Introduction
Exercise-induced bronchoconstriction (EIB) refers to narrowing of the airways during or shortly after physical activity.[1][2] While exercise is a common trigger of bronchoconstriction in individuals with asthma, EIB is also seen in up to 20% of individuals without a formal asthma diagnosis.[1][3][4] Despite the well-documented health benefits of regular physical activity, individuals with EIB may avoid exercise due to shortness of breath, coughing, chest tightness, and wheezing. This avoidance, particularly among adolescents, can lead to social isolation, obesity, and overall poor health.[3]
Paradoxically, regular exercise can reduce the severity of EIB, improve lung function, and decrease airway inflammation in affected individuals.[2][3] This improvement is often attributed to better overall conditioning and adaptations in the respiratory system that occur with consistent physical activity. Early recognition and diagnosis, confirmed by changes in lung function during or after exercise, and appropriate treatment can significantly improve quality of life, allowing individuals with EIB to remain active, including participation in elite-level sports.[3][5]
Indirect testing, which is more specific for EIB, can involve aerobic exercise in a controlled environment with cold, dry air, as these conditions are known to precipitate EIB in susceptible individuals. Alternatives to exercise testing include eucapnic voluntary hyperpnea and airway provocation tests, such as methacholine, hyperosmolar saline, or mannitol, which induce EIB by dehydrating the respiratory epithelium. The sensitivity and specificity of these methods are not well established and may vary by laboratory.[1][6][7]
Management strategies include nonpharmacological interventions, such as improving cardiovascular fitness, performing warm-up exercises, minimizing exposure to cold, dry air, pollutants, and allergens, and pharmacological treatments. Common medications, including short-acting β-agonists (SABAs), inhaled corticosteroids (ICSs), leukotriene receptor antagonists (LTRAs), and mast cell stabilizers (MCSAs), target the underlying pathophysiology of EIB and are generally well-tolerated, with minimal adverse events.
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
EIB refers to a temporary narrowing of the airways during physical activity. A rapid increase in airflow during exercise requires the airways to warm and humidify large volumes of incoming air, triggering the condition. In susceptible individuals, this process leads to inflammatory, neuronal, and vascular responses, resulting in bronchial smooth muscle contraction.
Epidemiology
EIB occurs in 40% to 90% of individuals with asthma and up to 20% of the general population without asthma.[1][2][3] Elite athletes have an increased prevalence, ranging from 30% to 70%, with higher rates observed in women and those participating in winter sports.[1][3][8] Asthma is a global health concern, affecting approximately 260 million people worldwide. EIB may serve as an indicator of poor asthma control.[3] Each year, asthma is responsible for nearly 420,000 deaths globally.[3][9][10][11]
Risk factors for EIB include a personal or family history of asthma, a personal history of atopy or allergic rhinitis, exposure to cigarette smoke, participating in high-risk sports, and living and practicing in areas with high levels of pollution.[12][13] Results from several small studies suggest sugar-sweetened beverages may increase risk by increasing inflammation and adiposity.[14][15] The likelihood of experiencing EIB varies depending on the type of sport. High-risk sports typically involve prolonged periods of continuous exercise lasting more than 5 to 8 minutes, often in environments with cold, dry air or exposure to irritants like chlorine. These sports include long-distance running, cycling, cross-country or downhill skiing, ice hockey, ice skating, high-altitude sports, swimming, water polo, and triathlons. Medium-risk sports, such as soccer, rugby, football, basketball, volleyball, baseball, cricket, and field hockey, generally involve shorter bursts of activity, with athletes rarely exercising continuously for more than 5 to 8 minutes. Low-risk sports, such as sprinting, tennis, fencing, gymnastics, boxing, golf, weightlifting, bodybuilding, and martial arts, are less likely to trigger EIB due to the intermittent or short duration of exertion.[2]
Pathophysiology
EIB arises from disruptions in normal lung physiology caused by evaporative water loss, temperature changes, and exposure to irritants during exercise-induced hyperpnea. During intense physical activity, minute ventilation can increase to 200 L/min, leading to airway cooling and mucosal dehydration.[1][16][17] As airway cells lose water, their osmolality increases, causing cell shrinkage, which triggers coughing, increased mucus production, and an epithelial barrier breakdown. In response, cells attempt to restore volume by drawing fluid from the submucosa, resulting in airway edema and releasing inflammatory mediators, such as histamine, leukotrienes, prostaglandins, and mast cell products.[16]
Bronchial blood flow also increases, generating reactive oxygen species, contributing to oxidative stress and lipid peroxidation.[1][6][9][16][17] Additionally, activation of sensory and autonomic nerves leads to both direct and neuronal pathways of bronchoconstriction.[1][17] As nasal breathing becomes insufficient during vigorous exercise, individuals switch to mouth breathing. This increases exposure of lung surfaces to pollutants, irritants, and allergens, further promoting reactive oxygen species and triggering a neutrophilic inflammatory response (see Image. Pathophysiology of Asthma).[2][3][14]
Some research suggests that individuals genetically unable to counteract oxidative stress with antioxidants such as glutathione may have an increased risk of developing EIB.[14] This impaired antioxidant defense may enhance susceptibility to airway inflammation and damage during high levels of respiratory stress. Alterations in mucus composition and impaired mucociliary clearance during exercise can contribute to mucus plugging and the growth of bacteria.[16] This environment creates favorable conditions for respiratory infections and may exacerbate airway inflammation in susceptible individuals.
Intense physical training can cause transient immune suppression, shifting the immune response toward a type 2 lymphocyte pattern. This shift is associated with increased rates of atopy and viral upper respiratory infections.[2] Prolonged exposure to airway stress can lead to epithelial damage, cell shedding, and airway remodeling that resembles asthma. However, this process is partially reversible, as study results show that lung damage and airway hyperresponsiveness can improve over weeks to years once exposure and intense exercise are reduced or discontinued.[2][3]
Evidence linking the severity of symptoms to the rate of airway water loss supports the osmotic theory of EIB. Inhaling fully humidified air at body temperature can help prevent EIB, whereas inhaling hyperosmolar saline can provoke bronchoconstriction.[16] Research also demonstrates elevated numbers of epithelial cells in sputum samples and increased exhaled nitric oxide levels in individuals with EIB, indicating heightened airway inflammation and epithelial injury.[1] Environmental exposure to pollutants, such as fossil fuels, ozone, and trichloramine, especially in ice rinks, practice fields near major roadways, and swimming pools, has been shown to exacerbate EIB.[1][2] High-pollen environments can also worsen symptoms.
History and Physical
Symptoms of EIB can range from mild to moderate symptoms of chest tightness, wheezing, coughing, and dyspnea. At the onset of exercise, patients with EIB have a period of bronchodilation that lasts approximately 5 to 8 minutes, followed by bronchoconstriction. Bronchoconstriction peaks within 10 to 15 minutes and typically resolves within 30 to 90 minutes, followed by a refractory period of 1 to 3 hours, during which continued exercise does not cause bronchoconstriction.[1][2][3] The refractory period is most likely due to inhibitory prostaglandins, like prostaglandin E2.[18]
Although rare, there have been reports of severe EIB symptoms leading to respiratory failure and death. Some individuals may remain asymptomatic, contributing to underdiagnosis. The physical examination is often unremarkable in clinical settings. Clinicians should look for signs of atopy, acute or chronic sinus or pulmonary infection, allergic disease, heart murmurs, edema, or abnormal lung sounds.
Evaluation
Except for patients with an established asthma diagnosis and typical asthma symptoms associated with exercise, establishing a clinical diagnosis based on symptoms alone has low sensitivity and specificity. Clinicians establish the diagnosis based on a combination of clinical symptoms and objective exercise or alternative challenge testing. Standardized testing for diagnosis includes both direct and indirect methods.[19] Usually, testing involves measuring changes in spirometry measurements of the forced expiratory volume in 1 second (FEV1), expressed as a percentage of the decrease from baseline following the challenge.[1][3][7]
Pulmonary Function Testing
The first step in evaluating suspected EIB is pulmonary function testing with spirometry. This test helps establish baseline pulmonary function, assess for other conditions that may mimic asthma, and identify evidence of obstructive or restrictive lung disease.[4] If baseline spirometry is normal, further challenge testing may be necessary to confirm the diagnosis of EIB.
Exercise Challenge Testing
An exercise challenge test is the preferred method for diagnosing EIB.[1] Testing should follow specific parameters, including achieving target ventilation and elevating heart rate to 80% to 90% of the predicted maximum. This exercise should be conducted in a controlled, dry environment, and last 6 to 10 minutes, typically using a treadmill or ergometer. To ensure test accuracy, patients must avoid entering the postexercise refractory period, withhold certain medications, such as bronchodilators, and refrain from consuming caffeine or engaging in vigorous exercise on the test day. These precautions help prevent false-negative results and ensure that, if present, bronchoconstriction is accurately detected during testing.
Spirometry, particularly FEV1, is measured at multiple intervals following exercise, typically at 5, 10, 15, and 30 minutes. A decrease in FEV1 of 10% or more confirms the diagnosis of EIB, with severity classified as mild (10% to <25%), moderate (25%- 50%), or severe (>50%). Some centers use a 15% decrease in FEV1 as a more specific diagnostic threshold. Because test reproducibility is approximately 76%, repeat testing may sometimes be necessary to confirm the diagnosis. Despite these limitations, the exercise challenge test remains the most direct and preferred method for diagnosing EIB.[1]
Alternative Provocation Tests
Clinicians can use alternative provocation tests, such as eucapnic voluntary hyperventilation, methacholine, histamine, or mannitol inhalation, when an exercise challenge is not feasible or when the test results are negative in a patient with a high clinical suspicion for EIB.[20] These tests are useful for confirming the diagnosis when the exercise challenge test is inconclusive. Eucapnic voluntary hyperventilation is the most sensitive alternative test for EIB. Additionally, methacholine's direct stimulation of smooth muscle receptors to induce bronchoconstriction is well established, with a sensitivity of 58.6% to 91.1%.[7]
The International Olympic Committee and the World Anti-Doping Agency require athletes to undergo a challenge test to document asthma before allowing the use of medications like bronchodilators during international competitions.[20] This policy is based on the need for objective evidence to confirm the diagnosis of asthma or EIB due to the poor predictive value of respiratory symptoms alone in athletes.[20][21]
Allergy Testing
Clinicians should consider skin allergen testing or a radioallergosorbent test to identify specific allergens contributing to symptoms. Identifying these triggers can guide patients in avoiding causative antigens and inform decisions about initiating allergen-specific immunotherapy if indicated.
Fractional Excretion of Nitric Oxide Testing
Results from several smaller studies suggest that fractional excretion of nitric oxide (FENO) may replace FEV1 in diagnosing and measuring the severity of EIB. FENO testing can be used alongside direct or indirect challenge tests and is particularly helpful for younger children due to its ease of administration. FENO measures the T helper cell type 2 (Th2)-mediated airway inflammation, offering a different diagnostic perspective than airway responsiveness; this may make it more effective in distinguishing EIB from other causes of respiratory symptoms. Experts propose cut-off values of 27 to 46 parts per billion (ppb) FENO as diagnostic, with greater than 46 ppb being 100% specific.[22][23][24]
Additional Testing
The patient's history and physical examination should guide the selection of additional testing. Additional considerations are based on clinical context:
- Serum thyroid-stimulating hormone: Useful if anxiety is suspected to be mimicking asthma symptoms
- Chest and lateral neck radiographs: Recommended for patients older than 40 with new-onset asthma, or those presenting with atypical symptoms such as hemoptysis, persistent, localized wheezing, fever, weight loss, dysphonia, concern for foreign body, or significant hypoxemia
- Echocardiography: Indicated in patients with chest pain, cyanosis, a heart murmur, or syncope to assess cardiac valvular function and overall cardiac contractility
- Continuous laryngoscopy during exercise: Useful for evaluating exercise-induced laryngeal obstruction (EILO), which is typically characterized by inspiratory stridor, dyspnea that occurs only at the peak of vigorous exercise, and rapid symptom resolution upon cessation of activity
- EILO is identified by moderate to severe adduction of laryngeal structures during exertion.
- Laryngoscopy: Appropriate for assessing potential causes such as gastroesophageal reflux, foreign body, or inducible laryngeal obstruction
- Upper endoscopy: Recommended for patients with suspected reflux and alarm symptoms like new onset reflux in a patient 60 or older, dysphagia, unexplained weight loss, iron deficiency anemia, gastrointestinal cancer in a first-degree relative, and gastrointestinal bleeding
Treatment / Management
Clinicians monitor the response to therapy based on clinical symptoms and the patient's ability to tolerate exercise. Objective measures, such as pre- and postexercise FEV1, can provide valuable information. However, this is often substituted with peak expiratory flow measurement in clinical practice. See StatPearls' companion topic, "Peak Flow Rate Measurement," for an in-depth discussion on the proper technique and clinical uses of peak flow measurement. A repeat exercise challenge test may be used to evaluate the effectiveness of therapy and guide ongoing management.
Nonpharmacologic Interventions
Management of EIB begins with nonpharmacological strategies.[1] Improving cardiovascular fitness, building endurance, and promoting weight loss in patients with obesity can help mitigate symptoms. A Cochrane review and results from other studies show that regular exercise improves pulmonary function, decreases airway inflammation, and reduces the severity of EIB, particularly in patients with asthma.[1][2][3][25][26] (A1)
Study results are conflicting regarding the effectiveness of preexercise warm-ups; some evidence suggests that variable and high-intensity warm-ups reduce the fall in FEV1.[16][27] Breathing warm, humid air lessens the degree of bronchoconstriction. Athletes should consider wearing a thin scarf or a mask in cold or dry climates. Heat and moisture exchanger masks, which warm and humidify inspired air, may reduce symptom severity and reduce medication need.[27][28]
Patients should also avoid exercising in environments with high pollen, chlorine, ozone, or other pollutants. Wearing a mechanical barrier mask or selecting lower-risk sports can minimize exposure. In aquatic sports, alternative pool disinfection methods may help reduce symptom exacerbation.[3][27] Additional supportive measures include preexercise caffeine intake, which may reduce bronchoconstriction, lower ventilatory dead space, and lessen exercise-induced hypoxemia and respiratory muscle fatigue. Results from studies investigating several dietary modifications for treating EIB reveal a potential benefit of a low-sodium diet. However, fish oil, lycopene, vitamin C, and vitamin E have not demonstrated consistent benefits.[1][27][29](A1)
Another treatment approach focuses on reducing the perception of symptoms. Breathing control techniques, including yoga or supervised breathing training, have been shown in some small studies to decrease symptoms, reduce medication use, decrease anxiety and depression associated with EIB, and increase quality of life. However, further research and adaptation are needed before these methods can be widely recommended for patients with EIB.[27]
Respiratory muscle training involves using proper breathing techniques and devices to strengthen both inspiratory and expiratory muscles. A Cochrane review found no evidence for or against respiratory muscle training in patients with EIB. Nevertheless, respiratory muscle training is promising for patients with chronic obstructive pulmonary disease, EILO, and stridor, making it a promising, low-cost adjunctive therapy for individuals with EIB.[25][27]
Pharmacologic Interventions
Before initiating pharmacologic therapy, clinicians must ensure patients can use inhalers correctly. Improper technique can significantly reduce medication delivery to the lungs and diminish therapeutic effectiveness.
Short-acting ß-agonists
All patients with EIB should have access to a SABA, such as albuterol. SABAs work by relaxing airway smooth muscles and inhibiting mast cell degranulation.[30] The usual dose is 2 to 4 puffs; patients with frequent symptoms of EIB should use 2 puffs of albuterol as a preventative measure, 5 to 20 minutes before exercise.[1] Alternatively, 1 puff of budesonide-formoterol 160 µg/4.5 µg is an appropriate option. (A1)
With frequent SABA use, tolerance can develop, likely due to the downregulation of the ß2 receptors. Ipratropium is not recommended for rapid symptom relief due to its delayed onset of action.[1][31] However, it can be a viable option for pretreatment for patients unable to tolerate SABAs. Ultimately, the goal is adequate control of underlying asthma so that routine SABA use before exercise becomes unnecessary.(A1)
Inhaled corticosteroids
Study results estimate that 15% to 20% of the patients will not respond to SABA treatment alone. If symptoms remain uncontrolled or if the patient is using a SABA daily, this often indicates poorly controlled asthma. Before escalating therapy, clinicians should confirm the patient's adherence and correct use of current medications. Step-up therapy should be implemented according to the severity of asthma in patients with underlying asthma. See StatPearls' companion topics, "Pediatric Asthma" and "Asthma," for an in-depth discussion on diagnosing and treating asthma in adults and children.
Daily leukotriene receptor antagonists (LTRAs) or inhaled corticosteroids (ICSs) are first-line choices for patients with EIB without underlying asthma who require additional therapy. Additionally, patients who engage in prolonged exercise, exercise multiple times a day, or children with irregular schedules who play at various times risk developing tachyphylaxis from repeated dosing of ß2-agonists. These patients should receive daily LTRAs or ICSs in addition to SABAs.
Leukotriene receptor antagonists
Daily LTRAs address the release of inflammatory mediators involved in EIB.[1][31] Agents such as montelukast, zafirlukast, and zileuton provide longer-lasting bronchodilation and are not associated with tolerance. A single dose of LTRAs can provide protection within 2 hours. The choice between ICS and LTRA is patient-specific.[1] (A1)
Mast cell stabilizing agents
Mast cell degranulation plays a key role in EIB pathology. The American Thoracic Society (ATS) recommends adding an inhaled mast cell stabilizing agent, such as sodium cromoglycate or nedocromil sodium, before exercise in patients who continue to experience frequent symptoms despite using an inhaled SABA.[1] These medications are unavailable in the United States, but are readily accessible in many other countries.[1](A1)
Antihistamine
The ATS recommends adding a daily antihistamine in patients with allergies who continue to experience frequent symptoms despite using an inhaled SABA before exercise.[1] Antihistamines provide no additional benefit in nonatopic individuals. (A1)
Noninvasive Positive Pressure Ventilation
Results from a recent study exploring noninvasive positive pressure ventilation (NIPPV) as a nonpharmacologic treatment for EIB revealed that airway stretching activates inhibitory pathways that reduce inflammation and promote bronchodilation. Participants underwent ten 1-hour sessions over 5 weeks, beginning with 20 minutes of respiratory exercises in seated and supine positions. They then received continuous positive airway pressure (CPAP) at 8 cm H2O, inspiratory muscle training, or bilevel positive airway pressure (BiPAP) at 8 to 12 cm H2O. Outcome measures include exercise-induced changes in FEV1 according to ATS criteria, fractional exhaled nitric oxide to assess airway inflammation, and symptom scoring via questionnaire. CPAP and BiPAP significantly reduce EIB, while respiratory muscle training decreases medication use and improves respiratory muscle strength. These findings suggest that noninvasive positive pressure ventilation and respiratory training may offer effective nonpharmacologic options for managing EIB.[17](B3)
Differential Diagnosis
Although chest tightness, wheezing, coughing, and shortness of breath during exercise may suggest EIB, these symptoms can also result from other conditions involving the upper or lower airways, cardiovascular and gastrointestinal systems, or dysfunctional breathing patterns.[32] Healthcare professionals must consider a broad list of diagnoses when evaluating patients with exertional dyspnea. Diagnosing EIB can be particularly challenging due to the possible absence of other asthma signs, normal baseline spirometry, and lack of response to bronchodilator pretreatment.
Nasal Airway
- Exercise-induced rhinosinusitis
- Allergic rhinitis
- Upper airway cough syndrome
- Upper respiratory infection
- Anatomic abnormalities
The nasal airway helps filter, humidify, and regulate airway resistance. Conditions affecting the nasal airway are often comorbid with EIB.[8][33] Management of upper airway diseases includes avoiding triggers and irritants, as well as MCSAs and LTRAs, using intranasal corticosteroids, taking decongestants, and undergoing immunotherapy.[1][2][4][8][33]
Pharynx and Larynx
Lower Airways
- Airway obstruction due to malignancy
- Interstitial lung disease
- Chronic obstructive pulmonary disease
- Infection [1][2][4][8]
Cardiac
- Coronary artery disease
- Poor cardiovascular conditioning
- Exercise-induced dysrhythmias [6]
Others
- Exercise-induced anaphylaxis
- Anxiety disorder
- Exercise-induced pulmonary edema
- Pulmonary embolism
- Saltwater aspiration syndrome [4]
Prognosis
The prognosis of EIB is generally favorable, especially when the condition is accurately diagnosed and effectively managed. Most individuals experience mild to moderate symptoms such as chest tightness, coughing, wheezing, and shortness of breath, which may impair athletic performance but rarely lead to severe respiratory distress. With appropriate pharmacologic and nonpharmacologic interventions, including preexercise bronchodilator use and environmental modifications, patients can often fully participate in physical activity and excel in competitive sports at an elite level.
However, clinicians must recognize that severe episodes of EIB, though rare, can lead to significant respiratory compromise, including isolated cases of respiratory failure and death. Early identification, patient education, and individualized management strategies are, therefore, critical to optimizing outcomes and preventing complications. Ongoing monitoring and reassessment are also important to ensure sustained control of symptoms and to support long-term health and physical performance.
Complications
EIB can lead to significant complications if not properly managed. Commonly, individuals may experience limitations in physical activity, which can reduce overall fitness and contribute to a sedentary lifestyle. While most cases are mild to moderate, rare severe episodes can result in significant respiratory distress or, in extreme cases, respiratory failure.
The United States Food and Drug Administration has issued a boxed warning for montelukast, an LTRA, regarding the risk of behavior and mood-related changes. Reported symptoms are agitation, depression, insomnia, suicidal thoughts, and actions. However, research findings are mixed, and some experts believe that worsening asthma control in those who require LTRA initiation may play a role in behavior and mood changes. Results from a recent nationwide register-based cohort study from Sweden found no association between the use of montelukast and the risk of neuropsychiatric adverse events.[34] Results from other observational studies, however, reported a small increased risk of neuropsychiatric changes.[35][36]
Deterrence and Patient Education
EIB, marked by airway narrowing during or shortly after physical activity, affects the general population and individuals with underlying asthma, though it is more common in those with asthma. Despite the well-documented health benefits of regular exercise, many people with EIB avoid physical activity due to shortness of breath, coughing, chest tightness, and wheezing. This avoidance can lead to social withdrawal, weight gain, and declining overall health. Interestingly, regular exercise helps reduce the severity of EIB, improve lung function, and decrease airway inflammation, making patient education on the importance of staying active a key component of care.
Encouraging physical activity is essential to reducing fear and empowering patients to participate in physical activity safely. Healthcare professionals should counsel patients on the importance of early recognition and accurate diagnosis, confirmed through objective testing such as exercise challenge or airway provocation tests. Indirect testing methods, including eucapnic voluntary hyperpnea and pharmacologic challenges like methacholine, can help identify EIB in controlled settings.
Management strategies should combine nonpharmacologic interventions with pharmacologic treatments, such as improving cardiovascular fitness, performing warm-up exercises, avoiding cold, dry air, and reducing exposure to environmental irritants. SABAs, ICSs, LTRAs, and MCSAs are effective and generally well-tolerated. Educating patients on correct medication use and adherence, and recognizing when to seek medical attention, can prevent severe episodes and support a healthy and active lifestyle. This enables many individuals with EIB to participate in sports and physical activity safely.
Pearls and Other Issues
Key facts to keep in mind about EIB include the following:
- EIB is characterized by transient airway narrowing during or after exercise, leading to coughing, wheezing, dyspnea, and chest tightness; it is common in individuals with asthma, but can also occur in those without asthma.
- The primary mechanism involves airway dehydration and cooling during hyperventilation, leading to a hyperosmolar environment that triggers mast cell degranulation and release of inflammatory mediators like leukotrienes, histamine, and prostaglandins, causing bronchoconstriction.
- Diagnosis requires objective testing, as respiratory symptoms alone have poor predictive value. Recommended tests include exercise challenge tests and eucapnic voluntary hyperpnea. A significant drop in FEV1 indicates a positive test.
- SABAs, like albuterol, are the first-line treatment, taken 15 to 30 minutes before exercise.
- ICS and LTRAs are used for long-term control, especially in patients with frequent symptoms or poor response to SABAs alone.
- Preexercise warm-up, nasal breathing, and face masks in cold environments can help reduce symptoms.
- EIB can occur without asthma, and its prevalence is higher among elite athletes. Regular follow-up and re-evaluation of therapy are essential due to variability in response to treatment and potential development of tolerance to β2-agonists.
Clinicians who treat elite athletes must be aware of the World Anti-Doping Agency guidelines regarding medications for athletes who compete in international competitions. Some medications utilized for EIB may require a therapeutic use exemption (TUE). ICS, LTRAs, MCSAs, inhaled anticholinergics, SABA (including albuterol and formoterol), antibiotics, first-generation antihistamines with or without oral decongestants, nasal ipratropium, dextromethorphan, nasal corticosteroids, topical decongestants, and proton pump inhibitors in appropriate doses do not enhance performance and therefore do not require a TUE.[1] However, oral steroids and terbutaline are banned substances and require a TUE.[1][4] Additionally, nebulized ß-agonists may elevate levels over acceptable amounts (ie, urinary albuterol levels >1000 ng/mL).
Enhancing Healthcare Team Outcomes
Managing EIB effectively requires a team approach that includes advanced clinicians, nurses, pharmacists, and other healthcare professionals. Key strategies involve making an accurate diagnosis through tests like eucapnic voluntary hyperpnea, and creating personalized treatment plans that use medication and lifestyle changes. Clinicians must stay current with guidelines, such as those from the American Academy of Allergy, Asthma & Immunology, which recommend regularly reviewing treatment plans to account for individual variations in patient response to therapy.[37]
Ethically, athletes must receive appropriate medical treatment while ensuring compliance with anti-doping regulations established by organizations such as the World Anti-Doping Agency. Communication between healthcare professionals is vital to coordinate care, including teaching patients how to use inhalers properly, make environmental adjustments, and follow their treatment plans. Pharmacists are key in advising on medication use and potential side effects, while nurses help monitor symptoms and educate patients. Effective teamwork enhances patient care, improves treatment outcomes, and promotes safety by reducing the risk of complications.
Media
(Click Image to Enlarge)
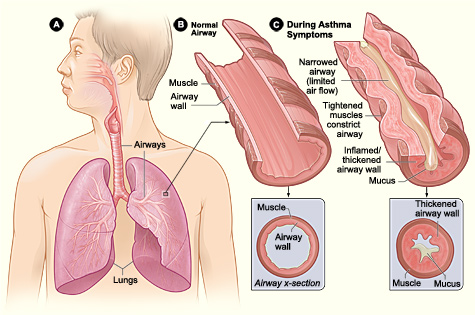
Pathophysiology of Asthma. Figure A displays the location of the lungs and airways in the body. Figure B shows a cross section of a normal airway. Figure C illustrates a cross section of an airway during asthma symptoms.
National Institutes of Health, Public Domain, via Wikimedia Commons
References
Parsons JP, Hallstrand TS, Mastronarde JG, Kaminsky DA, Rundell KW, Hull JH, Storms WW, Weiler JM, Cheek FM, Wilson KC, Anderson SD, American Thoracic Society Subcommittee on Exercise-induced Bronchoconstriction. An official American Thoracic Society clinical practice guideline: exercise-induced bronchoconstriction. American journal of respiratory and critical care medicine. 2013 May 1:187(9):1016-27. doi: 10.1164/rccm.201303-0437ST. Epub [PubMed PMID: 23634861]
Level 1 (high-level) evidenceBonini M, Silvers W. Exercise-Induced Bronchoconstriction: Background, Prevalence, and Sport Considerations. Immunology and allergy clinics of North America. 2018 May:38(2):205-214. doi: 10.1016/j.iac.2018.01.007. Epub 2018 Mar 2 [PubMed PMID: 29631730]
Jayasinghe H, Kopsaftis Z, Carson K. Asthma Bronchiale and Exercise-Induced Bronchoconstriction. Respiration; international review of thoracic diseases. 2015:89(6):505-12. doi: 10.1159/000433559. Epub 2015 Jun 11 [PubMed PMID: 26068579]
Boulet LP, Turmel J, Irwin RS, CHEST Expert Cough Panel. Cough in the Athlete: CHEST Guideline and Expert Panel Report. Chest. 2017 Feb:151(2):441-454. doi: 10.1016/j.chest.2016.10.054. Epub 2016 Nov 16 [PubMed PMID: 27865877]
Vlietstra RE, Holmes DR Jr. PTCA in acute ischemic syndromes. Current problems in cardiology. 1987 Dec:12(12):703-62 [PubMed PMID: 2963730]
Aggarwal B, Mulgirigama A, Berend N. Exercise-induced bronchoconstriction: prevalence, pathophysiology, patient impact, diagnosis and management. NPJ primary care respiratory medicine. 2018 Aug 14:28(1):31. doi: 10.1038/s41533-018-0098-2. Epub 2018 Aug 14 [PubMed PMID: 30108224]
Dreßler M, Friedrich T, Lasowski N, Herrmann E, Zielen S, Schulze J. Predictors and reproducibility of exercise-induced bronchoconstriction in cold air. BMC pulmonary medicine. 2019 May 16:19(1):94. doi: 10.1186/s12890-019-0845-3. Epub 2019 May 16 [PubMed PMID: 31097027]
Olin JT, Hull JH. Exercise and the Total Airway: A Call to Action. Immunology and allergy clinics of North America. 2018 May:38(2):xv-xix. doi: 10.1016/j.iac.2018.02.001. Epub 2018 Feb 19 [PubMed PMID: 29631744]
Wilkinson M, Hart A, Milan SJ, Sugumar K. Vitamins C and E for asthma and exercise-induced bronchoconstriction. The Cochrane database of systematic reviews. 2014 Jun 17:2014(6):CD010749. doi: 10.1002/14651858.CD010749.pub2. Epub 2014 Jun 17 [PubMed PMID: 24936673]
Level 1 (high-level) evidenceGBD 2019 Diseases and Injuries Collaborators. Global burden of 369 diseases and injuries in 204 countries and territories, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet (London, England). 2020 Oct 17:396(10258):1204-1222. doi: 10.1016/S0140-6736(20)30925-9. Epub [PubMed PMID: 33069326]
Level 1 (high-level) evidenceGBD 2021 Asthma and Allergic Diseases Collaborators. Global, regional, and national burden of asthma and atopic dermatitis, 1990-2021, and projections to 2050: a systematic analysis of the Global Burden of Disease Study 2021. The Lancet. Respiratory medicine. 2025 May:13(5):425-446. doi: 10.1016/S2213-2600(25)00003-7. Epub 2025 Mar 24 [PubMed PMID: 40147466]
Level 1 (high-level) evidenceKang N, Koh E, Lee JY, Song WJ, Choi DC, Lee BJ. Cut-off value for exercise-induced bronchoconstriction based on the features of the airway obstruction. PloS one. 2022:17(5):e0268969. doi: 10.1371/journal.pone.0268969. Epub 2022 May 26 [PubMed PMID: 35617173]
Van Meerbeke SW, McCarty M, Petrov AA, Schonffeldt-Guerrero P. The Impact of Climate, Aeroallergens, Pollution, and Altitude on Exercise-Induced Bronchoconstriction. Immunology and allergy clinics of North America. 2025 Feb:45(1):77-88. doi: 10.1016/j.iac.2024.09.004. Epub 2024 Oct 20 [PubMed PMID: 39608881]
Rundell KW, Smoliga JM, Bougault V. Exercise-Induced Bronchoconstriction and the Air We Breathe. Immunology and allergy clinics of North America. 2018 May:38(2):183-204. doi: 10.1016/j.iac.2018.01.009. Epub [PubMed PMID: 29631729]
Emerson SR, Rosenkranz SK, Rosenkranz RR, Kurti SP, Harms CA. The potential link between sugar-sweetened beverage consumption and post-exercise airway narrowing across puberty: a longitudinal cohort study. Public health nutrition. 2016 Sep:19(13):2435-40. doi: 10.1017/S1368980015003109. Epub 2015 Oct 30 [PubMed PMID: 26514591]
Kippelen P, Anderson SD, Hallstrand TS. Mechanisms and Biomarkers of Exercise-Induced Bronchoconstriction. Immunology and allergy clinics of North America. 2018 May:38(2):165-182. doi: 10.1016/j.iac.2018.01.008. Epub [PubMed PMID: 29631728]
David MMC, Gomes ELFD, Mello MC, Costa D. Noninvasive ventilation and respiratory physical therapy reduce exercise-induced bronchospasm and pulmonary inflammation in children with asthma: randomized clinical trial. Therapeutic advances in respiratory disease. 2018 Jan-Dec:12():1753466618777723. doi: 10.1177/1753466618777723. Epub [PubMed PMID: 29865929]
Level 3 (low-level) evidenceManning PJ, Watson RM, O'Byrne PM. Exercise-induced refractoriness in asthmatic subjects involves leukotriene and prostaglandin interdependent mechanisms. The American review of respiratory disease. 1993 Oct:148(4 Pt 1):950-4 [PubMed PMID: 8214949]
Mohning MP, Meneses-Tamayo E, Rodríguez Flores C. Diagnostic Testing in Exercise-Induced Bronchoconstriction. Immunology and allergy clinics of North America. 2025 Feb:45(1):89-99. doi: 10.1016/j.iac.2024.08.010. Epub 2024 Oct 20 [PubMed PMID: 39608882]
Fitch KD, Sue-Chu M, Anderson SD, Boulet LP, Hancox RJ, McKenzie DC, Backer V, Rundell KW, Alonso JM, Kippelen P, Cummiskey JM, Garnier A, Ljungqvist A. Asthma and the elite athlete: summary of the International Olympic Committee's consensus conference, Lausanne, Switzerland, January 22-24, 2008. The Journal of allergy and clinical immunology. 2008 Aug:122(2):254-60, 260.e1-7. doi: 10.1016/j.jaci.2008.07.003. Epub [PubMed PMID: 18678340]
Level 3 (low-level) evidenceBoulet LP, O'Byrne PM. Asthma and exercise-induced bronchoconstriction in athletes. The New England journal of medicine. 2015 Feb 12:372(7):641-8. doi: 10.1056/NEJMra1407552. Epub [PubMed PMID: 25671256]
Dreßler M, Salzmann-Manrique E, Zielen S, Schulze J. Exhaled NO as a predictor of exercise-induced asthma in cold air. Nitric oxide : biology and chemistry. 2018 Jun 1:76():45-52. doi: 10.1016/j.niox.2018.03.004. Epub 2018 Mar 8 [PubMed PMID: 29526567]
Kim K, Cho HJ, Yoon JW, Choi SH, Sheen YH, Han MY, Baek H. Exhaled nitric oxide and mannitol test to predict exercise-induced bronchoconstriction. Pediatrics international : official journal of the Japan Pediatric Society. 2018 Aug:60(8):691-696. doi: 10.1111/ped.13599. Epub [PubMed PMID: 29786927]
Alving K. FeNO and the Prediction of Exercise-Induced Bronchoconstriction. The journal of allergy and clinical immunology. In practice. 2018 May-Jun:6(3):863-864. doi: 10.1016/j.jaip.2017.12.038. Epub [PubMed PMID: 29747989]
Weatherald J, Lougheed MD, Taillé C, Garcia G. Mechanisms, measurement and management of exertional dyspnoea in asthma: Number 5 in the Series "Exertional dyspnoea" Edited by Pierantonio Laveneziana and Piergiuseppe Agostoni. European respiratory review : an official journal of the European Respiratory Society. 2017 Jun 30:26(144):. doi: 10.1183/16000617.0015-2017. Epub 2017 Jun 14 [PubMed PMID: 28615308]
Arce SC, Benítez-Pérez RE. Breathing Easy During Training. Strategies for Managing Exercise-Induced Bronchoconstriction. Immunology and allergy clinics of North America. 2025 Feb:45(1):101-111. doi: 10.1016/j.iac.2024.09.005. Epub 2024 Oct 24 [PubMed PMID: 39608872]
Dickinson J, Amirav I, Hostrup M. Nonpharmacologic Strategies to Manage Exercise-Induced Bronchoconstriction. Immunology and allergy clinics of North America. 2018 May:38(2):245-258. doi: 10.1016/j.iac.2018.01.012. Epub [PubMed PMID: 29631733]
Beuther DA, Martin RJ. Efficacy of a heat exchanger mask in cold exercise-induced asthma. Chest. 2006 May:129(5):1188-93 [PubMed PMID: 16685008]
Sogard AS, Emerson TS, Chandler CA, Cobb EA, Shei RJ, Paris HL, Lindley MR, Mickleborough TD. The role of nutritional factors in exercise-induced bronchoconstriction: a narrative review. American journal of physiology. Regulatory, integrative and comparative physiology. 2025 Jun 1:328(6):R651-R684. doi: 10.1152/ajpregu.00249.2024. Epub 2025 Apr 21 [PubMed PMID: 40257056]
Level 2 (mid-level) evidenceBonini M, Di Mambro C, Calderon MA, Compalati E, Schünemann H, Durham S, Canonica GW. Beta₂-agonists for exercise-induced asthma. The Cochrane database of systematic reviews. 2013 Oct 2:2013(10):CD003564. doi: 10.1002/14651858.CD003564.pub3. Epub 2013 Oct 2 [PubMed PMID: 24089311]
Level 1 (high-level) evidenceBacker V, Mastronarde J. Pharmacologic Strategies for Exercise-Induced Bronchospasm with a Focus on Athletes. Immunology and allergy clinics of North America. 2018 May:38(2):231-243. doi: 10.1016/j.iac.2018.01.011. Epub [PubMed PMID: 29631732]
Pelgröm AT, de Jongh FHC, van den Aardweg JG, van Veen IHPAA. Analysis of the breathing pattern in patients with asthma during physical exercise: A cross-sectional study. Respiratory medicine. 2025 Apr-May:240():108037. doi: 10.1016/j.rmed.2025.108037. Epub 2025 Mar 11 [PubMed PMID: 40081671]
Level 2 (mid-level) evidenceSteelant B, Hox V, Hellings PW, Bullens DM, Seys SF. Exercise and Sinonasal Disease. Immunology and allergy clinics of North America. 2018 May:38(2):259-269. doi: 10.1016/j.iac.2018.01.014. Epub 2018 Mar 2 [PubMed PMID: 29631734]
Wintzell V, Brenner P, Halldner L, Rhedin S, Gong T, Almqvist C. Montelukast Use and the Risk of Neuropsychiatric Adverse Events in Children. JAMA pediatrics. 2025 Apr 1:179(4):418-427. doi: 10.1001/jamapediatrics.2024.5429. Epub [PubMed PMID: 39836401]
Paljarvi T, Forton JT, Thompson C, Luciano S, Herttua K, Fazel S. Neuropsychiatric diagnoses after montelukast initiation in paediatric patients with asthma. Thorax. 2024 Dec 23:80(1):9-15. doi: 10.1136/thorax-2024-221590. Epub 2024 Dec 23 [PubMed PMID: 39578088]
Jo YW, Kwon HS, Min J, Her Y, Kwon JW. Neuropsychiatric Events Related to Montelukast and Pranlukast in Adults With Asthma and Rhinitis: A 10-Year Nationwide Population-Based Study. The journal of allergy and clinical immunology. In practice. 2025 Apr:13(4):893-902. doi: 10.1016/j.jaip.2024.09.010. Epub 2024 Sep 17 [PubMed PMID: 39299666]
Weiler JM, Brannan JD, Randolph CC, Hallstrand TS, Parsons J, Silvers W, Storms W, Zeiger J, Bernstein DI, Blessing-Moore J, Greenhawt M, Khan D, Lang D, Nicklas RA, Oppenheimer J, Portnoy JM, Schuller DE, Tilles SA, Wallace D. Exercise-induced bronchoconstriction update-2016. The Journal of allergy and clinical immunology. 2016 Nov:138(5):1292-1295.e36. doi: 10.1016/j.jaci.2016.05.029. Epub 2016 Sep 21 [PubMed PMID: 27665489]