Introduction
Antepartum infections are a significant contributor to maternal and fetal morbidity and mortality. Populations in countries with developing economies bear the brunt of the burden of these infections.[1][2] However, vaccination hesitancy in wealthier countries has resulted in a resurgence of some of these preventable infections.[3][4]
Understanding the infections and their manifestations is critical not only for the diagnosis and management but also for providing anticipatory care and guidance to pregnant individuals and their families. The following is a discussion of vertically-transmitted antepartum infections, infections that affect the uterus and birth canal, and infections that disproportionately affect pregnant people. Any infection present in the general population can present in those who are pregnant and consequently impact the pregnancy.
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
Pregnancy results in a state of immune alteration, which is necessary to prevent the maternal immune system from destroying the fetus. Infections acquired during pregnancy can be passed vertically to the developing fetus through the placenta and the birthing process. The etiology of antepartum infections is multifactorial and varies based on the pathogen, maternal health status, and environmental factors.
Infections can arise from ascending genital tract colonization, hematogenous spread, or direct exposure to contaminated food, water, or vectors. Maternal factors such as immunosuppression, poor nutritional status, and preexisting medical conditions increase susceptibility. Additionally, socioeconomic determinants, including inadequate prenatal care and limited access to sanitation, further contribute to the risk of antepartum infections, particularly in resource-limited settings.
Bacterial Infections
- Group A and B Streptococcus
- Chlamydia trachomatis
- Listeria monocytogenes
- Treponema pallidum (syphilis)
Common bacterial causes of chorioamnionitis include the following:
- Escherichia coli
- Ureaplasma urealyticum
- Ureaplasma pyogenes
- Mycoplasma hominis
- Gardnerella vaginalis
Viral Infections
- Herpes simplex virus
- Cytomegalovirus
- Zika virus
- Chikungunya
- Rubella
- Hepatitis A, B, C, D, and E
- Human immunodeficiency virus (HIV)
- SARS-CoV-2 virus (COVID)
- Parvovirus
- Monkeypox
- Coxsackievirus
Protozoal Infections:
- Toxoplasmosis gondii
- Malaria
Toxoplasmosis, Other, Rubella, Cytomegalovirus, Herpes Simplex Virus; TORCH:
- The TORCH organisms are causes of congenital infections that may be acquired through vertical transmission.[5]
- These organisms are responsible for roughly 35.7% of neonatal deaths, 15.6% of neonatal infections, and 10.5% of congenital abnormalities.[6]
- Ideally, serologic screening of biological women should be completed before pregnancy to establish an individual's serologic status, which is crucial for managing infections that occur through vertical transmission.
Epidemiology
Low- and middle-income countries (LMICs) continue to face challenges with preventing and managing maternal infections, as limited resources and restricted access to healthcare heighten the risk of adverse pregnancy outcomes. According to a systematic analysis by the World Health Organization (WHO), direct obstetric infections remain a significant cause of maternal mortality, responsible for approximately 10.7% of maternal deaths. This mortality rate is notably higher in LMICs compared to high-income countries (HICs), where the rate stands at 4.7%.[7]
Worldwide, sepsis is a leading cause of maternal morbidity and mortality, as there is frequently a delay in diagnosis and suboptimal medical management.[8] To address this issue, the WHO launched the Global Maternal Sepsis and Neonatal Initiative in collaboration with its partners in 2016. This initiative led to the implementation of the GLOSS (Global Maternal Sepsis Study) in 2017, aiming to reduce the burden of maternal sepsis and related infections. While some progress has been made in alleviating the impact of maternal sepsis and maternal infections (MSMI) across 131 LMICs, the disease burden remains significantly higher in low-income countries compared to lower-middle- and upper-middle-income countries. Factors such as socioeconomic status and access to universal health coverage play a crucial role in shaping the severity of MSMI in these regions.[7]
Infections during pregnancy pose a significant global public health concern. However, many of these infections are preventable.[9] Vertically transmitted infections, in particular, are disproportionately prevalent in regions with developing economies. For instance, the prevalence of toxoplasmosis varies significantly by region, with 11% of women of childbearing age in Europe testing seropositive compared to 77% in a comparable population in South America. Certain infections are notably more common in specific geographic areas, including high rates of syphilis in Central Africa, HIV in Southern Africa, cytomegalovirus (CMV) in South America, hepatitis B in West Sub-Saharan Africa, hepatitis C in the Middle East and East Asia, hepatitis E in India, Southeast Asia, the Middle East, and Africa.[10]
Antepartum infections can significantly impact maternal and fetal outcomes, including risks such as preterm birth, growth restriction, and fetal loss. Understanding the types, implications, and management is essential for optimizing prenatal care. The following discussion explores the various specific types of antepartum infections.
Antepartum Infections
Gonorrhea and chlamydia
In 2022, there were 2.5 million cases of gonorrhea, chlamydia, and syphilis reported to the Centers for Disease Control and Prevention (CDC), with chlamydia being one of the most common sexually transmitted infections, even in more developed countries.[11][12] Gonorrhea is the second most commonly reported sexually transmitted bacterial disease in the United States, with an estimated over 820,000 new Neisseria gonorrhoeae infections occurring each year.[13] Gonorrhea in pregnancy has been associated with chorioamnionitis, premature rupture of membranes, preterm labor, low birth weight, and small for gestational age infants.[14]
Perinatal transmission of gonorrhea can lead to ophthalmia neonatorum, which can cause blindness. Systemic neonatal infections such as sepsis, arthritis, and meningitis are rare complications of gonorrhea in pregnancy.[15][16] Similar complications of pregnancy are seen with chlamydia infection, including preterm birth, preterm prelabor rupture of membranes, low birth weight, and stillbirth.[14][17] Neonates exposed to chlamydia may develop conjunctivitis and chlamydial pneumonitis, occurring in up to 30% of exposed infants.[18]
Parvovirus B19
Primary infection with parvovirus B19 occurs through exposure to respiratory droplets or infected blood products. Congenital infection is transplacental.[10] When parvovirus B19 infection occurs in the first half of pregnancy, the rate of intrauterine transmission is 24% to 39%, with a risk of fetal hydrops and death of 4% to 10%.[6] There is no vaccine or medical treatment available for parvovirus.
Herpes simplex virus
Primary herpes simplex virus (HSV) infection occurs through sexual contact; vertical transmission occurs primarily through contact with active lesions in the maternal birth canal during parturition. Less common modes of transmission include transplacental infection and postnatal infection.[19] The highest risk of vertical transmission (30% to 50%) is when the primary infection with HSV occurs near term. Alternatively, the risk of vertical transmission in the first half of pregnancy is less than 1%.[6]
Syphilis
The microaerophilic, gram-negative spirochete Treponema pallidum causes syphilis. Maternal infection occurs through sexual contact, and congenital infection is transmitted transplacentally with increased risk in mothers with high spirochete titers, an early stage of infection at the time of pregnancy, and late or incomplete treatment.[10] Many neonates are born with no symptoms, but symptoms manifest after 3 months of age. Due to the hypoxemia caused by placental damage, 30% of fetuses with congenital syphilis are stillborn.[20] From 2015 to 2018, syphilis cases increased by 30%, with congenital syphilis cases rising as well.[6]
Zika virus
Zika virus is a significant infectious disease due to its impact on fetal neurodevelopment. Initially identified in tropical and subtropical regions, such as certain areas of South America, Zika has since spread to various parts of the world where Aedes mosquitoes are prevalent, as these mosquitoes serve as the primary vectors for the transmission of the disease. The peak incidence of Zika virus infections occurred in 2016. Prenatal exposure to the virus can lead to a spectrum of complications, ranging from severe microcephaly to more subtle neurodevelopmental delays that may not become apparent until later in childhood, even in the absence of obvious defects at birth.[21]
The mosquitoes in the Aedes genus spread the Zika virus, and an unknown primate reservoir is theorized to exist.[22] The virus has been found in the semen and vaginal secretions of infected individuals and sexual transmission of the virus has been documented.[23] Nonsexual human-to-human transmission has also been reported, although the mechanism of infection in these cases has not been elucidated.[24][25] Vertical transmission occurs in utero through infection or injury of the placenta, resulting in the transplacental transmission of the virus to the fetal brain.[26]
Chikungunya virus
Chikungunya virus (CHIKV), a member of the Togaviridae family, is another mosquito-borne virus transmitted by Aedes mosquitoes. Alongside dengue and Zika viruses, CHIKV has now spread worldwide. Due to overlapping symptoms, distinguishing among these viral infections can be challenging.[27][28] CHIKV infection can cause an acute febrile illness accompanied by rash and joint pain, with potential consequences for both maternal and neonatal health. Maternal-to-fetal transmission has been associated with severe neonatal complications, including enterocolitis, encephalitis, seizures, and even mortality. Additionally, cases of early fetal loss before 22 weeks of gestation have been reported among pregnant individuals with antepartum CHIKV infection, suggesting intrauterine transmission as a possible contributing factor.[28]
Malaria
According to the WHO, there are 85 countries in which malaria is endemic, mostly in sub-Saharan Africa. In 2020, there were 241 million cases of malaria. Over 11 million patients were thus exposed to malaria during pregnancy. Plasmodium falciparum malaria is most commonly related to pregnancy morbidity, followed by Plasmodium vivax, and some patients have both strains. Both placental malaria and gestational malaria infections can occur during pregnancy, resulting in abortion, premature delivery, maternal anemia, fetal growth restriction, stillbirth, and low birth weight. Approximately 100,000 infants die each year due to low birth weight in areas of Africa where malaria is endemic.[29]
Cytomegalovirus
Cytomegalovirus (CMV) is the leading cause of congenital viral infections, affecting approximately 1 in 150 live births, or 0.67% of all neonates. However, only about 10% of affected newborns exhibit symptoms at birth.[30] Primary CMV is acquired through contact with infected bodily fluids, including saliva, urine, cervical or vaginal secretions, semen, blood, and breast milk. The risk of vertical transmission is estimated to be approximately 35% and can occur transplacentally or during birth. Infection early in gestation is less common but associated with more severe manifestations.[30]
Primary infection with CMV is usually asymptomatic, making the diagnosis in pregnant women difficult. Symptoms can include a prolonged febrile illness resembling mononucleosis, sore throat, rash, and transaminitis. Cerebral manifestations of congenital CMV infection include periventricular calcifications, microcephaly, parenchymal echogenic foci, cerebral pseudocysts, hypoplastic corpus callosum, and abnormal gyri. These findings are often visible on fetal ultrasound before delivery.[31] Common systemic manifestations in neonates include intrauterine growth restriction (IUGR), thrombocytopenic purpura, microcephaly, hepatosplenomegaly, and pneumonia. Long-term complications are common, with up to 90% of symptomatic infants and 5% to 15% of asymptomatic cases developing conditions such as hearing impairment, neurological abnormalities (including structural brain defects, hydrocephalus, and seizures), and developmental delays.[30]
Rubella
Infection during pregnancy occurs when a non-immune individual is exposed to aerosolized viral particles. Primary infection with rubella in adults is often asymptomatic. Still, this condition can manifest with low-grade fevers, sore throat, conjunctivitis, and a macular rash that starts on the face and spreads downward. Transmission to the fetus is transplacental, and the highest risk of transmission is with maternal infection in the first trimester.[10][32] Congenital rubella syndrome (CRS) can include deafness, cardiac defects, and a variety of ophthalmic and ocular findings, including microphthalmia, cataracts, and glaucoma.[10]
Toxoplasma gondii
Toxoplasmosis is one of the most prevalent zoonotic diseases worldwide. This vector is a protozoan that can cause human infection through ingestion of raw or undercooked meat or contaminated products, contact with cat feces or contaminated food or soil, transfusion of infected blood products or donor organs. Vertical transmission of toxoplasmosis is through transplacental infection. The protozoan first infects maternal leukocytes, then invades trophoblasts, and lastly, the fetal vasculature.[20] The risk of congenital infection increases linearly with gestational age at the time of primary maternal infection.[10]
Maternal primary toxoplasmosis is frequently asymptomatic; approximately 70% to 90% of congenitally-infected infants are likewise asymptomatic at birth. Chorioretinitis, hydrocephalus, and periventricular calcifications are the classic, but rare, triad of symptoms (see Image. Congenital Toxoplasmosis). More common early manifestations of congenital toxoplasmosis include anemia and thrombocytopenia, hepatosplenomegaly, seizures, and jaundice. Late manifestations in untreated disease include hearing loss, microcephaly, seizures, motor and cerebellar dysfunction, and intellectual disability.[10]
Hepatitis viruses
While the hepatitis A virus has been documented in perinatal transmission, serious complications are uncommon, and chronic infection does not occur. Worldwide, perinatal transmission of hepatitis B virus (HBV) is the largest cause of chronic infection and can occur in up to 90% of cases when maternal antiviral treatment and neonatal prophylaxis are not used. In 2019, only 217 perinatal hepatitis C virus infection cases were reported. Hepatitis C (HCV) is associated with an increased risk of IUGR, intrahepatic cholestasis of pregnancy, and preterm labor and delivery.
Hepatitis D is usually transmitted through blood, and being an incomplete virus, hepatitis D only causes disease when the hepatitis B virus is also present. Hepatitis E virus (HEV) can cause severe effects on pregnant individuals. Although rarely seen in the United States (US), the hepatitis E virus is a waterborne disease, and poor sanitation promotes transmission. The mean incubation period is 40 days, with a higher risk to pregnant people and maternal mortality as high as 20% to 30%. Vertical transmission is as high as 50%, with fetal mortality as high as 35% in Asia and Africa.[33]
Primary infection with HBV is through contact with infected bodily fluids, with sexual contact being the most common cause of primary infection in the US.[34] Vertical transmission accounts for approximately a third of HBV infections worldwide and results primarily from contact with contaminated bodily fluids during labor and delivery. Transplacental infection accounts for a minority of congenital HBV infections.[10]
Primary HCV is transmitted through contact with infected body fluids, and the mechanism of its vertical transmission remains unclear.[10] Primary infection with HEV occurs through contaminated food and water or contact with infected blood. HEV is notable for its severity in pregnant individuals, with high mortality during the third trimester.[35] In endemic areas, it is a major contributor to maternal death, fulminant hepatic failure, and fetal loss.[36]
Human immunodeficiency virus
HIV infection occurs through contact with infected body fluids, including through sexual contact, needle sticks, or blood transfusions. Vertical transmission is most common in utero but can also occur intrapartum. High maternal viral plasma load, low maternal CD4 count, and acute maternal infection during pregnancy increase the risk of in-utero transmission.[10]
Listeria monocytogenes
A gram-positive bacterium, this vector is obtained through the consumption of contaminated food products. Chorioamnionitis is subsequently caused by the hematogenous transplacental spread of infection from the mother to the fetus.[37] Chorioamnionitis occurs in the setting of intact fetal membranes.[38] Placental pathology typically reveals abscesses, with organisms visible within these abscesses. The US has a low incidence of Listeria, which is commonly an asymptomatic disease.[20]
Monkeypox
Similar to smallpox, monkeypox has recently experienced a surge, prompting the WHO to declare a Public Health Emergency of International Concern on August 14, 2024. Monkeypox, a zoonotic disease that has now been shown to have human-to-human transmission, can be vertically transmitted to the fetus by intrauterine transmission. Like smallpox, monkeypox increases the risks of miscarriage, stillbirth, and severe disease in pregnant persons.[39]
Group B streptococci
Infections of the female urogenital tract can impact birth outcomes, even though they are not always vertically transmitted to the newborn. Such infections include group B streptococci (GBS), which may colonize the genitourinary tracts in many people but can cause significant fetal morbidity and mortality if prophylaxis is not used during labor. Neonatal infection can occur through ascending vaginal infection or contact during parturition.[40]
Group A streptococcus
Worldwide, group A Streptococcus (GAS, eg, Streptococcus pyogenes) infection is the third leading cause of mortality in pregnant individuals. This bacterium normally resides on human skin and mucus membranes. Ascending infection from the genital tract can cause multiorgan failure and septic shock. In pregnant individuals, there is a 20-fold increased risk of invasive GAS infection as compared to nonpregnant patients. This is a significant cause of miscarriage and morbidity from sepsis in pregnant people. Recently, the virulence of group A streptococcus has increased due to the predominance of certain genotypes and the rise in patient risk factors for infection, such as obesity.[41]
Coxsackievirus
Hand, foot, and mouth disease is caused by coxsackievirus, a type of enterovirus that causes painful, red, blister-like lesions on the hands, feet, and mouth. While some people remain asymptomatic, others may experience symptoms such as high fever, muscle aches, headache, sore throat, conjunctivitis, abdominal pain, and nausea. The fecal-oral route is the primary mode of transmission for this virus. During pregnancy, it can cause preterm labor, encephalitis, fetal growth restriction, and myocarditis. Long-term outcomes for the fetus may include cardiac anomalies and type 1 diabetes.[42]
Ureaplasmas
Ureaplasmas are mycoplasmas that colonize the urogenital tract of 40% to 80% of sexually active women.[20] Most cases of chorioamnionitis occur secondary to ascending infection with maternal urogenital organisms in the setting of ruptured fetal membranes. Other commonly implicated organisms in chorioamnionitis include GBS, Escherichia coli, Gardnerella vaginalis, and Mycoplasma hominis.[37][43] The association between ascending urogenital infection and ruptured membranes remains uncertain—whether due to opportunistic infection by these organisms following the loss of the anatomic barrier, or because infection itself weakens the fetal membranes and leads to premature rupture. [37]
Pathophysiology
Pregnancy alters immune and physiological functions, impacting the replication of infectious organisms, disease severity, and the risk of transmission to the fetus. The placenta serves as a protective barrier against infections and a site of immune tolerance for the developing fetus. While some viruses, such as CMV, Zika, and rubella, can bypass these defenses and infect the fetus, others, like influenza and SARS-CoV-2, may cause placental inflammation and damage without direct infection.[44]
Additionally, the hormonally-mediated physiologic changes of pregnancy increase maternal risk for certain infections and may likewise increase the severity of disease in pregnant patients.[45] Vertical transmission to the fetus can occur through transplacental migration of the organism prenatally or contact with maternal blood and vaginal secretions during labor.[10] As discussed, the mechanism of initial maternal infection varies with the etiologic agent.
The risk of chorioamnionitis is highest in pregnancies that end at earlier gestational ages, with neonates delivered during the periviable period having a 94% likelihood of developing the infection. Several maternal factors increase the risk of chorioamnionitis, including nulliparity, immunocompromised conditions, bacterial vaginosis, GBS or ureaplasma colonization, and substance use such as tobacco, alcohol, or drugs. In the US, 15% to 35% of women of childbearing age carry GBS in the vagina or rectum.[46]
Being a member of the Black population is also associated with an increased risk due to a combination of biological, socioeconomic, and healthcare-related factors. Labor-related factors that contribute to a higher risk of chorioamnionitis include preterm labor and prolonged preterm prelabor rupture of membranes.[37] Rare mechanisms of infection due to chorioamnionitis include direct iatrogenic inoculation of the amniotic fluid during invasive procedures such as amniocentesis or chorionic villous sampling, and the spread of peritoneal infection to the fetal membranes through the fallopian tubes. Patients with chronic liver and kidney disease are at particular risk for the spread of infection from the peritoneum through the fallopian tubes.[37]
Histopathology
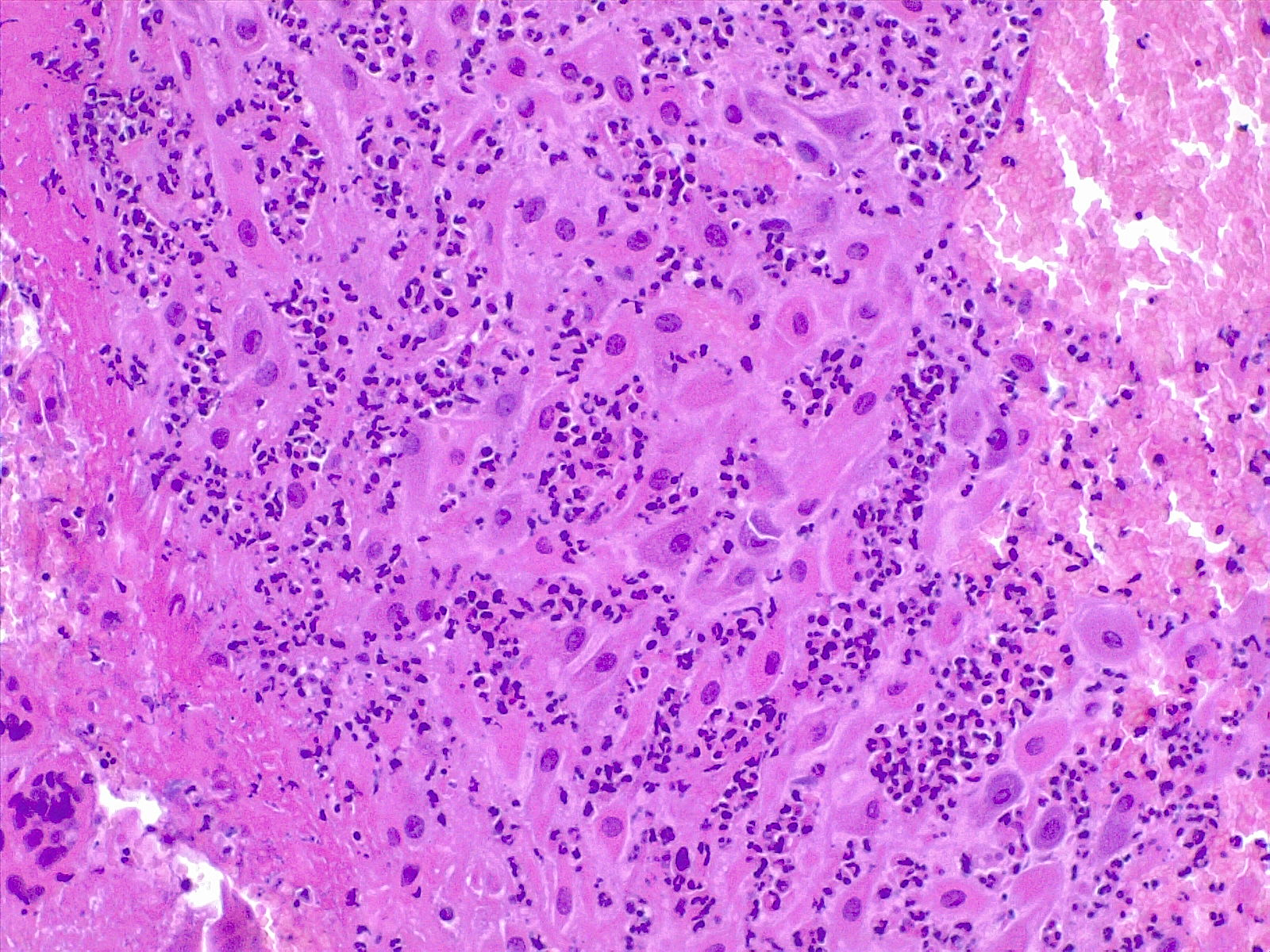
Recognizing the source of the inflammatory response in antepartum infections is crucial, as it may originate from the mother, the fetus, or both. Maternal inflammatory responses include chorioamnionitis, deciduitis, and inflammation of the chorionic plate (see Image. Deciduitis). In contrast, fetal inflammatory responses involve choriositis and inflammation of the umbilical cord and chorionic vessels on the chorionic plate. Fetal inflammatory responses are associated with an increased risk of neurologic impairment in offspring.
Results from clinical studies have shown that chorioamnionitis is strongly linked to low birth weight and cerebral palsy, suggesting a causal relationship between prematurity, inflammation, and adverse outcomes. Severe fetal inflammation, characterized by the presence of neutrophils and eosinophils, is predictive of poor neonatal outcomes, particularly in preterm infants. Current pathogenic models propose that fetal premyelinating oligodendrocytes are especially vulnerable to cytokine-mediated inflammation, which contributes to a strong association between neonatal infection and sensorineural auditory impairment.[47][48][49][50][51][52]
There are specific grading and staging systems for the maternal and fetal inflammatory response (eg, Amsterdam [53] and Redline [49] criteria).
- Stage of inflammatory response refers to the anatomical progression of the process.
- Stage 1: The presence of neutrophils in the chorion or subchorionic space
- Stage 2: The presence of neutrophils in the chorionic connective tissue or amnion
- Stage 3: Necrotizing chorioamnionitis and epithelial necrosis of the amnion
- Grade of inflammatory response refers to the intensity of the acute inflammation.
- Grade 1: Small clusters of maternal neutrophils, found in the chorion laeve, chorionic plate, subchorionic fibrin, and amnion
- Grade 2: Three or more confluent groups of 10 to 20 neutrophils (microabscesses)
Subchorial inflammation is the earliest form of inflammation and is defined by the presence of neutrophils between the decidua and chorion leave. This inflammation does not necessarily imply the presence of chorioamnionitis. In the early phases, a patchy accumulation of leukocytes and fibrin occurs at the choriodecidual interface. Extension of the infection into the chorionic layer is called chorionitis and can be followed by the involvement of the amniotic layer (chorioamnionitis).
Chorioamnionitis refers to the presence of inflammation in the chorion and amnion. This is usually the result of ascending microbial infection from the lower genital tract to the sterile amniotic cavity, potentially endangering the fetus.[54] Neutrophils migrate from the decidua in cases of ascending or blood-borne infection. Chorioamnionitis is diagnosed by microscopic examination of placental sections, with the typical findings of acute neutrophilic inflammation. In acute chorioamnionitis, the leukocytes are of maternal origin.
Acute villitis is a specific gross and histologic feature of acute inflammation of the placental villi. This condition is diagnosed histologically with the presence of neutrophilic abscesses in the placental parenchyma, often associated with neutrophilic chorioamnionitis. Acute villitis is a typical feature of bacterial infections, such as those caused by Listeria or Streptococcus species.
Funisitis is the inflammatory response occurring in the umbilical cord. Topographically, this condition can present with neutrophils in the umbilical vein, in the umbilical arteries, or infiltration into the Wharton jelly. Acute funisitis usually begins with inflammation of the umbilical vein. Arteritis is evidence of an advanced inflammatory response.
Congenital neonatal infection refers to microbial colonization of the amniotic cavity, which can result in fetal invasion of the respiratory tract, gastrointestinal tract, skin, or the ear through the auditory canal to the middle ear. The fetal examination can reveal severe dermatitis, pneumonitis, and encephalitis. The progression of the microbial agent to fetal circulation can lead to systemic disease. The rate of congenital neonatal sepsis is higher in preterm neonates than in term neonates, likely due to bacterial infection following preterm rupture of membranes.[55]
History and Physical
A thorough history and physical examination are essential first steps in evaluating suspected antepartum infections. These assessments help identify risk factors, detect early signs of infection, and guide the selection of appropriate diagnostic testing and management.
History
- Maternal risk factors
- Previous sexually transmitted infection
- Immunocompromised state
- Poor prenatal care
- History of preterm birth
- Symptoms
- Fever
- Chills
- Myalgias and arthralgias
- Pharyngitis with nasal congestion and cough
- Fatigue
- Shortness of breath
- Pelvic pain
- Painful urination
- Nausea
- Loss of smell and taste
- Travel history (eg, malaria or Zika virus in endemic areas)
Physical Examination Findings
- May be asymptomatic
- Hepatosplenomegaly
- Abnormal vaginal discharge
- Bilateral nontender cervical lymphadenopathy (toxoplasmosis)
- Abdominal or uterine tenderness
- Uterine contractions
- Abnormal fetal heart rate tracing or other signs of fetal distress
- Maculopapular rash with conjunctivitis
- Genital lesions
- Leukocytosis
- Thrombocytopenia
- Transaminitis
- Anemia
Key Fetal Ultrasound Findings
- Zika virus
- Microcephaly
- Partially collapsed skull with overlapping sutures and redundant skin folds
- Varicella, HSV, and Zika
- Limb hypoplasia or contractures
- Toxoplasmosis and varicella
- Intrahepatic calcifications
- Toxoplasmosis, CMV, Zika, and Listeria
- Intracranial calcifications
- Varicella and Rubella
- Cataracts
- Syphilis, parvovirus, malaria, and Zika
- Anemia causing abnormal MCA dopplers
Fetal Loss
- Untreated syphilis: 40%
- Zika: 5% to 10%
- Toxoplasmosis: 1.5%
When discussing the history and physical examination findings of antepartum infections, it is crucial to distinguish between the findings in primary (maternal) infections and those in congenital infections. Also note that the manifestations of congenital infection may not be present immediately following birth. Several findings are common to many congenital infections. These include hepatomegaly, splenomegaly, thrombocytopenia, and a violaceous macular or papular rash, commonly referred to as a "blueberry muffin rash," which results from extramedullary hematopoiesis (see Image. Congenital Rubella).[10] Other manifestations may include ascites, hepatosplenomegaly, hydrops, pericardial effusion, and placental enlargement.[31]
Primary infection with HIV is usually asymptomatic, although acute infection can present with a flu-like illness. Similarly, congenital infection is usually asymptomatic in the acute phase. Failure to thrive, oral candidiasis, developmental delay, and frequent opportunistic infections are common presenting complaints.[56] Noncongenital syphilis infection has 3 stages: primary, secondary, and tertiary. The primary stage is characterized by the development of a painless genital or rectal ulcer, known as a chancre.[57] A rash involving the palms and soles is the most characteristic symptom of secondary syphilis and can be macular, papular, ulcerating, or pustular. Other symptoms of secondary syphilis are nonspecific and can include constitutional symptoms, gastrointestinal ulcerations, musculoskeletal inflammation, and ocular complaints.[57]
Tertiary syphilis can manifest with cardiovascular complications such as aortitis and neurological abnormalities like tabes dorsalis or paresis.[58] Early manifestations of congenital syphilis include hepatosplenomegaly, nasal secretions (called "snuffles"), osteochondritis, pseudoparalysis, rash, anemia, and thrombocytopenia. Untreated congenital syphilis progresses to involve the central nervous and musculoskeletal systems, resulting in developmental abnormalities as well as abnormalities of the bones, joints, teeth, eyes, and skin.[10]
Active herpes infection is characterized by grouped, painful vesicular lesions. Congenital HSV can present with low birth weight, microcephaly, chorioretinitis, and hydrocephalus.[59] Characteristic vesicular lesions may also be present in neonatal infections. The feared manifestation of HSV in neonates is viral encephalitis, which can result in significant and permanent blindness, neurodevelopmental delay, and death.[60]
Maternal infection with parvovirus can be asymptomatic or may present with a distinctive "slapped cheeks" rash, preceded by a flu-like illness characterized by fever, malaise, arthralgias, and myalgias.[10] Manifestations of congenital infection include nonimmune hydrops fetalis secondary to high-output heart failure and viral myocarditis, severe anemia and thrombocytopenia, and maternal mirror syndrome.[10]
Acute manifestations of primary HBV include subclinical (anicteric) or icteric hepatitis, as well as fulminant hepatitis and cirrhosis. Symptoms of congenital HBV are usually absent at birth. A minority of affected neonates develop clinical hepatitis in the first few months of life and can present with jaundice, failure to thrive, and vomiting. More commonly, congenital HBV results in subclinical hepatitis and an immune-tolerant carrier state, and progression to chronic infection is uncommon.[10]
Acute primary HCV infection presents similarly to acute primary HBV infection. Most cases of congenital infection are asymptomatic, but, in contrast to congenital HBV infection, the majority of cases of congenital HCV result in chronic infection.[10] Primary HEV infection usually results in mild, subclinical hepatitis in the general population. Still, it has a high risk of progression to fulminant hepatitis and death in pregnant women, particularly in the third trimester.[36] Vertical transmission has been documented but is rare.[61] Primary infection with the Zika virus, an arbovirus, can be asymptomatic or present with a "dengue-like" syndrome of nonpurulent conjunctivitis, arthralgias, headache, maculopapular rash, extremity edema, and gastrointestinal upset.[22] Congenital Zika syndrome is characterized by severe microcephaly and neurological abnormalities.[62]
Monkeypox presents clinically with a characteristic rash accompanied by fever and lymphadenopathy. Mucosal lesions can be highly painful and lead to dehydration as well as hospitalization for pain control. The illness progresses in 2 phases: a prodromal phase lasting up to 5 days, characterized by fever (most common), headache, fatigue, muscle and back pain, and swollen lymph nodes, followed by a rash phase that appears 1 to 3 days after the onset of fever. The rash has a distinctive presentation, beginning as painful and/or pruritic maculopapular lesions that progress into a vesiculopustular rash. A secondary fever may indicate worsening. Severe cases can result in encephalitis, sepsis, or acute respiratory distress, which contribute to mortality. Most reported cases involve sexual contact, including anal and oral sex.[63]
While definitionally, intraamniotic infection is reserved for cases where there is evidence of pathogens in the amniotic fluid along with intraamniotic inflammation, the diagnosis of clinical chorioamnionitis is usually made based on clinical findings.[43] Unfortunately, the symptoms of chorioamnionitis are nonspecific and poorly sensitive for diagnosis; therefore, attention to the complete clinical picture is critical for making this diagnosis. Findings suggestive of chorioamnionitis include maternal fever, uterine tenderness, maternal or fetal tachycardia, and purulent or malodorous discharge.[37]
In July 2024, the American College of Obstetricians and Gynecologists (ACOG) issued a Clinical Practice Update stating that a suspected intraamniotic infection is diagnosed when a maternal temperature reaches 39 °C (102.2 °F) or higher. This condition can also be identified if the maternal temperature falls between 38.0 °C (100.4 °F) and 38.9 °C (102 °F), and at least 1 additional clinical risk factor is observed. In some cases, the diagnosis may be made even without maternal fever if other relevant clinical signs and symptoms are present.
Evaluation
Current guidelines by ACOG recommend universal screening for hepatitis B, hepatitis C, syphilis, gonorrhea, chlamydia, and asymptomatic bacteriuria at the first prenatal visit, which should occur at 8 to 10 weeks of gestation.[64] Universal recommendations also include an HIV screen at the first visit using an "opt-out" approach, where testing is done unless the patient declines it. All pregnant women should have a rectovaginal swab for GBS at 35 to 37 weeks of gestation; patients at high risk for preterm labor should be tested before 35 weeks.[65]
Additional recommended screening for antepartum infections includes repeat testing for HIV, chlamydia, and gonorrhea in the third trimester in populations at high risk for the acquisition of these infections, including adolescents and younger people.[64] These infections may be missed if repeat screenings are not performed in the third trimester.[66] ACOG also supports additional recommendations for syphilis screening 3 times during pregnancy, regardless of the patient's risk and the population prevalence.[12]
Pregnant individuals who test positive for hepatitis B surface antigen should have quantitative testing for hepatitis B virus deoxyribonucleic acid and maternal antiviral treatment during pregnancy when the viral load is over 200,000 IU/mL to help prevent perinatal transmission of hepatitis B. Infants born to HIV-positive mothers or mothers with unknown HIV status should be tested for the disease using nucleic acid tests. The recommended schedule includes testing within 48 hours of birth, at 2 weeks of age, between 4 and 6 weeks of age, and between 4 and 6 months of age.[10]
Chorioamnionitis is a pathologic diagnosis made by examination of the fetal membranes for a neutrophilic infiltrate.[37] However, this diagnosis is usually made on a clinical basis, and treatment should not be delayed for the postpartum pathologic examination of the placenta. Maternal leukocytosis supports the diagnosis of chorioamnionitis but is neither sensitive nor specific. Infants exposed to chorioamnionitis who meet criteria on sepsis calculators should have blood cultures, a complete blood count, and a C-reactive protein (CRP) drawn at birth to evaluate for neonatal sepsis, for which they are at increased risk.[37]
The United States Preventive Services Task Force (USPSTF) and ACOG recommend against routine screening for bacterial vaginosis in asymptomatic pregnant patients.[67] In symptomatic patients, testing is best done by vaginal pathogen swabbing, although microscopic evaluation of vaginal discharge obtained through a vaginal swab may be performed. The presence of clue cells is diagnostic of bacterial vaginosis.[68] USPSTF also recommends against routine serologic screening for HSV in asymptomatic individuals, including pregnant persons.[69]
According to ACOG, routine CMV serologic screening during pregnancy is not advised. Most positive immunoglobulin M (IgM) results, nearly 90%, are false positives, often caused by cross-reactivity with non–CMV antibodies. Additionally, maternal immunity does not fully protect against fetal infection. The absence of an approved vaccine or an effective treatment to prevent congenital transmission further reduces the benefits of universal screening.[70] With a known maternal exposure, serum CMV IgM and IgG may be tested. If all are negative, repeat testing of IgM and IgG may help identify seroconversion.[70] At least 6 weeks after maternal CMV infection is confirmed, an amniocentesis for CMV polymerase chain reaction (PCR) is recommended if at least 21 weeks of gestation.
However, a consensus recommendation from the European Congenital Infection Initiative advises conducting CMV serology testing as early as possible during the first trimester of pregnancy. For women who test seronegative, follow-up testing should be performed every 4 weeks until 14 to 16 weeks of gestation. Routine CMV serology is not recommended beyond 16 weeks unless ultrasound findings suggest a CMV-related anomaly.[71]
Treatment / Management
Effective treatment of antepartum infections is critical to minimizing risks to both mother and fetus. Management strategies should be guided by the specific pathogen involved, gestational age, and current evidence-based recommendations to ensure optimal outcomes.
Medical Interventions
- Antibiotics for GBS, chlamydia, and other bacterial infections
- Antiviral therapy for HSV (eg, acyclovir) and CMV (eg, valganciclovir in severe cases)
- Antimalarial drugs for malaria
Surgical Interventions
Cesarean delivery for active genital HSV or other severe maternal infections with risk to the fetus
National and International Guidelines
- CDC guidelines for GBS screening and treatment during pregnancy (see Image. Intrapartum Antibiotic Prophylaxis, Indications and Nonindications)
- WHO guidelines for preventing and managing maternal infections (eg, malaria, HIV)
- Guidelines for Zika virus management, including travel restrictions and testing
Toxoplasmosis
In toxoplasma seropositive pregnant individuals diagnosed before 18 weeks of gestation, treatment should begin with spiramycin until ultrasonography and PCR can evaluate for the presence of congenital fetal infection.[10] If fetal infection is present, spiramycin should be continued, and pyrimethamine, sulfadiazine,[8] and folinic acid should be added to the regimen. Observational data suggest a decrease in fetal infection and less severe neurologic sequelae with these regimens, although there is no trial data on the topic.[10](B2)
Rubella
There is no specific therapy for rubella in primary or congenital infections. The cornerstone of management is, therefore, prevention. All children and adolescents without contraindications to the measles, mumps, rubella (MMR) vaccine should receive it. MMR administration is contraindicated in pregnancy, however. Pregnant women found to be nonimmune to rubella should be counseled on the importance of vaccination and vaccinated upon completion of the pregnancy.[72] Women of childbearing age who receive the MMR vaccine should be cautioned to avoid becoming pregnant within 28 days of vaccine administration.[10]
Human immunodeficiency virus
Antiretroviral prophylaxis should be given to all infants born to HIV-positive mothers.[10] A 4- or 6-week course of zidovudine can be used for infant prophylaxis in cases where the mother was adherent with prepartum combination antiretroviral therapy (cART) and had effective viral suppression throughout the pregnancy.[10] In cases where prenatal cART was not used, infants should receive a 6-week course of zidovudine as well as 3 total doses of nevirapine within 8 hours after birth, at 48 hours following the first dose, and 96 hours following the second dose.
The cART should be immediately initiated in HIV-positive infants. Early cART initiation is associated with reduced mortality and improved developmental milestones and gross motor skills in these infants.[10] Vaginal delivery can be considered if the risk of transmission to the fetus is low, as evidenced by low maternal viral loads; otherwise, cesarean section is recommended to reduce the risk of intrapartum HIV transmission to the infant. HIV-positive mothers should not breastfeed.[73](B3)
Syphilis
The only treatment for syphilis in pregnant women is intramuscular penicillin.[10] Penicillin-allergic patients should be desensitized before treatment. Treatment for congenital syphilis is 10 days of parenteral penicillin.[74]
Herpes simplex virus
A primary HSV outbreak in pregnancy should be treated with acyclovir, famciclovir, or valacyclovir. Oral therapy is typically sufficient, but parenteral treatment may be needed in severe or disseminated cases. Oral HSV suppressive therapy should be offered at 36 weeks to women with genital herpes. Cesarean section should only be performed if genital lesions are present at the time of delivery.[75] Infants suspected or confirmed to have congenital HSV should receive intravenous acyclovir.[76]
Hepatitis E
Treatment, both for the primary infection in pregnant people and congenital cases, is supportive.[77]
Hepatitis B
In cases of high maternal hepatitis B viral load, the recommended strategy for preventing mother-to-child transmission involves initiating maternal antiviral therapy at 28 weeks' gestation, in conjunction with administering a full hepatitis B vaccination series and hepatitis B immune globulin (HBIG) to the neonate immediately after birth.[78] At the beginning of the third trimester, if viral loads exceed 200,000 IU/mL of hepatitis B virus deoxyribonucleic acid, tenofovir disoproxil fumarate should be initiated to prevent neonatal hepatitis B infection.(A1)
Infants should be encouraged to breastfeed, and cesarean delivery should be done only for obstetrical indications.[33] In areas with limited access to hepatitis B immune globulin (HBIG), results from a recent randomized controlled trial showed that initiating tenofovir therapy at 16 weeks, combined with infant hepatitis B vaccination, was not inferior to standard treatment for patients with high viral loads.[78] Additionally, ACOG recommends vaccination against the hepatitis A and B viruses during pregnancy if necessary. Prepregnancy screening and treatment are ideal.(A1)
Hepatitis C
Although direct antiviral agents for HCV are now available, due to a lack of data on their safety in pregnant patients, the Society for Maternal-Fetal Medicine and ACOG do not recommend medical treatment for Hepatitis C with antivirals during pregnancy or lactation.[79] A 12- to 24-week course of antiviral medications for virologic cure should be completed before pregnancy when possible.[33] Treatment of HCV should be deferred in children until at least 3 years of age to allow for potential spontaneous resolution and avoid the adverse effects of the treatment medications in the infant.[80] (A1)
Parvovirus B19
Treatment of primary parvovirus infection in pregnant individuals is supportive. Treatment of congenital infection is likewise primarily supportive, and in utero fetal transfusions have become a cornerstone of therapy in the management of the associated severe fetal anemia.[10]
Zika virus
Treatment of both primary and congenital Zika virus infection is supportive, so prevention is vital. Several vaccines are in development. Pregnant people and people desiring to become pregnant should avoid visiting countries where the Zika virus is circulating. Although the transmission of the Zika virus is predominantly vector-borne, sexual transmission has been described.[81]
Chorioamnionitis
Patients with preterm rupture of membranes should receive antibiotics targeting the most common organisms implicated in chorioamnionitis.[37] With preterm rupture of membranes, antenatal antibiotics are associated with a decreased risk of neonatal sepsis, respiratory distress syndrome, and necrotizing enterocolitis.[37] In these patients, antibiotic use is also associated with an increase in latency time until delivery; recommended antibiotic regimens include ampicillin and gentamicin.[37]
If signs of chorioamnionitis develop, urgent delivery is indicated regardless of gestational age. Neonates exposed to chorioamnionitis should be started on empiric antibiotics pending the results of the blood cultures and laboratory testing described above. The duration of antibiotic use in these neonates has not been delineated. However, experts suggest that, in the setting of reassuring laboratory results and negative blood cultures, antibiotics can be discontinued after 48 hours.[37]
Group B Streptococcus
Pregnant individuals colonized with GBS should receive intrapartum antibiotics, with penicillin G being the medication of choice in nonallergic patients.[65] Cefazolin can be used in patients having a non-life-threatening penicillin allergy. In contrast, clindamycin should be used in patients with a history of urticaria, angioedema, respiratory distress, or anaphylaxis with penicillin administration, assuming the bacteria are sensitive to clindamycin.[65](B3)
Group A Streptococcus
Blood and vaginal cultures should be considered if GAS is in the differential diagnosis. GAS remains highly sensitive to penicillins, and treatment includes intravenous penicillin plus clindamycin. GAS infection plus organ dysfunction should be surgically managed, commonly requiring a hysterectomy, to control the source of infection.[41](A1)
Monkeypox
Tecovirimat, an antiviral therapy that is US Food and Drug Administration (FDA)-approved for the treatment of smallpox, is recommended for pregnant people who have severe monkeypox disease. Monkeypox can result in severe disease in pregnant people and their newborns. The WHO recommends administration of the smallpox vaccine, eg, modified vaccinia Ankara–Bavarian Nordic (MVA-BN), which contains live, nonreplicating virus, to pregnant people who are at high risk of monkeypox. Smallpox is another orthopox virus, and cross-immunity to monkeypox is expected with vaccination. Further research is needed before clinical guidelines and policies for monkeypox in pregnancy are developed.[39]
Cytomegalovirus
In cases of maternal primary infection with CMV occurring around conception or during the first trimester, the European Congenital Infection Initiative consensus recommendation supports oral valacyclovir at a dosage of 8 g/day, starting as soon as the infection is diagnosed and continuing until CMV PCR results from amniocentesis are available. For fetuses confirmed to be infected, fetal ultrasound and magnetic resonance imaging (MRI) evaluation in the third trimester are recommended to assess CMV-related abnormalities and provide prognostic insights.[71](B3)
In contrast, the FDA has not approved any treatments for maternal or fetal CMV infection. Antiviral drugs like ganciclovir, valganciclovir, and foscarnet are FDA-approved solely for managing CMV infections in individuals with acquired immunodeficiency syndrome (AIDS) or those who have undergone organ transplantation. Valacyclovir to prevent fetal infection after maternal primary infection is not FDA-approved and not recommended by ACOG at this time.[70]
Chlamydia and Gonorrhea
Since doxycycline is contraindicated in pregnancy, azithromycin is the preferred treatment for chlamydia in pregnancy.[82] A test of cure is recommended 4 weeks after treatment.[16] For gonorrhea, the CDC and ACOG recommend the use of ceftriaxone as the first-line regimen for the treatment of gonorrhea in the United States.[13] Pregnant people with antenatal gonococcal infection should have a test of cure in 4 weeks and also be retested in the third trimester.(A1)
Coxsackievirus
While there is no current treatment for coxsackievirus, therapies are emerging with promising results. Infection control and hygiene measures to decrease transmission rates are highly recommended during coxsackievirus outbreaks.[42]
Maternal sepsis
With sepsis in pregnancy, broad-spectrum antibiotics should be administered within the first hour of recognition. Aggressive fluid resuscitation is required with a target mean arterial blood pressure greater than 65 mm Hg. Hypotension should be managed with vasopressors when necessary, with norepinephrine as first-line therapy since it is more effective than phenylephrine in pregnant individuals. Intravenous immunoglobulin (IVIG) should be considered for use in severe cases of streptococcal or staphylococcal infections that are resistant to other therapies.[8](B2)
Differential Diagnosis
High maternal temperature has a broad differential diagnosis that, in addition to antepartum infections, includes infections common to the general population. Differential diagnoses that should be considered in patients with fever from prenatal infection include the following:
- Respiratory infection or pneumonia
- Cellulitis
- Soft tissue infection from mastitis, Bartholin gland, or other skin injury
- Urinary tract infections
- Pulmonary embolism
- Sepsis
- Gastroenteritis
- Hyperthermic toxidromes
- Placental abruption
- Sexually transmitted infections
- Appendicitis
Prognosis
Antenatal infections can lead to serious complications that affect both the mother and the developing fetus. Recognizing these potential outcomes is crucial for timely intervention and improving pregnancy outcomes.
Maternal Prognosis
Maternal prognosis following antenatal infections varies widely, influenced by the type of infection, its severity, and the timing during pregnancy. While many infections are mild and manageable, some can lead to serious complications. For instance, infections like influenza can increase the risk of pneumonia and hospitalization, especially in the second trimester, and may lead to preterm labor or even maternal death in severe cases. Chorioamnionitis is associated with risks such as preterm birth, postpartum complications, hemorrhage, and sepsis. Severe infections during pregnancy are linked to higher odds of complications, with sepsis being a leading cause of maternal mortality.
With the notable exception of HEV, the antepartum infections discussed above have a favorable prognosis for the mother in immunocompetent individuals. Maternal sequelae from toxoplasmosis, rubella, CMV, parvovirus, and Zika virus infection are rare.[10] Progression of acute hepatitis B and C to chronic infection is possible and increases the risk for hepatic failure and hepatocellular carcinoma in these patients.[10][83] The risk of fulminant liver failure and mortality in pregnant patients with HEV ranges from 30% to 100%.[5] Finally, with appropriate antiviral therapy, individuals with HIV infection have a comparable life expectancy to their noninfected counterparts.[84]
Fetal Prognosis
The severity of the antenatal infection and the timing of intervention play crucial roles in fetal outcomes. Some infections may have minimal effects, while others can lead to serious complications such as miscarriage, stillbirth, preterm birth, or long-term developmental issues. Certain infections, like CMV, rubella, syphilis, and toxoplasmosis, are known to cross the placenta and affect fetal development. These infections can result in conditions such as hearing loss, vision problems, intellectual disabilities, or growth restriction. The severity often depends on when the infection occurs during pregnancy; earlier infections can be more detrimental.
Bacterial infections, such as GBS and chorioamnionitis, can also pose risks. GBS can lead to neonatal sepsis, pneumonia, or meningitis, particularly if not identified and managed during labor. Since chorioamnionitis is associated with preterm birth, this may increase the risk of cerebral palsy in the newborn.
Complications
Complications of antenatal infections can significantly impact both maternal and fetal health, leading to increased morbidity and mortality. Understanding these potential outcomes is vital for timely intervention and the prevention of long-term adverse events.
Maternal complications include the following:
- Sepsis, postpartum hemorrhage, uterine rupture, and disseminated intravascular coagulation
- Increased risk of preterm labor and delivery
Fetal complications include the following:
- Premature birth, low birth weight, and developmental delays
- Congenital effects (eg, microcephaly, congenital heart defects, vision or hearing loss in CMV, Zika)
- Neonatal infection and mortality in severe cases
The most severe fetal complication of congenital infection is fetal demise and spontaneous abortion, but the incidence of this is difficult to estimate. Congenital toxoplasmosis, Rubella, CMV, and HSV can result in low birth weight, failure to thrive, and significant neurodevelopmental and motor delays in affected infants.[10] HSV encephalitis is also associated with blindness. Congenital parvovirus is associated with fetal myocarditis and heart failure.[10]
Complications of congenital HBV include progression to fulminant hepatitis or, rarely, chronic HBV infection. Congenital HCV carries a high risk of progression to chronic HCV, leading to cirrhosis and an increased risk of hepatocellular carcinoma.[10] HEV infection is associated with a significant risk of fulminant hepatic failure and death in pregnant women compared to the general population.[10] Untreated bacterial vaginosis is associated with an increased risk of preterm birth, chorioamnionitis, and endometritis.[67]
Chorioamnionitis is associated with an increased risk of preterm birth, neonatal sepsis, intraventricular hemorrhage, periventricular leukomalacia, and cerebral palsy.[37] Data regarding the relationship between chorioamnionitis and bronchopulmonary dysplasia or respiratory distress syndrome is mixed.[37] Maternal complications from chorioamnionitis include a 2- to 3-fold increase in the risk of cesarean section and its associated complications, as well as increased risk of endometritis, wound infection, postpartum hemorrhage, bacteremia and sepsis, and postpartum hemorrhage.[85][86]
Deterrence and Patient Education
There is a growing anti-vaccination movement that has resulted in the resurgence of diseases that were previously near eradication. Consequently, many women of childbearing age are currently unvaccinated and at increased risk for antepartum infections. Screening for vaccination concerns during appointments can help identify individuals at risk.[87] While this is best done before the patient becomes pregnant, discussing and addressing vaccination concerns in pregnant individuals can not only protect subsequent pregnancies through vaccination of the patient but also encourage the patient to vaccinate her children, providing additional protection for the general population and future pregnant patients.[87][88][89]
HIV, HSV, and CMV can be vertically transmitted through breast milk.[10] Prior recommendations from the American Academy of Pediatrics that all HIV-positive mothers should not breastfeed to prevent vertical transmission have been revised.[90] In mothers who have HIV who are on antiretroviral therapy (ART) with viral suppression, the risk of HIV transmission from breastfeeding is estimated to be less than 1%.[91] Mothers should be made aware that not breastfeeding is the only known way to eliminate the risk of HIV transmission to the infant through breast milk. Mothers who are HIV-positive and who are not on ART or who are not virally suppressed should be counseled against breastfeeding.[91]
Mothers with known HSV but no active breast lesions may breastfeed, but should be counseled to discontinue breastfeeding if any lesions develop on the breast to prevent spread to the infant.[92] There are no recommendations against breastfeeding for mothers who are CMV-seropositive, as otherwise, healthy infants who become infected after birth generally show minimal to no symptoms. However, mothers should be counseled that infants born before 30 weeks' gestation and less than 1500 g birth weight are at increased risk of developing a sepsis-like syndrome if infected with CMV.[93] Additionally, CMV in breast milk may be associated with alterations in breast milk composition and the infant microbiome, although the clinical consequences of this are unknown.[94]
Medical professionals should help mothers weigh the risks of CMV transmission versus the benefits of breastfeeding in these cases. Freezing and pasteurizing breast milk can decrease the risk of transmission in these cases. Pregnant women should also be instructed to avoid travel to locations where HEV and Zika virus are endemic and to avoid high-risk behaviors that increase the risk of contracting syphilis, HIV, HSV, HBV, and HCV.
Enhancing Healthcare Team Outcomes
Effective management of antepartum infections requires strong clinical skills, evidence-based strategies, and adherence to ethical principles to prioritize maternal and fetal well-being. Advanced clinicians, nurses, pharmacists, and other healthcare professionals must collaborate through clear interprofessional communication to ensure accurate diagnosis, timely treatment, and coordinated care. Responsibilities include patient education, monitoring for complications, and preventing the transmission of infections. Ethical considerations focus on informed consent, confidentiality, and equitable care. Care coordination optimizes resource use, enhances patient safety, and improves maternal and neonatal outcomes, strengthening overall team performance in patient-centered care.
Media
(Click Image to Enlarge)
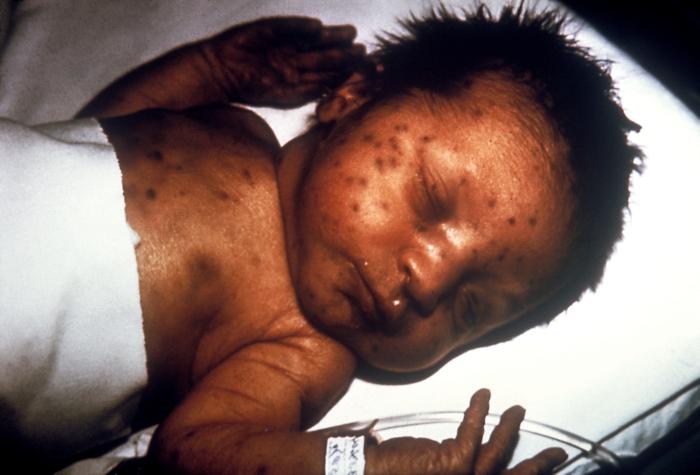
Congenital Rubella. This image demonstrates the blueberry muffin-like skin lesions indicative of congenital rubella.
Public Health Image Library, Public Domain, Centers for Disease Control and Prevention
(Click Image to Enlarge)
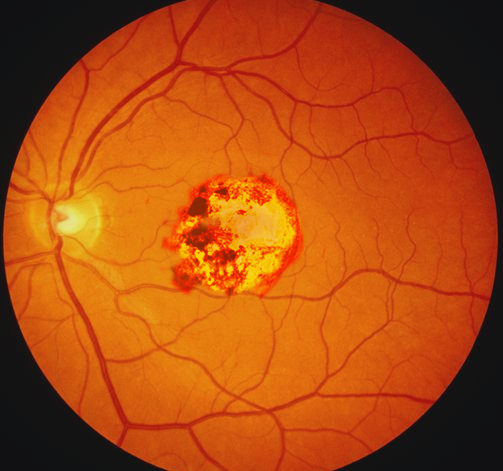
Congenital Toxoplasmosis. This infection is caused by a protozoan that can be transmitted to humans through the ingestion of raw or undercooked meat or contaminated products, contact with cat feces or contaminated food or soil, or transfusion of infected blood products or donor organs. Vertical transmission of toxoplasmosis is through transplacental infection.
Contributed by O Chaigasame, MD
(Click Image to Enlarge)
(Click Image to Enlarge)
References
Chou VB, Walker N, Kanyangarara M. Estimating the global impact of poor quality of care on maternal and neonatal outcomes in 81 low- and middle-income countries: A modeling study. PLoS medicine. 2019 Dec:16(12):e1002990. doi: 10.1371/journal.pmed.1002990. Epub 2019 Dec 18 [PubMed PMID: 31851685]
Level 2 (mid-level) evidenceSina BJ. Pregnancy and the global disease burden. Reproductive health. 2017 Dec 14:14(Suppl 3):170. doi: 10.1186/s12978-017-0420-4. Epub 2017 Dec 14 [PubMed PMID: 29297407]
Benecke O, DeYoung SE. Anti-Vaccine Decision-Making and Measles Resurgence in the United States. Global pediatric health. 2019:6():2333794X19862949. doi: 10.1177/2333794X19862949. Epub 2019 Jul 24 [PubMed PMID: 31384629]
Phadke VK, Bednarczyk RA, Salmon DA, Omer SB. Association Between Vaccine Refusal and Vaccine-Preventable Diseases in the United States: A Review of Measles and Pertussis. JAMA. 2016 Mar 15:315(11):1149-58. doi: 10.1001/jama.2016.1353. Epub [PubMed PMID: 26978210]
Navaneethan U, Al Mohajer M, Shata MT. Hepatitis E and pregnancy: understanding the pathogenesis. Liver international : official journal of the International Association for the Study of the Liver. 2008 Nov:28(9):1190-9. doi: 10.1111/j.1478-3231.2008.01840.x. Epub 2008 Jul 25 [PubMed PMID: 18662274]
Level 3 (low-level) evidencePavia G, Licata F, Marascio N, Giancotti A, Tassone MT, Costa C, Scarlata GGM, Prestagiacomo LE, Gigliotti S, Trecarichi EM, Torti C, Bianco A, Quirino A, Matera G. Seroprevalence and age-related susceptibility of TORCH infections in childbearing age women: A 5-year cross-sectional retrospective study and a literature review. Journal of infection and public health. 2024 Oct:17(10):102537. doi: 10.1016/j.jiph.2024.102537. Epub 2024 Aug 30 [PubMed PMID: 39255545]
Level 2 (mid-level) evidenceQin C, Liu M, Liu J. Trends and disparities of disease burden in infections among pregnant women in 131 low-income and middle-income countries, 1990-2019. Journal of global health. 2024 Sep 6:14():04130. doi: 10.7189/jogh.14.04130. Epub 2024 Sep 6 [PubMed PMID: 39238362]
Level 2 (mid-level) evidenceGiouleka S, Boureka E, Tsakiridis I, Lallas K, Papazisis G, Mamopoulos A, Kalogiannidis I, Athanasiadis A, Dagklis T. Sepsis in Pregnancy and the Puerperium: A Comparative Review of Major Guidelines. Obstetrical & gynecological survey. 2023 Apr:78(4):237-248. doi: 10.1097/OGX.0000000000001108. Epub [PubMed PMID: 37043300]
Level 2 (mid-level) evidenceAleem S, Bhutta ZA. Infection-related stillbirth: an update on current knowledge and strategies for prevention. Expert review of anti-infective therapy. 2021 Sep:19(9):1117-1124. doi: 10.1080/14787210.2021.1882849. Epub 2021 Feb 10 [PubMed PMID: 33517816]
Neu N, Duchon J, Zachariah P. TORCH infections. Clinics in perinatology. 2015 Mar:42(1):77-103, viii. doi: 10.1016/j.clp.2014.11.001. Epub 2014 Dec 20 [PubMed PMID: 25677998]
Salari N, Olfat N, Ghasemi H, Larti M, Beiromvand M, Mohammadi M. The global prevalence of Chlamydia trachomatis genital infection in pregnant women: a meta-analysis. Archives of gynecology and obstetrics. 2025 Feb:311(2):529-542. doi: 10.1007/s00404-024-07928-x. Epub 2025 Jan 17 [PubMed PMID: 39821423]
Level 1 (high-level) evidenceHufstetler K, Llata E, Miele K, Quilter LAS. Clinical Updates in Sexually Transmitted Infections, 2024. Journal of women's health (2002). 2024 Jun:33(6):827-837. doi: 10.1089/jwh.2024.0367. Epub 2024 May 21 [PubMed PMID: 38770770]
. Committee Opinion No. 645: Dual Therapy for Gonococcal Infections. Obstetrics and gynecology. 2016 May:127(5):e95-9. doi: 10.1097/AOG.0000000000001149. Epub [PubMed PMID: 27548425]
Level 3 (low-level) evidenceVallely LM, Egli-Gany D, Wand H, Pomat WS, Homer CSE, Guy R, Silver B, Rumbold AR, Kaldor JM, Vallely AJ, Low N. Adverse pregnancy and neonatal outcomes associated with Neisseria gonorrhoeae: systematic review and meta-analysis. Sexually transmitted infections. 2021 Mar:97(2):104-111. doi: 10.1136/sextrans-2020-054653. Epub 2021 Jan 12 [PubMed PMID: 33436505]
Level 1 (high-level) evidenceCommittee on Adolescence, Society for Adolescent Health and Medicine. Screening for nonviral sexually transmitted infections in adolescents and young adults. Pediatrics. 2014 Jul:134(1):e302-11. doi: 10.1542/peds.2014-1024. Epub [PubMed PMID: 24982099]
Workowski KA, Bachmann LH, Chan PA, Johnston CM, Muzny CA, Park I, Reno H, Zenilman JM, Bolan GA. Sexually Transmitted Infections Treatment Guidelines, 2021. MMWR. Recommendations and reports : Morbidity and mortality weekly report. Recommendations and reports. 2021 Jul 23:70(4):1-187. doi: 10.15585/mmwr.rr7004a1. Epub 2021 Jul 23 [PubMed PMID: 34292926]
He W, Jin Y, Zhu H, Zheng Y, Qian J. Effect of Chlamydia trachomatis on adverse pregnancy outcomes: a meta-analysis. Archives of gynecology and obstetrics. 2020 Sep:302(3):553-567. doi: 10.1007/s00404-020-05664-6. Epub 2020 Jul 8 [PubMed PMID: 32643040]
Level 1 (high-level) evidenceWiesenfeld HC. Screening for Chlamydia trachomatis Infections in Women. The New England journal of medicine. 2017 Feb 23:376(8):765-773. doi: 10.1056/NEJMcp1412935. Epub [PubMed PMID: 28225683]
. Management of Genital Herpes in Pregnancy: ACOG Practice Bulletin Summary, Number 220. Obstetrics and gynecology. 2020 May:135(5):1236-1238. doi: 10.1097/AOG.0000000000003841. Epub [PubMed PMID: 32332408]
Khullar P, Hon JD, Sethi S, Kim J, Iqbal M, Chavez MR. Placental Infections. Clinical obstetrics and gynecology. 2025 Mar 1:68(1):119-129. doi: 10.1097/GRF.0000000000000919. Epub 2024 Dec 18 [PubMed PMID: 39690484]
Yates EF, Mulkey SB. Viral infections in pregnancy and impact on offspring neurodevelopment: mechanisms and lessons learned. Pediatric research. 2024 Jul:96(1):64-72. doi: 10.1038/s41390-024-03145-z. Epub 2024 Mar 20 [PubMed PMID: 38509227]
Ioos S, Mallet HP, Leparc Goffart I, Gauthier V, Cardoso T, Herida M. Current Zika virus epidemiology and recent epidemics. Medecine et maladies infectieuses. 2014 Jul:44(7):302-7. doi: 10.1016/j.medmal.2014.04.008. Epub 2014 Jul 4 [PubMed PMID: 25001879]
Level 3 (low-level) evidenceMead PS, Hills SL, Brooks JT. Zika virus as a sexually transmitted pathogen. Current opinion in infectious diseases. 2018 Feb:31(1):39-44. doi: 10.1097/QCO.0000000000000414. Epub [PubMed PMID: 29176348]
Level 3 (low-level) evidenceSwaminathan S, Schlaberg R, Lewis J, Hanson KE, Couturier MR. Fatal Zika Virus Infection with Secondary Nonsexual Transmission. The New England journal of medicine. 2016 Nov 10:375(19):1907-1909 [PubMed PMID: 27681699]
Brent C, Dunn A, Savage H, Faraji A, Rubin M, Risk I, Garcia W, Cortese M, Novosad S, Krow-Lucal ER, Crain J, Hill M, Atkinson A, Peterson D, Christensen K, Dimond M, Staples JE, Nakashima A. Preliminary Findings from an Investigation of Zika Virus Infection in a Patient with No Known Risk Factors - Utah, 2016. MMWR. Morbidity and mortality weekly report. 2016 Sep 16:65(36):981-2. doi: 10.15585/mmwr.mm6536e4. Epub 2016 Sep 16 [PubMed PMID: 27631467]
Arora HS. A to Z of Zika Virus: A Comprehensive Review for Clinicians. Global pediatric health. 2020:7():2333794X20919595. doi: 10.1177/2333794X20919595. Epub 2020 May 27 [PubMed PMID: 32529004]
Qin C, Wang Y, Liu M, Liu J. Global burden and incidence trends of zika virus infection among women aged 15-49 years from 2011 to 2021: A systematic analysis. Journal of infection and public health. 2024 Nov:17(11):102557. doi: 10.1016/j.jiph.2024.102557. Epub 2024 Sep 27 [PubMed PMID: 39353399]
Level 1 (high-level) evidenceSagay AS, Hsieh SC, Dai YC, Chang CA, Ogwuche J, Ige OO, Kahansim ML, Chaplin B, Imade G, Elujoba M, Paul M, Hamel DJ, Furuya H, Khouri R, Boaventura VS, de Moraes L, Kanki PJ, Wang WK. Chikungunya virus antepartum transmission and abnormal infant outcomes in a cohort of pregnant women in Nigeria. International journal of infectious diseases : IJID : official publication of the International Society for Infectious Diseases. 2024 Feb:139():92-100. doi: 10.1016/j.ijid.2023.11.036. Epub 2023 Dec 4 [PubMed PMID: 38056689]
Das JK, Lakhani S, Rahman AR, Siddiqui F, Ali Padhani Z, Rashid Z, Mahmud O, Naqvi SK, Amir Naseem H, Jehanzeb H, Kumar S, Beg MA. Malaria in pregnancy: Meta-analyses of prevalence and associated complications. Epidemiology and infection. 2024 Feb 13:152():e39. doi: 10.1017/S0950268824000177. Epub 2024 Feb 13 [PubMed PMID: 38347721]
Rossen JL, Hindi A, Rahmani S, Bohnsack BL. Incidence of ophthalmic manifestations in congenital cytomegalovirus (CMV). BMC ophthalmology. 2025 Jan 24:25(1):45. doi: 10.1186/s12886-024-03792-0. Epub 2025 Jan 24 [PubMed PMID: 39856594]
Pass RF, Arav-Boger R. Maternal and fetal cytomegalovirus infection: diagnosis, management, and prevention. F1000Research. 2018:7():255. doi: 10.12688/f1000research.12517.1. Epub 2018 Mar 1 [PubMed PMID: 29560263]
Lambert N, Strebel P, Orenstein W, Icenogle J, Poland GA. Rubella. Lancet (London, England). 2015 Jun 6:385(9984):2297-307. doi: 10.1016/S0140-6736(14)60539-0. Epub 2015 Jan 8 [PubMed PMID: 25576992]
. Viral Hepatitis in Pregnancy: ACOG Clinical Practice Guideline No. 6. Obstetrics and gynecology. 2023 Sep 1:142(3):745-759. doi: 10.1097/AOG.0000000000005300. Epub [PubMed PMID: 37590986]
Level 1 (high-level) evidenceBartholomew ML, Lee MJ. Management of Hepatitis B Infection in Pregnancy. Clinical obstetrics and gynecology. 2018 Mar:61(1):137-145. doi: 10.1097/GRF.0000000000000331. Epub [PubMed PMID: 29252923]
Sultana R, Humayun S. Fetomaternal outcome in acute hepatitis e. Journal of the College of Physicians and Surgeons--Pakistan : JCPSP. 2014 Feb:24(2):127-30 [PubMed PMID: 24491009]
Level 2 (mid-level) evidenceWu C, Wu X, Xia J. Hepatitis E virus infection during pregnancy. Virology journal. 2020 Jun 10:17(1):73. doi: 10.1186/s12985-020-01343-9. Epub 2020 Jun 10 [PubMed PMID: 32522266]
Ericson JE, Laughon MM. Chorioamnionitis: implications for the neonate. Clinics in perinatology. 2015 Mar:42(1):155-65, ix. doi: 10.1016/j.clp.2014.10.011. Epub 2014 Nov 21 [PubMed PMID: 25678002]
Park JH, Ahn JH, Seo KJ, Choi SK, Park IY, Kim YH. Challenging management of chorioamnionitis with placental listeriosis: lessons from 2 cases. Obstetrics & gynecology science. 2018 Nov:61(6):688-692. doi: 10.5468/ogs.2018.61.6.688. Epub 2018 Oct 11 [PubMed PMID: 30474016]
Level 3 (low-level) evidenceNachega JB, Mohr EL, Dashraath P, Mbala-Kingebeni P, Anderson JR, Myer L, Gandhi M, Baud D, Mofenson LM, Muyembe-Tamfum JJ, Mpox Research Consortium (MpoxReC). Mpox in Pregnancy - Risks, Vertical Transmission, Prevention, and Treatment. The New England journal of medicine. 2024 Oct 10:391(14):1267-1270. doi: 10.1056/NEJMp2410045. Epub 2024 Aug 28 [PubMed PMID: 39197097]
Patras KA, Nizet V. Group B Streptococcal Maternal Colonization and Neonatal Disease: Molecular Mechanisms and Preventative Approaches. Frontiers in pediatrics. 2018:6():27. doi: 10.3389/fped.2018.00027. Epub 2018 Feb 22 [PubMed PMID: 29520354]
Harris K, Proctor LK, Shinar S, Philippopoulos E, Yudin MH, Murphy KE. Outcomes and management of pregnancy and puerperal group A streptococcal infections: A systematic review. Acta obstetricia et gynecologica Scandinavica. 2023 Feb:102(2):138-157. doi: 10.1111/aogs.14500. Epub 2023 Jan 12 [PubMed PMID: 36636775]
Level 1 (high-level) evidenceLongo C, Saito M, Castro PT, Traina E, Werner H, Elito Júnior J, Araujo Júnior E. Coxsackievirus Group B Infections during Pregnancy: An Updated Literature Review. Journal of clinical medicine. 2024 Aug 21:13(16):. doi: 10.3390/jcm13164922. Epub 2024 Aug 21 [PubMed PMID: 39201064]
Jung E, Romero R, Suksai M, Gotsch F, Chaemsaithong P, Erez O, Conde-Agudelo A, Gomez-Lopez N, Berry SM, Meyyazhagan A, Yoon BH. Clinical chorioamnionitis at term: definition, pathogenesis, microbiology, diagnosis, and treatment. American journal of obstetrics and gynecology. 2024 Mar:230(3S):S807-S840. doi: 10.1016/j.ajog.2023.02.002. Epub 2023 Mar 21 [PubMed PMID: 38233317]
Creisher PS, Klein SL. Pathogenesis of viral infections during pregnancy. Clinical microbiology reviews. 2024 Jun 13:37(2):e0007323. doi: 10.1128/cmr.00073-23. Epub 2024 Feb 29 [PubMed PMID: 38421182]
Kourtis AP, Read JS, Jamieson DJ. Pregnancy and infection. The New England journal of medicine. 2014 Sep 11:371(11):1077. doi: 10.1056/NEJMc1408436. Epub [PubMed PMID: 25207782]
Level 3 (low-level) evidenceSchuchat A. Epidemiology of group B streptococcal disease in the United States: shifting paradigms. Clinical microbiology reviews. 1998 Jul:11(3):497-513 [PubMed PMID: 9665980]
Redline RW. Villitis of unknown etiology: noninfectious chronic villitis in the placenta. Human pathology. 2007 Oct:38(10):1439-46 [PubMed PMID: 17889674]
Jones MH, Corso AL, Tepper RS, Edelweiss MI, Friedrich L, Pitrez PM, Stein RT. Chorioamnionitis and subsequent lung function in preterm infants. PloS one. 2013:8(12):e81193. doi: 10.1371/journal.pone.0081193. Epub 2013 Dec 5 [PubMed PMID: 24339909]
Redline RW, Faye-Petersen O, Heller D, Qureshi F, Savell V, Vogler C, Society for Pediatric Pathology, Perinatal Section, Amniotic Fluid Infection Nosology Committee. Amniotic infection syndrome: nosology and reproducibility of placental reaction patterns. Pediatric and developmental pathology : the official journal of the Society for Pediatric Pathology and the Paediatric Pathology Society. 2003 Sep-Oct:6(5):435-48 [PubMed PMID: 14708737]
Shevell A, Wintermark P, Benini R, Shevell M, Oskoui M. Chorioamnionitis and cerebral palsy: lessons from a patient registry. European journal of paediatric neurology : EJPN : official journal of the European Paediatric Neurology Society. 2014 May:18(3):301-7. doi: 10.1016/j.ejpn.2013.12.005. Epub 2014 Jan 2 [PubMed PMID: 24412077]
Level 2 (mid-level) evidenceLee JD, Park HJ, Park ES, Oh MK, Park B, Rha DW, Cho SR, Kim EY, Park JY, Kim CH, Kim DG, Park CI. Motor pathway injury in patients with periventricular leucomalacia and spastic diplegia. Brain : a journal of neurology. 2011 Apr:134(Pt 4):1199-210. doi: 10.1093/brain/awr021. Epub 2011 Mar 7 [PubMed PMID: 21385750]
Smilga AS, Garfinkle J, Ng P, Andersen J, Buckley D, Fehlings D, Kirton A, Wood E, van Rensburg E, Shevell M, Oskoui M. Neonatal Infection in Children With Cerebral Palsy: A Registry-Based Cohort Study. Pediatric neurology. 2018 Mar:80():77-83. doi: 10.1016/j.pediatrneurol.2017.11.006. Epub 2017 Dec 13 [PubMed PMID: 29428154]
Khong TY, Mooney EE, Ariel I, Balmus NC, Boyd TK, Brundler MA, Derricott H, Evans MJ, Faye-Petersen OM, Gillan JE, Heazell AE, Heller DS, Jacques SM, Keating S, Kelehan P, Maes A, McKay EM, Morgan TK, Nikkels PG, Parks WT, Redline RW, Scheimberg I, Schoots MH, Sebire NJ, Timmer A, Turowski G, van der Voorn JP, van Lijnschoten I, Gordijn SJ. Sampling and Definitions of Placental Lesions: Amsterdam Placental Workshop Group Consensus Statement. Archives of pathology & laboratory medicine. 2016 Jul:140(7):698-713. doi: 10.5858/arpa.2015-0225-CC. Epub 2016 May 25 [PubMed PMID: 27223167]
Level 3 (low-level) evidenceKim CJ, Romero R, Chaemsaithong P, Chaiyasit N, Yoon BH, Kim YM. Acute chorioamnionitis and funisitis: definition, pathologic features, and clinical significance. American journal of obstetrics and gynecology. 2015 Oct:213(4 Suppl):S29-52. doi: 10.1016/j.ajog.2015.08.040. Epub [PubMed PMID: 26428501]
Simonsen KA, Anderson-Berry AL, Delair SF, Davies HD. Early-onset neonatal sepsis. Clinical microbiology reviews. 2014 Jan:27(1):21-47. doi: 10.1128/CMR.00031-13. Epub [PubMed PMID: 24396135]
Luzuriaga K, Mofenson LM. Challenges in the Elimination of Pediatric HIV-1 Infection. The New England journal of medicine. 2016 Feb 25:374(8):761-70. doi: 10.1056/NEJMra1505256. Epub [PubMed PMID: 26933850]
DANBOLT N, CLARK EG, GJESTLAND T. The Oslo study of untreated syphilis; a re-study of the Boeck-Bruusgaard material concerning the fate of syphilitics who receive no specific treatment; a preliminary report. Acta dermato-venereologica. 1954:34(1-2):34-8 [PubMed PMID: 13157853]
CLARK EG, DANBOLT N. The Oslo study of the natural history of untreated syphilis; an epidemiologic investigation based on a restudy of the Boeck-Bruusgaard material; a review and appraisal. Journal of chronic diseases. 1955 Sep:2(3):311-44 [PubMed PMID: 13252075]
McPherson CC. Neonatal Herpes Simplex Virus: The Long Road to Improved Outcomes. Neonatal network : NN. 2020 Mar 1:39(2):92-98. doi: 10.1891/0730-0832.39.2.92. Epub [PubMed PMID: 32317339]
AK AK, Bhutta BS, Mendez MD. Herpes Simplex Encephalitis. StatPearls. 2025 Jan:(): [PubMed PMID: 32491575]
Khuroo MS, Kamili S, Jameel S. Vertical transmission of hepatitis E virus. Lancet (London, England). 1995 Apr 22:345(8956):1025-6 [PubMed PMID: 7723501]
Calvet GA, Santos FB, Sequeira PC. Zika virus infection: epidemiology, clinical manifestations and diagnosis. Current opinion in infectious diseases. 2016 Oct:29(5):459-66. doi: 10.1097/QCO.0000000000000301. Epub [PubMed PMID: 27496713]
Level 3 (low-level) evidenceAl-Dabbagh J, Mohammad Deeb E, Younis R, Eissa R. The dermatological manifestations and differential diagnosis of monkeypox: A narrative review. Medicine. 2024 Nov 1:103(44):e40359. doi: 10.1097/MD.0000000000040359. Epub [PubMed PMID: 39496026]
Level 3 (low-level) evidencePollock L, Cohan D, Pecci CC, Mittal P. ACOG Committee Opinion No. 752: Prenatal and Perinatal Human Immunodeficiency Virus Testing. Obstetrics and gynecology. 2019 Jan:133(1):187. doi: 10.1097/AOG.0000000000003048. Epub [PubMed PMID: 30575656]
Level 3 (low-level) evidence. Prevention of Group B Streptococcal Early-Onset Disease in Newborns: ACOG Committee Opinion, Number 797. Obstetrics and gynecology. 2020 Feb:135(2):e51-e72. doi: 10.1097/AOG.0000000000003668. Epub [PubMed PMID: 31977795]
Level 3 (low-level) evidenceGovender V, Moodley D, Naidoo M, Connoly C, Ngcapu S, Abdool Karim Q. Sexually transmitted infections in pregnancy and adverse pregnancy outcomes: A retrospective cohort study. International journal of gynaecology and obstetrics: the official organ of the International Federation of Gynaecology and Obstetrics. 2024 Jul:166(1):62-70. doi: 10.1002/ijgo.15529. Epub 2024 Apr 4 [PubMed PMID: 38573181]
Level 2 (mid-level) evidenceUS Preventive Services Task Force, Owens DK, Davidson KW, Krist AH, Barry MJ, Cabana M, Caughey AB, Donahue K, Doubeni CA, Epling JW Jr, Kubik M, Ogedegbe G, Pbert L, Silverstein M, Simon MA, Tseng CW, Wong JB. Screening for Bacterial Vaginosis in Pregnant Persons to Prevent Preterm Delivery: US Preventive Services Task Force Recommendation Statement. JAMA. 2020 Apr 7:323(13):1286-1292. doi: 10.1001/jama.2020.2684. Epub [PubMed PMID: 32259236]
Kairys N, Carlson K, Garg M. Bacterial Vaginosis. StatPearls. 2025 Jan:(): [PubMed PMID: 29083654]
Asher GN, Feltner C, Harrison WN, Schwimmer E, Rains C, Jonas DE. Serologic Screening for Genital Herpes: Updated Evidence Report and Systematic Review for the US Preventive Services Task Force. JAMA. 2023 Feb 14:329(6):510-512. doi: 10.1001/jama.2022.20356. Epub [PubMed PMID: 36786798]
Level 1 (high-level) evidenceCoskun E, Kakkar F, Riley LE, Ciaranello AL, Prabhu M. Evaluation and Management of Congenital Cytomegalovirus Infection. Obstetrics and gynecology. 2025 Mar 1:145(3):297-306. doi: 10.1097/AOG.0000000000005840. Epub 2025 Jan 23 [PubMed PMID: 39847776]
Leruez-Ville M, Chatzakis C, Lilleri D, Blazquez-Gamero D, Alarcon A, Bourgon N, Foulon I, Fourgeaud J, Gonce A, Jones CE, Klapper P, Krom A, Lazzarotto T, Lyall H, Paixao P, Papaevangelou V, Puchhammer E, Sourvinos G, Vallely P, Ville Y, Vossen A. Consensus recommendation for prenatal, neonatal and postnatal management of congenital cytomegalovirus infection from the European congenital infection initiative (ECCI). The Lancet regional health. Europe. 2024 May:40():100892. doi: 10.1016/j.lanepe.2024.100892. Epub 2024 Apr 1 [PubMed PMID: 38590940]
Level 3 (low-level) evidenceMoniz MH, Beigi RH. Maternal immunization. Clinical experiences, challenges, and opportunities in vaccine acceptance. Human vaccines & immunotherapeutics. 2014:10(9):2562-70. doi: 10.4161/21645515.2014.970901. Epub 2014 Oct 30 [PubMed PMID: 25483490]
. ACOG Committee Opinion No. 751: Labor and Delivery Management of Women With Human Immunodeficiency Virus Infection. Obstetrics and gynecology. 2018 Sep:132(3):e131-e137. doi: 10.1097/AOG.0000000000002820. Epub [PubMed PMID: 30134427]
Level 3 (low-level) evidenceWorkowski KA, Bolan GA, Centers for Disease Control and Prevention. Sexually transmitted diseases treatment guidelines, 2015. MMWR. Recommendations and reports : Morbidity and mortality weekly report. Recommendations and reports. 2015 Jun 5:64(RR-03):1-137 [PubMed PMID: 26042815]
. Management of Genital Herpes in Pregnancy: ACOG Practice Bulletinacog Practice Bulletin, Number 220. Obstetrics and gynecology. 2020 May:135(5):e193-e202. doi: 10.1097/AOG.0000000000003840. Epub [PubMed PMID: 32332414]
Kimberlin DW. Neonatal herpes simplex infection. Clinical microbiology reviews. 2004 Jan:17(1):1-13 [PubMed PMID: 14726453]
Jilani N, Das BC, Husain SA, Baweja UK, Chattopadhya D, Gupta RK, Sardana S, Kar P. Hepatitis E virus infection and fulminant hepatic failure during pregnancy. Journal of gastroenterology and hepatology. 2007 May:22(5):676-82 [PubMed PMID: 17444855]
Pan CQ, Dai E, Mo Z, Zhang H, Zheng TQ, Wang Y, Liu Y, Chen T, Li S, Yang C, Wu J, Chen X, Zou H, Mei S, Zhu L. Tenofovir and Hepatitis B Virus Transmission During Pregnancy: A Randomized Clinical Trial. JAMA. 2025 Feb 4:333(5):390-399. doi: 10.1001/jama.2024.22952. Epub [PubMed PMID: 39540799]
Level 1 (high-level) evidenceGupta N, Hiebert L, Saseetharran A, Chappell C, El-Sayed MH, Hamid S, Jhaveri R, Judd A, Kushner T, Badell M, Biondi M, Buresh M, Prasad M, Price JC, Ward JW. Best practices for hepatitis C linkage to care in pregnant and postpartum women: perspectives from the Treatment In Pregnancy for Hepatitis C Community of Practice. American journal of obstetrics and gynecology. 2024 Oct:231(4):377-385. doi: 10.1016/j.ajog.2024.06.028. Epub 2024 Jul 2 [PubMed PMID: 38960017]
Level 3 (low-level) evidenceKim NG, Kullar R, Khalil H, Saab S. Meeting the WHO hepatitis C virus elimination goal: Review of treatment in paediatrics. Journal of viral hepatitis. 2020 Aug:27(8):762-769. doi: 10.1111/jvh.13317. Epub 2020 May 25 [PubMed PMID: 32386099]
Consigny PH. [Zika virus infection: sexual transmission and implications for prevention]. Medecine tropicale et sante internationale. 2024 Jun 30:4(2):. pii: mtsi.v4i2.2024.502. doi: 10.48327/mtsi.v4i2.2024.502. Epub 2024 Apr 2 [PubMed PMID: 39099710]
Cluver C, Novikova N, Eriksson DO, Bengtsson K, Lingman GK. Interventions for treating genital Chlamydia trachomatis infection in pregnancy. The Cochrane database of systematic reviews. 2017 Sep 22:9(9):CD010485. doi: 10.1002/14651858.CD010485.pub2. Epub 2017 Sep 22 [PubMed PMID: 28937705]
Level 1 (high-level) evidenceBeudeker BJB, Boonstra A. Circulating biomarkers for early detection of hepatocellular carcinoma. Therapeutic advances in gastroenterology. 2020:13():1756284820931734. doi: 10.1177/1756284820931734. Epub 2020 Jun 29 [PubMed PMID: 32647536]
Level 3 (low-level) evidenceHodgkinson LM, Abwalaba RA, Arudo J, Barry M. Ten-year survival with analysis of gender difference, risk factors, and causes of death during 13 years of public antiretroviral therapy in rural Kenya. Medicine. 2020 May 22:99(21):e20328. doi: 10.1097/MD.0000000000020328. Epub [PubMed PMID: 32481319]
Woodd SL, Montoya A, Barreix M, Pi L, Calvert C, Rehman AM, Chou D, Campbell OMR. Incidence of maternal peripartum infection: A systematic review and meta-analysis. PLoS medicine. 2019 Dec:16(12):e1002984. doi: 10.1371/journal.pmed.1002984. Epub 2019 Dec 10 [PubMed PMID: 31821329]
Level 1 (high-level) evidenceDaifotis HA, Smith MM, Denoble AE, Dotters-Katz SK. Risk Factors for Postpartum Maternal Infection Following Spontaneous Vaginal Delivery Complicated by Chorioamnionitis. AJP reports. 2020 Apr:10(2):e159-e164. doi: 10.1055/s-0040-1709983. Epub 2020 May 15 [PubMed PMID: 32426175]
Veerasingam P, Grant CC, Chelimo C, Philipson K, Gilchrist CA, Berry S, Carr PA, Camargo CA Jr, Morton S. Vaccine Education During Pregnancy and Timeliness of Infant Immunization. Pediatrics. 2017 Sep:140(3):. pii: e20163727. doi: 10.1542/peds.2016-3727. Epub 2017 Aug 18 [PubMed PMID: 28821625]
O'Leary ST, Riley LE, Lindley MC, Allison MA, Albert AP, Fisher A, Jiles AJ, Crane LA, Hurley LP, Beaty B, Brtnikova M, Kempe A. Obstetrician-Gynecologists' Strategies to Address Vaccine Refusal Among Pregnant Women. Obstetrics and gynecology. 2019 Jan:133(1):40-47. doi: 10.1097/AOG.0000000000003005. Epub [PubMed PMID: 30531564]
Clarke RM, Sirota M, Paterson P. Do previously held vaccine attitudes dictate the extent and influence of vaccine information-seeking behavior during pregnancy? Human vaccines & immunotherapeutics. 2019:15(9):2081-2089. doi: 10.1080/21645515.2019.1638203. Epub 2019 Aug 23 [PubMed PMID: 31291160]
Abuogi L, Noble L, Smith C, COMMITTEE ON PEDIATRIC AND ADOLESCENT HIV, SECTION ON BREASTFEEDING. Infant Feeding for Persons Living With and at Risk for HIV in the United States: Clinical Report. Pediatrics. 2024 Jun 1:153(6):. pii: e2024066843. doi: 10.1542/peds.2024-066843. Epub [PubMed PMID: 38766700]
Flynn PM, Taha TE, Cababasay M, Fowler MG, Mofenson LM, Owor M, Fiscus S, Stranix-Chibanda L, Coutsoudis A, Gnanashanmugam D, Chakhtoura N, McCarthy K, Mukuzunga C, Makanani B, Moodley D, Nematadzira T, Kusakara B, Patil S, Vhembo T, Bobat R, Mmbaga BT, Masenya M, Nyati M, Theron G, Mulenga H, Butler K, Shapiro DE, PROMISE Study Team. Prevention of HIV-1 Transmission Through Breastfeeding: Efficacy and Safety of Maternal Antiretroviral Therapy Versus Infant Nevirapine Prophylaxis for Duration of Breastfeeding in HIV-1-Infected Women With High CD4 Cell Count (IMPAACT PROMISE): A Randomized, Open-Label, Clinical Trial. Journal of acquired immune deficiency syndromes (1999). 2018 Apr 1:77(4):383-392. doi: 10.1097/QAI.0000000000001612. Epub [PubMed PMID: 29239901]
Level 1 (high-level) evidenceRenesme L. [Neonatal herpes: Epidemiology, clinical manifestations and management. Guidelines for clinical practice from the French College of Gynecologists and Obstetricians (CNGOF)]. Gynecologie, obstetrique, fertilite & senologie. 2017 Dec:45(12):691-704. doi: 10.1016/j.gofs.2017.10.005. Epub 2017 Nov 11 [PubMed PMID: 29132771]
Angueyra C, Abou Hatab H, Pathak A. Congenital Cytomegalovirus and Zika Infections. Indian journal of pediatrics. 2020 Oct:87(10):840-845. doi: 10.1007/s12098-020-03260-9. Epub 2020 Apr 13 [PubMed PMID: 32281058]
Johnson KE, Hernandez-Alvarado N, Blackstad M, Heisel T, Allert M, Fields DA, Isganaitis E, Jacobs KM, Knights D, Lock EF, Rudolph MC, Gale CA, Schleiss MR, Albert FW, Demerath EW, Blekhman R. Human cytomegalovirus in breast milk is associated with milk composition and the infant gut microbiome and growth. Nature communications. 2024 Jul 23:15(1):6216. doi: 10.1038/s41467-024-50282-4. Epub 2024 Jul 23 [PubMed PMID: 39043677]