Introduction
Neuroendocrine tumors (NETs) are a diverse group of neoplasms that arise from neuroendocrine cells and can develop in various organs, including the gastrointestinal (GI) tract, lungs, ovaries, adrenal glands, and thyroid. The terms “carcinoid tumor” and “carcinoid” historically refer to well-differentiated NETs, particularly those originating in the GI tract and lungs, and have often been used interchangeably with “NETs.” For clarity and consistency, this course discussion will use the term “neuroendocrine tumor (NET)” throughout, except when referring to historical nomenclature or specific tumor subsets (eg, typical carcinoid, atypical carcinoid).
Pulmonary NETs account for 1% to 2% of all lung malignancies in adults and approximately 25% to 30% of all NETs, making the lung the second most common site after the GI tract.[1] Pulmonary NETs can be classified into 2 subcategories: typical and atypical carcinoids, with the latter comprising only 10% to 15% of cases. Typical carcinoids and atypical carcinoids are considered low- and intermediate-grade, respectively. Thymic neuroendocrine tumors follow a similar histopathologic spectrum, though their classification is less well-defined and often associated with more aggressive clinical behavior. The grading criteria for these tumors are defined by the World Health Organization (WHO) and will be reviewed in the histopathology section of this activity.
Approximately 80% of pulmonary NETs are centrally within the lung, while 20% arise in peripheral regions.[2] All pulmonary NETs are malignant and have the potential to metastasize.[3] Unlike many other lung cancers, pulmonary NETs are not associated with smoking. These tumors are typically small and tend to occur in central locations. Lymph node involvement is observed in less than 10% of cases. Carcinoid syndrome, which is characterized by a collection of paraneoplastic symptoms resulting from bioactive chemicals released by the tumor—such as serotonin, histamine, chromogranin, and somatostatin—can occur.[4] Symptoms may include flushing, asthma, diarrhea, and right-sided valvular heart disease. However, carcinoid syndrome is uncommon in pulmonary NETs, with an estimated incidence of 2% to 12% among patients, and typically arises only in cases with extensive hepatic or extrahepatic metastasis. This is due to first-pass metabolism, which inactivates vasoactive substances.[5]
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
The etiology of NETs is not fully understood, and although some studies suggest a potential link with smoking, the causal relationship remains uncertain. Evidence indicates that more patients with atypical carcinoids are smokers compared to those with typical carcinoids. Other research studies have identified certain air pollutants and chemicals as risk factors.[6] Pulmonary NETs are hypothesized to have associations with various comorbidities and malignancies, indicating potential shared risk factors. Furthermore, research suggests that individuals diagnosed with pulmonary NETs may exhibit an elevated risk of developing secondary primary cancers relative to the general population. This interplay between pulmonary NETs and other neoplastic conditions underscores the complexity of their etiological profile and necessitates further investigation into the underlying mechanisms involved.[7]
In rare cases, pulmonary NETs can be associated with multiple endocrine neoplasia type 1 (MEN-1) syndrome. Furthermore, there have been reports of sporadic instances in which the MEN-1 gene is found to be inactivated, suggesting a potential genetic component to the development of these tumors.[8] The etiology of thymic NETs remains unclear. MEN-1 syndrome is associated with 25% of cases of thymic NETs and develops predominantly in males.[9] No association with carcinogens or environmental factors has been established for sporadic cancer cases. However, there is evidence of a higher incidence of thymic NETs in MEN-1 patients who are heavy smokers.[10] Pulmonary NETs are relatively rare compared to thymic NETs and are predominantly observed in females with MEN-1 syndrome.[11]
Epidemiology
NETs are uncommon malignancies that can affect the lungs. While they can occur at any age between 5 and 90, the mean age of presentation of typical carcinoids is 58.8. Notably, atypical carcinoids (AC) present approximately 10 years earlier than typical carcinoids (TC). Approximately 8% of these tumors are diagnosed during the second decade of life, making them the most common primary pulmonary tumor in childhood. The WHO classification recognizes 4 major types of pulmonary neuroendocrine tumors: TC, AC, large cell neuroendocrine carcinoma (LCNEC), and small cell lung cancer (SCLC). The most common pulmonary NET is SCLC (20%), followed by LCNEC (3%), TC (2%), and AC (0.2%).[12]
Some data suggest a higher incidence of NETs among individuals of white ethnicity and women than men.[13] In recent decades, the incidence of NETs, including pulmonary NETs, has demonstrated an upward trend. This increase can be attributed to several factors, such as advancements in medical investigations and improved detection rates.[14]
Pathophysiology
The precise mechanisms underlying the development and progression of NETs are not fully understood. However, in some cases, it is believed that these tumors may arise from proliferating pulmonary neuroendocrine cells through a condition known as diffuse idiopathic pulmonary neuroendocrine cell hyperplasia (DIPNECH) and tumorlets.[15] Carcinoid tumorlets are histologically identical to NETs but are smaller, measuring less than 0.5 cm in diameter. The exact significance of tumorlets is unknown, but they are often associated with inflammatory or fibrosing conditions. DIPNECH is a rare condition characterized by the widespread presence of pulmonary carcinoid tumorlets and, occasionally, tumors. This condition occurs almost exclusively in women and can cause symptoms when the lungs are extensively involved.[16]
The exact pathophysiology of these tumors' development is not fully understood. Although rare, thymic NETs have been associated with various paraneoplastic syndromes. Up to one-third present concomitantly with paraneoplastic conditions. The most common being Cushing syndrome, presenting in 30% to 35% cases, in which thymic NETs may secrete ectopic adrenocorticotropic hormone.[17][18]
Humoral hypercalcemia of malignancy secondary to parathyroid hormone-related peptide secretion can be present in up to 30% of patients.[18] Unlike NETs involving other organs, thymic NETs rarely present with carcinoid syndrome.[19] Acromegaly secondary to ectopic production of growth hormone-releasing hormone and syndrome of inappropriate antidiuretic hormone, albeit uncommon, have been described in the literature.[20] As previously mentioned, MEN-1 syndrome is associated with 25% of cases of thymic NETs, and roughly 8% of patients with MEN-1 syndrome develop a thymic NET.[9] Recent studies have shown that frequent 11q deletions in TC and AC include the MEN-1 gene locus, suggesting this is a distinct genetic event in pulmonary NETs. Further losses, specifically at 10q and 13q, have been associated with AC, which indicates that genetic factors may be driving their more aggressive behavior.[21]
Histopathology
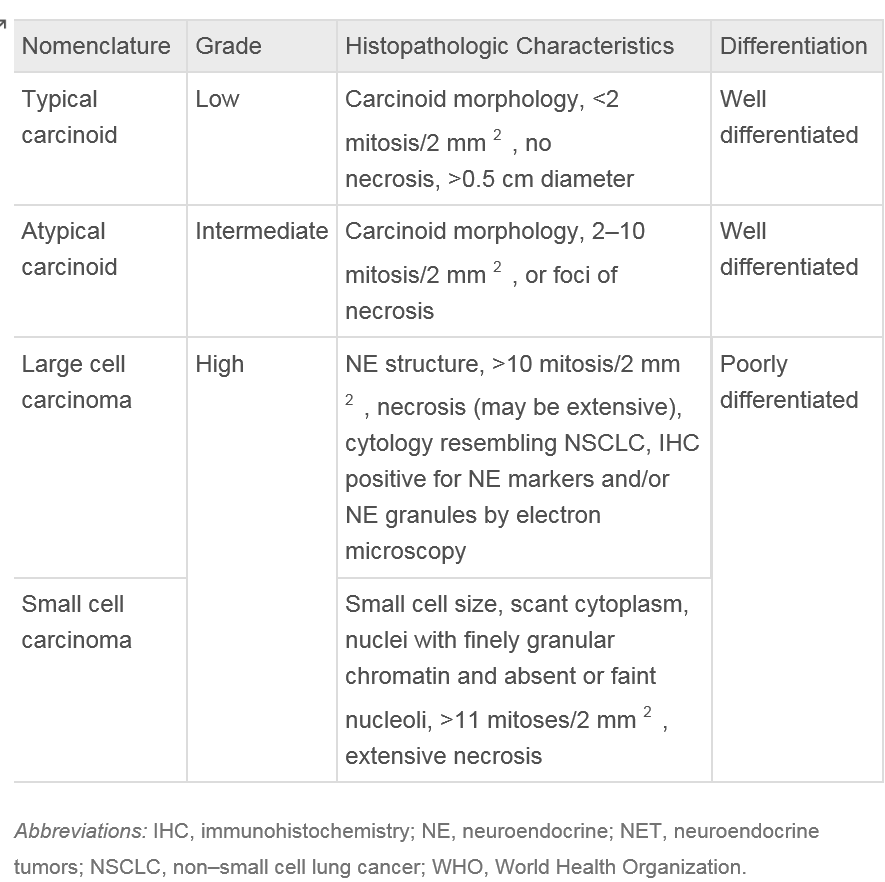
Pulmonary NETs are cataloged based on the WHO classification: low-grade (typical) NETs (TCs), intermediate-grade (atypical) NETs (ACs), LCNEC, and SCLC.[22] Histological classification is based upon the presence or absence of necrosis, mitotic activity, and large cell or small cell cytology. The nuclear chromatin, in general, is finely granular, while LCNEC may show prominent nucleoli. Tumor cells may form trabeculae, rosettes, festoons, glands, and solid sheets.[22] TCs and ACs are both classified as well-differentiated. However, AC exhibits more aggressive clinical behavior compared to TC. LCNEC and SCLC represent 2 distinct types of poorly differentiated neuroendocrine carcinomas, classified separately. Despite differences in grade and clinical behavior, all pulmonary neuroendocrine neoplasms share key features, including the ability to synthesize neuropeptides and the presence of submicroscopic cytoplasmic dense-core granules.[23]
TCs generally have a more benign course, whereas SCLC has a more aggressive course. ACs and LCNECs fall in the middle of the spectrum. These pathological classifications indicate a correlation with prognosis.[18][24] Factors significantly affecting prognosis include primary tumor size greater than 3 cm, histological atypia, and nodal metastases.[25]
Macroscopic Findings
Central pulmonary NETs exhibit well-defined boundaries and have a round to oval shape. They can be either sessile or pedunculated. Histologically, these tumors display a finely granular chromatin pattern and moderate to abundant eosinophilic cytoplasm. They often occupy and fill the bronchial lumen.[26]
As central pulmonary NETs grow, they may extend between the cartilaginous plates into adjacent tissues. Pulmonary NETs can range widely in size, from small nodules measuring approximately 0.5 cm to larger masses up to 9 to 10 cm in diameter at the time of diagnosis. On average, ACs are larger than TCs.[26] In 1 retrospective series of 121 patients who underwent surgical resection of pulmonary NETs, patients with ACs demonstrated a significantly larger tumor size than patients with TCs. Patients with ACs also had an increased number of lymph node metastases when compared with patients with TCs.[27]
Histopathology
The growth patterns of NETs suggest neuroendocrine differentiation. The most common patterns observed are organoid and trabecular arrangements. However, other growth patterns may also be present, such as rosette formation, papillary growth, pseudoglandular growth, and follicular growth. The tumor cells typically appear uniform, with finely granular nuclear chromatin, inconspicuous nucleoli, and moderate to abundant eosinophilic cytoplasm.[12]
The stroma surrounding NETs is highly vascularized but can also display extensive hyalinization, cartilage, or bone formation. There have been reports of stromal amyloid deposition and prominent mucinous stroma within these tumors. TCs are characterized by having fewer than 2 mitoses per 2 mm², lacking necrosis, and measuring at least 0.5 cm in diameter. In contrast, ACs exhibit similar histological features to TCs but are defined by the presence of 2 to 10 mitoses per 2 mm² or necrosis.[28] Necrosis in ACs is usually punctate. Mitotic activity should be counted in the area with the highest number of mitoses, encompassing as many viable tumor cells as possible. The counting should be reported per 2 mm² rather than based on a specific number of high-power fields.[12]
Immunohistochemistry
Immunohistochemistry is crucial in diagnosing NETs, especially in small biopsy samples. A recommended antibody panel for immunostaining includes chromogranin A, synaptophysin, and CD56. However, none of these markers can differentiate between typical and atypical carcinoids. Additionally, most NETs react to pan-cytokeratin antibodies, commonly used to identify epithelial cells. Some peripheral tumors may also exhibit staining with thyroid transcription factor 1, although this marker is less specific for NETs. In evaluating biopsy or cytology samples, the Ki-67 labeling index is valuable, especially in cases where crush artifacts make assessing the mitotic index challenging. By measuring the Ki-67 labeling index, which indicates the percentage of cells undergoing active cell division, misdiagnosis of NETs as high-grade neuroendocrine carcinomas can be avoided.[12]
History and Physical
Approximately 25% of individuals with pulmonary NETs may not experience any noticeable disease symptoms. These tumors are often incidentally discovered during diagnostic tests or screenings for unrelated conditions.
The most common presenting symptoms of bronchopulmonary NETs are:
- Coughing or wheezing
- Hemoptysis
- Dyspnea
- Recurrent pneumonia
- Symptoms related to the consequences of collapse or pneumonia distal to airway obstruction [2]
- Sometimes patients may present with stridor.
Since pulmonary NETs are primarily neuroendocrine in origin, some patients may present with symptoms of carcinoid syndrome:
- Facial flushing
- Shortness of breath
- Hyper- or hypotension
- Weight gain
- Hirsutism
- Asthma-like symptoms
- Bronchospasm
- Electrolyte disturbances (eg, hyponatremia, hypokalemia)
Small NETs may be asymptomatic and are usually discovered incidentally during medical examinations or imaging tests conducted for unrelated reasons. In most cases, pulmonary NETs do not exhibit endocrine symptoms at the clinical level. These tumors are often considered "endocrinologically silent" because they do not commonly produce significant amounts of hormones or bioactive substances that result in noticeable clinical effects. Clinical syndromes secondary to peptide production are uncommon; they include carcinoid syndrome, Cushing syndrome, and acromegaly.[2]
Thymic NETs present as a mass located in the anterior mediastinum. Thymic NETs are rare, accounting for less than 5% of all anterior mediastinal neoplasms, and occur more frequently in males, typically in their fourth and fifth decades of life. In most cases, thymic NETs go unrecognized until they reach advanced stages, as patients often remain asymptomatic or symptoms at presentation are ambiguous.[29] These tumors exhibit aggressive behavior, with a tendency to invade locally. Hence, 50% of patients present with invasion to local organs at the time of diagnosis, and 20% to 30% have distant metastatic disease, which occurs via lymphatic or hematogenous spread.[30][9]
Most patients present with symptoms of mass compression to local structures, while only a third of cases are asymptomatic. Symptoms can vary depending on the extent of invasion. Common complaints include cough, dyspnea, chest pain, and hoarseness from recurrent laryngeal nerve invasion.[9] Superior vena cava syndrome has been observed in up to 20% of patients at presentation.[9]
Evaluation
Biochemical Evaluation
Plasma chromogranin A (CGA) levels, synaptophysin, neuron-specific enolase (NSE), blood count, electrolytes, and liver and kidney function are tests indicated in the diagnostic process and follow-up of NETs [31].
Specific tests are performed when symptoms suggesting hormonal secretion are present. They include:
- Urinary 5-hydroxyindoleacetic acid, a metabolite of serotonin, is often elevated in patients with carcinoid syndrome.
- Serum cortisol levels and 24-hour urine free cortisol levels will be elevated.
- Adrenocorticotropic hormone levels are indicated in patients with suspected Cushing disease. These will be increased.
- Plasma growth hormone-releasing hormone and insulin growth factor-I levels are indicated in patients with signs of acromegaly. These will be elevated.[32]
Laboratory Studies
As previously discussed, most NET cases do not produce hormones. Therefore, the utility of laboratory evaluation is limited to specific circumstances. CGA levels, if initially elevated at diagnosis, may help monitor disease activity, especially in patients with metastatic disease.[33] However, CGA is not a diagnostic tool due to its insufficient sensitivity and specificity.[34] Molecular profiling of tumor tissue may be considered, particularly for ACs. Genetic counseling and testing should be regarded as essential for individuals with inherited genetic syndromes.
In cases of paraneoplastic syndromes, such as Cushing syndrome, a high cortisol concentration can be detected in the serum, and 24-hour urine cortisol levels are also elevated. Hypercalcemia and hypophosphatemia can be observed in humoral hypercalcemia of malignancy, along with elevated levels of parathyroid hormone–related peptide concentration, and may also be used to monitor disease recurrence.[33]
Radiological Assessment
NETs can be visible on a standard chest x-ray in 40% of cases. However, the gold standard for the radiological detection and evaluation of pulmonary NETs is computed tomography (CT) with intravenous contrast. Both chest x-rays and CT scans typically show well-defined, round, and sometimes slightly lobulated masses in cases of pulmonary NETs. Central pulmonary NETs can exhibit calcification. When the tumor involves the bronchial airway, secondary effects can be observed, including atelectasis, bronchiectasis, and areas of hyperlucency on CT scans.[35]
Fluorodeoxyglucose positron emission tomography may reveal higher uptake in ACs than TCs, reflecting differences in metabolic activity. This technique is most helpful in discerning well-differentiated NETs from high-grade neuroendocrine carcinomas, such as small-cell or large-cell neuroendocrine tumors, which typically demonstrate intense FDG uptake.[36] Octreotide single-photon emission CT and other novel imaging techniques, such as gallium-labeled somatostatin analogs, have shown promise in improving the detection sensitivity of pulmonary NETs.[37] Imaging for somatostatin receptors using indium-111-labeled octreotide may increase sensitivity for the diagnosis, staging, and follow-up of recurrence.[38] Thymic NETs pose a diagnostic challenge as symptoms at presentation are typically equivocal. Therefore, clinicians must attain a high level of suspicion. Occasionally, thymic NETs may be found incidentally during workup for other conditions.
Imaging Studies
Initial evaluation with CT of the chest, using intravenous (IV) contrast, is the test of choice for assessing anterior mediastinal masses. Cross-sectional imaging, combined with IV contrast, helps characterize the mass and improve localization, while providing information regarding local invasion or regional metastases. Findings of a large, heterogeneous, lobulated, and most often invasive, anterior mediastinal mass with areas of hemorrhage or necrosis suggest thymic NETs. Magnetic resonance imaging (MRI), specifically cardiac MRI, can provide more detailed information about invasion into nearby structures such as the proximal great vessels, heart structures, or pericardium.
Over the past decade, somatostatin receptor-based imaging has become more popular for diagnosing and staging NETs. Thymic NETs, like other NETs, demonstrate an overexpression of somatostatin receptors; thus, the use of radiolabeled somatostatin analogs, such as the gallium Ga-68 DOTATOC (OctreoScan), allows thymic NETs to be identified and distinguished from other malignancies that arise in the anterior mediastinum.[39] Other advantages of these techniques include distinguishing between primary malignancies in the thymus and metastatic disease, as they can scan the entire body. In addition, the sole recognition of somatostatin receptors on the tumor allows the early utilization of peptide receptor radioligand therapy in advanced cases.[40][41] Ruling out lymphomas or germ cell tumors is critical, as these malignancies are typically treated medically rather than surgically, which is the case for thymic NETs.
Bronchoscopy
Bronchoscopy is crucial in diagnosing pulmonary NETs, as these tumors are usually centrally located and visible during bronchoscopic evaluation.[42] Bronchoscopic evaluation with tissue biopsy is the gold standard for diagnosing pulmonary NETs. However, due to the highly vascular nature of these tumors, bronchoscopic biopsy carries a risk of bleeding.
Biopsy
Based on CT chest findings and characteristics of the mass and level of invasion, medical teams can determine if surgical resection or tissue biopsy is indicated. For small, encapsulated masses, surgical resection of the tumor should be attempted. For larger masses with unclear margins, a tissue biopsy is recommended before attempting surgical resection to ascertain the need for a possible role of chemotherapy.[34][43]
Most biopsies are performed through a CT-guided core needle biopsy, which can diagnose a thymic NET in most cases.[43] In alternative situations, where there may not be enough tumor cells to secure a definitive diagnosis through immunohistochemistry or flow cytometry, or the tumor location does not allow for a CT-guided approach, endoscopic or transbronchial ultrasound-guided fine needle biopsy or endobronchial ultrasound-guided transbronchial needle aspiration may be preferred.[9] These techniques are also preferred when mediastinal lymph node metastatic disease is present.
If biopsy results remain inconclusive, a surgical approach is recommended. The location of the tumor will determine the type of surgical approach. Anterior mediastinotomy is indicated in cases where the mass invades the anterior chest wall. On the other hand, if the mass has invaded either side of the chest, a video-assisted thoracoscopic (VATS) approach is warranted.[9] A VATS approach is also helpful if there are concerns for possible lung metastases.
Treatment / Management
Despite having a good prognosis, NETs can be challenging to diagnose due to their nonspecific symptoms. Patients with NETs may present with cough, fever, and shortness of breath. The differential diagnosis is broad, including chronic obstructive airway disease, pneumonia, tuberculosis, sarcoidosis, adenocarcinoma of the lung, squamous carcinoma of the lung, and heart failure. In some cases, the tumor may be discovered incidentally during imaging studies for unrelated reasons.
Collaboration across disciplines is essential in providing patient-centered care for individuals with thoracic NETs, which present unique challenges due to their rarity, biologic heterogeneity, and variable clinical behavior. A multi-disciplinary, team-based approach enhances healthcare delivery and patient outcomes by facilitating comprehensive evaluation, personalized treatment planning, and longitudinal surveillance. Given the broad range of differential diagnoses and the need for a definitive diagnosis, a biopsy is typically necessary. The workup may involve collaboration between pulmonologists, radiologists, and pathologists to appropriately evaluate and interpret clinical findings, imaging studies, and tissue samples. Meticulous staging and planning are required before surgery. Smoking cessation and rigorous chest physiotherapy should be advised for smokers. Calculation of post-operative pulmonary reserve requires careful evaluation by a respiratory clinician.
The interventional radiologist can assist with the biopsy of peripherally located lesions. The biopsy of centrally located lung masses usually requires invasive techniques, such as bronchoscopy; therefore, it is often omitted to preserve surgical fields and avoid procedures that require anesthesia. This poses a particular problem for NETs, as the scope of surgery and adjuvant treatment is not fully understood, leading to overtreatment. The involvement of a pathologist could alleviate this problem. After administering anesthesia to the patient, a bronchoscopy and needle biopsy of the lung lesion and suspected lymph nodes should be performed. The pathologist could then create frozen sections and readily analyze them to help define the extent of resection, thereby avoiding overtreatment.
Pharmacists are crucial in the healthcare team and can help manage post-operative pain. They collaborate with the medical team to prescribe appropriate pain medication, considering individual patient factors and potential drug interactions. Physiotherapists and pulmonary rehabilitation specialists play a crucial role in the post-operative care of patients undergoing lung surgery. They can help with early mobilization, breathing exercises, and respiratory rehabilitation.
Guidelines issued by professional societies, such as the Society of Thoracic Surgeons and the Society of Surgical Oncology, provide evidence-based recommendations for treating pulmonary NETs. These guidelines are valuable resources for healthcare professionals in treatment planning and surveillance. They help ensure patients receive optimal, standardized care based on the best available evidence.
The outcome of pulmonary NETs is favorable and depends on timely intervention. Prompt consultation with interprofessional teams improves patient recovery and survival. Interprofessional collaboration for the care of thoracic NET aligns diagnostic precision, surgical expertise, oncologic therapy, and long-term follow-up. This ultimately enhances outcomes by providing safer interventions, tailored treatment plans, and durable surveillance.
Surgery
Surgical resection is the primary treatment of choice for patients with thoracic NETs. The goal is to remove the tumor while preserving as much healthy lung tissue as possible.[32] The extent of the resection depends on several factors, including the type of NET (eg, typical or atypical) and the involvement of surrounding lung tissue.(A1)
Extensive resections may be necessary in cases of ACs or when the distal lung parenchyma is destroyed. The surgical technique of choice is often lobectomy. In some instances, sleeve or wedge resection may also be performed. Since pulmonary NETs frequently arise from the main or lobar bronchi, bronchoplastic procedures are usually required during surgical resection to preserve lung function and maintain proper airway continuity. A wedge resection is favored in peripheral pulmonary NETs and is often sufficient.[2]
Systemic Therapy
Systemic therapy is used for advanced or metastatic pulmonary NETs. The choice of systemic treatment depends on several factors, including the tumor grade, the extent of metastasis, and individual patient characteristics. The goal of medical management is to control hormone-related symptoms and prevent disease progression.
Adjuvant Therapy
The use of adjuvant therapy in pulmonary NETs is an ongoing debate, and there is no consensus on its role in managing these tumors.[2] The National Comprehensive Cancer Network (NCCN) guidelines recommend considering adjuvant chemotherapy with cisplatin and etoposide, with or without radiation, in stage III ACs.[2] The European Neuroendocrine Tumor Society also recommends adjuvant treatment for ACs with positive lymph nodes, but not for TCs.[2]
Localized Disease
Official guidelines for treating thymic NETs are unavailable, as data from prospective trials are lacking and are mainly based on case reports and case series. Complete surgical resection with negative margins, which remains the therapy of choice, entails the resection of the entire anterior mediastinal compartment, including hilar and mediastinal lymph nodes, given the high rate of nodal metastasis.[30][9] Unfortunately, due to their size, most thymic NETs are deemed not amenable to resection at diagnosis. The role of neoadjuvant chemotherapy or radiation therapy (RT) remains unclear, as data are based solely on case reports.[9][30]
In cases of local invasion, despite total complete surgical resection, microscopic resection may not be achieved, and the rate of recurrence is high.[9] Adjuvant chemotherapy and RT are reasonable options following total resection for control of the residual disease. According to the NCCN, in patients who have had an incomplete resection, RT, preferably with chemotherapy based on cisplatin or carboplatin plus etoposide, is recommended for patients with moderately or poorly differentiated disease.[9] However, the evidence supporting the use of adjuvant RT or chemotherapy is limited.
Metastatic or Recurrent Disease
Even in the instance of recurrent disease, if the tumor is deemed to be resectable, surgical resection should be attempted.[30] In cases of unresectable or metastatic disease, systemic therapy is favored.[44] For tumors with evidence of somatostatin receptor expression, somatostatin analogs are chosen, although data regarding the benefits of this therapy for thymic NETs are scant. In such circumstances, octreotide and lanreotide are considered reasonable options, especially in the rare instance of patients presenting with carcinoid syndrome.[45](A1)
A platinum-based chemotherapy regimen, such as carboplatin and etoposide, is used for intermediate to poorly differentiated tumors, based again on treatment guidelines for poorly differentiated NETs of the gastrointestinal tract and lung.[46] Other systemic therapies, such as everolimus, which have shown survival benefits in patients with advanced, nonfunctional lung and GI NETs, have been adopted as a treatment option for thymic NETs, due to their similarities.[47] The role of RT remains palliative for symptomatic patients with recurrent or metastatic disease.(B2)
Differential Diagnosis
The differential diagnoses of pulmonary NET include:
- Metastatic NETs from other locations, especially those originating from the GI tract
- SCLC
- Salivary gland-type tumors
- Metastases of lobular breast carcinoma, paraganglioma, and glomangioma
- Tumorlets
- Thymic carcinoma
- Thymoma
- Paraganglioma
- Lymphoma
- Germ cell tumors [12]
Surgical Oncology
Stage I to III NETs are treated with surgical resection, the only curative treatment modality for pulmonary NETs. The extent of surgical resection depends on the tumor characteristics, location, and stage of the disease. For stage I pulmonary NETs, which are localized and confined to the lung without the involvement of lymph nodes, surgical resection with lobectomy is often the treatment of choice. However, pneumonectomy or bilobectomy may be needed for centrally located lesions.[48] In cases where the tumor is smaller and located in the peripheral regions of the lung, segmentectomy or wedge resection may be considered an alternative.
TCs, which have favorable histology and are localized without lymph node involvement, can often be managed with more limited resections and still achieve a cure. However, for ACs, which have a higher risk of recurrence and metastasis, the surgical guidelines for NSCLC are followed. Lobectomy is often considered for lesions smaller than 2 cm, and mediastinal lymph node dissection is performed in all patients. The role of mediastinal lymph node dissection in pulmonary NETs is still debatable, with limited evidence supporting its benefits in reducing local recurrence and improving survival. However, performing mediastinal lymph node dissection in all patients with pulmonary NETs is generally recommended, as it allows for accurate staging and helps guide further treatment decisions.[49]
Historically, open thoracotomy was the standard approach for pulmonary resection. In recent years,video-assisted thoracoscopic surgery (VATS) has gained popularity and become the preferred technique for resection of pulmonary NETs. The advantages of VATS include reduced morbidity, shorter hospital stays, and faster recovery. Numerous studies have demonstrated VATS's short-term and long-term benefits, making it the standard approach for surgical procedures.[50][51] Even in cases where an open thoracotomy is performed, the technique has evolved and improved. The incisions are smaller and muscle-sparing, resulting in reduced trauma to the chest and enhanced postoperative recovery. As most pulmonary NETs are intrabronchial in location and tend to grow slowly, there has been an emphasis on developing parenchymal-sparing procedures for pulmonary resection. These include sleeve lobectomy and sleeve pneumonectomy, which have been performed with excellent long-term results.
Radiation Oncology
NETs are less responsive to RT than SCLC; therefore, it has a limited role in managing pulmonary NETs. However, in some instances, RT may be used as part of the treatment approach for pulmonary NETs.The NCCN guidelines recommend using adjuvant cisplatin and etoposide with or without radiation in stage III ACs.[2] The European Neuroendocrine Tumor Society also advocates adjuvant treatment in ACs with positive lymph nodes, but not for TCs.[2] Chemoradiotherapy is often recommended for the treatment of locally advanced, unresectable diseases. A commonly used dose for radiotherapy in this setting is 60 Gy.
Medical Oncology
Adjuvant chemotherapy is not recommended for stage I to III NETs as the risk of recurrence is low. This treatment is, however, recommended for stage III ACs.[2] If surgery is not an option for locally advanced ACs, chemoradiotherapy is recommended. Chemotherapy forms the backbone of metastatic stage IV NET treatment, although efficacy data are lacking due to the rarity of these cases. Independent trials of pulmonary NETs are rare. NCCN guidelines recommend close radiological surveillance with chest CT with IV contrast and multiphasic abdominal and pelvic CT or MRI every 3 to 6 months for cases with low tumor burden and low-grade NETs. Systemic therapy is initiated at the time of progression. Various options for systemic treatment include somatostatin analogs, chemotherapy, mammalian target of rapamycin (mTOR) inhibitors, and anti-vascular endothelial growth factor agents.
Somatostatin Analogs
The use of somatostatin analogs in pulmonary NETs is extrapolated from the experience with GI tract NETs. Small studies show that despite low response rates, somatostatin receptor-targeted therapies improve the symptoms of carcinoid syndrome and disease progression. The PROMID study demonstrated the activity of long-acting somatostatin in midgut NETs. The effect was most pronounced in patients with low hepatic tumor burden and those who underwent primary tumor resection. Compared to the placebo, the time to disease progression was prolonged; however, the overall survival benefit remains unclear.[52]
Chemotherapy
NETs are less responsive to chemotherapy compared to more aggressive counterparts, such as SCLCs. However, without more effective alternatives, chemotherapy regimens used in SCLC are sometimes used to treat advanced or metastatic pulmonary NETs. Cisplatin and etoposide showed an overall response rate of 44% in small case series with a disease control rate of 75%.[53] The combination of temozolomide and capecitabine has also demonstrated activity in advanced pulmonary NETs.[54]
Mammalian Target of Rapamycin-Directed Therapy (mTOR)
The mTOR pathway has been implicated in the pathogenesis of NETs. The RADIANT-4 trial studied the role of everolimus in nonfunctional pulmonary and GI NETs. Out of 90 patients with pulmonary NETs, the median progression-free survival PFS was 9.2 months for everolimus compared to 3.6 months for placebo. The toxicities of everolimus included hyperglycemia, infections, and stomatitis.[47]
Staging
In pulmonary NETs, the classification system designates TC as a low-grade tumor.[55] The staging of TCs and ACs follows the TNM staging of lung cancer.[56] Most TCs are stage 1a.[55] AC is classified as intermediate grade. ACs are typically at a more advanced stage at presentation than TC.[55] See Image. Bronchial Carcinoid Tumors and Lung Carcinomas Tumor–Node–Metastasis (TNM) Staging, Table.
Prognosis
The prognosis of pulmonary NETs is influenced by tumor size, grade, disease stage, and older age. The 5-year survival rate varies depending on the stage of the disease. Generally, the prognosis improves with earlier-stage diagnosis. The approximate 5-year survival rates for stages I to IV are 93%, 85%, 75%, and 57%, respectively. TC has a better prognosis than atypical cases. The 5-year survival rate for patients with TC is 90%, while for AC, it is approximately 60%.[57] Nodal involvement is considered a poor prognostic factor. However, the impact of nodal involvement on prognosis remains uncertain.
Prognosis is determined by tumor TNM stage, resectability, histologic grade, proliferation index, and size.[58][59][60] Other factors associated with a poorer prognosis in TC include incomplete resection, age, gender, tumor site (central versus peripheral), and performance status. A 2015 European Association of Thoracic Surgeons NETs Working Group article revealed higher mortality for patients with increased age, male gender, central tumor location, higher TNM stage, and lower functional status.[61]
Thymic NETs' prognosis is worse than that of their counterparts in the GI tract or the lungs.[30] These tumors exhibit aggressive behavior, with a tendency to metastasize or recur locally. Their poor prognosis can also be attributed to delayed tumor detection. In an analysis of the Surveillance, Epidemiology, and End Results database, the overall survival for localized disease was estimated to be 110 months, compared to significantly lower survival rates for patients with regional spread and metastatic disease, at 59 and 35 months, respectively.[58]
Complications
Pulmonary NETs often involve major bronchi. This could lead to massive, potentially life-threatening hemoptysis. While carcinoid syndrome, Cushing syndrome, and acromegaly are potential complications of NETs, they are relatively infrequent. These syndromes occur when the tumor produces ectopic peptides or hormones, leading to specific clinical manifestations such as flushing, diarrhea, wheezing, and hormonal imbalances.[62][63] Vasoactive substances, such as serotonin, secreted by pulmonary NETs generally undergo first-pass metabolism in the lungs, inactivating them before they reach the systemic circulation. Carcinoid syndrome usually occurs in the setting of extensive hepatic tumor burden, extrahepatic metastasis, or the presence of a bronchial-to-systemic venous shunt that allows bypass of the pulmonary circulation.[4] These symptoms are more commonly associated with GI NETs than pulmonary NETs.
Smokers have an increased risk of developing a second primary lung or other smoking-related cancer. Though rarely administered, chest irradiation increases the risk of secondary cancers and coronary artery disease. Other tobacco-related diseases like cardiovascular disease, stroke, and chronic obstructive pulmonary disease also impact survival. Disease-related complications include carcinoid or paraneoplastic syndromes such as Cushing or humoral hypercalcemia of malignancy.[30] Treatment and intervention-related complications may arise following thymectomy, including phrenic and recurrent laryngeal nerve injury, surgical site infection, mediastinitis, and wound dehiscence.[64][65]
Deterrence and Patient Education
Patients should be thoroughly counseled on the potential recurrence and metastatic spread of the disease, even following complete surgical resection. Given the rare nature, variable biologic behavior, and often prolonged disease course of thoracic NETs, education should be personalized, multidisciplinary, and ongoing throughout the continuum of care. At the time of initial diagnosis, it is essential that providers clearly define the diagnosis, explain whether the tumor is typical or atypical, and describe the implications for tumor behavior, prognosis, and treatment options, while also clarifying the non-benign nature of thoracic NETs.
Preoperative counseling for surgical patients should involve defining the goals of resection (curative versus cytoreductive), the possibility of lymph node dissection, the risks of recurrence even after an apparent complete resection, the potential for functional tumor-related syndromes, a plan for postoperative monitoring, and the importance of long-term surveillance. If the tumor is functional, patients should be educated on syndrome-specific symptoms, such as Cushingoid features and carcinoid syndrome, and be provided instructions on recognizing biochemical recurrence. Endocrinology experts may be engaged for hormone-specific monitoring and management.
Structured oncologic follow-up and surveillance are conducted similarly to those for pulmonary carcinoma after resection. Patients are followed clinically with history and physical examination, chest and abdominal CT scans with contrast for primary lung and thymic NETs (with pelvic imaging as clinically indicated), and follow-up with biochemical markers as clinically indicated for functional tumors every 2 to 3 months for the first year after surgery, earlier if patients are symptomatic. Based on National Comprehensive Cancer Network guidelines, yearly follow-up is then advised for 10 years.
Lifelong monitoring may be considered in patients with high-risk features, incomplete resection, or poorly differentiated histology. The goal of follow-up is early detection of recurrence and timely management of hormone-related syndromes in functional tumors. Patients should also be offered psychosocial support, as many patients experience anxiety due to the potential for late recurrence. The current guidelines recommend no surveillance for node-negative, low-grade (typical) NETs unless the tumor is greater than 3 cm, close margins are noted on resected tumor pathology, or multifocal disease is present.
Enhancing Healthcare Team Outcomes
Due to their diverse presentation and variable biologic behavior, managing NETs requires advanced clinical skills and strategic coordination among multiple disciplines. Physicians and advanced clinicians must be proficient in recognizing subtle clinical manifestations, interpreting imaging, and applying evidence-based diagnostic strategies, while pathologists contribute critical expertise in histopathologic and immunohistochemical characterization. Surgical teams require technical skill in thoracic resections, and oncologists must integrate systemic therapies tailored to tumor grade and molecular profile. Nurses and pharmacists are vital in monitoring treatment-related toxicities, ensuring accurate medication administration, and supporting patient adherence to complex regimens.
Optimal care for patients with thoracic NETs hinges on interprofessional communication and coordinated care planning across specialties. Regular tumor board discussions involving pulmonology, thoracic surgery, oncology, radiology, and pathology ensure individualized treatment strategies align with patient goals. Nurses provide patient education and symptom management, pharmacists advise on safe and effective systemic therapy use, and allied health professionals assist with nutrition, rehabilitation, and psychosocial support. This collaborative approach enhances patient safety, reduces delays in diagnosis or treatment, and improves oncologic outcomes and quality of life, while fostering a culture of high-performing, patient-centered team care.
Media
(Click Image to Enlarge)
References
Rindi G, Klimstra DS, Abedi-Ardekani B, Asa SL, Bosman FT, Brambilla E, Busam KJ, de Krijger RR, Dietel M, El-Naggar AK, Fernandez-Cuesta L, Klöppel G, McCluggage WG, Moch H, Ohgaki H, Rakha EA, Reed NS, Rous BA, Sasano H, Scarpa A, Scoazec JY, Travis WD, Tallini G, Trouillas J, van Krieken JH, Cree IA. A common classification framework for neuroendocrine neoplasms: an International Agency for Research on Cancer (IARC) and World Health Organization (WHO) expert consensus proposal. Modern pathology : an official journal of the United States and Canadian Academy of Pathology, Inc. 2018 Dec:31(12):1770-1786. doi: 10.1038/s41379-018-0110-y. Epub 2018 Aug 23 [PubMed PMID: 30140036]
Level 3 (low-level) evidenceGosain R, Mukherjee S, Yendamuri SS, Iyer R. Management of Typical and Atypical Pulmonary Carcinoids Based on Different Established Guidelines. Cancers. 2018 Dec 12:10(12):. doi: 10.3390/cancers10120510. Epub 2018 Dec 12 [PubMed PMID: 30545054]
Rosado de Christenson ML, Abbott GF, Kirejczyk WM, Galvin JR, Travis WD. Thoracic carcinoids: radiologic-pathologic correlation. Radiographics : a review publication of the Radiological Society of North America, Inc. 1999 May-Jun:19(3):707-36 [PubMed PMID: 10336200]
Menon G, Pandit S, Annamaraju P, Bhusal K. Carcinoid Syndrome. StatPearls. 2025 Jan:(): [PubMed PMID: 28846309]
Granberg D, Juhlin CC, Falhammar H, Hedayati E. Lung Carcinoids: A Comprehensive Review for Clinicians. Cancers. 2023 Nov 16:15(22):. doi: 10.3390/cancers15225440. Epub 2023 Nov 16 [PubMed PMID: 38001701]
Davini F, Gonfiotti A, Comin C, Caldarella A, Mannini F, Janni A. Typical and atypical carcinoid tumours: 20-year experience with 89 patients. The Journal of cardiovascular surgery. 2009 Dec:50(6):807-11 [PubMed PMID: 19935614]
Level 2 (mid-level) evidenceBuikhuisen WA, Steinbusch LC, Kodach LL, Tesselaar MET, Damhuis RAM. Risk of second primary malignancies among patients with carcinoid of the lung. Lung cancer (Amsterdam, Netherlands). 2021 Jan:151():5-7. doi: 10.1016/j.lungcan.2020.11.017. Epub 2020 Nov 19 [PubMed PMID: 33278670]
Debelenko LV, Brambilla E, Agarwal SK, Swalwell JI, Kester MB, Lubensky IA, Zhuang Z, Guru SC, Manickam P, Olufemi SE, Chandrasekharappa SC, Crabtree JS, Kim YS, Heppner C, Burns AL, Spiegel AM, Marx SJ, Liotta LA, Collins FS, Travis WD, Emmert-Buck MR. Identification of MEN1 gene mutations in sporadic carcinoid tumors of the lung. Human molecular genetics. 1997 Dec:6(13):2285-90 [PubMed PMID: 9361035]
Litvak A, Pietanza MC. Bronchial and Thymic Carcinoid Tumors. Hematology/oncology clinics of North America. 2016 Feb:30(1):83-102. doi: 10.1016/j.hoc.2015.09.003. Epub [PubMed PMID: 26614370]
Ferolla P, Falchetti A, Filosso P, Tomassetti P, Tamburrano G, Avenia N, Daddi G, Puma F, Ribacchi R, Santeusanio F, Angeletti G, Brandi ML. Thymic neuroendocrine carcinoma (carcinoid) in multiple endocrine neoplasia type 1 syndrome: the Italian series. The Journal of clinical endocrinology and metabolism. 2005 May:90(5):2603-9 [PubMed PMID: 15713725]
Sachithanandan N, Harle RA, Burgess JR. Bronchopulmonary carcinoid in multiple endocrine neoplasia type 1. Cancer. 2005 Feb 1:103(3):509-15 [PubMed PMID: 15611976]
Rekhtman N. Neuroendocrine tumors of the lung: an update. Archives of pathology & laboratory medicine. 2010 Nov:134(11):1628-38 [PubMed PMID: 21043816]
Stankovic B, Aamodt H, Bjørhovde HAK, Müller E, Hammarström C, Brustugun OT, Helland Å, Øynebråten I, Corthay A. The immune microenvironment in typical carcinoid lung tumour, a brief report of four cases. Scandinavian journal of immunology. 2020 Aug:92(2):e12893. doi: 10.1111/sji.12893. Epub 2020 Jun 9 [PubMed PMID: 32433774]
Level 3 (low-level) evidenceHallet J, Law CH, Cukier M, Saskin R, Liu N, Singh S. Exploring the rising incidence of neuroendocrine tumors: a population-based analysis of epidemiology, metastatic presentation, and outcomes. Cancer. 2015 Feb 15:121(4):589-97. doi: 10.1002/cncr.29099. Epub 2014 Oct 13 [PubMed PMID: 25312765]
Level 2 (mid-level) evidenceSwarts DR, Ramaekers FC, Speel EJ. Molecular and cellular biology of neuroendocrine lung tumors: evidence for separate biological entities. Biochimica et biophysica acta. 2012 Dec:1826(2):255-71. doi: 10.1016/j.bbcan.2012.05.001. Epub 2012 May 10 [PubMed PMID: 22579738]
Level 3 (low-level) evidenceDavies SJ, Gosney JR, Hansell DM, Wells AU, du Bois RM, Burke MM, Sheppard MN, Nicholson AG. Diffuse idiopathic pulmonary neuroendocrine cell hyperplasia: an under-recognised spectrum of disease. Thorax. 2007 Mar:62(3):248-52 [PubMed PMID: 17099078]
Wick MR, Rosai J. Neuroendocrine neoplasms of the thymus. Pathology, research and practice. 1988 Apr:183(2):188-99 [PubMed PMID: 3290867]
Bohnenberger H, Dinter H, König A, Ströbel P. Neuroendocrine tumors of the thymus and mediastinum. Journal of thoracic disease. 2017 Nov:9(Suppl 15):S1448-S1457. doi: 10.21037/jtd.2017.02.02. Epub [PubMed PMID: 29201448]
Soga J, Yakuwa Y, Osaka M. Evaluation of 342 cases of mediastinal/thymic carcinoids collected from literature: a comparative study between typical carcinoids and atypical varieties. Annals of thoracic and cardiovascular surgery : official journal of the Association of Thoracic and Cardiovascular Surgeons of Asia. 1999 Oct:5(5):285-92 [PubMed PMID: 10550713]
Level 2 (mid-level) evidenceJansson JO, Svensson J, Bengtsson BA, Frohman LA, Ahlman H, Wängberg B, Nilsson O, Nilsson M. Acromegaly and Cushing's syndrome due to ectopic production of GHRH and ACTH by a thymic carcinoid tumour: in vitro responses to GHRH and GHRP-6. Clinical endocrinology. 1998 Feb:48(2):243-50 [PubMed PMID: 9579239]
Level 3 (low-level) evidenceWalch AK, Zitzelsberger HF, Aubele MM, Mattis AE, Bauchinger M, Candidus S, Präuer HW, Werner M, Höfler H. Typical and atypical carcinoid tumors of the lung are characterized by 11q deletions as detected by comparative genomic hybridization. The American journal of pathology. 1998 Oct:153(4):1089-98 [PubMed PMID: 9777940]
Level 2 (mid-level) evidenceArora R, Gupta R, Sharma A, Dinda AK. Primary neuroendocrine carcinoma of thymus: a rare cause of Cushing's syndrome. Indian journal of pathology & microbiology. 2010 Jan-Mar:53(1):148-51. doi: 10.4103/0377-4929.59210. Epub [PubMed PMID: 20090249]
Level 3 (low-level) evidenceKayser K, Kayser C, Rahn W, Bovin NV, Gabius HJ. Carcinoid tumors of the lung: immuno- and ligandohistochemistry, analysis of integrated optical density, syntactic structure analysis, clinical data, and prognosis of patients treated surgically. Journal of surgical oncology. 1996 Oct:63(2):99-106 [PubMed PMID: 8888802]
Goto K, Kodama T, Matsuno Y, Yokose T, Asamura H, Kamiya N, Shimosato Y. Clinicopathologic and DNA cytometric analysis of carcinoid tumors of the thymus. Modern pathology : an official journal of the United States and Canadian Academy of Pathology, Inc. 2001 Oct:14(10):985-94 [PubMed PMID: 11598168]
McCaughan BC, Martini N, Bains MS. Bronchial carcinoids. Review of 124 cases. The Journal of thoracic and cardiovascular surgery. 1985 Jan:89(1):8-17 [PubMed PMID: 2981373]
Level 3 (low-level) evidenceSaber M, Ismail Y, Alieldin N, Loay I, El Zawahry M. Neuroendocrine tumors of the lung: A five-year retrospective experience of Egyptian NCI (2010-2014). Journal of the Egyptian National Cancer Institute. 2018 Dec:30(4):151-158. doi: 10.1016/j.jnci.2018.10.005. Epub 2018 Nov 22 [PubMed PMID: 30470605]
Level 2 (mid-level) evidenceOkereke IC, Taber AM, Griffith RC, Ng TT. Outcomes after surgical resection of pulmonary carcinoid tumors. Journal of cardiothoracic surgery. 2016 Mar 2:11():35. doi: 10.1186/s13019-016-0424-0. Epub 2016 Mar 2 [PubMed PMID: 26935588]
Zeng Y, Zhu Y, Ding Y, Xu L, Zhai B, Zhang X, Ge Q, Li J, Song Q, Li X, Zhang Z. Analysis of lung biopsies using the 2015 WHO criteria and detection of sensitizing mutations--a single-institution experience of 5032 cases. Diagnostic pathology. 2020 May 19:15(1):59. doi: 10.1186/s13000-020-00975-3. Epub 2020 May 19 [PubMed PMID: 32429938]
Level 3 (low-level) evidenceChaer R, Massad MG, Evans A, Snow NJ, Geha AS. Primary neuroendocrine tumors of the thymus. The Annals of thoracic surgery. 2002 Nov:74(5):1733-40 [PubMed PMID: 12440652]
Gaude GS, Hattiholi V, Malur PR, Hattiholi J. Primary neuroendocrine carcinoma of the thymus. Nigerian medical journal : journal of the Nigeria Medical Association. 2013 Jan:54(1):68-71. doi: 10.4103/0300-1652.108904. Epub [PubMed PMID: 23661903]
Ferolla P. Medical treatment of advanced thoracic neuroendocrine tumors. Thoracic surgery clinics. 2014 Aug:24(3):351-5. doi: 10.1016/j.thorsurg.2014.05.006. Epub [PubMed PMID: 25065936]
Caplin ME, Baudin E, Ferolla P, Filosso P, Garcia-Yuste M, Lim E, Oberg K, Pelosi G, Perren A, Rossi RE, Travis WD, ENETS consensus conference participants. Pulmonary neuroendocrine (carcinoid) tumors: European Neuroendocrine Tumor Society expert consensus and recommendations for best practice for typical and atypical pulmonary carcinoids. Annals of oncology : official journal of the European Society for Medical Oncology. 2015 Aug:26(8):1604-20. doi: 10.1093/annonc/mdv041. Epub 2015 Feb 2 [PubMed PMID: 25646366]
Level 1 (high-level) evidenceOronsky B, Ma PC, Morgensztern D, Carter CA. Nothing But NET: A Review of Neuroendocrine Tumors and Carcinomas. Neoplasia (New York, N.Y.). 2017 Dec:19(12):991-1002. doi: 10.1016/j.neo.2017.09.002. Epub 2017 Nov 5 [PubMed PMID: 29091800]
Gibril F, Chen YJ, Schrump DS, Vortmeyer A, Zhuang Z, Lubensky IA, Reynolds JC, Louie A, Entsuah LK, Huang K, Asgharian B, Jensen RT. Prospective study of thymic carcinoids in patients with multiple endocrine neoplasia type 1. The Journal of clinical endocrinology and metabolism. 2003 Mar:88(3):1066-81 [PubMed PMID: 12629087]
Queiroz RM, Santana DBF, Nastri Filho R, Landell GAM, Félix PR, Valentin MVN. Endobronchial carcinoid tumor: Radiological findings of a clinical case. Revista da Associacao Medica Brasileira (1992). 2018 Jan:64(1):15-18. doi: 10.1590/1806-9282.64.01.15. Epub [PubMed PMID: 29561937]
Level 3 (low-level) evidenceDaniels CE, Lowe VJ, Aubry MC, Allen MS, Jett JR. The utility of fluorodeoxyglucose positron emission tomography in the evaluation of carcinoid tumors presenting as pulmonary nodules. Chest. 2007 Jan:131(1):255-60 [PubMed PMID: 17218584]
Level 2 (mid-level) evidenceVenkitaraman B, Karunanithi S, Kumar A, Khilnani GC, Kumar R. Role of 68Ga-DOTATOC PET/CT in initial evaluation of patients with suspected bronchopulmonary carcinoid. European journal of nuclear medicine and molecular imaging. 2014 May:41(5):856-64. doi: 10.1007/s00259-013-2659-5. Epub 2014 Jan 17 [PubMed PMID: 24435773]
Erasmus JJ, McAdams HP, Patz EF Jr, Coleman RE, Ahuja V, Goodman PC. Evaluation of primary pulmonary carcinoid tumors using FDG PET. AJR. American journal of roentgenology. 1998 May:170(5):1369-73 [PubMed PMID: 9574618]
Level 2 (mid-level) evidenceGuidoccio F, Grosso M, Maccauro M, Orsini F, Perri M, Boni G, Banti E, Grassetto G, Rubello D, Mariani G, Volterrani D. Current role of 111In-DTPA-octreotide scintigraphy in diagnosis of thymic masses. Tumori. 2011 Mar-Apr:97(2):191-5 [PubMed PMID: 21617714]
Granberg D, Sundin A, Janson ET, Oberg K, Skogseid B, Westlin JE. Octreoscan in patients with bronchial carcinoid tumours. Clinical endocrinology. 2003 Dec:59(6):793-9 [PubMed PMID: 14974924]
Level 2 (mid-level) evidenceNilsson O, Kölby L, Wängberg B, Wigander A, Billig H, William-Olsson L, Fjälling M, Forssell-Aronsson E, Ahlman H. Comparative studies on the expression of somatostatin receptor subtypes, outcome of octreotide scintigraphy and response to octreotide treatment in patients with carcinoid tumours. British journal of cancer. 1998 Feb:77(4):632-7 [PubMed PMID: 9484822]
Level 2 (mid-level) evidenceFilosso PL, Rena O, Donati G, Casadio C, Ruffini E, Papalia E, Oliaro A, Maggi G. Bronchial carcinoid tumors: surgical management and long-term outcome. The Journal of thoracic and cardiovascular surgery. 2002 Feb:123(2):303-9 [PubMed PMID: 11828290]
Level 2 (mid-level) evidenceKaiser LR. Surgical treatment of thymic epithelial neoplasms. Hematology/oncology clinics of North America. 2008 Jun:22(3):475-88. doi: 10.1016/j.hoc.2008.03.009. Epub [PubMed PMID: 18514128]
Kvols LK. Therapy of the malignant carcinoid syndrome. Endocrinology and metabolism clinics of North America. 1989 Jun:18(2):557-68 [PubMed PMID: 2663485]
Oberg K, Hellman P, Kwekkeboom D, Jelic S, ESMO Guidelines Working Group. Neuroendocrine bronchial and thymic tumours: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Annals of oncology : official journal of the European Society for Medical Oncology. 2010 May:21 Suppl 5():v220-2. doi: 10.1093/annonc/mdq191. Epub [PubMed PMID: 20555085]
Level 1 (high-level) evidenceWang DY, Chang DB, Kuo SH, Yang PC, Lee YC, Hsu HC, Luh KT. Carcinoid tumours of the thymus. Thorax. 1994 Apr:49(4):357-60 [PubMed PMID: 8202907]
Level 2 (mid-level) evidenceFazio N, Buzzoni R, Delle Fave G, Tesselaar ME, Wolin E, Van Cutsem E, Tomassetti P, Strosberg J, Voi M, Bubuteishvili-Pacaud L, Ridolfi A, Herbst F, Tomasek J, Singh S, Pavel M, Kulke MH, Valle JW, Yao JC. Everolimus in advanced, progressive, well-differentiated, non-functional neuroendocrine tumors: RADIANT-4 lung subgroup analysis. Cancer science. 2018 Jan:109(1):174-181. doi: 10.1111/cas.13427. Epub 2017 Nov 9 [PubMed PMID: 29055056]
Harpole DH Jr, Feldman JM, Buchanan S, Young WG, Wolfe WG. Bronchial carcinoid tumors: a retrospective analysis of 126 patients. The Annals of thoracic surgery. 1992 Jul:54(1):50-4; discussion 54-5 [PubMed PMID: 1610254]
Level 2 (mid-level) evidenceMineo TC, Guggino G, Mineo D, Vanni G, Ambrogi V. Relevance of lymph node micrometastases in radically resected endobronchial carcinoid tumors. The Annals of thoracic surgery. 2005 Aug:80(2):428-32 [PubMed PMID: 16039178]
Level 2 (mid-level) evidenceZhang Z, Zhang Y, Feng H, Yao Z, Teng J, Wei D, Liu D. Is video-assisted thoracic surgery lobectomy better than thoracotomy for early-stage non-small-cell lung cancer? A systematic review and meta-analysis. European journal of cardio-thoracic surgery : official journal of the European Association for Cardio-thoracic Surgery. 2013 Sep:44(3):407-14. doi: 10.1093/ejcts/ezt015. Epub 2013 Jan 30 [PubMed PMID: 23371973]
Level 1 (high-level) evidencePark HS, Detterbeck FC, Boffa DJ, Kim AW. Impact of hospital volume of thoracoscopic lobectomy on primary lung cancer outcomes. The Annals of thoracic surgery. 2012 Feb:93(2):372-9. doi: 10.1016/j.athoracsur.2011.06.054. Epub 2011 Sep 25 [PubMed PMID: 21945225]
Rinke A, Müller HH, Schade-Brittinger C, Klose KJ, Barth P, Wied M, Mayer C, Aminossadati B, Pape UF, Bläker M, Harder J, Arnold C, Gress T, Arnold R, PROMID Study Group. Placebo-controlled, double-blind, prospective, randomized study on the effect of octreotide LAR in the control of tumor growth in patients with metastatic neuroendocrine midgut tumors: a report from the PROMID Study Group. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2009 Oct 1:27(28):4656-63. doi: 10.1200/JCO.2009.22.8510. Epub 2009 Aug 24 [PubMed PMID: 19704057]
Level 1 (high-level) evidencePuliafito I, Chillari F, Russo A, Cantale O, Sciacca D, Castorina L, Colarossi C, Franchina T, Vitale MP, Ricciardi GRR, Adamo V, Esposito F, Giuffrida D. Therapeutic efficacy of platinum/etoposide regimens in the treatment of advanced poorly differentiated neuroendocrine carcinomas of the lung: A retrospective analysis. Frontiers in endocrinology. 2023:14():1065599. doi: 10.3389/fendo.2023.1065599. Epub 2023 Jan 30 [PubMed PMID: 36793289]
Level 2 (mid-level) evidencePapaxoinis G, Kordatou Z, McCallum L, Nasralla M, Lamarca A, Backen A, Nonaka D, Mansoor W. Capecitabine and Temozolomide in Patients with Advanced Pulmonary Carcinoid Tumours. Neuroendocrinology. 2020:110(5):413-421. doi: 10.1159/000502864. Epub 2019 Aug 23 [PubMed PMID: 31437838]
Lim E, Yap YK, De Stavola BL, Nicholson AG, Goldstraw P. The impact of stage and cell type on the prognosis of pulmonary neuroendocrine tumors. The Journal of thoracic and cardiovascular surgery. 2005 Oct:130(4):969-72 [PubMed PMID: 16214506]
Level 2 (mid-level) evidenceLababede O, Meziane MA. The Eighth Edition of TNM Staging of Lung Cancer: Reference Chart and Diagrams. The oncologist. 2018 Jul:23(7):844-848. doi: 10.1634/theoncologist.2017-0659. Epub 2018 Apr 12 [PubMed PMID: 29650687]
Mezzetti M, Raveglia F, Panigalli T, Giuliani L, Lo Giudice F, Meda S, Conforti S. Assessment of outcomes in typical and atypical carcinoids according to latest WHO classification. The Annals of thoracic surgery. 2003 Dec:76(6):1838-42 [PubMed PMID: 14667595]
Gaur P, Leary C, Yao JC. Thymic neuroendocrine tumors: a SEER database analysis of 160 patients. Annals of surgery. 2010 Jun:251(6):1117-21. doi: 10.1097/SLA.0b013e3181dd4ec4. Epub [PubMed PMID: 20485130]
Cardillo G, Rea F, Lucchi M, Paul MA, Margaritora S, Carleo F, Marulli G, Mussi A, Granone P, Graziano P. Primary neuroendocrine tumors of the thymus: a multicenter experience of 35 patients. The Annals of thoracic surgery. 2012 Jul:94(1):241-5; discussion 245-6. doi: 10.1016/j.athoracsur.2012.03.062. Epub 2012 May 26 [PubMed PMID: 22632882]
Weksler B, Holden A, Sullivan JL. Impact of Positive Nodal Metastases in Patients with Thymic Carcinoma and Thymic Neuroendocrine Tumors. Journal of thoracic oncology : official publication of the International Association for the Study of Lung Cancer. 2015 Nov:10(11):1642-7. doi: 10.1097/JTO.0000000000000660. Epub [PubMed PMID: 26317915]
Travis WD, Giroux DJ, Chansky K, Crowley J, Asamura H, Brambilla E, Jett J, Kennedy C, Rami-Porta R, Rusch VW, Goldstraw P, International Staging Committee and Participating Institutions. The IASLC Lung Cancer Staging Project: proposals for the inclusion of broncho-pulmonary carcinoid tumors in the forthcoming (seventh) edition of the TNM Classification for Lung Cancer. Journal of thoracic oncology : official publication of the International Association for the Study of Lung Cancer. 2008 Nov:3(11):1213-23. doi: 10.1097/JTO.0b013e31818b06e3. Epub [PubMed PMID: 18978555]
Halperin DM, Shen C, Dasari A, Xu Y, Chu Y, Zhou S, Shih YT, Yao JC. Frequency of carcinoid syndrome at neuroendocrine tumour diagnosis: a population-based study. The Lancet. Oncology. 2017 Apr:18(4):525-534. doi: 10.1016/S1470-2045(17)30110-9. Epub 2017 Feb 24 [PubMed PMID: 28238592]
Scanagatta P, Montresor E, Pergher S, Mainente M, Bonadiman C, Benato C, Feil B, Destefanis ML, Pea M, Spinoso A, Scilanga L, Francia G. Cushing's syndrome induced by bronchopulmonary carcinoid tumours: a review of 98 cases and our experience of two cases. Chirurgia italiana. 2004 Jan-Feb:56(1):63-70 [PubMed PMID: 15038649]
Level 3 (low-level) evidenceJaretzki A 3rd. Injury to the phrenic and recurrent nerves needs to be avoided in the performance of thymectomy for myasthenia gravis. The Annals of thoracic surgery. 2002 Aug:74(2):633; author reply 634 [PubMed PMID: 12173873]
Level 3 (low-level) evidenceMachens A, Emskötter T, Busch C, Izbicki JR. Postoperative infection after transsternal thymectomy for myasthenia gravis: a retrospective analysis of 125 cases. Surgery today. 1998:28(8):808-10 [PubMed PMID: 9719001]
Level 2 (mid-level) evidence