Introduction
Carotid sinus hypersensitivity (CSH) often manifests as syncope and results from an exaggerated response of hypersensitive baroreceptors within the carotid sinus when stimulated.[1] The carotid sinus, located at the bifurcation of the common carotid artery into the internal and external carotid arteries, functions as a neurovascular structure housing pressure-sensitive baroreceptors.[2][3] These receptors, which respond to stretch or pressure (baro originates from the Greek word for pressure), play a central role in blood pressure regulation (see Image. Pathophysiology of Carotid Sinus Hypersensitivity).
Baroreceptor stimulation triggers afferent neural signals via the glossopharyngeal nerve to the solitary nucleus of the medulla. In response to increased pressure or stretch, this neural pathway activates parasympathetic output, resulting in vasodilation, bradycardia, and hypotension. The resulting decline in blood pressure reduces baroreceptor stimulation and ultimately terminates the reflex response.
CSH is defined clinically as an exaggerated autonomic reaction to mechanical stimulation of the carotid sinus, leading to transient cerebral hypoperfusion and subsequent presyncope or syncope.[4] Three subtypes are recognized. The cardioinhibitory response involves a pause in cardiac activity (asystole) lasting 3 seconds or longer on continuous monitoring. The vasodepressor response is characterized by a drop in systolic blood pressure of 50 mm Hg or greater without marked bradycardia. The mixed response includes both cardioinhibitory and vasodepressor components (see Image. Types of Carotid Sinus Hypersensitivity).
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
The exact cause of CSH has not been established. Incidence increases with advancing age, and the condition is uncommon before 50 years. CSH occurs more frequently in men, particularly those with chronic conditions such as hypertension, coronary artery disease, diabetes, and valvular heart disease.[5] An association has also been observed between CSH and neurodegenerative disorders, with increased prevalence in patients with Parkinson disease, Alzheimer disease, and dementia with Lewy bodies. Degenerative changes in the medullary autonomic nuclei, which modulate baroreceptor input, likely contribute to an exaggerated autonomic response, resulting in hypotension or bradycardia. Although some studies have investigated possible genetic contributions to CSH, no specific gene has been definitively implicated.[6][7]
During the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pandemic, several reports described cases of autonomic dysfunction. However, interpretation of these cases requires caution due to patients’ complex and prolonged clinical histories. Syncope related to autonomic dysfunction associated with coronavirus disease 2019 (COVID-19) warrants comprehensive evaluation. A diagnosis of CSH should be considered only after other etiologies have been thoroughly excluded.[8][9]
Epidemiology
The incidence of CSH in the United States is estimated at 35 to 40 cases per million individuals annually. This figure rises with advancing age, and the condition is virtually absent in younger populations. CSH occurs more frequently in men, with a male-to-female ratio of 4 to 1.[10] Prevalence also increases with age, affecting approximately 2.4% of individuals in their sixth decade, 9.1% in the seventh, and 40% of those older than 80 years.[11]
Globally, CSH accounts for nearly 30% of cases of unexplained syncope in older adults and affects 14% of nursing home residents. Overlap with other syncope-related conditions, including vasovagal syncope, orthostatic hypotension, and various autonomic disturbances, complicates accurate epidemiologic estimates. Since syncope may result from multiple overlapping mechanisms, attributing symptoms to a single etiology introduces potential error in calculating incidence and prevalence. Among CSH subtypes, the cardioinhibitory form occurs in approximately 70% of cases, the vasodepressor form in 10%, and the mixed type in 20%. Recognition of these subtypes guides diagnostic evaluation, particularly during carotid sinus massage with continuous hemodynamic monitoring.
Pathophysiology
Baroreceptors play a central role in maintaining the balance of blood pressure and heart rate. These pressure-sensitive receptors, located in the carotid sinus, aortic arch, and great vessels, relay information to the autonomic nervous system.[12] The baroreflex arc consists of afferent and efferent limbs. The afferent limb involves baroreceptors detecting changes in arterial wall stretch and transmitting signals via the glossopharyngeal nerve (from the carotid sinus) and the vagus nerve (from the aortic arch) to the solitary nucleus of the medulla. In response, the efferent limb activates either the sympathetic or parasympathetic branches. Sympathetic activation occurs with decreased blood pressure or reduced wall stretch, resulting in vasoconstriction and tachycardia. Parasympathetic activation follows increased pressure or stretch, producing vasodilation, bradycardia, and hypotension.
The precise mechanism underlying CSH remains unclear, but dysfunction may occur at any point along this reflex pathway. What appears consistent is that mechanical stimulation or stretch of the carotid sinus leads to exaggerated signaling, culminating in bradycardia, hypotension, or both.[13][14] Recent findings challenge earlier hypotheses that implicated reduced arterial compliance from carotid atherosclerosis and compensatory α2-adrenoreceptor upregulation in the brainstem. Studies in patients with CSH revealed intact afferent baroreflex function and no attenuation of reflex sensitivity following administration of yohimbine, an α2-adrenoreceptor antagonist. These results suggest that α2-receptor upregulation does not contribute meaningfully to the pathophysiology of CSH.[15]
Histopathology
Histological studies using specialized stains and techniques have identified τ protein hyperphosphorylation and α-synuclein accumulation in the medulla among patients with CSH associated with neurodegenerative disorders. These findings suggest a possible mechanistic link between CSH and neurodegeneration, potentially explaining the observed association in clinical populations.[16]
History and Physical
Patients with CSH often have a medical history that includes hypertension, diabetes, coronary artery disease, and neurodegenerative disorders such as Parkinson disease or Alzheimer disease. Although these comorbidities may be present, physical examination frequently reveals no abnormalities besides findings related to those underlying conditions. Symptomatic CSH typically presents with presyncope or syncope, unexplained falls, and transient visual disturbances such as a darkened visual field preceding loss of consciousness. On examination, hypotension, pallor, bradycardia, and bruising consistent with fall-related trauma may be observed.
Identifying the etiology of syncope or falls in older adults is essential, as failure to do so may result in missed diagnoses of serious cardiac or neurologic conditions such as aortic stenosis, arrhythmias, seizures, or stroke. Undiagnosed CSH can have life-threatening consequences in high-risk scenarios, such as syncope occurring while driving, swimming, diving, parachuting, or operating heavy machinery. Conversely, CSH may be entirely asymptomatic and detected only during carotid sinus massage. Notably, the diagnostic criteria for CSH may be met even without clinical symptoms during testing. Rare cases involving head and neck tumors have demonstrated exaggerated carotid sinus responses due to tumor compression of baroreceptors. Management in such cases depends on tumor type, stage, patient age, and overall prognosis.[17]
Evaluation
Diagnosis of CSH begins with a detailed history and is confirmed through carotid sinus massage performed under appropriate monitoring conditions. Further diagnostic evaluation is warranted to exclude alternative causes of syncope or transient loss of consciousness in patients whose presentation does not align with the typical features of CSH. Assessment should begin with a comprehensive event history, focusing on known triggers associated with specific types of syncope, such as prolonged standing in vagally mediated episodes or exertion in arrhythmia-related syncope. A past medical and surgical history, along with a thorough family history, aids in identifying inherited cardiomyopathies or familial arrhythmia syndromes.
A focused physical examination should follow, including vital signs with orthostatic measurements and a brief neurologic assessment. Initial laboratory testing typically includes a complete blood count, basic metabolic panel, and capillary glucose level to screen for systemic or metabolic contributors such as hypoglycemia. Electrocardiography is essential in all cases. In selected patients, transthoracic echocardiography may be indicated to assess for structural heart disease. Additional testing, such as a tilt table test, can help differentiate vasovagal syncope or orthostatic hypotension. Brain imaging or electroencephalography may be necessary to evaluate for seizures, transient ischemic attack, or other central nervous system pathology in patients with suspected neurologic involvement.
Additional diagnostic tools such as Holter monitoring and cardiac electrophysiologic studies may aid in evaluating patients with unexplained syncope. However, a monitored carotid sinus massage is the most direct and effective method for confirming CSH. The clinical history should guide the diagnostic strategy to rule out other potential syncope causes appropriately. Careful evaluation is necessary to avoid misdiagnosis and prevent recurrence of potentially injurious syncopal episodes in older adults, especially those presenting with unexplained or recurrent falls.
Carotid sinus massage is performed with the patient in the supine position. The examiner locates the carotid sinus just anterior to the sternocleidomastoid muscle at the level of the upper thyroid cartilage. The right carotid sinus is massaged first using the index and middle fingers, as it typically elicits a stronger baroreceptor response. The massage is applied in a circular motion for 5 to 10 seconds.
The left side may be tested if no diagnostic response is observed, provided no contraindications exist. A positive test is defined as asystole lasting 3 seconds or longer or a decrease in systolic blood pressure of 50 mm Hg or more, regardless of symptom reproduction. Repeating the maneuver with the patient upright or during tilt table testing may improve yield if initial testing is nondiagnostic. Emergency equipment and trained personnel must be available during the procedure.[18][19]
Carotid sinus massage is contraindicated in specific clinical settings due to the risk of precipitating serious adverse events. Absolute contraindications include a history of stroke, transient ischemic attack, or myocardial infarction within the previous 3 months. Relative contraindications involve the presence of certain arrhythmias, particularly ventricular tachycardia or ventricular fibrillation, and the detection of a carotid artery bruit on physical examination. In cases where a bruit is present, a carotid duplex ultrasound should be performed to assess the degree of stenosis. Carotid massage is contraindicated if the study reveals 70% or greater narrowing. However, the procedure may be considered in patients with 50% and 69% stenosis, provided appropriate precautions are in place.[19][20]
Treatment / Management
Contemporary management of CSH centers on trigger avoidance and the prevention or control of symptomatic episodes. This approach typically combines lifestyle modifications, pharmacologic therapy, and, in selected cases, device-based interventions such as pacemaker implantation. Treatment is tailored according to CSH subtype, whether vasodepressor, cardioinhibitory, or mixed, and symptom severity.
General Approach
Patient education forms the foundation of CSH management. Individuals should receive clear instructions about the condition, common triggers, and warning signs. Avoidance of tight collars, abrupt neck movements, and other activities that increase pressure on the carotid sinus is essential. Maintaining adequate hydration and awareness of prodromal symptoms can reduce the risk of syncope. Asymptomatic individuals who fulfill diagnostic criteria for CSH require no further intervention. Individuals who demonstrate a positive response to supervised carotid sinus massage without symptoms should focus on trigger avoidance and control of comorbidities such as hypertension and diabetes.
Treatment by Subtype
In the vasodepressor subtype, lifestyle changes such as increased fluid and salt intake, up to 6 grams of sodium chloride daily if not contraindicated, are generally sufficient. Pharmacologic agents such as fludrocortisone or midodrine may be considered in persistent cases, although data supporting their use are limited. Careful monitoring is necessary, especially in patients with hypertension or heart failure.[21][22] Cardioinhibitory CSH is best managed with dual-chamber pacemaker implantation in patients with recurrent syncope and documented asystolic pauses during carotid sinus massage. Mixed CSH may also benefit from pacing, but additional measures to address the vasodepressor component, such as lifestyle adjustments or pharmacotherapy, are required.[23][24](A1)
Comorbidities like sick sinus syndrome coexist with CSH in approximately 26% of cases. Dual-chamber pacing offers therapeutic benefit for both conditions.[25] Historical interventions such as surgical or radiologic carotid sinus denervation have been largely abandoned due to unacceptable complication rates. Treatment effectiveness, particularly with pharmacologic agents, varies among individuals. Management strategies must be patient-specific, with close follow-up to assess efficacy and monitor for adverse effects.
Differential Diagnosis
CSH may present as a syncopal episode, complicating clinical evaluation, particularly when carotid stimulation is not clearly evident, such as with abrupt neck movement or a sudden change in blood pressure. Patients may not report activities that triggered the episode, making diagnosis more challenging. The following conditions can mimic the presentation of CSH, necessitating careful differentiation:
- Vasovagal syncope
- Orthostatic hypotension
- Cardiac arrhythmias (eg, atrial fibrillation, ventricular tachycardia)
- Postural orthostatic tachycardia syndrome
- Myocardial infarction
- Transient ischemic attack
- Stroke (ischemic or hemorrhagic)
- Hypoglycemia
- Severe dehydration
- Medications (eg, antihypertensives, β-blockers, diuretics)
- Neurological conditions (eg, epilepsy, vestibular disorders)
- Aortic stenosis
- Pulmonary embolism
- Anemia
- Cerebral hypoperfusion
- Hyperthyroidism or thyrotoxicosis
- Severe bradycardia or heart block
- Adrenal insufficiency (Addison disease)
- Autonomic neuropathy (eg, diabetes, Parkinson disease)
- Hyperventilation syndrome
- Multiple system atrophy
- Dehydration or electrolyte imbalances (eg, hyponatremia)
- Intracranial hypertension (eg, due to mass effect or hydrocephalus)
- Carotid artery dissection or stenosis
- Neurocardiogenic syncope
A comprehensive and systematic diagnostic approach is essential to differentiate CSH from these other etiologies. Thorough evaluation with a structured plan increases diagnostic accuracy, although a subset of cases may remain unexplained.[26][27]
Prognosis
The mortality rate among patients with CSH does not exceed that of the general population, indicating that CSH itself is not inherently life-threatening. However, the clinical concern centers on syncope-related injuries. Nonaccidental falls or sudden loss of consciousness during critical activities may result in serious complications, including multiple fractures or intracranial hemorrhage. These risks are particularly pronounced in older adults, many of whom receive anticoagulant or antiplatelet therapy. In such cases, even minor trauma can lead to fatal outcomes.[28][29]
Complications
The primary complication of CSH is syncope, often resulting in nonaccidental falls that cause injuries such as fractures and head trauma. These events can severely affect mobility and independence, particularly in older adults with osteoporosis, poor surgical candidacy, anticoagulant use, or an increased risk of postoperative complications. Recurrent syncopal episodes diminish quality of life and contribute to psychological distress, including fear, anxiety, and social withdrawal. Inadequate evaluation before carotid sinus massage, such as failure to detect carotid bruits or significant stenosis, elevates the risk of stroke, underscoring the importance of thorough diagnostic assessment.[30]
Deterrence and Patient Education
Patient education plays a vital role in managing CSH by helping individuals understand the nature of the condition and how to avoid potential triggers. Preventive measures include avoiding tight collars and minimizing pressure near the carotid sinus, such as during shaving. Activities that require full consciousness, such as driving, operating heavy machinery, and engaging in sea-based activities like diving or swimming, should be avoided until proper treatment has been established.
Driving regulations vary by state, and clinicians are responsible for informing patients of relevant laws. Under local driving laws, patients with the cardioinhibitory subtype who undergo pacemaker implantation may resume driving after a week. In those with the vasodepressor subtype, stabilization of blood pressure may enable the safe return to previous activities.[31]
Enhancing Healthcare Team Outcomes
CSH is a frequent cause of syncope in older adults. Evaluation must prioritize the exclusion of life-threatening etiologies before initiating condition-specific testing. A monitored carotid sinus massage may be performed in appropriate clinical contexts, provided contraindications, such as carotid bruits or a recent history of stroke, are absent.
Management begins with patient education, which includes awareness of CSH triggers and potential consequences. Activities requiring full consciousness, including driving, must be addressed in detail. Symptomatic individuals should receive targeted treatment, and resumption of restricted activities should follow a symptom-free observation period under state-specific laws and clinical judgment. Optimal care often involves interprofessional collaboration. Accurate clinical assessment and judicious use of diagnostic tools are essential, as prompt identification of the underlying cause of syncope can substantially reduce associated morbidity and mortality.
Media
(Click Image to Enlarge)
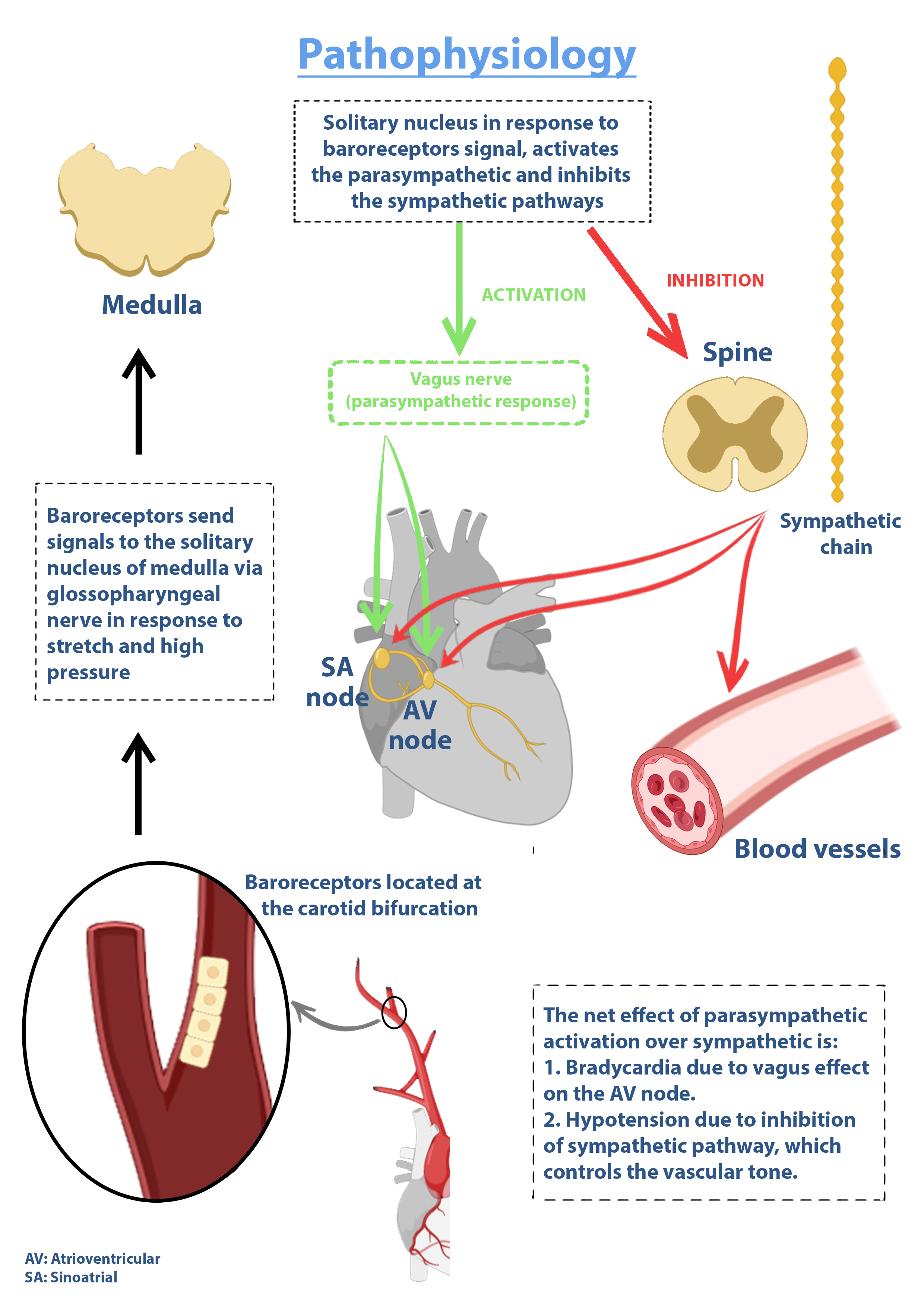
Pathophysiology of Carotid Sinus Hypersensitivity. The image illustrates the baroreflex arc involved in carotid sinus hypersensitivity, including afferent input from the carotid sinus baroreceptors to the medulla and efferent autonomic output resulting in vasodilation and bradycardia.
Contributed by Antoine Kharsa, MD with permission from graphic designer Mirna Roumieh to be published for Statpearls.
(Click Image to Enlarge)
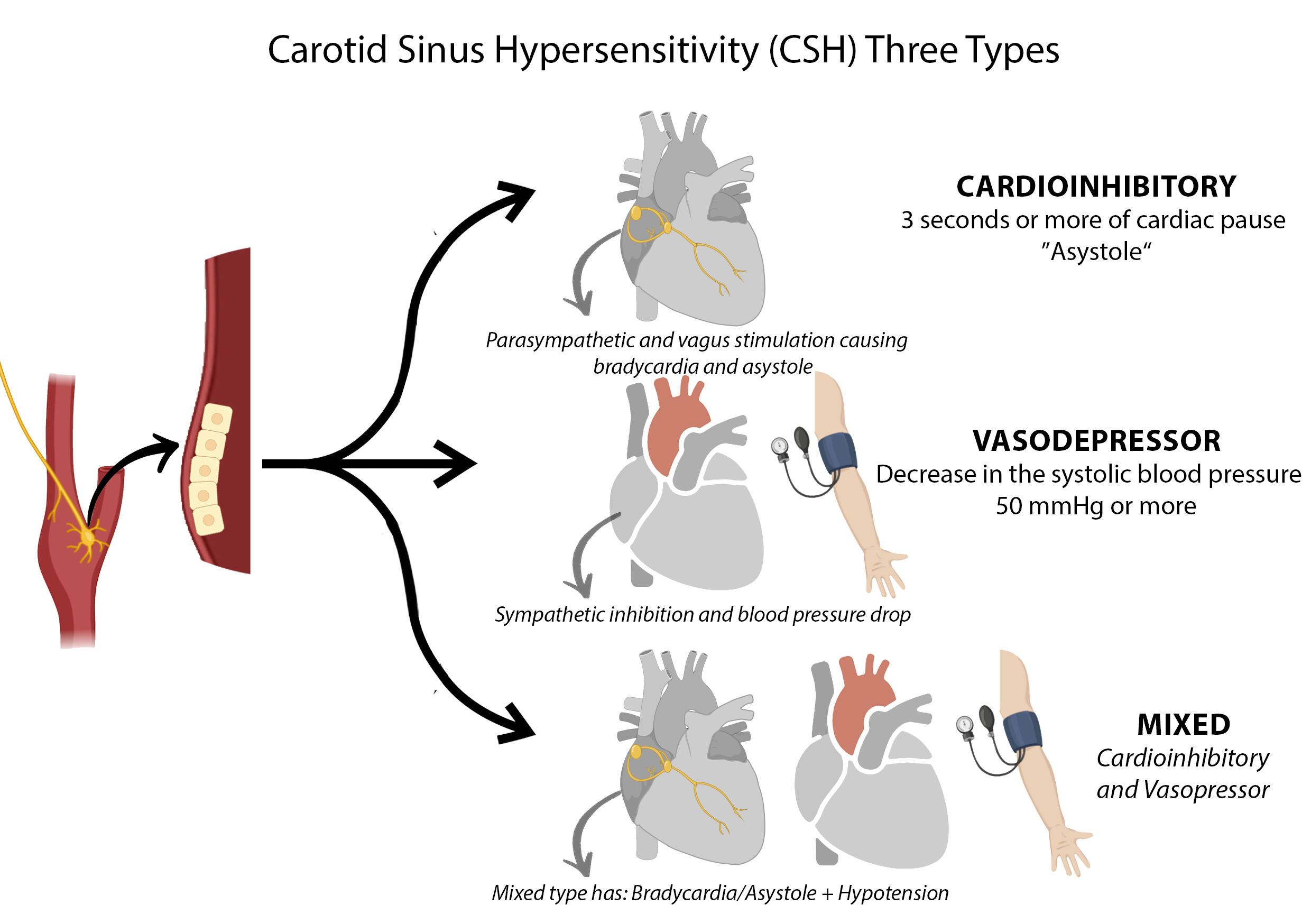
Types of Carotid Sinus Hypersensitivity. The illustration displays the cardioinhibitory, vasodepressor, and mixed subtypes of carotid sinus hypersensitivity, each associated with characteristic changes in heart rhythm and blood pressure. Three schematic hearts represent the distinct hemodynamic responses seen in each variant.
Contributed by Antoine Kharsa, MD with permission from graphic designer Mirna Roumieh to be published for Statpearls.
References
de Lange FJ, de Jong JSY, van Zanten S, Hofland WPME, Tabak R, Cammenga M, Francisco-Pascual J, Russo V, Fedorowski A, Deharo JC, Brignole M. Carotid sinus massage in clinical practice: the Six-Step-Method. Europace : European pacing, arrhythmias, and cardiac electrophysiology : journal of the working groups on cardiac pacing, arrhythmias, and cardiac cellular electrophysiology of the European Society of Cardiology. 2024 Nov 1:26(11):. doi: 10.1093/europace/euae266. Epub [PubMed PMID: 39397761]
Andani R, Khan YS. Anatomy, Head and Neck: Carotid Sinus. StatPearls. 2025 Jan:(): [PubMed PMID: 32119265]
Wu TC. What is the Real Clinical Significance of Carotid Sinus Hypersensitivity in Clinical Practice? A Dilemma Still Waiting for Answers. Arquivos brasileiros de cardiologia. 2020 Feb:114(2):254-255. doi: 10.36660/abc.20200005. Epub [PubMed PMID: 32215493]
Evidence Review Committee Members, Varosy PD, Chen LY, Miller AL, Noseworthy PA, Slotwiner DJ, Thiruganasambandamoorthy V. Pacing as a treatment for reflex-mediated (vasovagal, situational, or carotid sinus hypersensitivity) syncope: A systematic review for the 2017 ACC/AHA/HRS guideline for the evaluation and management of patients with syncope: A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Heart rhythm. 2017 Aug:14(8):e255-e269. doi: 10.1016/j.hrthm.2017.03.006. Epub 2017 Mar 9 [PubMed PMID: 28286245]
Level 1 (high-level) evidenceJędrzejczyk-Spaho J, Wileczek A, Nessler J, Kornaszewska M, Konarski Ł, Kustroń A, Zając M, Ratajska A, Śledź J, Stec S. Management of various spectra of patients with carotid sinus syndrome/hypersensitivity in the era of cardioneuroablation. Kardiologia polska. 2025:83(6):750-752. doi: 10.33963/v.phj.105108. Epub 2025 Mar 24 [PubMed PMID: 40126430]
Miller VM, Kenny RA, Slade JY, Oakley AE, Kalaria RN. Medullary autonomic pathology in carotid sinus hypersensitivity. Neuropathology and applied neurobiology. 2008 Aug:34(4):403-11 [PubMed PMID: 18005097]
2018 ESC Guidelines for the diagnosis and management of syncope., Brignole M,Moya A,de Lange FJ,Deharo JC,Elliott PM,Fanciulli A,Fedorowski A,Furlan R,Kenny RA,Martín A,Probst V,Reed MJ,Rice CP,Sutton R,Ungar A,van Dijk JG,, European heart journal, 2018 Mar 19 [PubMed PMID: 29562304]
Larsen NW, Stiles LE, Miglis MG. Preparing for the long-haul: Autonomic complications of COVID-19. Autonomic neuroscience : basic & clinical. 2021 Nov:235():102841. doi: 10.1016/j.autneu.2021.102841. Epub 2021 Jul 3 [PubMed PMID: 34265539]
Haloot J, Bhavaraju-Sanka R, Pillarisetti J, Verduzco-Gutierrez M. Autonomic Dysfunction Related to Postacute SARS-CoV-2 Syndrome. Physical medicine and rehabilitation clinics of North America. 2023 Aug:34(3):563-572. doi: 10.1016/j.pmr.2023.04.003. Epub 2023 Apr 18 [PubMed PMID: 37419532]
da Silva RM. Syncope: epidemiology, etiology, and prognosis. Frontiers in physiology. 2014:5():471. doi: 10.3389/fphys.2014.00471. Epub 2014 Dec 8 [PubMed PMID: 25538626]
Amin V, Pavri BB. Carotid sinus syndrome. Cardiology in review. 2015 May-Jun:23(3):130-4. doi: 10.1097/CRD.0000000000000041. Epub [PubMed PMID: 25211534]
Urroz Lopez M, Mitchell JR, Sheldon RS, Tyberg JV. Effector mechanisms in the baroreceptor control of blood pressure. Advances in physiology education. 2022 Jun 1:46(2):282-285. doi: 10.1152/advan.00160.2021. Epub 2022 Feb 24 [PubMed PMID: 35201919]
Level 3 (low-level) evidenceO'Callaghan S, Kenny RA. Neurocardiovascular Instability and Cognition. The Yale journal of biology and medicine. 2016 Mar:89(1):59-71 [PubMed PMID: 27505017]
Sutton R. Carotid sinus syndrome: Progress in understanding and management. Global cardiology science & practice. 2014:2014(2):1-8. doi: 10.5339/gcsp.2014.18. Epub 2014 Jun 18 [PubMed PMID: 25405171]
Level 3 (low-level) evidenceParry SW, Baptist M, Gilroy JJ, Steen N, Kenny RA. Central alpha2 adrenoceptors and the pathogenesis of carotid sinus hypersensitivity. Heart (British Cardiac Society). 2004 Aug:90(8):935-6 [PubMed PMID: 15253974]
Level 3 (low-level) evidencePolvikoski T, Kalaria RN, Perry R, Miller V, Kenny RA. Carotid sinus hypersensitivity associated with focal alpha-synucleinopathy of the autonomic nervous system. Journal of neurology, neurosurgery, and psychiatry. 2006 Sep:77(9):1064-6 [PubMed PMID: 16914754]
Level 3 (low-level) evidenceMuntz HR, Smith PG. Carotid sinus hypersensitivity: a cause of syncope in patients with tumors of the head and neck. The Laryngoscope. 1983 Oct:93(10):1290-3 [PubMed PMID: 6621227]
Level 3 (low-level) evidenceGauer RL. Evaluation of syncope. American family physician. 2011 Sep 15:84(6):640-50 [PubMed PMID: 21916389]
Parry SW, Richardson DA, O'Shea D, Sen B, Kenny RA. Diagnosis of carotid sinus hypersensitivity in older adults: carotid sinus massage in the upright position is essential. Heart (British Cardiac Society). 2000 Jan:83(1):22-3 [PubMed PMID: 10618329]
Kenny RA, O'Shea D, Parry SW. The Newcastle protocols for head-up tilt table testing in the diagnosis of vasovagal syncope, carotid sinus hypersensitivity, and related disorders. Heart (British Cardiac Society). 2000 May:83(5):564-9 [PubMed PMID: 10768910]
Moore A, Watts M, Sheehy T, Hartnett A, Clinch D, Lyons D. Treatment of vasodepressor carotid sinus syndrome with midodrine: a randomized, controlled pilot study. Journal of the American Geriatrics Society. 2005 Jan:53(1):114-8 [PubMed PMID: 15667387]
Level 1 (high-level) evidenceda Costa D, McIntosh S, Kenny RA. Benefits of fludrocortisone in the treatment of symptomatic vasodepressor carotid sinus syndrome. British heart journal. 1993 Apr:69(4):308-10 [PubMed PMID: 8489861]
Lopes R, Gonçalves A, Campos J, Frutuoso C, Silva A, Touguinha C, Freitas J, Maciel MJ. The role of pacemaker in hypersensitive carotid sinus syndrome. Europace : European pacing, arrhythmias, and cardiac electrophysiology : journal of the working groups on cardiac pacing, arrhythmias, and cardiac cellular electrophysiology of the European Society of Cardiology. 2011 Apr:13(4):572-5. doi: 10.1093/europace/euq455. Epub 2010 Dec 17 [PubMed PMID: 21169606]
Level 2 (mid-level) evidenceMadigan NP, Flaker GC, Curtis JJ, Reid J, Mueller KJ, Murphy TJ. Carotid sinus hypersensitivity: beneficial effects of dual-chamber pacing. The American journal of cardiology. 1984 Apr 1:53(8):1034-40 [PubMed PMID: 6702680]
Brignole M, Menozzi C, Lolli G, Oddone D, Gianfranchi L, Bertulla A. Pacing for carotid sinus syndrome and sick sinus syndrome. Pacing and clinical electrophysiology : PACE. 1990 Dec:13(12 Pt 2):2071-5 [PubMed PMID: 1704595]
Bădilă E, Negrea C, Rîpă A, Weiss E, Bartoş D, Tîrziu C. The Etiology of Syncope in an Emergency Hospital. Romanian journal of internal medicine = Revue roumaine de medecine interne. 2016 Sep 1:54(3):173-178. doi: 10.1515/rjim-2016-0023. Epub [PubMed PMID: 27658165]
Kerr SR, Pearce MS, Brayne C, Davis RJ, Kenny RA. Carotid sinus hypersensitivity in asymptomatic older persons: implications for diagnosis of syncope and falls. Archives of internal medicine. 2006 Mar 13:166(5):515-20 [PubMed PMID: 16534037]
Hampton JL, Brayne C, Bradley M, Kenny RA. Mortality in carotid sinus hypersensitivity: a cohort study. BMJ open. 2011 Jul 20:1(1):e000020. doi: 10.1136/bmjopen-2010-000020. Epub 2011 Jul 20 [PubMed PMID: 22021728]
Lacerda GC, Lorenzo AR, Tura BR, Santos MCD, Guimarães AEC, Lacerda RC, Pedrosa RC. Long-Term Mortality in Cardioinhibitory Carotid Sinus Hypersensitivity Patient Cohort. Arquivos brasileiros de cardiologia. 2020 Feb:114(2):245-253. doi: 10.36660/abc.20190008. Epub [PubMed PMID: 32215492]
van Munster CE, van Ballegoij WJ, Schroeder-Tanka JM, van den Berg-Vos RM. [A severe stroke following carotid sinus massage]. Nederlands tijdschrift voor geneeskunde. 2017:161():D826 [PubMed PMID: 28378696]
Sorajja D, Nesbitt GC, Hodge DO, Low PA, Hammill SC, Gersh BJ, Shen WK. Syncope while driving: clinical characteristics, causes, and prognosis. Circulation. 2009 Sep 15:120(11):928-34. doi: 10.1161/CIRCULATIONAHA.108.827626. Epub 2009 Aug 31 [PubMed PMID: 19720940]
Level 2 (mid-level) evidence