Introduction
Baroreceptors, a specialized type of mechanoreceptor, detect pressure and stretch within the blood vessels of the aortic arch and carotid sinus. These unique structures contribute to the regulation of mean arterial pressure by adjusting vascular tone and heart rate in response to physiological stimuli.
Baroreceptor activity returns to baseline upon restoration of homeostatic arterial pressure. The receptors form part of the afferent system, transmitting pressure signals via the glossopharyngeal (cranial nerve IX) and vagus (cranial nerve X) nerves to central regulatory centers, specifically the nucleus tractus solitarius in the medulla, involved in blood pressure modulation (see Image. Neural Pathways of Baroreceptor Signaling). The physiological principles governing baroreceptor function are clinically relevant in the context of carotid massage, carotid occlusion, and the Cushing reflex.[1][2]
Structure and Function
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Structure and Function
Peripheral baroreceptors are located in the aortic arch and carotid sinus. Baroreceptors within the carotid sinus reside at the bifurcation of the common carotid arteries and transmit afferent signals via the glossopharyngeal nerve to the solitary nucleus of the medulla.[3][4]
Stretch-sensitive fibers within the carotid sinus generate afferent signals based on the degree of vascular distension. Increases in arterial pressure result in greater stretch of these fibers, which enhances baroreceptor signaling. The nucleus solitarius, located in the dorsolateral medulla oblongata, serves as the central integration site for afferent input from carotid baroreceptors and initiates reflex responses to maintain hemodynamic stability.[5][6]
The vagus and glossopharyngeal nerves transmit afferent signals to the solitary nucleus of the medulla. In response, efferent signals are sent to the periphery, resulting in venous and arterial vasodilatation to lower blood pressure. This effect occurs through decreased sympathetic outflow, which reduces vasoconstriction and total peripheral resistance.
Parasympathetic efferent signaling to the sinoatrial node increases, reducing heart rate. Simultaneously, reduced sympathetic outflow decreases cardiac contractility and further lowers heart rate, leading to diminished cardiac output. Elevated blood pressure also prompts the kidneys to reduce salt and water retention, contributing to blood pressure reduction.
Efferent responses targeting the heart, vasculature, and kidneys serve as compensatory mechanisms in response to increased blood pressure. Baroreceptor signaling returns to baseline once homeostatic arterial pressure is restored.[7]
In contrast, hemorrhage causes a decline in arterial pressure. This decrease reduces afferent signaling from carotid baroreceptors to the nucleus solitarius via the glossopharyngeal nerve. The resulting efferent response includes increased sympathetic outflow, promoting vasoconstriction, enhanced cardiac contractility, and elevated heart rate. These effects reflect reduced parasympathetic efferent activity. Decreased blood pressure also stimulates renal retention of salt and water, which increases intravascular volume and supports blood pressure restoration. Efferent signals to the heart, vasculature, and kidneys serve as compensatory mechanisms in response to hypotension.[8]
Embryology
Baroreceptors are active during development. Neurotrophic interactions, potentially involving brain-derived neurotrophic factor (BDNF), may support the enhancement of synaptic plasticity within the baroreflex pathway. These sensory neurons originate from the neural crest, which is derived from the ectodermal sheet.
Clinical Significance
Baroreceptors in the aortic arch and carotid sinus play clinically important roles. Increased pressure on the carotid artery, such as during carotid massage, enhances stretch receptor activation and augments afferent baroreceptor signaling. The carotid sinus detects this heightened activity and transmits signals via the glossopharyngeal nerve, which the central nervous system interprets as elevated arterial pressure.
Compensatory efferent signaling through the nucleus solitarius induces venous and arterial dilation, decreases heart rate, prolongs the atrioventricular node refractory period, and reduces systemic blood pressure. This abrupt decline in perfusion pressure can lead to syncope, particularly in individuals with a history of syncope triggered by activities such as shaving or buttoning a shirt, which increase pressure on the carotid artery.[9]
In contrast, carotid occlusion eliminates blood flow to the carotid sinus. Reduced perfusion of the carotid artery diminishes stretch fiber activation, leading to decreased baroreceptor firing. The carotid sinus transmits this reduced afferent signaling via the glossopharyngeal nerve, which the central nervous system misinterprets as hypotension.
Compensatory efferent output from the nucleus solitarius promotes venous and arterial vasoconstriction, elevates heart rate, and increases systemic blood pressure. Carotid occlusive disease, such as carotid stenosis, may present with ischemic stroke due to impaired cerebral perfusion.[10]
The Cushing reaction represents another clinically relevant manifestation of baroreceptor-mediated regulation. This reflex is characterized by a triad of bradycardia, hypertension, and respiratory depression. In this context, elevated intracranial pressure compresses cerebral arterioles, causing ischemia. Resulting increases in arterial partial pressure of carbon dioxide and reductions in pH trigger sympathetic activation to elevate perfusion pressure through systemic hypertension. The associated rise in arterial pressure enhances peripheral baroreceptor stretch and afferent firing. Increased baroreceptor activity initiates a compensatory reflex bradycardia, completing the Cushing reflex triad.[11]
Media
(Click Image to Enlarge)
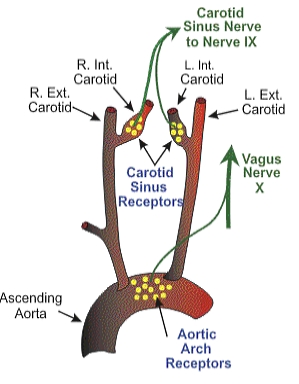
Neural Pathways of Baroreceptor Signaling. The illustration shows baroreceptors in the carotid sinus and aortic arch transmitting afferent signals via the glossopharyngeal (cranial nerve IX) and vagus (cranial nerve X) nerves to central regulatory centers involved in blood pressure modulation.
Contributed by Bruno Bordoni, PhD.
References
Lau EO, Lo CY, Yao Y, Mak AF, Jiang L, Huang Y, Yao X. Aortic Baroreceptors Display Higher Mechanosensitivity than Carotid Baroreceptors. Frontiers in physiology. 2016:7():384. doi: 10.3389/fphys.2016.00384. Epub 2016 Aug 31 [PubMed PMID: 27630578]
Montorfano L, Giambartolomei G, Funes DR, Lo Menzo E, Dip F, White KP, Rosenthal RJ. The Cushing reflex and the vasopressin-mediated hemodynamic response to increased intracranial pressure during acute elevations in intraabdominal pressure. Surgery. 2020 Feb:167(2):478-483. doi: 10.1016/j.surg.2019.10.006. Epub 2019 Dec 6 [PubMed PMID: 31813477]
Saku K, Kishi T, Sakamoto K, Hosokawa K, Sakamoto T, Murayama Y, Kakino T, Ikeda M, Ide T, Sunagawa K. Afferent vagal nerve stimulation resets baroreflex neural arc and inhibits sympathetic nerve activity. Physiological reports. 2014 Sep 1:2(9):. doi: 10.14814/phy2.12136. Epub 2014 Sep 4 [PubMed PMID: 25194023]
Porzionato A, Macchi V, Stecco C, De Caro R. The Carotid Sinus Nerve-Structure, Function, and Clinical Implications. Anatomical record (Hoboken, N.J. : 2007). 2019 Apr:302(4):575-587. doi: 10.1002/ar.23829. Epub 2018 May 2 [PubMed PMID: 29663677]
Raul L. Serotonin2 receptors in the nucleus tractus solitarius: characterization and role in the baroreceptor reflex arc. Cellular and molecular neurobiology. 2003 Oct:23(4-5):709-26 [PubMed PMID: 14514026]
Fahim M. Cardiovascular sensory receptors and their regulatory mechanisms. Indian journal of physiology and pharmacology... 2003 Apr:47(2):124-46 [PubMed PMID: 15255616]
Level 3 (low-level) evidenceLantelme P, Harbaoui B, Courand PY. [Resistant hypertension and carotid baroreceptors stimulation]. Presse medicale (Paris, France : 1983). 2015 Jul-Aug:44(7-8):730-6. doi: 10.1016/j.lpm.2015.03.020. Epub 2015 Jul 2 [PubMed PMID: 26144275]
Geerdes BP, Frederick KL, Brunner MJ. Carotid baroreflex control during hemorrhage in conscious and anesthetized dogs. The American journal of physiology. 1993 Jul:265(1 Pt 2):R195-202 [PubMed PMID: 8342687]
Level 3 (low-level) evidenceMateos JCP. Carotid Sinus Massage in Syncope Evaluation: A Nonspecific and Dubious Diagnostic Method. Arquivos brasileiros de cardiologia. 2018 Jul:111(1):92-93. doi: 10.5935/abc.20180134. Epub [PubMed PMID: 30110050]
Förster A, Wenz H, Böhme J, Groden C, Alonso A. Asymmetrical Gadolinium Leakage in Ocular Structures in Stroke Due to Internal Carotid Artery Stenosis or Occlusion. Clinical neuroradiology. 2020 Jun:30(2):221-228. doi: 10.1007/s00062-018-0754-5. Epub 2018 Dec 28 [PubMed PMID: 30593604]
Saleem S, Teal PD, Howe CA, Tymko MM, Ainslie PN, Tzeng YC. Is the Cushing mechanism a dynamic blood pressure-stabilizing system? Insights from Granger causality analysis of spontaneous blood pressure and cerebral blood flow. American journal of physiology. Regulatory, integrative and comparative physiology. 2018 Sep 1:315(3):R484-R495. doi: 10.1152/ajpregu.00032.2018. Epub 2018 Apr 18 [PubMed PMID: 29668325]
Level 2 (mid-level) evidence