Introduction
The celiac trunk, also termed the "celiac artery" or "celiac axis," constitutes a surgically significant splanchnic branch of the abdominal aorta, emerging anteriorly at approximately the T12 vertebral level.[1] This short artery provides the primary blood supply to the foregut and foregut-derived organs in the superior abdominal cavity, including the stomach, liver, spleen, pancreas, and proximal duodenum. The vessel arises from the ventral segmental branches of the abdominal aorta during fetal development, reflecting the vascular pattern of the primitive foregut. Innervation of the celiac trunk originates from the celiac plexus, conveying both sympathetic and parasympathetic input to the foregut vasculature.
Variations in the celiac trunk’s branching pattern can complicate procedures such as liver transplantation, pancreatic resection, or endovascular interventions. Pathology of this vessel may result in pancreatic impairment, liver failure, or hemorrhage. Understanding the celiac trunk’s structural and functional characteristics assists clinicians in avoiding intraoperative injury and optimizing diagnostic imaging interpretation.
Structure and Function
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Structure and Function
The celiac trunk is a short vessel arising from the abdominal aorta at approximately the level of the T12 vertebra. This vessel courses inferiorly, deep to the median arcuate ligament, and typically divides into 3 principal branches (see Image. Celiac Trunk Branches).
The left gastric artery, usually the smallest branch, supplies the proximal portion of the stomach along the lesser curvature and the distal esophagus, and forms an anastomosis with the right gastric artery, a branch of the common hepatic artery. The common hepatic artery gives rise to branches that supply the liver, pylorus of the stomach, distal portions of the greater and lesser curvatures, gallbladder, proximal duodenum, and pancreatic head. The splenic artery provides arterial supply to the proximal and middle segments of the greater curvature, the fundus of the stomach, a substantial portion of the pancreas, and the spleen, terminating at the splenic parenchyma.
The classic trifurcation of the celiac trunk, termed “tripus Halleri,” was first described by von Haller in 1917 and is observed in approximately 89% of individuals, irrespective of sex. Two forms of trifurcation have been defined. A “true” tripod occurs when the hepatic, left gastric, and splenic arteries share a common origin, forming a hepatogastrosplenic trunk. In contrast, a “false” tripod is present when at least 1 of these arteries arises separately before the remaining 2 along the course of the celiac trunk.[2][3]
The celiac artery typically measures 1.5 to 2 cm in length and provides the primary arterial supply to the foregut and foregut-derived structures, including the distal esophagus, stomach, proximal 1/3 of the duodenum, liver, pancreas, gallbladder, and spleen.[4][5] Adequate blood flow through the celiac trunk is essential for maintaining the physiological function of these organs.
Embryology
The arterial system undergoes numerous modifications in utero. During the intraembryonic period, paired dorsal aortae form the primitive arterial system and eventually fuse to constitute the abdominal aorta. Paired ventral segmental arteries develop from the dorsal aorta, with some fusing to form median vessels and temporarily interconnected by a ventral longitudinal anastomosis. The 10th, 13th, and 21st ventral segmental arteries persist to form the celiac trunk and the superior and inferior mesenteric arteries, respectively. Persistence of other ventral segmental arteries or of the ventral longitudinal anastomosis gives rise to the arterial variations observed in adults.
By the 4th week of embryonic development, the gastrointestinal tract differentiates into the foregut, midgut, and hindgut. The foregut extends from the esophagus superiorly to the proximal 1/3 of the duodenum inferiorly, just distal to the hepatopancreatic ampulla (ampulla of Vater). The midgut spans from the proximal duodenum to the proximal 2/3 of the transverse colon, terminating at the left colic (splenic) flexure. The hindgut extends from the left colic flexure to the rectum. Each gut segment receives a distinct arterial supply originating from the abdominal aorta, which serves to define the foregut, midgut, and hindgut territories. Incomplete fusion or malfusion of the vitelline arteries during development may account for the anatomical variations observed in the celiac trunk.[6][7]
Blood Supply and Lymphatics
Blood Supply
The 3 major celiac trunk branches are the left gastric, splenic, and common hepatic arteries, which collectively supply the foregut, foregut-derived organs, and the spleen. The left gastric artery gives off branches that supply the inferior portion of the esophagus, then courses along the lesser curvature of the stomach and anastomoses with the right gastric artery, forming an important collateral circulatory route. The splenic artery travels posterior to the stomach and gives rise to the left gastroepiploic artery, which supplies the proximal segment of the greater curvature.
Additional branches of the splenic artery provide arterial supply to the body and tail of the pancreas. The short gastric arteries, which diverge from the splenic artery to supply the stomach fundus, do not form anastomoses with other vessels. Obstruction or rupture of the splenic artery may result in ischemia of the stomach fundus due to the absence of collateral blood supply.
The common hepatic artery constitutes the liver's sole arterial supply. This blood vessel gives rise to the proper hepatic, gastroduodenal, and right gastric arteries. The proper hepatic artery divides into the right and left hepatic arteries. Some texts describe the right gastric artery as a branch of the proper hepatic artery. However, by definition, a “proper” artery exclusively supplies its namesake organ, which in this case is the liver.
The right gastric artery supplies the pylorus and the distal portion of the stomach’s lesser curvature. The right and left hepatic arteries perfuse the corresponding liver lobes, while the cystic artery, typically a branch of the right hepatic artery, supplies the gallbladder. The cystic artery originates from the left gastric artery in approximately 1.2% of individuals.[8]
The gastroduodenal artery gives rise to the right gastroepiploic and superior pancreaticoduodenal arteries. The right gastroepiploic artery supplies the distal portion of the stomach’s greater curvature. The superior pancreaticoduodenal artery delivers blood to the proximal head of the pancreas and to part of the duodenum. The pancreatic head is the most frequent site of pancreatic adenocarcinoma and is typically excised during a Whipple procedure.
Lymphatics
Celiac lymph nodes surrounding the celiac trunk collect lymph from the liver, gallbladder, stomach, spleen, and pancreas. Lymphatic vessels in this region converge on the cisterna chyli, which subsequently drains into the thoracic duct.
Nerves
The celiac ganglia consist of 2 large, irregularly shaped collections of neuronal cell bodies located lateral to the celiac trunk at approximately the T12 to L1 spinal levels. These ganglia are components of the autonomic nervous system, providing innervation to the foregut and foregut-derived abdominal organs. The celiac ganglia convey sympathetic input to the abdominal parenchyma and play a critical role in coordinating digestive functions, including motility, secretion, and absorption.[9] Axons from these neurons innervate the lower esophageal sphincter, stomach, upper small intestine, liver, and pancreas.
In the stomach and intestines, sympathetic output from the celiac ganglia induces sphincter contraction and reduces motility. In the pancreas, this input diminishes both exocrine secretion and insulin release. In the liver, celiac ganglion activity stimulates glycogenolysis and enhances gluconeogenesis.[10]
Physiologic Variants
Celiac trunk branching variations have been documented in the literature. Variants include bifurcation into the common hepatic and splenic arteries and the formation of a celiacomesenteric trunk, in which the celiac and superior mesenteric arteries share a common origin. In some cases, the inferior phrenic arteries arise from the celiac trunk rather than their usual origin from the abdominal aorta. The left gastric artery may also branch directly from the abdominal aorta.
A pentafurcation pattern has been described, consisting of 3 collateral branches—2 ascending and 1 descending—and 2 terminal branches. The ascending collateral branches comprise the left inferior phrenic artery and a secondary hepatogastric trunk, which further divides into a replaced left hepatic artery and the left gastric artery. The descending collateral branch corresponds to the dorsal pancreatic artery, while the terminal branches include the common hepatic and splenic arteries.[11][12]
Abdominal anastomoses are critical during surgical procedures requiring vessel ligation. Detailed knowledge of both normal celiac trunk anatomy and its variants allows surgeons to minimize complications. Variations in celiac trunk branching can present diagnostic and therapeutic challenges during the interpretation of selective angiograms, performance of interventional radiology procedures, including endovascular treatment of arterial aneurysms, angioplasty and stent placement for mesenteric ischemia, arterial embolization for hemorrhage control, and chemoembolization of liver tumors, and abdominal surgical interventions such as pancreatic tumor resection, management of visceral trauma, or organ transplantation.[13]
Surgical Considerations
Celiac vascular anomalies are predominantly asymptomatic. However, iatrogenic injury may result from ligation of an aberrant arterial branch or inadvertent transection of an accessory vessel. Comprehensive knowledge of this segment of the abdominal vasculature and its potential variations is essential to minimize blood loss during abdominal surgical procedures.[14][15]
Clinical Significance
Pathology of the celiac artery may result in hemorrhage or compromise of the organs it supplies. Clinical manifestations range from mild to life-threatening, depending on the type of injury, disease progression, and the patient’s overall health. The conditions discussed below involve this critical arterial structure.
Celiac Artery Compression Syndrome
Celiac artery compression syndrome (CACS), also referred to as "celiac axis syndrome," "median arcuate ligament syndrome," "Marable syndrome," or "Dunbar syndrome," typically presents as recurrent upper abdominal pain resulting from compression of the celiac artery by the diaphragmatic crura. The median arcuate ligament is a fibrous arch connecting the right and left diaphragmatic crura around the aorta at the diaphragm’s base, usually near the level of the 12th thoracic or 1st lumbar vertebra. Celiac artery compression may occur due to an abnormally cephalad origin of the artery or an unusually caudad insertion of the diaphragm.[16] A low-lying median arcuate ligament can impinge on the celiac trunk inferiorly, producing severe epigastric pain that is typically alleviated in the standing position and exacerbated in the supine position.
CACS most commonly affects adults aged 20 to 40. In some cases, the syndrome may lead to mesenteric ischemia, manifesting as postprandial epigastric pain and unintentional weight loss.
Computed tomography (CT) angiography may demonstrate focal stenosis of the celiac artery with a characteristic hook-shaped contour due to distortion of the superior vessel surface. Similar imaging findings may occur in asymptomatic individuals. Therefore, radiographic results must be interpreted in the context of clinical history. Definitive treatment involves laparoscopic decompression through division of the median arcuate ligament, which is typically reserved for symptomatic patients.
Celiac Aneurysm
Although rare, celiac artery aneurysms represent a subset of splanchnic artery aneurysms. These lesions are typically asymptomatic unless rupture occurs. Early identification is critical, as rupture is associated with high morbidity and mortality. Celiac artery aneurysms are often detected incidentally on diagnostic imaging, such as arteriography, or identified during postrupture autopsy examinations.
Celiac Trunk Dissection
Celiac trunk dissection is most commonly iatrogenic but may also occur in individuals with atherosclerosis, hypertension, or preexisting vascular disorders such as fibromuscular dysplasia. Additional predisposing factors include pregnancy, trauma, and sudden increases in intra-abdominal pressure.
Dissection of the celiac trunk is frequently asymptomatic. However, pain may develop if bowel ischemia occurs secondary to rupture of the foregut branches of the celiac artery or adjacent vessels, including the renal and superior mesenteric arteries.
Contrast-enhanced CT or CT angiography is the preferred diagnostic modality for celiac trunk dissection, although magnetic resonance angiography or ultrasonography may also be utilized. Typical imaging findings include intimal flaps and intraluminal mural thrombi.
Surgical intervention remains the definitive treatment, preventing acute complications such as rupture and intestinal ischemia, as well as chronic complications including arterial stenosis. Conservative management may be appropriate for limited dissections, with emphasis on preventing thromboembolic events through anticoagulation and blood pressure control. Endovascular stenting may be considered if collateral circulation is adequate.[17]
Peptic Ulcer Disease
Gastric and duodenal ulcers are painful mucosal lesions that commonly result from Helicobacter pylori infection, as well as long-term use of nonsteroidal anti-inflammatory drugs or tobacco. Patients typically present with epigastric pain and early satiety. Gastric ulcers are often associated with pain exacerbated by food intake, whereas duodenal ulcers usually cause pain that is relieved by eating. Gastric ulcers most frequently occur along the lesser curvature of the stomach, which is supplied by the right and left gastric arteries, while duodenal ulcers predominantly affect the posterior duodenal wall, an area perfused by the gastroduodenal artery.
Perforation of peptic ulcers may compromise branches of the celiac artery. Erosion of the superior mesenteric artery is an additional potential complication. Esophagogastroduodenoscopy is the gold standard for the diagnosis of peptic ulcer disease. Management generally involves pharmacologic therapy with H2-receptor antagonists and proton pump inhibitors.
Media
(Click Image to Enlarge)
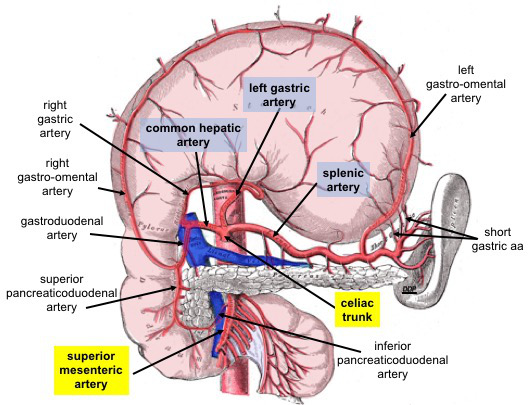
Celiac Trunk Branches. This anterior view shows the stomach reflected superiorly. Celiac trunk branches include the common hepatic, left gastric, and splenic arteries. The common hepatic artery divides into the right and left hepatic, right gastric, and gastroduodenal arteries. The gastroduodenal artery gives rise to the right gastro-omental and anterior and posterior superior pancreaticoduodenal arteries. The left gastric artery supplies the distal esophagus and lesser curvature of the stomach. The splenic artery supplies the spleen and gives rise to the left gastro-omental and short gastric arteries.
The superior mesenteric artery is an abdominal aortic branch inferior to the celiac trunk. The anterior and posterior inferior pancreaticoduodenal arteries arise from this vessel.
Contributed By Dennis M DePace, PhD - Own work, CC BY-SA 4.0, https://commons.wikimedia.org/w/index.php?curid=77732420
References
Mao Y, Yao Y, Li X, Zhang C, Chen X, Wang Y. Absence of the celiac trunk: Definition, classification, multidetector computed tomography angiographic findings, and their probable embryological mechanisms. Vascular. 2023 Dec:31(6):1214-1221. doi: 10.1177/17085381221106318. Epub 2022 May 30 [PubMed PMID: 35634715]
Vougadiotis I, Karampelias V, Chrysikos D, Antonopoulos I, Solia E, Spanidis Y, Tsakotos G, Troupis T. Anatomical Variations in the Celiac Trunk: A Short Review. Acta medica academica. 2023 Aug:52(2):134-141. doi: 10.5644/ama2006-124.413. Epub [PubMed PMID: 37933510]
Omar R, Kisansa M, Dehnavi AD. The prevalence of anatomical variants of the coeliac trunk and renal arteries on contrast-enhanced abdominal computed tomography scans at Dr George Mukhari Academic Hospital. SA journal of radiology. 2021:25(1):1990. doi: 10.4102/sajr.v25i1.1990. Epub 2021 Jan 25 [PubMed PMID: 33604070]
Lung K, Lui F. Anatomy, Abdomen and Pelvis: Arteries. StatPearls. 2025 Jan:(): [PubMed PMID: 30247834]
Shaikh H, Wehrle CJ, Khorasani-Zadeh A. Anatomy, Abdomen and Pelvis: Superior Mesenteric Artery. StatPearls. 2025 Jan:(): [PubMed PMID: 30137844]
Whitley A, Oliverius M, Kocián P, Havlůj L, Gürlich R, Kachlík D. Variations of the celiac trunk investigated by multidetector computed tomography: Systematic review and meta-analysis with clinical correlations. Clinical anatomy (New York, N.Y.). 2020 Nov:33(8):1249-1262. doi: 10.1002/ca.23576. Epub 2020 Feb 18 [PubMed PMID: 32012339]
Level 1 (high-level) evidencePrakash, Rajini T, Mokhasi V, Geethanjali BS, Sivacharan PV, Shashirekha M. Coeliac trunk and its branches: anatomical variations and clinical implications. Singapore medical journal. 2012 May:53(5):329-31 [PubMed PMID: 22584973]
Dandekar U, Dandekar K. Cystic Artery: Morphological Study and Surgical Significance. Anatomy research international. 2016:2016():7201858 [PubMed PMID: 27822387]
Candal R, Reddy V, Samra NS. Anatomy, Abdomen and Pelvis: Celiac Ganglia. StatPearls. 2025 Jan:(): [PubMed PMID: 30844156]
McCorry LK. Physiology of the autonomic nervous system. American journal of pharmaceutical education. 2007 Aug 15:71(4):78 [PubMed PMID: 17786266]
Ulualp K, Masnyj SV, Lee CJ, Gould JC. Median Arcuate Ligament Syndrome Related to Bodybuilding. Surgical laparoscopy, endoscopy & percutaneous techniques. 2019 Feb:29(1):e9-e11. doi: 10.1097/SLE.0000000000000592. Epub [PubMed PMID: 30395046]
Rusu MC, Manta BA. Pentafurcated Celiac Trunk. Annals of vascular surgery. 2021 Jan:70():567.e1-567.e6. doi: 10.1016/j.avsg.2020.08.007. Epub 2020 Aug 12 [PubMed PMID: 32795653]
Laleye CM, Ahouansou PY, Hounton SED, Videgla LB, Hadonou AA, Agossou AC, Attolou SG, Dibert-Bekoy-Nouganga E, Biaou O, Hounnou GM, Mehinto, Voyeme AK. Anatomical variants of the celiac trunk. Morphologie : bulletin de l'Association des anatomistes. 2021 Sep:105(350):227-236. doi: 10.1016/j.morpho.2020.10.003. Epub 2020 Nov 7 [PubMed PMID: 33172784]
Hemamalini. Variations in the branching pattern of the celiac trunk and its clinical significance. Anatomy & cell biology. 2018 Sep:51(3):143-149. doi: 10.5115/acb.2018.51.3.143. Epub 2018 Sep 28 [PubMed PMID: 30310705]
Coelho A, Monteiro P, Nogueira C, Gouveia R, Semião AC, Canedo A. Management of a rapidly expanding celiac artery aneurysm with the chimney technique. Journal of vascular surgery cases and innovative techniques. 2018 Sep:4(3):252-256. doi: 10.1016/j.jvscit.2018.05.006. Epub 2018 Aug 30 [PubMed PMID: 30186997]
Level 3 (low-level) evidenceMatsuura H, Okita A, Suganami Y. Intermittent Severe Epigastric Pain and Abdominal Bruit Varying With Respiration. Gastroenterology. 2020 Mar:158(4):e11-e12. doi: 10.1053/j.gastro.2019.09.022. Epub 2019 Sep 24 [PubMed PMID: 31560895]
Abugroun A, Natarajan A, Daoud H, Khalaf H. Spontaneous Celiac Artery Dissection Presenting With Splenic Infarction: A Case Report. Gastroenterology research. 2018 Oct:11(5):379-382. doi: 10.14740/gr1065w. Epub 2018 Oct 1 [PubMed PMID: 30344811]
Level 3 (low-level) evidence