Obesity as a Chronic Disease: Epidemiology and Pathophysiologic Insights
Obesity as a Chronic Disease: Epidemiology and Pathophysiologic Insights
Introduction
Understanding the prevalence, distribution, and biological mechanisms of obesity is vital for shaping effective public health measures and clinical care strategies. The recent National Health and Nutrition Examination Survey (NHANES) data from 2021 to 2023 indicate that obesity affects 40.3% of adults in the United States (US), with the highest prevalence among middle-aged adults (eg, 40–59 years), marked by persistent demographic and geographic disparities. Severe obesity continues to rise, now impacting 9.4% of adults, with notable differences in sex and substantial associated healthcare costs. Geographic patterns reveal an 18.7 percentage point difference between the highest and lowest state rates, driven by socioeconomic, environmental, and cultural factors. Disparities across racial, ethnic, and educational groups further underscore the role of social determinants of health in obesity prevalence.
Beyond epidemiology, advances in understanding the pathophysiology of obesity underscore its classification as a complex, chronic disease characterized by dysregulated neuroendocrine pathways, adaptive thermogenesis, and environmental interactions. Mechanisms, such as the set point and settling point models, alterations in the gut microbiome, epigenetic changes, and exposure to endocrine-disrupting chemicals, provide insight into disease persistence and treatment challenges. This knowledge forms the foundation for targeted, evidence-based prevention and management strategies that address both biological and social drivers of obesity.
Epidemiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Epidemiology
Understanding the scope and distribution of obesity across populations provides the foundation for effective public health interventions and clinical care strategies. Current epidemiological data reveal persistent high prevalence rates, accompanied by significant demographic and geographic disparities, which necessitate targeted approaches to prevention and treatment.
National Obesity Trends
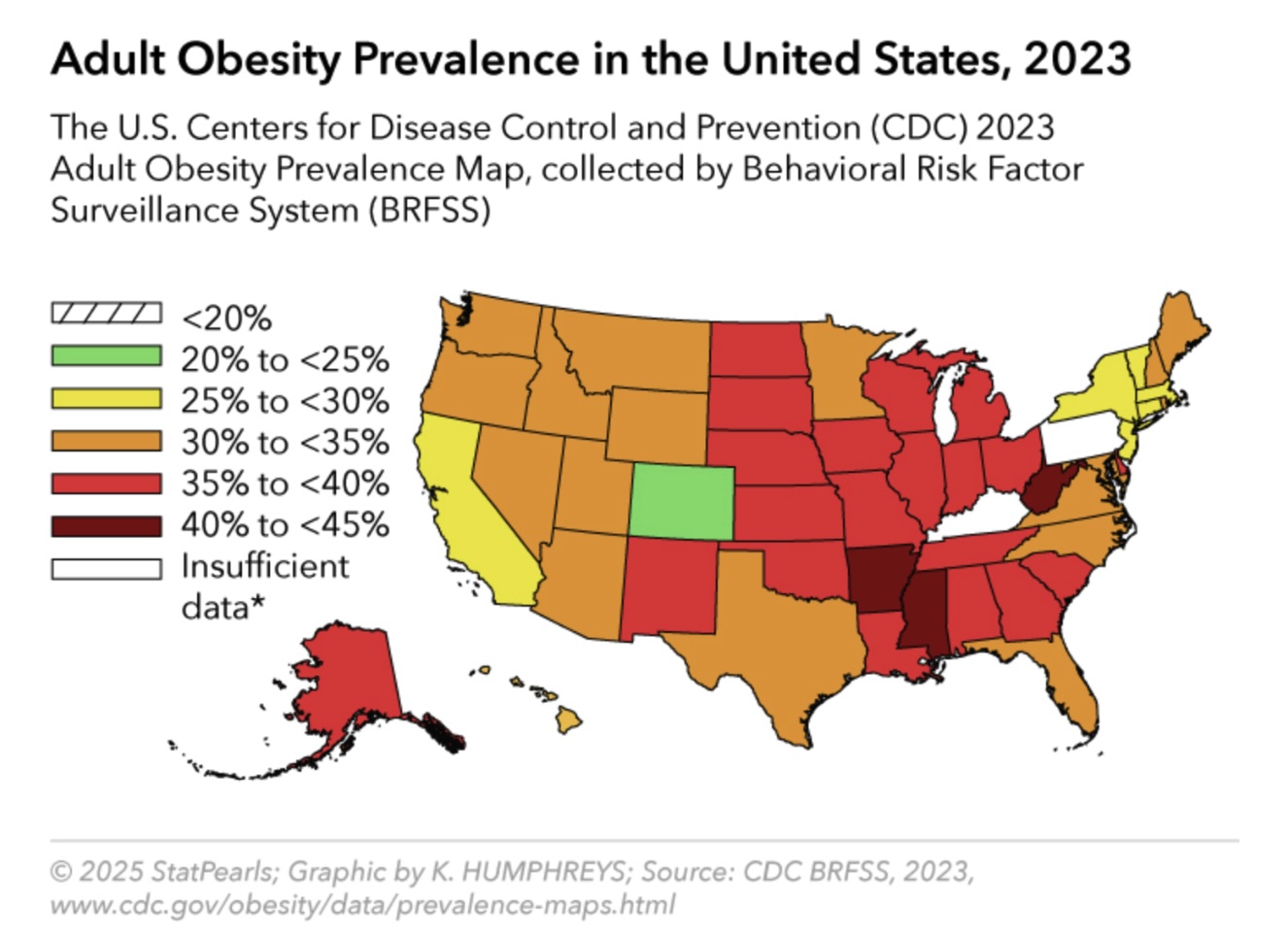
The most recent NHANES data, from August 2021 to August 2023, reveal concerning yet stable trends in obesity in the US. The overall prevalence of obesity, or a body mass index (BMI) of 30 kg/m² or higher, among adults in the US is 40.3%, representing no significant change from previous years but remaining substantially above the Healthy People 2030 goal of 36.0%.[1] This prevalence represents approximately 93.3 million US adults living with obesity, reflecting a substantial public health burden. See Image. Adult Obesity Prevalence in the United States.
Recent national data indicate that severe, or class 3, obesity (a BMI of 40 kg/m² or higher) affects 9.4% of adults nationwide. Sex-based analysis shows similar overall obesity rates, with 39.2% of men and 41.3% of women affected, revealing no statistically significant difference. Age-specific patterns demonstrate notable variation, with prevalence at 35.5% among adults aged 20 to 39, peaking at 46.4% in those aged 40 to 59, and decreasing slightly to 38.9% in adults aged 60 and older. This midlife peak underscores the influence of cumulative metabolic, lifestyle, and hormonal factors.
The absence of significant sex differences in overall obesity prevalence represents a notable shift from historical patterns. However, severe obesity shows significant gender disparities, with women demonstrating higher rates across all age groups. The peak prevalence of obesity in middle-aged adults reflects the cumulative effects of metabolic changes, lifestyle factors, and hormonal influences during this life stage.[2]
Clinical Significance of Obesity Trends
Obesity prevalence in the United States has reached a plateau; however, rates remain at historically high levels, demanding ongoing and sustained intervention efforts to prevent further escalation. The prevalence of severe obesity has risen markedly, with current estimates at 9.4%, up from 7.7% in 2013 to 2014. This trend reflects increasing disease severity and underscores the need for more intensive prevention and treatment strategies. The overall burden extends beyond individual health, as obesity-related medical expenses are estimated at $173 billion annually, placing a significant strain on the healthcare system and reinforcing the need for practical, population-wide interventions.
State-Level Variations and Geographic Disparities
Geographic variations in obesity prevalence reflect complex interactions among environmental, socioeconomic, and cultural factors that influence weight status across different regions. Understanding these patterns helps clinicians recognize population-level risk factors and implement targeted interventions. Current data reveal striking geographic variations in obesity prevalence across the US, with 23 states reporting adult obesity rates of 35% or higher (see Table 1). These statistics represent a concerning increase from 2012, when no state had an obesity rate above 35%.
Table 1. States with Highest and Lowest 2023 Adult Obesity Rates
|
West Virginia |
41.2 |
District of Columbia |
23.5 |
|
Mississippi |
40.1 |
Colorado |
24.9 |
|
Arkansas |
40.0 |
Hawaii |
26.1 |
|
Louisiana |
39.9 |
Massachusetts |
27.4 |
|
Alabama |
39.2 |
California |
27.7 |
Geographic Pattern Analysis
The 18.7 percentage point difference between the highest (West Virginia) and lowest (District of Columbia) obesity rates reflects complex interactions of the following factors:
- Socioeconomic factors: Income inequality, educational attainment, employment opportunities
- Food environment: Access to healthy foods, density of fast-food establishments, food pricing
- Built environment: Walkability, recreational facilities, urban planning, transportation options
- Cultural factors: Regional food traditions, attitudes toward physical activity, social norms
- Healthcare access: Clinician availability, insurance coverage, specialized obesity services
Demographic and socioeconomic disparities
Health disparities in obesity prevalence demonstrate the profound influence of social determinants of health on weight status. Obesity prevalence varies significantly across demographic groups, highlighting persistent health disparities that require targeted intervention strategies (see Table 2). These differences reflect complex interactions of genetic, environmental, socioeconomic, and cultural factors that must be addressed through culturally responsive care approaches.
Table 2. NHANES Obesity Prevalence by Race/Ethnicity, 2021–2023
|
Black Adults |
49.9 |
13.8 |
Food traditions, church-based interventions |
|
Hispanic Adults |
45.6 |
9.3 |
Family-centered approaches, traditional foods |
|
White Adults |
41.4 |
9.0 |
Individual versus community approaches |
|
Asian Adults |
16.1* |
2.0 |
Lower body mass index thresholds for health risks |
*Note: Health risks may begin at lower BMI levels in Asian populations (≥23 kg/m² for increased risk, ≥25 kg/m² for high risk) due to different body composition patterns.[3]
Educational and socioeconomic gradients
A clear inverse relationship exists between educational attainment and obesity prevalence, demonstrating the powerful influence of social determinants of health on weight status (see Table 3).
Table 3. Obesity Prevalence by Educational Attainment
|
Less than High School |
39.8 |
Health literacy enhancement |
|
High School Graduate |
37.8 |
Practical skill building |
|
Some College |
36.2 |
Motivation enhancement |
|
Bachelor's Degree or Higher |
26.5 |
Maintenance support |
Pediatric Obesity Trends and Implications
Childhood obesity patterns establish trajectories that often persist into adulthood, making early intervention critical for long-term health outcomes. Current NHANES data reveal that 19.3% of children and adolescents aged 2 to 19 years have obesity, with 6.1% having severe obesity.[CDC Obesity Prevalence Rates]
The following age-specific pediatric prevalence patterns have been documented:
- Ages 2 to 5 years: 13.4% obesity prevalence
- Ages 6 to 11 years: 20.3% obesity prevalence
- Ages 12 to 19 years: 21.2% obesity prevalence [CDC Obesity Prevalence Rates]
Clinical Significance of Obesity Epidemiology
A clear understanding of obesity epidemiology enables healthcare clinicians to identify high-risk populations that require targeted interventions. This knowledge supports the recognition of the multifactorial nature of obesity disparities, encompassing biological, environmental, socioeconomic, and cultural influences. Epidemiological insights also guide the implementation of culturally appropriate prevention and treatment strategies, ensuring that interventions align with the values, traditions, and needs of diverse communities. Clinicians can address social determinants of health contributing to obesity risk while advocating for policy changes that promote healthy environments, improve access to nutritious foods, and expand opportunities for physical activity.
| Pause and Reflect |
A 45-year-old Hispanic woman with obesity (BMI 32 kg/m²) presents to your clinic in West Virginia.
|
Pathophysiology
Recognizing obesity as a chronic disease fundamentally shifts the clinical approach from viewing weight as a lifestyle choice to defining it as a complex medical condition involving multiple interconnected biological systems. This pathophysiological framework provides the scientific foundation for evidence-based treatment approaches and helps explain the challenges inherent in obesity management.
Neuroendocrine Weight Regulation Mechanisms
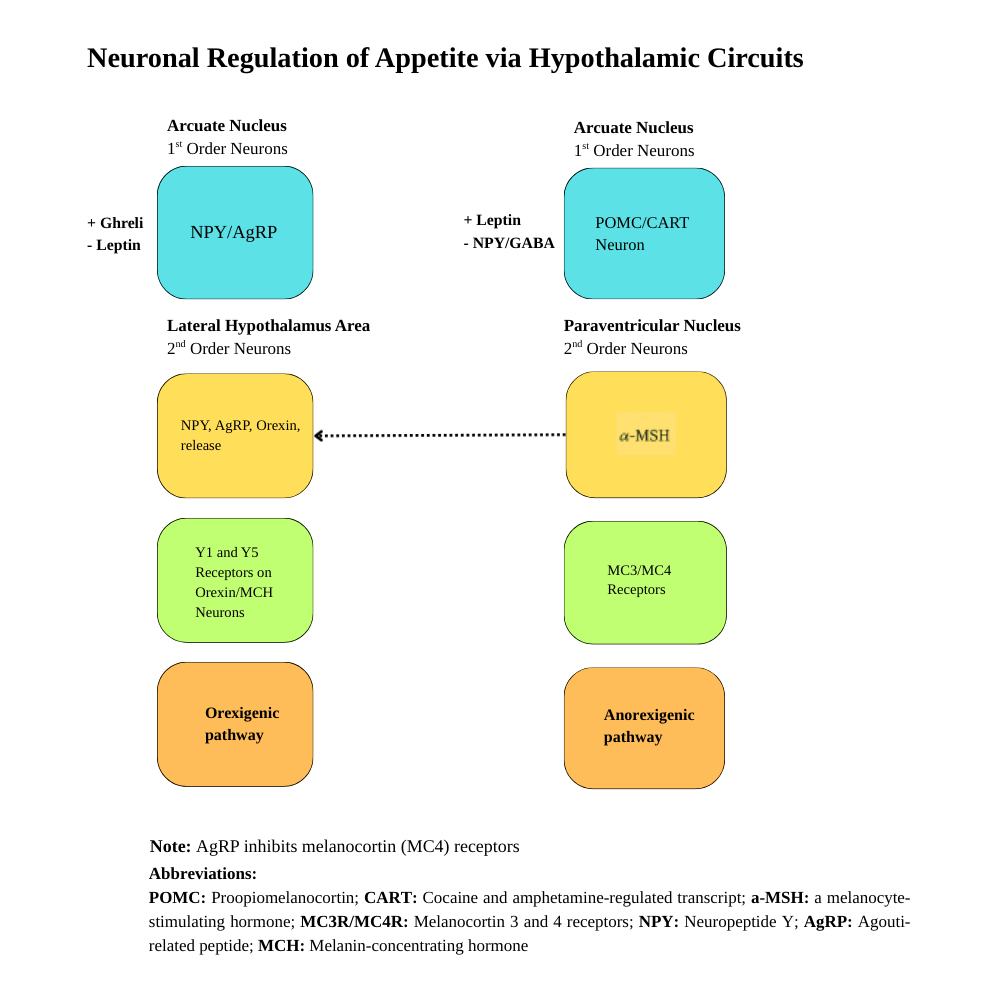
Complex neural and hormonal networks regulate energy balance through feedback mechanisms that more strongly defend against weight loss than weight gain. Understanding these mechanisms provides insight into why obesity develops and persists despite individual efforts to control weight. Obesity represents a complex, chronic disease characterized by the dysregulation of multiple interconnected physiological systems that govern energy homeostasis. The central nervous system, particularly the hypothalamus, is the primary integration center for signals regulating food intake, energy expenditure, and maintaining body weight.[4] See Image. Neuronal Regulation of Appetite Via Hypothalamic Circuits. Please refer to the StatPearls companion resource, "Obesity and SetPoint Theory," for further information.
Hypothalamic control networks
The hypothalamus's arcuate nucleus contains 2 functionally opposing neuronal populations that respond to circulating hormonal and nutrient signals (see Table 4). For further information, please refer to the StatPearls companion resource, "Pathophysiology of Obesity."
Anorexigenic (appetite-suppressing) pathway
The anorexigenic, or appetite-suppressing, pathway involves proopiomelanocortin neurons, which produce the hormone proopiomelanocortin. This precursor is cleaved into α-melanocyte-stimulating hormone (α-MSH). When α-MSH binds to melanocortin-4 receptors (MC4R) in downstream brain regions, it activates anorexigenic signaling pathways. These pathways lead to decreased food intake, increased energy expenditure, and enhanced thermogenesis, thereby contributing to maintaining energy balance and regulating weight.
Orexigenic (appetite-suppressing) pathway
Conversely, the orexigenic, or appetite-stimulating, pathway is mediated by neurons producing neuropeptide Y (NPY) and agouti-related peptide (AgRP). AgRP is an antagonist to MC4R signaling, while NPY actively promotes feeding behavior. This pathway increases food intake, reduces energy expenditure, and decreases thermogenesis, promoting energy storage and weight gain.
Table 4. Major Weight-Regulating Hormones and Their Functions
|
Leptin |
White adipose tissue |
|
Resistance develops, elevated levels |
|
Ghrelin |
Stomach fundus |
|
Elevated baseline levels |
|
Glucagon-like peptide-1 |
Intestinal L-cells |
|
Reduced postprandial response |
|
Insulin |
Pancreatic β-cells |
|
Resistance, compensatory hyperinsulinemia |
|
Cortisol |
Adrenal cortex |
|
Dysregulated circadian rhythm |
Adaptive Thermogenesis and Metabolic Adaptation
Biological mechanisms that defend against weight loss represent the primary challenge in long-term obesity management, explaining why sustainable weight maintenance requires ongoing medical intervention rather than short-term lifestyle changes. Adaptive thermogenesis is one of the most significant biological barriers to achieving and maintaining long-term weight management success. This phenomenon involves coordinated reductions in energy expenditure that exceed what would be predicted based solely on changes in body mass and composition.[5]
Molecular mechanisms of adaptive thermogenesis
Adaptive thermogenesis encompasses multiple coordinated physiological responses that collectively defend against energy deficit. The primary mechanism involves uncoupling protein 1 in brown adipose tissue, dissipating the proton gradient across mitochondrial membranes to produce heat instead of adenosine triphosphate.[6]
Quantitative assessment of metabolic adaptation
A systematic analysis of the results from controlled studies reveals that adaptive thermogenesis is a common feature in most weight loss interventions. Study results also show that an overcompensating mechanism in response to weight loss can trigger a 20% to 25% reduction in total energy expenditure (10%–15% beyond expected body composition changes).[7] Individual variation in adaptive thermogenesis is dramatic, with recent study results showing early adaptive thermogenesis within the first week of caloric restriction averaging -178 ± 137 kcal/day, suggesting rapid physiological responses to an energy deficit that may predict the magnitude of longer-term adaptation.[8]
Persistence and clinical implications
The persistence of metabolic adaptation varies significantly depending on the type of intervention, the magnitude of weight loss, and individual factors. The famous "Biggest Loser" study documented persistent adaptation (-500 kcal/day) at 6-year follow-up, while patients who have undergone bariatric surgery typically demonstrate adaptation resolution after 6 to 24 months.[9] Clinical management implications include recognizing that many patients exhibit metabolic adaptations, necessitating individualized treatment approaches rather than uniform protocols. Understanding adaptation helps explain weight loss plateaus, the need for progressive caloric restriction, and challenges with long-term weight maintenance.[10]
Set Point Theory and Settling Point Models
Theoretical frameworks explaining weight regulation help clinicians understand the biological basis for weight regain and inform realistic treatment expectations. These models provide a scientific foundation for developing long-term obesity management strategies.
Traditional set point theory
The set point theory proposes that body weight is regulated around a predetermined "set point" through homeostatic mechanisms that resist deviations from this weight. Supporting evidence includes animal studies demonstrating weight return to baseline levels after forced feeding or caloric restriction, and twin studies showing similar body weights among identical twins raised in different environments.[11]
Emerging settling point models
Contemporary evidence favors "settling point" models over rigid set point theory. The settling point concept suggests that body weight reaches equilibrium based on the interaction between biological predisposition and environmental factors, without requiring active defense of a specific weight. This model comprehensively explains population-level increases in obesity and individual success stories from weight maintenance registries.[12]
Emerging Factors in Obesity Pathogenesis
Contemporary research supports the concept that additional biological mechanisms contribute to the development and maintenance of obesity beyond traditional energy balance models. These emerging factors offer new insights into the pathogenesis of obesity and potential therapeutic targets.
Gut microbiome alterations
The gut microbiome has emerged as a critical focus in obesity research, supported by growing evidence of its influence on energy regulation, inflammatory processes, and overall metabolic health. Individuals with obesity often exhibit compositional shifts in their gut microbiota, frequently characterized by a higher Firmicutes-to-Bacteroidetes (F/B) ratio, although significant variability exists across studies. Several bacterial alterations consistently appear in the gut microbiome of individuals with obesity. Levels of Akkermansia muciniphila, a species that supports metabolic health and promotes energy expenditure, are commonly depleted; Faecalibacterium prausnitzii, a key butyrate producer contributing to intestinal health, is also often reduced. Conversely, populations of Proteobacteria and Fusobacteria frequently increase, correlating with heightened inflammation and impaired metabolic function. Overall microbial diversity tends to decline, as reflected by lower Shannon diversity indices, which may diminish the gut's resilience and functional capacity.[13]
The gut microbiome composition exhibits distinct alterations in obesity, with the most widely studied being an elevated F/B ratio compared to lean individuals. This shift reflects changes in the 2 dominant bacterial phyla in the human gut, where Firmicutes, including genera such as Lactobacillus, Clostridium, and Enterococcus, become proportionally more abundant while Bacteroidetes, including Bacteroides and Prevotella, decrease. The proposed mechanism involves Firmicutes' enhanced capacity for energy harvest from dietary polysaccharides through increased expression of genes encoding carbohydrate-active enzymes, potentially contributing to weight gain by extracting additional calories from the same food intake.
However, this paradigm has faced scrutiny due to substantial between-study variability, with some investigations reporting no significant F/B ratio differences or even contradictory findings. Methodological factors, including DNA extraction protocols, sequencing platforms, geographical populations studied, and confounding variables (eg, diet, medications, and comorbidities), likely contribute to this heterogeneity, emphasizing that obesity-associated dysbiosis extends beyond simple ratio measurements to encompass broader taxonomic and functional diversity changes.
Epigenetic mechanisms
DNA methylation patterns show consistent associations with obesity across multiple populations and tissue types. Tissue-specific epigenetic patterns reveal multiple genes exhibiting correlated methylation-expression changes that affect key metabolic pathways, including lipid metabolism, inflammation, and insulin signaling.[14]
Endocrine-disrupting chemicals
Endocrine-disrupting chemicals, including bisphenol A (BPA), phthalates, and certain pesticides, act as "obesogens" by interfering with normal hormonal signaling pathways. BPA is detected in 93% of children, while phthalates are ubiquitous in the environment.[15]
Chronic low-grade inflammation
Adipose tissue in obesity becomes a source of chronic inflammation through the infiltration of macrophages and the alteration of adipokine secretion. M1-polarized macrophages can comprise up to 40% of adipose tissue cells in severe obesity, secreting proinflammatory cytokines, including tumor necrosis factor-α, interleukin (IL)-6, and IL-1β.[16]
Clinical Implications of Obesity Pathophysiology
A thorough understanding of obesity pathophysiology enables clinicians to set realistic weight loss goals, often targeting an initial reduction of 5% to 10% of body weight. This knowledge allows clinicians to explain the biological mechanisms underlying weight loss plateaus and weight regain, thereby helping patients maintain motivation throughout their treatment. By recognizing the complex biological underpinnings of obesity, clinicians can justify the need for long-term treatment plans and sustained maintenance strategies. This perspective also supports reducing weight bias and stigma, shifting the focus from personal blame to the medical complexity of the condition. Furthermore, understanding specific physiological mechanisms guides the selection of therapeutic interventions most likely to achieve effective and lasting results.
| Pause and Reflect |
Your patient asks, "Why can't I keep the weight off? I lost 30 pounds last year but regained it all despite continuing my diet."
|
Adipokine Dysregulation and Inflammatory Signaling
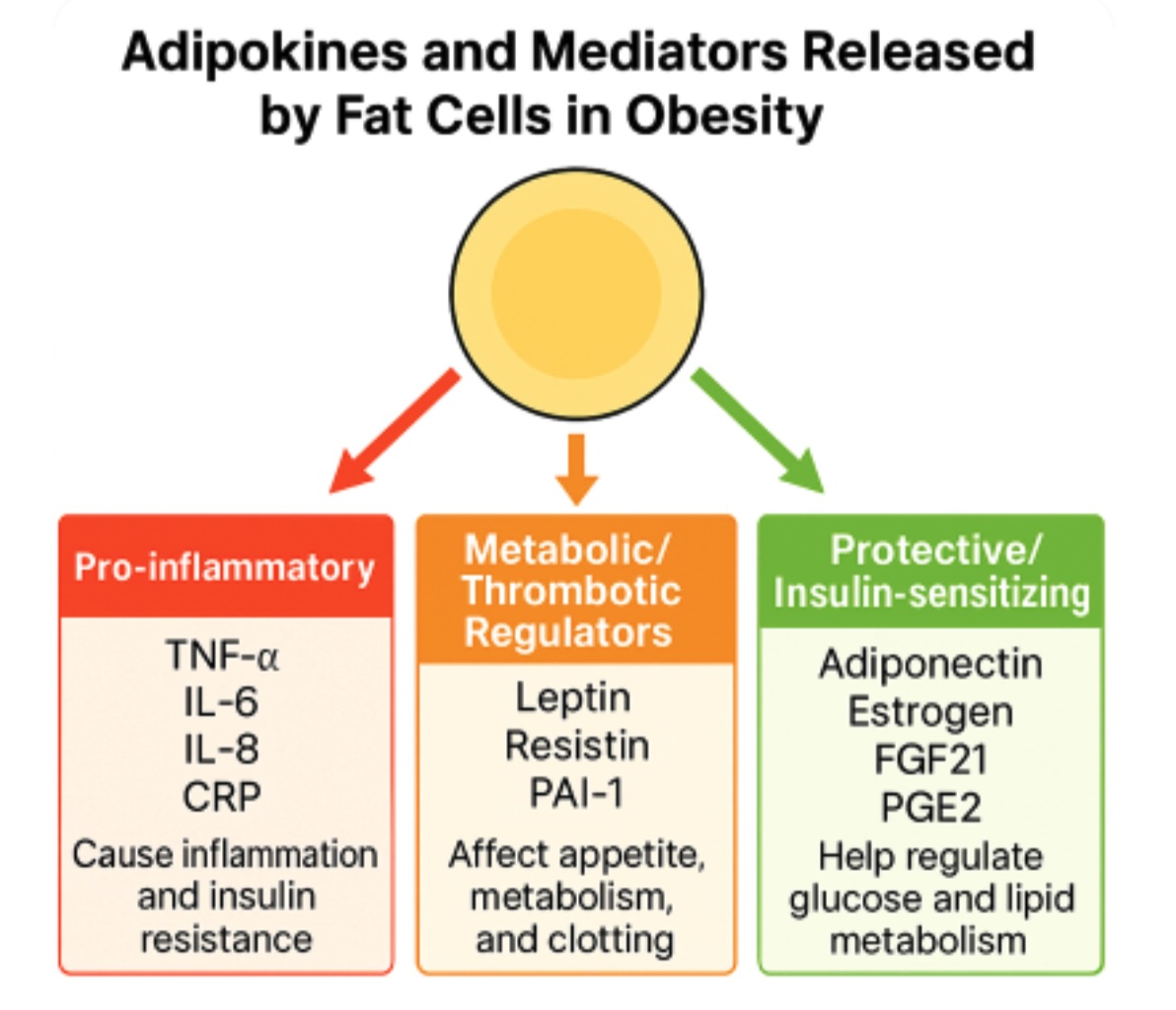
Adipose tissue functions as an active endocrine organ secreting numerous bioactive molecules called adipokines that regulate energy homeostasis, inflammation, and metabolic function. In obesity, adipose tissue undergoes significant remodeling characterized by adipocyte hypertrophy, macrophage infiltration, and altered adipokine secretion profiles that shift toward a proinflammatory state. This dysregulation represents a fundamental mechanism linking obesity to its associated comorbidities, including insulin resistance, type 2 diabetes, and cardiovascular disease.
The adipokine secretion profile in obesity demonstrates a characteristic pattern of increased proinflammatory mediators and decreased anti-inflammatory factors. Proinflammatory adipokines, including tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6), and interleukin-8 (IL-8), are elevated in obese individuals and contribute to systemic inflammation and insulin resistance by activating inflammatory signaling pathways, such as nuclear factor-κB (NF-κB) and c-Jun N-terminal kinase (JNK). Conversely, beneficial adipokines such as adiponectin, which normally enhance insulin sensitivity and exert anti-inflammatory effects, are paradoxically decreased in obesity despite increased adipose tissue mass. This imbalance creates a state of chronic low-grade inflammation that perpetuates metabolic dysfunction and increases cardiovascular risk.[17] See Image. Adipokine Dysregulation and Inflammatory Signaling.
Energy Expenditure Components and Metabolic Regulation
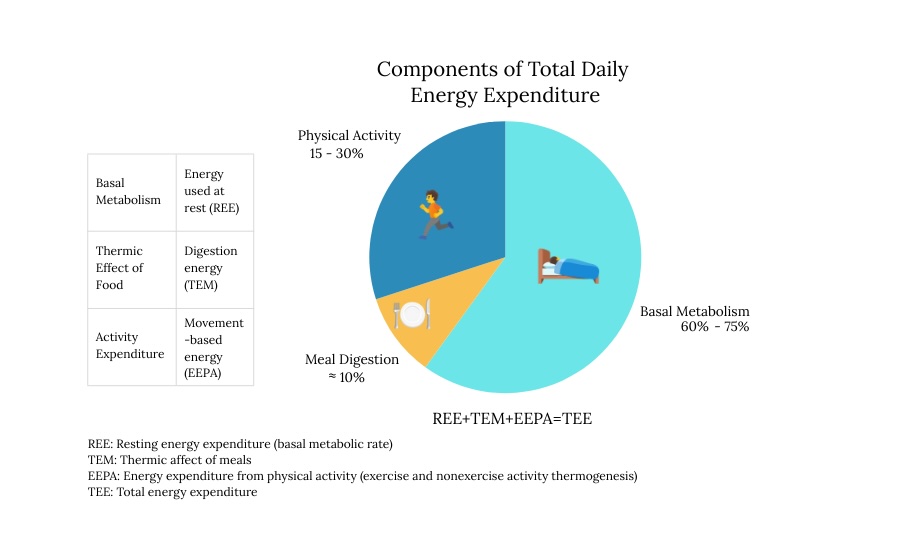
Total daily energy expenditure comprises several distinct components that collectively determine metabolic rate and energy balance. Resting metabolic rate (RMR), also known as resting energy expenditure (REE), represents the most significant component, accounting for approximately 60 to 75% of total energy expenditure in sedentary individuals. This component reflects the energy required for essential physiological functions, including cellular maintenance, organ function, protein synthesis, and ion transport. The thermic effect of food (TEF), which accounts for approximately 8% to 10% of total expenditure, encompasses the energy required for digesting, absorbing, transporting, and metabolizing nutrients. Physical activity accounts for the most variable component, ranging from 15% to 30% of total expenditure and including both exercise activity thermogenesis (EAT) and non-exercise activity thermogenesis (NEAT).
Understanding these energy expenditure components is crucial for obesity management as each responds differently to interventions and exhibits varying degrees of adaptive regulation. During caloric restriction, all components can undergo adaptive reductions that defend against weight loss, with RMR showing metabolic adaptation and NEAT demonstrating substantial decreases that contribute to weight loss plateaus. The relative contribution of each component varies significantly among individuals based on factors such as age, sex, body composition, genetics, and training status, necessitating individualized approaches to obesity treatment that account for these physiological variations.[18] See Image. Energy Expenditure Components and Metabolic Regulation.
Enhancing Healthcare Team Outcomes
Obesity is a complex chronic disease influenced by genetic, metabolic, hormonal, environmental, and behavioral factors. The pathophysiology of obesity involves dysregulation of energy balance, adaptive thermogenesis, hormonal changes, and biological mechanisms that defend against weight loss. Understanding these processes helps explain weight plateaus, weight regain, and the need for long-term management. Effective care requires evidence-based assessment, targeted treatment strategies, and reduction of weight bias to support sustainable outcomes.
Physicians, general and advanced practitioners, nurses, pharmacists, and allied health professionals share responsibility for delivering coordinated, patient-centered care for patients with obesity. Clinicians must apply current epidemiological knowledge, recognize high-risk populations, and set realistic weight loss goals. Strategies include comprehensive assessments, personalized interventions, ongoing maintenance planning, and sustained motivation to support long-term success. Interprofessional communication ensures that therapeutic approaches, medication management, and lifestyle counseling are aligned and integrated. Care coordination across disciplines enhances patient safety, optimizes adherence, and addresses social determinants of health. Through collaborative teamwork, healthcare team members can improve outcomes, empower patients, and bridge the gap between obesity science and clinical practice.
Media
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
References
Emmerich SD, Fryar CD, Stierman B, Ogden CL. Obesity and Severe Obesity Prevalence in Adults: United States, August 2021-August 2023. NCHS data brief. 2024 Sep:(508):. doi: 10.15620/cdc/159281. Epub [PubMed PMID: 39808758]
Hales CM, Carroll MD, Fryar CD, Ogden CL. Prevalence of Obesity and Severe Obesity Among Adults: United States, 2017-2018. NCHS data brief. 2020 Feb:(360):1-8 [PubMed PMID: 32487284]
Cohen AK, Rai M, Rehkopf DH, Abrams B. Educational attainment and obesity: a systematic review. Obesity reviews : an official journal of the International Association for the Study of Obesity. 2013 Dec:14(12):989-1005. doi: 10.1111/obr.12062. Epub 2013 Jul 25 [PubMed PMID: 23889851]
Level 1 (high-level) evidenceKent S, Fusco F, Gray A, Jebb SA, Cairns BJ, Mihaylova B. Body mass index and healthcare costs: a systematic literature review of individual participant data studies. Obesity reviews : an official journal of the International Association for the Study of Obesity. 2017 Aug:18(8):869-879. doi: 10.1111/obr.12560. Epub 2017 May 22 [PubMed PMID: 28544197]
Level 1 (high-level) evidenceRosenbaum M, Leibel RL. Adaptive thermogenesis in humans. International journal of obesity (2005). 2010 Oct:34 Suppl 1(0 1):S47-55. doi: 10.1038/ijo.2010.184. Epub [PubMed PMID: 20935667]
Cannon B, Nedergaard J. Brown adipose tissue: function and physiological significance. Physiological reviews. 2004 Jan:84(1):277-359 [PubMed PMID: 14715917]
Martins C, Roekenes J, Gower BA, Hunter GR. Metabolic adaptation is associated with less weight and fat mass loss in response to low-energy diets. Nutrition & metabolism. 2021 Jun 11:18(1):60. doi: 10.1186/s12986-021-00587-8. Epub 2021 Jun 11 [PubMed PMID: 34116675]
Nymo S, Coutinho SR, Torgersen LH, Bomo OJ, Haugvaldstad I, Truby H, Kulseng B, Martins C. Timeline of changes in adaptive physiological responses, at the level of energy expenditure, with progressive weight loss. The British journal of nutrition. 2018 Jul:120(2):141-149. doi: 10.1017/S0007114518000922. Epub 2018 May 7 [PubMed PMID: 29733003]
Fothergill E, Guo J, Howard L, Kerns JC, Knuth ND, Brychta R, Chen KY, Skarulis MC, Walter M, Walter PJ, Hall KD. Persistent metabolic adaptation 6 years after "The Biggest Loser" competition. Obesity (Silver Spring, Md.). 2016 Aug:24(8):1612-9. doi: 10.1002/oby.21538. Epub 2016 May 2 [PubMed PMID: 27136388]
Müller MJ, Enderle J, Bosy-Westphal A. Changes in Energy Expenditure with Weight Gain and Weight Loss in Humans. Current obesity reports. 2016 Dec:5(4):413-423 [PubMed PMID: 27739007]
Speakman JR, Levitsky DA, Allison DB, Bray MS, de Castro JM, Clegg DJ, Clapham JC, Dulloo AG, Gruer L, Haw S, Hebebrand J, Hetherington MM, Higgs S, Jebb SA, Loos RJ, Luckman S, Luke A, Mohammed-Ali V, O'Rahilly S, Pereira M, Perusse L, Robinson TN, Rolls B, Symonds ME, Westerterp-Plantenga MS. Set points, settling points and some alternative models: theoretical options to understand how genes and environments combine to regulate body adiposity. Disease models & mechanisms. 2011 Nov:4(6):733-45. doi: 10.1242/dmm.008698. Epub [PubMed PMID: 22065844]
Maclean PS, Bergouignan A, Cornier MA, Jackman MR. Biology's response to dieting: the impetus for weight regain. American journal of physiology. Regulatory, integrative and comparative physiology. 2011 Sep:301(3):R581-600. doi: 10.1152/ajpregu.00755.2010. Epub 2011 Jun 15 [PubMed PMID: 21677272]
Ley RE, Turnbaugh PJ, Klein S, Gordon JI. Microbial ecology: human gut microbes associated with obesity. Nature. 2006 Dec 21:444(7122):1022-3 [PubMed PMID: 17183309]
Dick KJ, Nelson CP, Tsaprouni L, Sandling JK, Aïssi D, Wahl S, Meduri E, Morange PE, Gagnon F, Grallert H, Waldenberger M, Peters A, Erdmann J, Hengstenberg C, Cambien F, Goodall AH, Ouwehand WH, Schunkert H, Thompson JR, Spector TD, Gieger C, Trégouët DA, Deloukas P, Samani NJ. DNA methylation and body-mass index: a genome-wide analysis. Lancet (London, England). 2014 Jun 7:383(9933):1990-8. doi: 10.1016/S0140-6736(13)62674-4. Epub 2014 Mar 13 [PubMed PMID: 24630777]
Vom Saal FS, Nagel SC, Coe BL, Angle BM, Taylor JA. The estrogenic endocrine disrupting chemical bisphenol A (BPA) and obesity. Molecular and cellular endocrinology. 2012 May 6:354(1-2):74-84. doi: 10.1016/j.mce.2012.01.001. Epub 2012 Jan 10 [PubMed PMID: 22249005]
Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW Jr. Obesity is associated with macrophage accumulation in adipose tissue. The Journal of clinical investigation. 2003 Dec:112(12):1796-808 [PubMed PMID: 14679176]
Al-Mansoori L, Al-Jaber H, Prince MS, Elrayess MA. Role of Inflammatory Cytokines, Growth Factors and Adipokines in Adipogenesis and Insulin Resistance. Inflammation. 2022 Feb:45(1):31-44. doi: 10.1007/s10753-021-01559-z. Epub 2021 Sep 18 [PubMed PMID: 34536157]
Levine JA. Measurement of energy expenditure. Public health nutrition. 2005 Oct:8(7A):1123-32 [PubMed PMID: 16277824]