosiderosis (plaque jaune), both of which are epileptogenic.
- Trauma-induced coagulopathy
- Acute and chronic hydrocephalus
- Dysautonomia
- Dyselectrolyetemia: Cerebral salt wasting and syndrome of inappropriate antidiuretic hormone secretion
- Superficial siderosis
- Persistent neurological deficits
- Pseudoaneurysm
- Vasospsam
- Dependency
- Coma
- Death
- Employment disability
- Impaired concentration, attention, and memory
- Cognitive deficits
- Personality changes
- Posttraumatic stress disorder and other mental health issues
- Chronic traumatic encephalopathy
- Complications related to chronic ICU stay
- Complications related to general anesthesia and surgery [15][53]
Consultations
Consultation with an interprofessional team, including the emergency clinician, trauma surgeon, neurosurgeon, intensivist, neuroradiologist, respiratory therapist, and specialists in physical and rehabilitation medicine, is essential for comprehensive management, timely intervention, and optimizing both acute and long-term outcomes in patients with cerebral contusions.
Deterrence and Patient Education
Brain contusion is a significant cause of disability in patients. This condition can be devastating, causing coma and death to the patient. Brain contusion places a substantial burden on the patient's relatives and incurs a high economic cost to the healthcare system. Most of these costs are related to prolonged hospitalizations and rehabilitation for the patient.
Specific recommendations to patients or parents for prevention include the following:
- Use of helmets by cyclists
- Use of automobile seatbelts
- Use of child restraints
- Supervise younger children at all times
- Avoid uneven or unpaved surfaces when cycling or skateboarding
- For some sports, use helmets
Pearls and Other Issues
Future Directions and Recent Advances
Recent investigations have focused on the hemorrhagic progression of a contusion, the spread of an initial contusion, or the spawning of new hemorrhagic foci after the initial insult. HPC has increasingly become known to be one of the main causes of TBI-related morbidity and mortality. Imaging investigations have also identified some initial head CT features that are predictive of HPC.[56][57] For instance, a 2024 meta-analysis concluded that initial subarachnoid hemorrhage, subdural hematoma, or CT angiography spot sign—a sign of active contrast extravasation—in association with a contusion were associated with markedly elevated odds of contusion enlargement.[57] Such findings also emphasize early close surveillance for those with returning scans that demonstrate multiple hemorrhagic foci or active contrast leak. There is also growing interest in applying machine learning algorithms to predict which patients with TBI are most likely to develop hematoma enlargement. Recent studies have employed algorithms, such as random forests and XGBoost, on clinical and imaging features to predict hemorrhage progression with high accuracy (area under the curve of approximately 0.90), which can inform more individualized decisions about ICU admission and repeat imaging.[56] These precision-medicine initiatives circumvent the heterogeneity of TBI by tailoring management to individual risk profiles. Furthermore, optimal current management of cerebral contusions increasingly entails early identification of those at risk of HPC and anticipatory measures to avoid secondary insult.[56]
New Therapeutic Strategies
In addition to improved risk stratification, novel therapy methods are being developed to decrease secondary brain injury from contusions. One notable development is the exploration of sulfonylurea receptor-1 (SUR1)-TRPM4 channel blockers as a means to reverse cerebral edema and hemorrhagic enlargement. Preclinical studies have shown that administration of a SUR1-TRPM4 blocker by glibenclamide—a substance initially designed to treat diabetes—powerfully decreases contusion edema, inhibits secondary hemorrhage enlargement, and improves neurological findings. Blocking this pathway extends upon prior findings that mechanical forces from trauma begin endothelial activation through transcription factors NF-κB and Sp1 in the contusion penumbra, which results in upregulation of the SUR1-TRPM4 channels and subsequent capillary disruption and blood-brain barrier permeability. Early-phase clinical studies have also shown encouraging findings: a small, blinded, randomized study found a trend toward diminished lesion enlargement in TBI cases treated with intravenous glibenclamide, and a noted but ongoing phase II study (ASTRAL study) is now evaluating its potential in preventing contusion enlargement and edema in the broader patient population.[56]
Another recent improvement in acute TBI management is the administration of tranexamic acid to reduce intracranial bleeding. International CRASH-3 trial (2019) demonstrated that administration of tranexamic acid within 3 hours of head trauma is safe and reduces TBI mortality, and most notably, in those who present with mild-to-moderate TBI (GCS >8) and overt hemorrhage by CT.[58] Although tranexamic acid lacks specificity for contusions, stabilizing clot formation may prevent the expansion of hemorrhagic contusions when administered early. Overall, these novel interventions, ranging from molecular targeting therapies such as glibenclamide to antifibrinolytic therapies such as tranexamic acid, represent a remarkable trend toward evidence-driven, targeted therapy in TBI, offering hope for improving outcomes in patients with cerebral contusions that were once deemed untreatable beyond supportive care.
Enhancing Healthcare Team Outcomes
There is currently a lack of consensus worldwide regarding the management of contusions.[15] This knowledge gap stems from inconsistent treatment approaches and inadequate care plans.[51] Establishing a universal definition for the hemorrhagic progression of contusions is essential. Surgical decisions should be made collaboratively by a multidisciplinary team, with clear communication involving the patient and their family members. The use of predictive models based on machine learning algorithms can enhance management plans and strategies.[51] Additionally, a systematic trauma data bank should be established to understand the natural progression of the condition, optimize treatment strategies, and predict clinical outcomes for cerebral contusions.
Although the emergency clinician and trauma surgeon are almost always involved in the initial care of patients with cerebral contusions associated with TBI, it is essential to consult with an interprofessional team of specialists that includes a neurosurgeon and intensivist. Prompt consultation with an interprofessional group of specialists is recommended to improve outcomes. Nurses are also vital members of the interprofessional team, as they monitor the patient's vital signs. The blood bank and clinical laboratory provide key elements in treatment and management decisions. If the patient is intubated, respiratory therapy is essential for managing ventilators, with coordination by the neurointensivist. The radiologist plays a vital role in radiological monitoring of imaging changes.
A significant complication in these patients, associated with high morbidity, is deep vein thrombosis. Many patients can be placed on medical prophylaxis if there is no evidence of the contusion's progression. Physical therapists must be consulted for early ambulation. An integrated care pathway, utilizing an evidence-based approach and adhering to management guidelines, is a crucial element for improving outcomes.
Media
(Click Image to Enlarge)
(Click Image to Enlarge)
References
Corrigan JD, Selassie AW, Orman JA. The epidemiology of traumatic brain injury. The Journal of head trauma rehabilitation. 2010 Mar-Apr:25(2):72-80. doi: 10.1097/HTR.0b013e3181ccc8b4. Epub [PubMed PMID: 20234226]
Bullock MR, Chesnut R, Ghajar J, Gordon D, Hartl R, Newell DW, Servadei F, Walters BC, Wilberger JE, Surgical Management of Traumatic Brain Injury Author Group. Surgical management of acute subdural hematomas. Neurosurgery. 2006 Mar:58(3 Suppl):S16-24; discussion Si-iv [PubMed PMID: 16710968]
McGinn MJ, Povlishock JT. Pathophysiology of Traumatic Brain Injury. Neurosurgery clinics of North America. 2016 Oct:27(4):397-407. doi: 10.1016/j.nec.2016.06.002. Epub 2016 Aug 10 [PubMed PMID: 27637392]
Lalwani S, Hasan F, Khurana S, Mathur P. Epidemiological trends of fatal pediatric trauma: A single-center study. Medicine. 2018 Sep:97(39):e12280. doi: 10.1097/MD.0000000000012280. Epub [PubMed PMID: 30278499]
Level 2 (mid-level) evidenceLu J, Marmarou A, Choi S, Maas A, Murray G, Steyerberg EW, Impact and Abic Study Group. Mortality from traumatic brain injury. Acta neurochirurgica. Supplement. 2005:95():281-5 [PubMed PMID: 16463866]
Level 2 (mid-level) evidenceNalliah RP, Anderson IM, Lee MK, Rampa S, Allareddy V, Allareddy V. Epidemiology of hospital-based emergency department visits due to sports injuries. Pediatric emergency care. 2014 Aug:30(8):511-5. doi: 10.1097/PEC.0000000000000180. Epub [PubMed PMID: 25062295]
Level 2 (mid-level) evidenceSchneider ALC, Wang D, Ling G, Gottesman RF, Selvin E. Prevalence of Self-Reported Head Injury in the United States. The New England journal of medicine. 2018 Sep 20:379(12):1176-1178. doi: 10.1056/NEJMc1808550. Epub [PubMed PMID: 30231228]
Bullock R, Chesnut RM, Clifton G, Ghajar J, Marion DW, Narayan RK, Newell DW, Pitts LH, Rosner MJ, Wilberger JW. Guidelines for the management of severe head injury. Brain Trauma Foundation. European journal of emergency medicine : official journal of the European Society for Emergency Medicine. 1996 Jun:3(2):109-27 [PubMed PMID: 9028756]
Level 1 (high-level) evidenceMaas AI, Stocchetti N, Bullock R. Moderate and severe traumatic brain injury in adults. The Lancet. Neurology. 2008 Aug:7(8):728-41. doi: 10.1016/S1474-4422(08)70164-9. Epub [PubMed PMID: 18635021]
Jiang JY, Chinese Head Trauma Study Collaborators. Head trauma in China. Injury. 2013 Nov:44(11):1453-7. doi: 10.1016/j.injury.2012.08.045. Epub 2012 Oct 13 [PubMed PMID: 23068139]
Parchani A, El-Menyar A, Al-Thani H, Tuma M, Zarour A, Abdulrahman H, Peralta R, Asim M, Latifi R. Recreational-related head injuries in Qatar. Brain injury. 2013:27(12):1450-3. doi: 10.3109/02699052.2013.823664. Epub 2013 Aug 7 [PubMed PMID: 23924056]
Level 2 (mid-level) evidenceLeijdesdorff HA, van Dijck JT, Krijnen P, Vleggeert-Lankamp CL, Schipper IB, Regional Trauma Center West-Netherlands’ Research Group. Injury pattern, hospital triage, and mortality of 1250 patients with severe traumatic brain injury caused by road traffic accidents. Journal of neurotrauma. 2014 Mar 1:31(5):459-65. doi: 10.1089/neu.2013.3111. Epub 2013 Dec 21 [PubMed PMID: 24093437]
Fernández-Abinader JA, González-Colón K, Feliciano CE, Mosquera-Soler AM. Traumatic Brain Injury Profile of an Elderly Population in Puerto Rico. Puerto Rico health sciences journal. 2017 Dec:36(4):237-239 [PubMed PMID: 29220069]
Egbohou P, Mouzou T, Tchetike P, Sama HD, Assenouwe S, Akala-Yoba G, Randolph L, Tomta K. Epidemiology of Pediatric Traumatic Brain Injury at Sylvanus Olympio University Hospital of Lomé in Togo. Anesthesiology research and practice. 2019:2019():4038319. doi: 10.1155/2019/4038319. Epub 2019 Aug 1 [PubMed PMID: 31467523]
Jirlow U, Hossain I, Korhonen O, Depreitere B, Rostami E. Cerebral contusions - Pathomechanism, predictive factors for progression and historical and current management. Brain & spine. 2024:4():103329. doi: 10.1016/j.bas.2024.103329. Epub 2024 Aug 28 [PubMed PMID: 39281852]
Cepeda S, Gómez PA, Castaño-Leon AM, Martínez-Pérez R, Munarriz PM, Lagares A. Traumatic Intracerebral Hemorrhage: Risk Factors Associated with Progression. Journal of neurotrauma. 2015 Aug 15:32(16):1246-53. doi: 10.1089/neu.2014.3808. Epub 2015 Apr 15 [PubMed PMID: 25752340]
Level 2 (mid-level) evidenceCepeda S, Castaño-León AM, Munarriz PM, Paredes I, Panero I, Eiriz C, Gómez PA, Lagares A. Effect of decompressive craniectomy in the postoperative expansion of traumatic intracerebral hemorrhage: a propensity score-based analysis. Journal of neurosurgery. 2020 May 1:132(5):1623-1635. doi: 10.3171/2019.2.JNS182025. Epub 2019 Apr 26 [PubMed PMID: 31026834]
Kurland D, Hong C, Aarabi B, Gerzanich V, Simard JM. Hemorrhagic progression of a contusion after traumatic brain injury: a review. Journal of neurotrauma. 2012 Jan 1:29(1):19-31. doi: 10.1089/neu.2011.2122. Epub 2011 Dec 5 [PubMed PMID: 21988198]
Juratli TA, Zang B, Litz RJ, Sitoci KH, Aschenbrenner U, Gottschlich B, Daubner D, Schackert G, Sobottka SB. Early hemorrhagic progression of traumatic brain contusions: frequency, correlation with coagulation disorders, and patient outcome: a prospective study. Journal of neurotrauma. 2014 Sep 1:31(17):1521-7. doi: 10.1089/neu.2013.3241. Epub 2014 Jul 8 [PubMed PMID: 24738836]
Level 2 (mid-level) evidenceZhang J, Jiang R, Liu L, Watkins T, Zhang F, Dong JF. Traumatic brain injury-associated coagulopathy. Journal of neurotrauma. 2012 Nov 20:29(17):2597-605. doi: 10.1089/neu.2012.2348. Epub 2012 Oct 31 [PubMed PMID: 23020190]
Level 3 (low-level) evidenceHang CH, Chen G, Shi JX, Zhang X, Li JS. Cortical expression of nuclear factor kappaB after human brain contusion. Brain research. 2006 Sep 13:1109(1):14-21 [PubMed PMID: 16857176]
Simard JM, Kilbourne M, Tsymbalyuk O, Tosun C, Caridi J, Ivanova S, Keledjian K, Bochicchio G, Gerzanich V. Key role of sulfonylurea receptor 1 in progressive secondary hemorrhage after brain contusion. Journal of neurotrauma. 2009 Dec:26(12):2257-67. doi: 10.1089/neu.2009.1021. Epub [PubMed PMID: 19604096]
Level 3 (low-level) evidenceMartínez-Valverde T, Vidal-Jorge M, Martínez-Saez E, Castro L, Arikan F, Cordero E, Rădoi A, Poca MA, Simard JM, Sahuquillo J. Sulfonylurea Receptor 1 in Humans with Post-Traumatic Brain Contusions. Journal of neurotrauma. 2015 Oct 1:32(19):1478-87. doi: 10.1089/neu.2014.3706. Epub 2015 Jun 3 [PubMed PMID: 26398596]
Gerzanich V, Stokum JA, Ivanova S, Woo SK, Tsymbalyuk O, Sharma A, Akkentli F, Imran Z, Aarabi B, Sahuquillo J, Simard JM. Sulfonylurea Receptor 1, Transient Receptor Potential Cation Channel Subfamily M Member 4, and KIR6.2:Role in Hemorrhagic Progression of Contusion. Journal of neurotrauma. 2019 Apr 1:36(7):1060-1079. doi: 10.1089/neu.2018.5986. Epub 2018 Oct 4 [PubMed PMID: 30160201]
Jha RM, Bell J, Citerio G, Hemphill JC, Kimberly WT, Narayan RK, Sahuquillo J, Sheth KN, Simard JM. Role of Sulfonylurea Receptor 1 and Glibenclamide in Traumatic Brain Injury: A Review of the Evidence. International journal of molecular sciences. 2020 Jan 9:21(2):. doi: 10.3390/ijms21020409. Epub 2020 Jan 9 [PubMed PMID: 31936452]
Simard JM, Kahle KT, Gerzanich V. Molecular mechanisms of microvascular failure in central nervous system injury--synergistic roles of NKCC1 and SUR1/TRPM4. Journal of neurosurgery. 2010 Sep:113(3):622-9. doi: 10.3171/2009.11.JNS081052. Epub [PubMed PMID: 20035575]
Level 3 (low-level) evidenceMoscote-Salazar LR, M Rubiano A, Alvis-Miranda HR, Calderon-Miranda W, Alcala-Cerra G, Blancas Rivera MA, Agrawal A. Severe Cranioencephalic Trauma: Prehospital Care, Surgical Management and Multimodal Monitoring. Bulletin of emergency and trauma. 2016 Jan:4(1):8-23 [PubMed PMID: 27162922]
Ragaisis V. [Brain contusion: morphology, pathogenesis and treatment]. Medicina (Kaunas, Lithuania). 2002:38(3):243-9; quiz 354 [PubMed PMID: 12474694]
Bouma GJ, Muizelaar JP. Cerebral blood flow in severe clinical head injury. New horizons (Baltimore, Md.). 1995 Aug:3(3):384-94 [PubMed PMID: 7496746]
Teasdale G, Jennett B. Assessment of coma and impaired consciousness. A practical scale. Lancet (London, England). 1974 Jul 13:2(7872):81-4 [PubMed PMID: 4136544]
Shetty VS, Reis MN, Aulino JM, Berger KL, Broder J, Choudhri AF, Kendi AT, Kessler MM, Kirsch CF, Luttrull MD, Mechtler LL, Prall JA, Raksin PB, Roth CJ, Sharma A, West OC, Wintermark M, Cornelius RS, Bykowski J. ACR Appropriateness Criteria Head Trauma. Journal of the American College of Radiology : JACR. 2016 Jun:13(6):668-79. doi: 10.1016/j.jacr.2016.02.023. Epub [PubMed PMID: 27262056]
Snider SB, Temkin NR, Sun X, Stubbs JL, Rademaker QJ, Markowitz AJ, Rosenthal ES, Diaz-Arrastia R, Fox MD, Manley GT, Jain S, Edlow BL, TRACK-TBI Investigators. Automated Measurement of Cerebral Hemorrhagic Contusions and Outcomes After Traumatic Brain Injury in the TRACK-TBI Study. JAMA network open. 2024 Aug 1:7(8):e2427772. doi: 10.1001/jamanetworkopen.2024.27772. Epub 2024 Aug 1 [PubMed PMID: 39212991]
Bullock MR, Chesnut R, Ghajar J, Gordon D, Hartl R, Newell DW, Servadei F, Walters BC, Wilberger J, Surgical Management of Traumatic Brain Injury Author Group. Surgical management of traumatic parenchymal lesions. Neurosurgery. 2006 Mar:58(3 Suppl):S25-46; discussion Si-iv [PubMed PMID: 16540746]
Hutchinson PJ, Kolias AG, Timofeev IS, Corteen EA, Czosnyka M, Timothy J, Anderson I, Bulters DO, Belli A, Eynon CA, Wadley J, Mendelow AD, Mitchell PM, Wilson MH, Critchley G, Sahuquillo J, Unterberg A, Servadei F, Teasdale GM, Pickard JD, Menon DK, Murray GD, Kirkpatrick PJ, RESCUEicp Trial Collaborators. Trial of Decompressive Craniectomy for Traumatic Intracranial Hypertension. The New England journal of medicine. 2016 Sep 22:375(12):1119-30. doi: 10.1056/NEJMoa1605215. Epub 2016 Sep 7 [PubMed PMID: 27602507]
Stiver SI. Complications of decompressive craniectomy for traumatic brain injury. Neurosurgical focus. 2009 Jun:26(6):E7. doi: 10.3171/2009.4.FOCUS0965. Epub [PubMed PMID: 19485720]
Level 2 (mid-level) evidenceCarney N, Totten AM, O'Reilly C, Ullman JS, Hawryluk GW, Bell MJ, Bratton SL, Chesnut R, Harris OA, Kissoon N, Rubiano AM, Shutter L, Tasker RC, Vavilala MS, Wilberger J, Wright DW, Ghajar J. Guidelines for the Management of Severe Traumatic Brain Injury, Fourth Edition. Neurosurgery. 2017 Jan 1:80(1):6-15. doi: 10.1227/NEU.0000000000001432. Epub [PubMed PMID: 27654000]
Hawryluk GWJ, Rubiano AM, Totten AM, O'Reilly C, Ullman JS, Bratton SL, Chesnut R, Harris OA, Kissoon N, Shutter L, Tasker RC, Vavilala MS, Wilberger J, Wright DW, Lumba-Brown A, Ghajar J. Guidelines for the Management of Severe Traumatic Brain Injury: 2020 Update of the Decompressive Craniectomy Recommendations. Neurosurgery. 2020 Sep 1:87(3):427-434. doi: 10.1093/neuros/nyaa278. Epub [PubMed PMID: 32761068]
Keirsey M, Niziolek GM. Management of post-injury anticoagulation in the traumatic brain injury patient: A scoping review. Injury. 2025 Feb:56(2):112159. doi: 10.1016/j.injury.2025.112159. Epub 2025 Jan 9 [PubMed PMID: 39799871]
Level 2 (mid-level) evidenceWaheed SM, Kudaravalli P, Hotwagner DT. Deep Venous Thrombosis. StatPearls. 2025 Jan:(): [PubMed PMID: 29939530]
Vrettou CS, Dima E, Karela NR, Sigala I, Korfias S. Severe Traumatic Brain Injury and Pulmonary Embolism: Risks, Prevention, Diagnosis and Management. Journal of clinical medicine. 2024 Aug 2:13(15):. doi: 10.3390/jcm13154527. Epub 2024 Aug 2 [PubMed PMID: 39124793]
Nakamura N, Yamaura A, Shigemori M, Ogawa T, Tokutomi T, Ono J, Kawamata T, Sakamoto T. Final report of the Japan Neurotrauma Data Bank project 1998-2001: 1,002 cases of traumatic brain injury. Neurologia medico-chirurgica. 2006 Dec:46(12):567-74 [PubMed PMID: 17185881]
Level 3 (low-level) evidenceWang XP, Zhong J, Lei T, Wang HJ, Zhu LN, Chu S, Liu L. Epidemiology of traumatic brain injury-associated epilepsy in western China: An analysis of multicenter data. Epilepsy research. 2020 Aug:164():106354. doi: 10.1016/j.eplepsyres.2020.106354. Epub 2020 May 11 [PubMed PMID: 32438297]
Brennan PM, Murray GD, Teasdale GM. Simplifying the use of prognostic information in traumatic brain injury. Part 1: The GCS-Pupils score: an extended index of clinical severity. Journal of neurosurgery. 2018 Jun:128(6):1612-1620. doi: 10.3171/2017.12.JNS172780. Epub 2018 Apr 10 [PubMed PMID: 29631516]
Murray GD, Brennan PM, Teasdale GM. Simplifying the use of prognostic information in traumatic brain injury. Part 2: Graphical presentation of probabilities. Journal of neurosurgery. 2018 Jun:128(6):1621-1634. doi: 10.3171/2017.12.JNS172782. Epub 2018 Apr 10 [PubMed PMID: 29631517]
Jennett B, Bond M. Assessment of outcome after severe brain damage. Lancet (London, England). 1975 Mar 1:1(7905):480-4 [PubMed PMID: 46957]
Marshall LF, Marshall SB, Klauber MR, Van Berkum Clark M, Eisenberg H, Jane JA, Luerssen TG, Marmarou A, Foulkes MA. The diagnosis of head injury requires a classification based on computed axial tomography. Journal of neurotrauma. 1992 Mar:9 Suppl 1():S287-92 [PubMed PMID: 1588618]
Maas AI, Hukkelhoven CW, Marshall LF, Steyerberg EW. Prediction of outcome in traumatic brain injury with computed tomographic characteristics: a comparison between the computed tomographic classification and combinations of computed tomographic predictors. Neurosurgery. 2005 Dec:57(6):1173-82; discussion 1173-82 [PubMed PMID: 16331165]
Huang YH, Deng YH, Lee TC, Chen WF. Rotterdam computed tomography score as a prognosticator in head-injured patients undergoing decompressive craniectomy. Neurosurgery. 2012 Jul:71(1):80-5. doi: 10.1227/NEU.0b013e3182517aa1. Epub [PubMed PMID: 22382208]
Level 2 (mid-level) evidenceCharry JD, Falla JD, Ochoa JD, Pinzón MA, Tejada JH, Henriquez MJ, Solano JP, Calvache C. External Validation of the Rotterdam Computed Tomography Score in the Prediction of Mortality in Severe Traumatic Brain Injury. Journal of neurosciences in rural practice. 2017 Aug:8(Suppl 1):S23-S26. doi: 10.4103/jnrp.jnrp_434_16. Epub [PubMed PMID: 28936067]
Level 1 (high-level) evidenceLiu H, Su Y, Peng M, Zhang D, Wang Q, Zhang M, Ge R, Xu H, Chang J, Shao X. Prediction of prognosis in patients with cerebral contusions based on machine learning. Scientific reports. 2024 Dec 30:14(1):31993. doi: 10.1038/s41598-024-83481-6. Epub 2024 Dec 30 [PubMed PMID: 39738368]
Li C, Su Z, Deng S, Zhang B, Qin J, Wu K, Zhao Y, Liu Y. Factors affecting prognosis in traumatic cerebral contusions: A protocol for a systematic review and meta-analysis. PloS one. 2025:20(2):e0319146. doi: 10.1371/journal.pone.0319146. Epub 2025 Feb 25 [PubMed PMID: 39999086]
Level 1 (high-level) evidenceAdatia K, Newcombe VFJ, Menon DK. Contusion Progression Following Traumatic Brain Injury: A Review of Clinical and Radiological Predictors, and Influence on Outcome. Neurocritical care. 2021 Feb:34(1):312-324. doi: 10.1007/s12028-020-00994-4. Epub [PubMed PMID: 32462411]
Zhu Y, Jin X, Xu L, Han P, Lin S, Lu Z. Establishment and validation of prognosis model for patients with cerebral contusion. BMC neurology. 2021 Nov 29:21(1):463. doi: 10.1186/s12883-021-02482-4. Epub 2021 Nov 29 [PubMed PMID: 34844563]
Level 1 (high-level) evidenceSmith PD, Shukla I, Azam F, Trautmann D, Gee E, Korb M, Pitonak M, Srinivasan S, Caruso JP, Caldwell C, Hall K, Tamimi MA, Reisch J, Bedros NM, Aoun SG. Predictive factors for traumatic cerebral contusion volume, expansion, and outcomes. Journal of neurosurgery. 2025 Jun 1:142(6):1616-1624. doi: 10.3171/2024.8.JNS241051. Epub 2025 Jan 3 [PubMed PMID: 39752651]
Bodanapally UK, Shanmuganathan K, Issa G, Dreizin D, Li G, Sudini K, Fleiter TR. Dual-Energy CT in Hemorrhagic Progression of Cerebral Contusion: Overestimation of Hematoma Volumes on Standard 120-kV Images and Rectification with Virtual High-Energy Monochromatic Images after Contrast-Enhanced Whole-Body Imaging. AJNR. American journal of neuroradiology. 2018 Apr:39(4):658-662. doi: 10.3174/ajnr.A5558. Epub 2018 Feb 8 [PubMed PMID: 29439124]
Jha RM, Simard JM. Glibenclamide for Brain Contusions: Contextualizing a Promising Clinical Trial Design that Leverages an Imaging-Based TBI Endotype. Neurotherapeutics : the journal of the American Society for Experimental NeuroTherapeutics. 2023 Oct:20(6):1472-1481. doi: 10.1007/s13311-023-01389-x. Epub 2023 Jun 12 [PubMed PMID: 37306928]
Peng J, Luo T, Li X, Li B, Cheng Y, Huang Q, Su J. Imaging predictors of hemorrhagic progression of a contusion after traumatic brain injury: a systematic review and meta-analysis. Scientific reports. 2024 Mar 12:14(1):5961. doi: 10.1038/s41598-024-56232-w. Epub 2024 Mar 12 [PubMed PMID: 38472247]
Level 1 (high-level) evidenceCRASH-3 trial collaborators. Effects of tranexamic acid on death, disability, vascular occlusive events and other morbidities in patients with acute traumatic brain injury (CRASH-3): a randomised, placebo-controlled trial. Lancet (London, England). 2019 Nov 9:394(10210):1713-1723. doi: 10.1016/S0140-6736(19)32233-0. Epub 2019 Oct 14 [PubMed PMID: 31623894]
Level 1 (high-level) evidence