Introduction
Anal carcinoma is a rare neoplasm; however, its incidence has been rising at an annual rate of approximately 2.7% over the past decade. Among its histologic subtypes, squamous cell carcinoma (SCC) is the most predominant, comprising approximately 85% of cases. At the time of diagnosis, around 48% of anal carcinomas remain localized to the primary site, 32% exhibit regional lymphatic involvement, and 13% present with distant metastases.[1][2][3]
A thorough understanding of the anatomy of the anal canal, perianal skin, and lymphatic drainage pathways is essential for the accurate diagnosis and effective management of anal carcinoma. The anal region consists of the anal canal and the perianal skin within a 5 cm radius of the anal verge. While the diagnostic evaluation for anal canal and perianal malignancies is similar, treatment strategies differ based on tumor staging and anatomical location.[4]
Significant advancements have been made in the therapeutic landscape of anal carcinoma. Preventive measures, particularly HPV vaccination, are crucial in reducing disease incidence. Current definitive treatment options include chemoradiation therapy (CRT), organ-preserving surgical approaches, and, in select cases, abdominoperineal resection (APR). CRT remains the standard-of-care for SCC of the anal canal, with surgical intervention reserved for persistent or recurrent disease. In contrast, early-stage perianal SCC is often amenable to local excision, whereas advanced cases typically necessitate CRT. Additionally, emerging systemic therapies, including immunotherapy for metastatic disease and metastasectomy for select patients, are reshaping the management paradigm for advanced anal carcinoma.[1]
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
An increasing number of risk factors for anal carcinoma have been identified, with global social and cultural shifts over recent decades contributing to heightened exposure to these risk factors, which include:
-
Human papillomavirus (HPV) infection: Persistent infection with HPV, a nonenveloped, double-stranded DNA virus that infects the squamous epithelium, is the most significant risk factor for anal squamous cell carcinoma (SCC). Chronic infection with high-risk HPV subtypes, particularly HPV-16 and HPV-18, is strongly associated with anogenital malignancies, including cervical, vaginal, vulvar, penile, and oropharyngeal cancers, as well as anal carcinoma. HPV is primarily transmitted through direct skin-to-skin contact, with sexual activity increasing the likelihood of exposure, although transmission can occur independently of sexual contact.[5]
-
Age: The incidence of anal carcinoma increases with advancing age, with a median age at diagnosis of 62. While the disease is rare in individuals younger than 35, incidence rates are rising among younger adults. The highest prevalence is observed in women aged 75 to 79 and men aged 80 to 84. Age is also a critical factor in the progression to high-grade anal intraepithelial neoplasia, particularly in both HIV-positive and HIV-negative males. This trend is primarily attributed to the prolonged latency period associated with HPV-related carcinogenesis.[6]
-
Gender: Anal carcinoma exhibits a higher prevalence in women than in men, though the underlying mechanisms for this gender disparity remain incompletely understood. The higher HPV infection rates among females and the anatomical proximity of the genital and anal regions, facilitating cross-site viral transmission, may contribute to this discrepancy. Additionally, preclinical models suggest that female sex hormones may influence HPV-independent anal SCC pathogenesis, with estrogen and progesterone withdrawal potentially exerting a protective effect against tumor development.[7]
-
Human immunodeficiency virus (HIV): HIV-positive individuals face a markedly increased risk of anal carcinoma, with incidence rates up to 40 times higher than those observed in the general population. This population also demonstrates a heightened prevalence of anal dysplasia and invasive carcinoma, often with earlier age at diagnosis. The precise mechanisms by which HIV-related immunosuppression differentially affects specific HPV subtypes and their oncogenic potential remain unclear. However, studies have demonstrated a strong correlation between lower CD4+ T-cell counts (<200/mm³) or a low CD4 nadir and an increased risk of progression from low-grade squamous intraepithelial lesions (LSIL) to high-grade squamous intraepithelial lesions (HSIL) or invasive anal carcinoma.[8]
-
Sexual activity: Sexual behavior significantly influences the risk of anal carcinoma. Factors, eg, multiple lifetime sexual partners, early initiation of sexual activity, and engagement in receptive anal intercourse are associated with increased rates of anal HPV infection, thereby elevating the risk of malignant transformation.[9]
-
Immunosuppression: Individuals with immunosuppressive conditions—including organ transplant recipients and patients with autoimmune disorders such as rheumatoid arthritis, systemic lupus erythematosus, Crohn’s disease, and ulcerative colitis—are at elevated risk for anal carcinoma. Long-term systemic corticosteroid therapy further contributes to this vulnerability. The increased incidence in immunosuppressed individuals is attributed to impaired immune surveillance, reducing the host’s ability to clear persistent HPV infections.[10]
-
Smoking: Tobacco use is a significant independent risk factor for anal carcinoma, with risk escalating in direct correlation with the number of pack-years smoked. Smoking is believed to interfere with apoptotic pathways that facilitate the elimination of dysplastic or malignant cells. Furthermore, tobacco use may impair immune function, thereby diminishing the body’s capacity to clear persistent HPV infections and dysplastic anal epithelial cells.[11]
Epidemiology
Anal carcinoma is a relatively uncommon malignancy, comprising approximately 2.8% of all newly diagnosed digestive system cancers in the United States, with a female-to-male incidence ratio of 2.1:1. Each year, an estimated 10,000 new cases are identified in the United States, with over 50,000 cases reported globally. Despite its relative rarity, the incidence of anal carcinoma has been steadily increasing worldwide. The disease is infrequent in individuals younger than 35, with the majority of cases occurring in older adults, typically around the early 60s. Epidemiological data indicate a higher prevalence among White women and Black men. At the time of diagnosis, approximately 48% of cases remain localized to the primary site, 32% exhibit regional lymphatic involvement, and 13% present with distant metastases.[2][12][13]
Pathophysiology
The pathophysiology of anal SCC is strongly associated with chronic inflammatory processes triggered by persistent HPV infection. HPV preferentially targets the squamocolumnar transition zone within the anal canal, where viral particles infect the basal epithelial cells. This leads to the expression of early viral oncoproteins, eg, E6 and E7, which drive cellular proliferation, disrupt tumor suppressor pathways, and promote malignant transformation. Persistent infection with high-risk HPV serotypes, particularly HPV-16 and HPV-18, is a well-established precursor to anal intraepithelial neoplasia (AIN), a premalignant condition characterized by dysplastic changes in the squamous epithelium. AIN is classified into grades AIN 1, AIN 2, and AIN 3, based on the extent of epithelial abnormalities, mitotic activity, and nuclear atypia. Progression from AIN to invasive SCC occurs in approximately 10% to 11% of cases, with a significantly higher malignant transformation risk in HIV-positive individuals due to impaired immune surveillance.[1]
Molecular profiling of anal SCC has identified recurrent mutations in the PI3K/AKT/mTOR signaling pathway, as well as alterations in chromatin-modifying genes MLL2 and MLL3, which may contribute to tumor initiation and progression. Additionally, genetic alterations in key tumor suppressor genes, including TP53 and CDKN2A, have been observed, with CDKN2A mutations being more prevalent in HPV-negative tumors, which are associated with worse clinical outcomes. Furthermore, programmed death-ligand 1 (PD-L1) expression is detected in approximately 30% of HPV-positive and 40% of HPV-negative anal SCC cases. This suggests a potential role for immune checkpoint dysregulation in tumor pathogenesis, which may have therapeutic implications, particularly in the setting of immunotherapy-based treatment strategies.[14][15][16]
Histopathology
Premalignant Lesions
The WHO classification includes low- and high-grade intraepithelial neoplasia or dysplasia, Bowen disease, and perianal squamous intraepithelial neoplasia. Other systems, eg, the American Joint Commission on Cancer (AJCC), use terms like carcinoma in situ, high-grade squamous intraepithelial lesion (HSIL), and AIN grades 2 to 3.[17]
LSIL-AIN 1 is characterized by the proliferation of metaplastic squamous cells with nuclear irregularities confined to the lower third of the epithelium or by koilocytotic alterations in a papillary structure. In contrast, HSIL-AIN 2-3 is identified by a thickened epithelial layer containing atypical cells with disrupted nuclear polarity, significant nuclear pleomorphism, a high nuclear-to-cytoplasmic ratio, pronounced nuclear hyperchromatism, and elevated mitotic activity.[18]
Malignant Tumors
Types of malignancies associated with anal cancer include:
- Squamous cell carcinoma: The most common anal malignancy (85%), SCC typically arises at the transformation zone between squamous and columnar epithelium. The cells may exhibit large, pale, eosinophilic squamous features, with or without regions of keratinization. Alternatively, tumor-cell clusters may display prominent nuclear palisading. These cells can form tumor nests, and well-differentiated tumors might show peripheral nuclear palisading or central areas of keratinization.
- Verrucous carcinoma: Known as the Buschke-Löwenstein tumor, this locally aggressive lesion resembles condyloma but has destructive potential.
- Adenocarcinoma: Originating in mucosa or anal glands, adenocarcinoma may be associated with Paget or Crohn’s disease.
- Melanoma: Rare and aggressive, anal melanomas resemble cutaneous melanomas but are associated with poor prognosis.
- Neuroendocrine tumors: Accounting for 1% of anal cancers, these rapidly growing tumors are diagnosed with immunohistochemical markers like chromogranin and synaptophysin.[19][1]
Other Rare Tumors
Less common types include lipomas, Kaposi sarcoma, leiomyosarcomas, and basal cell carcinomas, which are often limited to the anal skin.[19]
Immunohistochemistry and Molecular Features
SCC typically exhibits markers including CK5/6, CK13/19, and p63. In contrast, CK7 is generally present in adenocarcinoma but is usually absent in SCC, except in cases with adenoid cystic patterns, where CK7 may be expressed. Neuroendocrine tumors are characterized by positive staining for classic neuroendocrine markers, melanomas by melanocytic markers, and lymphomas by a specific panel of lymphoid markers. For primary perianal Paget disease, CK and gross cystic disease fluid protein 15 (GCDFP15) are commonly expressed. However, in cases involving pagetoid extension from colorectal or urothelial adenocarcinoma, the pagetoid cells often coexpress CK20 and lack GCDFP-15.[20]
History and Physical
Clinical History
Assessing a patient for suspected anal carcinoma begins with a thorough clinical history and physical examination. Clinicians should obtain detailed information regarding the duration, progression, and nature of symptoms, including rectal bleeding, unexplained weight loss, fecal incontinence, and anal or perirectal pain. Anal carcinoma most commonly presents with rectal or anal bleeding in nearly 50% of cases, followed by anal pain and the presence of a palpable mass. Additional symptoms may include pruritus, tenesmus, mucopurulent discharge, changes in stool caliber, and alterations in bowel continence. Some patients may exhibit clinical features resembling benign anorectal conditions, eg, anal fissures or fistulas, whereas others remain asymptomatic until the disease has reached an advanced stage.[21][22]
The duration of symptoms does not generally correlate with prognosis, except in cases where symptoms persist for less than 1 month, which has been associated with improved outcomes. The type of presenting symptom is more strongly correlated with the tumor's T stage rather than its duration. A comprehensive history should also include an assessment of prior or current HPV and HIV infections, as both are significant risk factors for anal carcinoma.[23]
Physical Examination
A digital rectal examination (DRE) and an evaluation for inguinal and femoral lymphadenopathy are essential. The precise location of the lesion should be identified, differentiating between the following:
- Skin lesions: Located >5 cm from the anal verge and visible without the need for traction.
- Perianal lesions: Found within 5 cm of the anus, fully visible with gentle traction.
- Anal canal lesions: Wholly or partially nonvisible even with traction, requiring DRE for identification.
In women, performing a vaginal exam and evaluating the cervix to rule out HPV related cervical pathology, which can coexist, is essential.[23]
During staging assessment, tumor size, mobility, and rectal tone should be carefully documented. Advanced disease may present with inguinal or femoral lymphadenopathy, particularly in cases where the tumor is distal to the dentate line. Lymph node involvement is a critical prognostic indicator, as nodal metastasis significantly reduces survival rates in locally advanced disease.[23]
Evaluation
Anal carcinoma evaluation comprises several laboratory, imaging, and histologic studies for initial diagnosis as well as posttreatment monitoring.
Laboratory Studies
The following laboratory studies are recommended in the evaluation of anal carcinoma:
- HIV testing as anal cancer has a strong association with HPV and is more common in HIV-positive patients.
- Complete blood count (CBC) to assess for anemia or other hematologic abnormalities.
- Liver function to evaluate for possible liver metastases.
- High-risk HPV subtypes (HPV-16, HPV-18) are implicated in most cases of anal squamous cell carcinoma. Testing for p16 protein (a surrogate marker for HPV-related cancer) may provide prognostic information.[24]
Gynecologic and Fertility Assessment
Infertility risks and fertility preservation counseling should be discussed before treatment begins.[24]
Imaging Studies
Imaging plays a pivotal role in staging, treatment planning, and response assessment in anal cancer, predominantly squamous cell carcinoma. The primary objectives include defining tumor extent (T-staging), assessing nodal involvement (N-staging), and detecting distant metastases (M-staging).
High-resolution anoscopy
High-resolution anoscopy (HRA) enhances an anoscope with a high-magnification colposcope, offering a more detailed imaging approach for identifying precancerous squamous cell changes. A large, multicenter randomized controlled trial (ANCHOR) found that detecting HSIL through biopsy and treating with local ablation, topical fluorouracil, or topical imiquimod significantly reduces the risk of developing anal cancer, thereby highlighting the advantages of early detection and intervention.[23][25]
Magnetic resonance imaging
Magnetic resonance imaging (MRI) with high-resolution T2-weighted (T2WI), diffusion-weighted (DWI), and dynamic contrast-enhanced (DCE) sequences is the preferred modality for T and N staging. It provides superior soft-tissue contrast, allowing precise delineation of tumor invasion into the sphincters, levator ani, and adjacent structures. The sensitivity for primary tumor detection approaches 95%, with specificity for distinguishing viable tumors from fibrosis postchemoradiation reaching 85% to 90%.MRI criteria for nodal involvement include size >10 mm, irregular borders, central necrosis, and restricted diffusion. The sensitivity and specificity for detecting metastatic lymph nodes are 85% and 78%, respectively. The National Comprehensive Cancer Network (NCCN), American Society of Clinical Oncology, and European Society for Medical Oncology guidelines endorse MRI as the primary staging modality.[24][26][27]
Computed tomography
Contrast-enhanced CT (CECT) of the chest, abdomen, and pelvis is essential for M-staging, particularly for the lungs and liver, which are common metastatic sites. Sensitivity for pulmonary metastases is 80% to 90%, while for liver metastases, it ranges from 85% to 95%. Although inferior to MRI in local staging, CT is often used when MRI is unavailable or contraindicated.
Positron emission tomography-computed tomography
FDG positron emission tomography-computed tomography (PET-CT) is highly sensitive for nodal metastases (90% to 95%) and superior to CT for distant disease detection, with a specificity of 85% to 90%. PET-CT benefits small, borderline lymph nodes and distant metastases undetected by MRI or CT (see Image. Anal Squamous Cell Carcinoma). PET-CT plays a crucial role in posttreatment response assessment, distinguishing viable tumor from radiation-induced fibrosis, where MRI alone has limitations.NCCN recommends PET-CT in cases of suspected metastases, particularly in T3/T4 or node-positive disease.[24][28][29]
Endoanal ultrasound
For early-stage (T1-T2) tumors, endoanal ultrasound provides high-resolution visualization of tumor depth and sphincter invasion, with a sensitivity of 85% to 95% and specificity of 75% to 85%. However, endoanal ultrasound is operator-dependent and limited in larger or locally advanced tumors, where MRI is superior.[30]
Inguinal lymph node ultrasound with fine-needle aspiration
Ultrasound-guided fine-needle aspiration is critical for inguinal lymph node evaluation, particularly when MRI or CT findings are indeterminate. Hypoechoic texture, loss of fatty hilum, and irregular margins characterize suspicious lymph nodes. Fine-needle aspiration confirms metastases with a diagnostic accuracy of 90% and is recommended by the NCCN when inguinal adenopathy is present.[24]
Imaging in Treatment Response and Surveillance
MRI remains the modality of choice for posttreatment assessment, though postradiation fibrosis versus residual disease remains a challenge (specificity ~80%). With its ability to detect metabolic activity, PET-CT improves accuracy in distinguishing recurrence, mainly when conventional imaging is inconclusive.[31]
Treatment / Management
Historically, the treatment for anal cancer involved surgical resection via abdominoperial resection, which achieved a cure rate of approximately 50% but was associated with significant morbidity. Today, the standard treatment for localized anal cancer has shifted to a combination of chemotherapy and radiation, which offers improved outcomes with reduced morbidity.[32]
The diagnostic evaluation and workup for anal canal cancer and perianal cancer are similar; however, treatment strategies differ based on the stage and anatomical location of the disease. For SCC of the anal canal, chemoradiation is the primary treatment, with surgery typically reserved for cases of persistent or recurrent disease. In contrast, early-stage perianal SCC (T1N0 and select T2N0 cases) can often be treated effectively with local excision, while more advanced stages usually require chemoradiation.[33]
Close posttreatment surveillance is essential to monitor the response to therapy after chemoradiation. Anal cancers tend to regress slowly over time, with complete responses often taking up to 26 weeks. Progressive disease during or after this period should be evaluated with a biopsy, and salvage surgical resection should be considered when necessary. For patients with metastatic disease, treatment is palliative and includes chemotherapy regimens such as fluorouracil, mitomycin-C, cisplatin, and paclitaxel. Additionally, ongoing clinical trials are exploring the role of immunotherapy in the management of anal SCC, offering potential new avenues for treatment.[34][35](B3)
Differential Diagnosis
Differential diagnoses that should also be considered when evaluating anal carcinoma include:
- Condylomata acuminatum
- Hemorrhoid
- Skin tag
- Anal fissure/fistula
- Chancroid
- Psoriasis
Surgical Oncology
Early-Stage Anal Cancer
Local excision is a therapeutic option for superficially invasive squamous cell carcinoma (SISCCA), which meets specific histopathologic criteria: tumor invasion of ≤3 mm into the basement membrane and a horizontal spread of ≤7 mm (T1, NX). SISCCA is frequently detected incidentally during the biopsy or excision of lesions presumed to be benign, eg, condylomas, hemorrhoids, or anal skin tags.
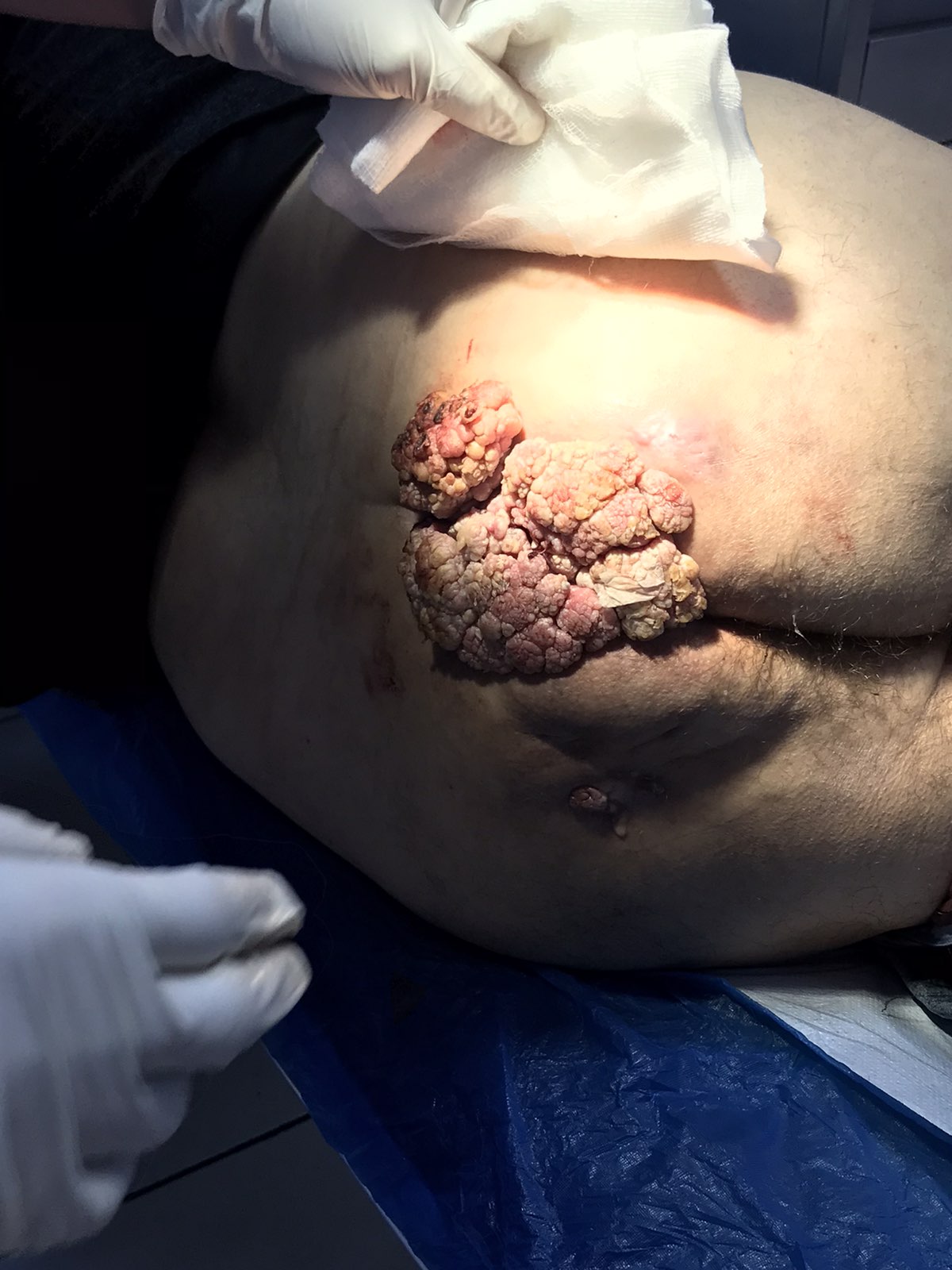
In cases where the carcinoma is completely excised with histologically confirmed negative margins, local excision may suffice as definitive treatment, as long as the site is surveyed closely. Close follow-up is essential, as studies indicate a 74% incidence of HSIL following local excision (see Image. Anal High-Grade Squamous Intraepithelial Lesions). A retrospective study of 17 patients with completely excised invasive anal carcinoma, including 7 with SISCCA, demonstrated excellent outcomes, reporting a 5-year overall survival (OS) rate of 100% and recurrence-free survival of 87% after a median follow-up of 45 months.[36]
Local excision is also a potential treatment modality for select perianal carcinomas, specifically T1, N0 or well-differentiated T2, N0 tumors that do not involve the anal sphincter complex. A surgical margin of at least 1 cm is recommended in these cases. However, thorough evaluation for anal canal involvement is necessary, as many patients with T1 or select T2 perianal carcinomas also harbor HSIL within the anal canal, a factor that must be considered when opting for conservative management.[24]
Locally Advanced and Recurrent Anal Cancer
Chemo-radiotherapy (chemoRT) remains the standard first-line treatment for anal carcinoma; however, locoregional failure rates range from 10% to 30%. Risk factors for tumor recurrence following chemoRT include higher T-stage and nodal metastasis (N-stage). If disease progression is suspected based on DRE findings, further biopsy and imaging (eg, CT or PET/CT) assessment is warranted. In patients with biopsy-confirmed locally progressive disease, radical surgical resection via abdominoperineal resection (APR) may be considered.
For select patients with locally advanced or recurrent disease, immunotherapy with nivolumab or pembrolizumab may be an alternative to surgery. However, mature clinical data to support this approach are not yet available.[24][37]
Radiation Oncology
Radiation doses for anal cancer treatment are derived from the RTOG 98-11 trial, which established dosing protocols tailored to tumor size, stage, and risk of nodal involvement. These doses are individualized based on individual factors. Additional radiation fractions are often prescribed for high-risk areas or involved nodal basins to ensure adequate tumor control while minimizing radiation-induced toxicity.
Intensity-modulated radiation therapy (IMRT) has become the preferred technique over 3-dimensional conformal radiation therapy (3D-CRT) due to its ability to optimize tumor coverage, reduce radiation exposure to adjacent normal tissues, and lower treatment-related toxicity. IMRT also allows for dose painting, where different regions receive customized radiation intensities, improving local tumor control and reducing adverse effects such as dermatitis, proctitis, and enteritis.[38]
A standard radiation protocol typically includes a minimum dose of 45 Gy to the primary tumor across all disease stages, ensuring adequate coverage of the gross tumor volume (GTV) and areas at risk for microscopic disease. The initial phase of treatment delivers 30.6 Gy, encompassing the pelvis, anus, perineum, and inguinal lymph nodes, as these areas are at high risk for subclinical disease. Patients with T2-T4 tumors or clinically positive lymph nodes require an additional boost of 9 to 14 Gy, further escalating the total dose to approximately 54 to 59 Gy in high-risk regions. In cases of persistent or recurrent disease, dose escalation beyond 59 Gy may be considered. Concurrent chemotherapy with radiation is typically used to increase efficacy.[24][39]
Medical Oncology
Nonmetastatic Disease
The current standard protocol consists of 5-fluorouracil (5-FU)-based chemotherapy combined with mitomycin-C (MMC) and concurrent radiation (RT). The approach known as the Nigro regimen revolutionized anal cancer treatment by showing that chemoradiation (5-FU + MMC + RT) could achieve high cure rates without surgery. This regimen used 5-FU (1,000 mg/m²/day, continuous infusion on days 1 to 4 and 29 to 32) and MMC (10 to 15 mg/m², single dose on day 1) with RT. Later trials refined the regimen, confirming MMC (10 mg/m² capped at 20 mg, day 1 and 29) + 5-FU (1,000 mg/m²/day, days 1 to 4 and 29 to 32) + RT as the gold standard for locally advanced anal cancer. Complete tumor regression was seen in 80% to 90% of cases, significantly outperforming radiation therapy alone, with a response rate of 45% to 56%. The best outcomes are observed in patients with T1/T2 tumors and no nodal involvement.[40][41][24]
Capecitabine, an oral alternative to 5-FU, has been investigated and demonstrated similar clinical outcomes but lower hematologic toxicity in retrospective studies, particularly reduced rates of neutropenia. However, nonhematologic toxicities, eg, hand-foot syndrome and gastrointestinal adverse effects, may be more frequent with capecitabine. The NCCN panel recommends mitomycin/capecitabine plus RT as an alternative for stages I to III.[42][43]
Cisplatin has been studied as a substitute for 5-FU or mitomycin. The ACT II Trial found no significant difference between 5-FU + MMC + RT and 5-FU + cisplatin + RT regarding complete response, colostomy-free survival, or overall survival, confirming no advantage of cisplatin over MMC. However, the RTOG 98-11 trial showed that 5-FU + MMC + RT had superior 5-year overall survival (78% versus 70%) and lower locoregional failure rates compared to 5-FU + cisplatin + RT, establishing MMC as the preferred agent.[40][41] Studies also suggest no added benefit from induction chemotherapy or radiation dose boosts in chemoRT protocols for localized anal cancer.
Emerging treatments include biologics like PD-1 inhibitors and EGFR-targeting therapies, eg, cetuximab. While promising, cetuximab has shown significant toxicity in trials, limiting its applicability. For older or less fit patients unable to tolerate mitomycin, options such as cisplatin-based regimens, capecitabine, or RT alone are under consideration, though data remain insufficient for definitive recommendations.[44][24]
Metastatic Disease
Metastatic anal cancer most commonly spreads to the liver, lungs, and extrapelvic lymph nodes. Although this type of cancer is rare, with only 10% to 20% of cases presenting as metastatic outside the pelvis, systemic therapy has demonstrated potential benefits. Palliative chemoRT to the primary tumor site can be considered for local control in symptomatic, bulky tumors following initial chemotherapy. Data from the National Cancer Database indicate that combining chemotherapy with definitive pelvic RT is associated with longer median OS than chemotherapy alone (21.3 versus 15.9 months). Additionally, retrospective studies suggest that surgical removal or ablation of liver metastases may improve long-term outcomes, although this strategy is not yet included in standard guidelines.[45]
First-Line Chemotherapy
The phase II InterAACT trial identified carboplatin combined with paclitaxel as the preferred first-line regimen for metastatic anal cancer due to its favorable toxicity profile compared to cisplatin and 5-FU. While response rates were similar (approximately 59%), carboplatin plus paclitaxel demonstrated longer progression-free survival (PFS) and OS (8.1 and 20 months, respectively) compared to cisplatin and 5-FU (5.7 and 12.3 months, respectively). Other treatment options include FOLFCIS (5-FU, Leucovorin, Cisplatin), FOLFOX (Folinic acid, 5-FU, Oxaliplatin), and modified DCF (Docetaxel, Cisplatin and 5-FU) regimens, each showing varying efficacy and safety profiles. For instance, FOLFOX is noted for manageable safety, while modified DCF shows promise but comes with higher toxicity concerns.[46]
Ongoing research is exploring the integration of immune checkpoint inhibitors to enhance treatment outcomes. The phase III POD1UM-303/InterAACT 2 trial is evaluating the addition of retifanlimab to carboplatin and paclitaxel in patients with inoperable locally recurrent or metastatic squamous cell carcinoma of the anal canal. Preliminary results indicate a significant improvement in progression-free survival with the inclusion of retifanlimab. EA2176 trial is a Phase III clinical study evaluating the addition of nivolumab to the chemotherapy regimen of carboplatin and paclitaxel for patients with metastatic anal cancer.[47]
Second-Line Chemotherapy
Checkpoint inhibitors have shown promise for patients with refractory metastatic anal cancer. Trials of nivolumab and pembrolizumab demonstrated response rates of 24% and 17%, respectively, with manageable toxicity. Pembrolizumab also showed benefits in a microsatellite instability-high (MSI-H)/deficient mismatch repair (dMMR) cancer cohort, with an objective response rate of 11%. Further research is underway, including trials evaluating nivolumab alone or in combination with ipilimumab and other novel agents. Current guidelines recommend PD-1 inhibitors like nivolumab and pembrolizumab as preferred second-line therapies for patients who have progressed on initial chemotherapy.[48][49]
Staging
Anal cancer staging established by the AJCC ninth edition uses the following tumor, node, metastasis criteria:
- T (tumor) stage
- TX Primary tumor not assessed
- T0 No evidence of primary tumor
- Tis high-grade squamous intraepithelial lesion (previously termed carcinoma in situ, Bowen disease, anal intraepithelial neoplasia II to III, high-grade anal intraepithelial neoplasia)
- T1 Tumor ≤2 cm
- T2 Tumor >2 cm but ≤5 cm
- T3 Tumor >5 cm
- T4 Tumor of any size invading adjacent organs, eg, the vagina, urethra, or bladder
- N (node) stage
- NX Regional lymph nodes cannot be assessed
- N0 No regional lymph node metastasis
- N1 Metastasis in inguinal, mesorectal, internal iliac, or external iliac nodes
- N1a Metastasis in inguinal, mesorectal, or internal iliac lymph nodes
- N1b Metastasis in external iliac lymph nodes
- N1c Metastasis in external iliac (N1b) and any N1a nodes
- M (metastasis) stage:
- M0 No distant metastasis
- cM1 Distant metastasis
- pM1 microscopic confirmation of distant metastasis [24]
Prognosis
The prognosis of anal carcinoma is primarily determined by tumor size and the presence of lymph node metastases. At the time of diagnosis, approximately 50% of patients present with localized disease (T1-T2, N0), which is associated with a 5-year OS rate of 80%. However, 29% of patients exhibit regional lymph node involvement (N1-N3), which reduces the 5-year survival rate to 60% due to the increased risk of locoregional recurrence and distant dissemination. Approximately 12% of cases present with distant metastases (M1), leading to a reduced 5-year survival rate of 30.5%.[21]
A comprehensive analysis of over 600 patients from the RTOG 98-11 trial reinforced the prognostic significance of tumor and nodal classification in predicting OS, disease-free survival, and colostomy-free survival. The study highlighted that patients with T4, N0 disease and T3-T4, N+ disease had the poorest outcomes, demonstrating higher rates of treatment failure, locoregional recurrence, and the need for colostomy.[50][24]
Complications
Treatment-related toxicities are a well-documented consequence of chemoradiation for anal SCC. These complications, which can involve both superficial and deep pelvic structures, often have a profound impact on patients' quality of life. In some cases, they necessitate planned or unplanned interruptions in therapy, potentially compromising treatment outcomes. Research indicates that up to 80% of patients require treatment breaks due to these toxicities.[51][33]
Radiation-induced toxicities are given the following classifications of acute if they occur within 3 months of treatment or late if they arise beyond this period:
- Acute toxicity complications (< 3 months posttreatment): Anoproctitis, bleeding, cystitis, diarrhea, dehydration, enteritis, neutropenia, pelvic/perineal pain, and perineal dermatitis.
- Late toxicity complications (>3 months posttreatment): Anorectal or perineal pain, bowel obstructions, decreased sexual desire, fecal incontinence, fistulas, impotence, non-healing wounds, osteomyelitis, osteonecrosis, pelvic fractures, stricture, urinary incontinence, and vaginal stenosis.
Encouragingly, several quality-of-life studies have demonstrated that symptoms associated with acute toxicities often improve or resolve within 3 months following the completion of therapy.[32]
Postoperative and Rehabilitation Care
A thorough surveillance program should evaluate a patient's quality of life after treatment completion. Early follow-up should focus on perianal skin care, as acute ano-proctitis from chemoradiation can lead to severe rectal pain, drainage, and perianal skin ulcers. Chronic perineal dermatitis is a common adverse effect of radiation therapy. Using topical barrier creams is a simple yet effective way to relieve symptoms.
Patients should also be monitored for severe radiation-related complications like anal stenosis, mucosal necrosis, and rectal strictures, which may require a colostomy. Long-term follow-up should address late radiation toxicity, including bowel, sexual, and urinary dysfunction. Survivors of anal cancer often experience significant reductions in overall quality of life, with common symptoms, eg, fatigue, shortness of breath, nausea, appetite loss, pain, and insomnia. Regular screening for distress in these patients is also essential.[52]
Deterrence and Patient Education
Anal cancer, primarily linked to HPV infection, occurs in the tissues of the anus, with symptoms like bleeding, lumps, or pain in the area. Risk factors include HPV infection, a weakened immune system (eg, HIV or organ transplants), a history of certain cancers, multiple sexual partners, receptive anal intercourse, and smoking. Early detection involves tests, including a digital rectal exam, anoscopy, and biopsies. Staging determines treatment, including surgery, radiation, chemotherapy, or newer therapies like immunotherapy. Patients should be educated on the importance of regular screenings, understanding risk factors, and potential adverse effects of treatments. Prompt consultation with a healthcare practitioner is key for early detection and effective management.
Enhancing Healthcare Team Outcomes
Effective management of anal cancer requires an interprofessional approach to ensure optimal patient-centered care, safety, and outcomes. Physicians, including oncologists, surgeons, and radiologists, must utilize advanced diagnostic and therapeutic skills, applying evidence-based guidelines for staging and treatment. Advanced practitioners provide patient education, symptom management, and continuity of care. Nurses deliver essential supportive care, monitor treatment responses, and facilitate communication between the care team and the patient. Pharmacists ensure safe and effective chemotherapy and pain management, advising on drug interactions and adverse effects.
Healthcare professionals must prioritize ethical principles such as patient autonomy, beneficence, and nonmaleficence. Informed consent, shared decision-making, and respect for patient values are crucial. Clinicians must ensure equitable access to care and advocate for individualized treatment approaches, particularly for vulnerable populations. Effective communication among team members enhances patient safety and treatment efficiency. Regular tumor board meetings, shared electronic health records, and structured handoffs minimize errors and streamline care transitions. Clear documentation and communication of treatment plans prevent medication errors, redundant testing, and misinterpretation of findings.
Coordinated care improves survival rates, treatment adherence, and quality of life. Managing toxicities from chemoradiation, mitigating complications like infection or radiation proctitis, and providing psychosocial support are critical. Nursing interventions in skin care and bowel management, pharmacist-led medication optimization, and palliative care services enhance patient safety and comfort. By fostering teamwork, leveraging expertise, and maintaining ethical integrity, healthcare teams can optimize outcomes for patients with anal cancer while upholding high standards of patient-centered care.
Media
(Click Image to Enlarge)
(Click Image to Enlarge)
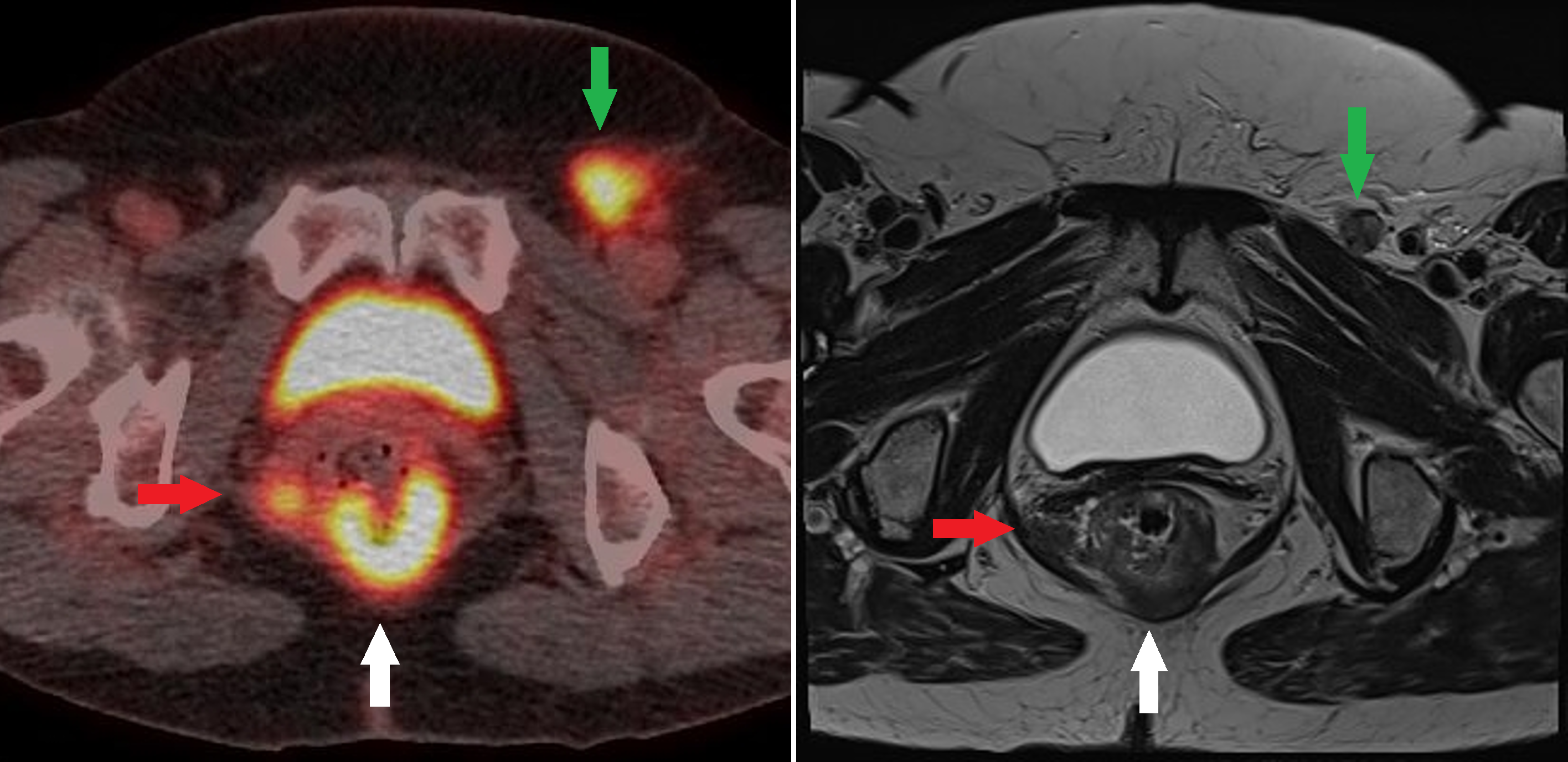
Anal Squamous Cell Carcinoma. Fused fluorodeoxyglucose positron emission tomography/computed tomography and T2 axial magnetic resonance images at the same level of an anorectal junction tumor (white arrow) with a fluorodeoxyglucose-avid nodal deposit in the right mesorectum (red arrow) and the left groin (green arrow).
Contributed by A Ramzan, FRCR
References
Gondal TA, Chaudhary N, Bajwa H, Rauf A, Le D, Ahmed S. Anal Cancer: The Past, Present and Future. Current oncology (Toronto, Ont.). 2023 Mar 11:30(3):3232-3250. doi: 10.3390/curroncol30030246. Epub 2023 Mar 11 [PubMed PMID: 36975459]
Stewart DB, Gaertner WB, Glasgow SC, Herzig DO, Feingold D, Steele SR, Prepared on Behalf of the Clinical Practice Guidelines Committee of the American Society of Colon and Rectal Surgeons. The American Society of Colon and Rectal Surgeons Clinical Practice Guidelines for Anal Squamous Cell Cancers (Revised 2018). Diseases of the colon and rectum. 2018 Jul:61(7):755-774. doi: 10.1097/DCR.0000000000001114. Epub [PubMed PMID: 29878949]
Level 1 (high-level) evidenceEng C, Ciombor KK, Cho M, Dorth JA, Rajdev LN, Horowitz DP, Gollub MJ, Jácome AA, Lockney NA, Muldoon RL, Washington MK, O'Brian BA, Benny A, Lebeck Lee CM, Benson AB 3rd, Goodman KA, Morris VK. Anal Cancer: Emerging Standards in a Rare Disease. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2022 Aug 20:40(24):2774-2788. doi: 10.1200/JCO.21.02566. Epub 2022 Jun 1 [PubMed PMID: 35649196]
Ahmed A, Arbor TC, Qureshi WA. Anatomy, Abdomen and Pelvis: Anal Canal. StatPearls. 2024 Jan:(): [PubMed PMID: 32119418]
Lin C, Franceschi S, Clifford GM. Human papillomavirus types from infection to cancer in the anus, according to sex and HIV status: a systematic review and meta-analysis. The Lancet. Infectious diseases. 2018 Feb:18(2):198-206. doi: 10.1016/S1473-3099(17)30653-9. Epub 2017 Nov 17 [PubMed PMID: 29158102]
Level 1 (high-level) evidenceTong WW, Jin F, McHugh LC, Maher T, Sinclair B, Grulich AE, Hillman RJ, Carr A. Progression to and spontaneous regression of high-grade anal squamous intraepithelial lesions in HIV-infected and uninfected men. AIDS (London, England). 2013 Sep 10:27(14):2233-43. doi: 10.1097/QAD.0b013e3283633111. Epub [PubMed PMID: 24157904]
Walcheck MT, Matkowskyj KA, Turco A, Blaine-Sauer S, Nukaya M, Noel J, Ronnekleiv OK, Ronnekleiv-Kelly SM. Sex-dependent development of Kras-induced anal squamous cell carcinoma in mice. PloS one. 2021:16(11):e0259245. doi: 10.1371/journal.pone.0259245. Epub 2021 Nov 4 [PubMed PMID: 34735515]
Colón-López V, Shiels MS, Machin M, Ortiz AP, Strickler H, Castle PE, Pfeiffer RM, Engels EA. Anal Cancer Risk Among People With HIV Infection in the United States. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2018 Jan 1:36(1):68-75. doi: 10.1200/JCO.2017.74.9291. Epub 2017 Nov 15 [PubMed PMID: 29140774]
Kang YJ, Smith M, Canfell K. Anal cancer in high-income countries: Increasing burden of disease. PloS one. 2018:13(10):e0205105. doi: 10.1371/journal.pone.0205105. Epub 2018 Oct 19 [PubMed PMID: 30339668]
Song M, Engels EA, Clarke MA, Kreimer AR, Shiels MS. Autoimmune disease and the risk of anal cancer in the US population aged 66 years and over. Journal of the National Cancer Institute. 2024 Feb 8:116(2):309-315. doi: 10.1093/jnci/djad187. Epub [PubMed PMID: 37701981]
McMahon KR, Gemma N, Clapp M, Sanchez-Montejo P, Dibello J, Laipply E. Relationship between anal cancer recurrence and cigarette smoking. World journal of clinical oncology. 2023 Jul 24:14(7):259-264. doi: 10.5306/wjco.v14.i7.259. Epub [PubMed PMID: 37583947]
Siegel RL, Giaquinto AN, Jemal A. Cancer statistics, 2024. CA: a cancer journal for clinicians. 2024 Jan-Feb:74(1):12-49. doi: 10.3322/caac.21820. Epub 2024 Jan 17 [PubMed PMID: 38230766]
Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I, Jemal A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: a cancer journal for clinicians. 2024 May-Jun:74(3):229-263. doi: 10.3322/caac.21834. Epub 2024 Apr 4 [PubMed PMID: 38572751]
Hamza A, Masliah-Planchon J, Neuzillet C, Lefèvre JH, Svrcek M, Vacher S, Bourneix C, Delaye M, Goéré D, Dartigues P, Samalin E, Hilmi M, Lazartigues J, Girard E, Emile JF, Rigault E, Dangles-Marie V, Rioux-Leclercq N, de la Fouchardière C, Tougeron D, Casadei-Gardini A, Mariani P, Peschaud F, Cacheux W, Lièvre A, Bièche I. Pathogenic alterations in PIK3CA and KMT2C are frequent and independent prognostic factors in anal squamous cell carcinoma treated with salvage abdominoperineal resection. International journal of cancer. 2024 Feb 1:154(3):504-515. doi: 10.1002/ijc.34781. Epub 2023 Oct 31 [PubMed PMID: 37908048]
Holliday EB, Peddireddy A, Morris VK. Prognostic and Predictive Markers for Patients With Anal Cancer. Journal of the National Comprehensive Cancer Network : JNCCN. 2023 Jun:21(6):678-684. doi: 10.6004/jnccn.2023.7031. Epub [PubMed PMID: 37308122]
Chung JH, Sanford E, Johnson A, Klempner SJ, Schrock AB, Palma NA, Erlich RL, Frampton GM, Chalmers ZR, Vergilio J, Rubinson DA, Sun JX, Chmielecki J, Yelensky R, Suh JH, Lipson D, George TJ Jr, Elvin JA, Stephens PJ, Miller VA, Ross JS, Ali SM. Comprehensive genomic profiling of anal squamous cell carcinoma reveals distinct genomically defined classes. Annals of oncology : official journal of the European Society for Medical Oncology. 2016 Jul:27(7):1336-41. doi: 10.1093/annonc/mdw152. Epub 2016 Apr 6 [PubMed PMID: 27052656]
Loughrey MB, Shepherd NA. Anal and Perianal Preneoplastic Lesions. Gastroenterology clinics of North America. 2024 Mar:53(1):201-220. doi: 10.1016/j.gtc.2023.09.007. Epub 2023 Oct 16 [PubMed PMID: 38280748]
Roberts JR, Siekas LL, Kaz AM. Anal intraepithelial neoplasia: A review of diagnosis and management. World journal of gastrointestinal oncology. 2017 Feb 15:9(2):50-61. doi: 10.4251/wjgo.v9.i2.50. Epub [PubMed PMID: 28255426]
Cruz-Ramos PA, Nguyen S, Hayman AV. Uncommon Anal Cancers. Surgical oncology clinics of North America. 2025 Jan:34(1):103-113. doi: 10.1016/j.soc.2024.07.006. Epub 2024 Aug 17 [PubMed PMID: 39547762]
Hoff PM, Coudry R, Moniz CM. Pathology of Anal Cancer. Surgical oncology clinics of North America. 2017 Jan:26(1):57-71. doi: 10.1016/j.soc.2016.07.013. Epub [PubMed PMID: 27889037]
Ryan DP,Compton CC,Mayer RJ, Carcinoma of the anal canal. The New England journal of medicine. 2000 Mar 16 [PubMed PMID: 10717015]
Sauter M, Keilholz G, Kranzbühler H, Lombriser N, Prakash M, Vavricka SR, Misselwitz B. Presenting symptoms predict local staging of anal cancer: a retrospective analysis of 86 patients. BMC gastroenterology. 2016 Apr 6:16():46. doi: 10.1186/s12876-016-0461-0. Epub 2016 Apr 6 [PubMed PMID: 27048435]
Level 2 (mid-level) evidenceHong JS, Yuan V, Patron-Lozano R, Chao SY. Diagnosis of Anal Cancer: Symptoms, Imaging, and Endoscopy. Surgical oncology clinics of North America. 2025 Jan:34(1):37-48. doi: 10.1016/j.soc.2024.06.002. Epub 2024 Jul 23 [PubMed PMID: 39547767]
Benson AB, Venook AP, Al-Hawary MM, Azad N, Chen YJ, Ciombor KK, Cohen S, Cooper HS, Deming D, Garrido-Laguna I, Grem JL, Hecht JR, Hoffe S, Hubbard J, Hunt S, Hussan H, Jeck W, Johung KL, Joseph N, Kirilcuk N, Krishnamurthi S, Maratt J, Messersmith WA, Meyerhardt J, Miller ED, Mulcahy MF, Nurkin S, Overman MJ, Parikh A, Patel H, Pedersen K, Saltz L, Schneider C, Shibata D, Skibber JM, Sofocleous CT, Stotsky-Himelfarb E, Tavakkoli A, Willett CG, Williams G, Algieri F, Gurski L, Stehman K. Anal Carcinoma, Version 2.2023, NCCN Clinical Practice Guidelines in Oncology. Journal of the National Comprehensive Cancer Network : JNCCN. 2023 Jun:21(6):653-677. doi: 10.6004/jnccn.2023.0030. Epub [PubMed PMID: 37308125]
Level 1 (high-level) evidencePalefsky JM, Lee JY, Jay N, Goldstone SE, Darragh TM, Dunlevy HA, Rosa-Cunha I, Arons A, Pugliese JC, Vena D, Sparano JA, Wilkin TJ, Bucher G, Stier EA, Tirado Gomez M, Flowers L, Barroso LF, Mitsuyasu RT, Lensing SY, Logan J, Aboulafia DM, Schouten JT, de la Ossa J, Levine R, Korman JD, Hagensee M, Atkinson TM, Einstein MH, Cracchiolo BM, Wiley D, Ellsworth GB, Brickman C, Berry-Lawhorn JM, ANCHOR Investigators Group. Treatment of Anal High-Grade Squamous Intraepithelial Lesions to Prevent Anal Cancer. The New England journal of medicine. 2022 Jun 16:386(24):2273-2282. doi: 10.1056/NEJMoa2201048. Epub [PubMed PMID: 35704479]
Morris VK, Kennedy EB, Amin MA, Aranha O, Benson AB 3rd, Dorth JA, Horowitz DP, Kennecke HF, Kim S, Kreppel L, Mettu NB, Rajdev L, Riechelmann R, Sio TT, Eng C. Systemic Therapy for Stage I-III Anal Squamous Cell Carcinoma: ASCO Guideline. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2025 Feb 10:43(5):605-615. doi: 10.1200/JCO-24-02120. Epub 2024 Dec 16 [PubMed PMID: 39680825]
Rao S, Guren MG, Khan K, Brown G, Renehan AG, Steigen SE, Deutsch E, Martinelli E, Arnold D, ESMO Guidelines Committee. Electronic address: clinicalguidelines@esmo.org. Anal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up(☆). Annals of oncology : official journal of the European Society for Medical Oncology. 2021 Sep:32(9):1087-1100. doi: 10.1016/j.annonc.2021.06.015. Epub 2021 Jun 24 [PubMed PMID: 34175386]
Level 1 (high-level) evidenceMahmud A, Poon R, Jonker D. PET imaging in anal canal cancer: a systematic review and meta-analysis. The British journal of radiology. 2017 Dec:90(1080):20170370. doi: 10.1259/bjr.20170370. Epub 2017 Oct 3 [PubMed PMID: 28972796]
Level 1 (high-level) evidenceAlbertsson P, Alverbratt C, Liljegren A, Björkander E, Strandell A, Samuelsson O, Palm S, Hallqvist A. Positron emission tomography and computed tomographic (PET/CT) imaging for radiation therapy planning in anal cancer: A systematic review and meta-analysis. Critical reviews in oncology/hematology. 2018 Jun:126():6-12. doi: 10.1016/j.critrevonc.2018.03.013. Epub 2018 Mar 31 [PubMed PMID: 29759568]
Level 1 (high-level) evidenceReginelli A, Granata V, Fusco R, Granata F, Rega D, Roberto L, Pellino G, Rotondo A, Selvaggi F, Izzo F, Petrillo A, Grassi R. Diagnostic performance of magnetic resonance imaging and 3D endoanal ultrasound in detection, staging and assessment post treatment, in anal cancer. Oncotarget. 2017 Apr 4:8(14):22980-22990. doi: 10.18632/oncotarget.14946. Epub [PubMed PMID: 28152518]
Kim K, Mercer J, John V, Mathew S, Kochhar R. Imaging Features of Anal Carcinoma after Chemoradiation. Radiographics : a review publication of the Radiological Society of North America, Inc. 2025 Apr:45(4):e240119. doi: 10.1148/rg.240119. Epub [PubMed PMID: 40080437]
Kouzy R, Abi Jaoude J, Lin D, El Alam MB, Minsky BD, Koay EJ, Das P, Holliday EB, Klopp AH, Colbert LE, Taniguchi CM. Patient-Reported GI Outcomes in Patients With Anal Cancer Receiving Modern Chemoradiation. JCO oncology practice. 2020 Dec:16(12):e1524-e1531. doi: 10.1200/OP.20.00122. Epub 2020 Jul 1 [PubMed PMID: 32609585]
Stapler SJ, Gunnells DJ Jr, Hollis RH. Management of Localized and Locally Advanced Anal Cancer. Surgical oncology clinics of North America. 2025 Jan:34(1):59-67. doi: 10.1016/j.soc.2024.07.009. Epub [PubMed PMID: 39547769]
Pricolo VE, Bonvini M, Abelli CF. Patterns of care for anal cancer in the United States - a comparison between academic and community cancer centers. BMC cancer. 2018 May 16:18(1):567. doi: 10.1186/s12885-018-4488-1. Epub 2018 May 16 [PubMed PMID: 29769057]
PDQ Adult Treatment Editorial Board. Anal Cancer Treatment (PDQ®): Health Professional Version. PDQ Cancer Information Summaries. 2002:(): [PubMed PMID: 26389221]
Arana R, Fléjou JF, Si-Mohamed A, Bauer P, Etienney I. Clinicopathological and virological characteristics of superficially invasive squamous-cell carcinoma of the anus. Colorectal disease : the official journal of the Association of Coloproctology of Great Britain and Ireland. 2015 Nov:17(11):965-72. doi: 10.1111/codi.12951. Epub [PubMed PMID: 25784158]
Mullen JT, Rodriguez-Bigas MA, Chang GJ, Barcenas CH, Crane CH, Skibber JM, Feig BW. Results of surgical salvage after failed chemoradiation therapy for epidermoid carcinoma of the anal canal. Annals of surgical oncology. 2007 Feb:14(2):478-83 [PubMed PMID: 17103253]
Ajani JA,Winter KA,Gunderson LL,Pedersen J,Benson AB 3rd,Thomas CR Jr,Mayer RJ,Haddock MG,Rich TA,Willett C, Fluorouracil, mitomycin, and radiotherapy vs fluorouracil, cisplatin, and radiotherapy for carcinoma of the anal canal: a randomized controlled trial. JAMA. 2008 Apr 23 [PubMed PMID: 18430910]
Level 1 (high-level) evidenceMyerson RJ, Garofalo MC, El Naqa I, Abrams RA, Apte A, Bosch WR, Das P, Gunderson LL, Hong TS, Kim JJ, Willett CG, Kachnic LA. Elective clinical target volumes for conformal therapy in anorectal cancer: a radiation therapy oncology group consensus panel contouring atlas. International journal of radiation oncology, biology, physics. 2009 Jul 1:74(3):824-30. doi: 10.1016/j.ijrobp.2008.08.070. Epub 2008 Dec 29 [PubMed PMID: 19117696]
Level 3 (low-level) evidenceGunderson LL, Winter KA, Ajani JA, Pedersen JE, Moughan J, Benson AB 3rd, Thomas CR Jr, Mayer RJ, Haddock MG, Rich TA, Willett CG. Long-term update of US GI intergroup RTOG 98-11 phase III trial for anal carcinoma: survival, relapse, and colostomy failure with concurrent chemoradiation involving fluorouracil/mitomycin versus fluorouracil/cisplatin. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2012 Dec 10:30(35):4344-51. doi: 10.1200/JCO.2012.43.8085. Epub 2012 Nov 13 [PubMed PMID: 23150707]
Level 1 (high-level) evidenceJames RD, Glynne-Jones R, Meadows HM, Cunningham D, Myint AS, Saunders MP, Maughan T, McDonald A, Essapen S, Leslie M, Falk S, Wilson C, Gollins S, Begum R, Ledermann J, Kadalayil L, Sebag-Montefiore D. Mitomycin or cisplatin chemoradiation with or without maintenance chemotherapy for treatment of squamous-cell carcinoma of the anus (ACT II): a randomised, phase 3, open-label, 2 × 2 factorial trial. The Lancet. Oncology. 2013 May:14(6):516-24. doi: 10.1016/S1470-2045(13)70086-X. Epub 2013 Apr 9 [PubMed PMID: 23578724]
Level 1 (high-level) evidenceOliveira SC, Moniz CM, Riechelmann R, Alex AK, Braghirolli MI, Bariani G, Nahas C, Hoff PM. Phase II Study of Capecitabine in Substitution of 5-FU in the Chemoradiotherapy Regimen for Patients with Localized Squamous Cell Carcinoma of the Anal Canal. Journal of gastrointestinal cancer. 2016 Mar:47(1):75-81. doi: 10.1007/s12029-015-9790-4. Epub [PubMed PMID: 26691173]
Meulendijks D, Dewit L, Tomasoa NB, van Tinteren H, Beijnen JH, Schellens JH, Cats A. Chemoradiotherapy with capecitabine for locally advanced anal carcinoma: an alternative treatment option. British journal of cancer. 2014 Oct 28:111(9):1726-33. doi: 10.1038/bjc.2014.467. Epub 2014 Aug 28 [PubMed PMID: 25167226]
Level 2 (mid-level) evidenceGarg MK, Zhao F, Sparano JA, Palefsky J, Whittington R, Mitchell EP, Mulcahy MF, Armstrong KI, Nabbout NH, Kalnicki S, El-Rayes BF, Onitilo AA, Moriarty DJ, Fitzgerald TJ, Benson AB 3rd. Cetuximab Plus Chemoradiotherapy in Immunocompetent Patients With Anal Carcinoma: A Phase II Eastern Cooperative Oncology Group-American College of Radiology Imaging Network Cancer Research Group Trial (E3205). Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2017 Mar:35(7):718-726. doi: 10.1200/JCO.2016.69.1667. Epub 2017 Jan 9 [PubMed PMID: 28068178]
Cummings BJ. Metastatic anal cancer: the search for cure. Onkologie. 2006 Feb:29(1-2):5-6 [PubMed PMID: 16514247]
Rao S, Sclafani F, Eng C, Adams RA, Guren MG, Sebag-Montefiore D, Benson A, Bryant A, Peckitt C, Segelov E, Roy A, Seymour MT, Welch J, Saunders MP, Muirhead R, O'Dwyer P, Bridgewater J, Bhide S, Glynne-Jones R, Arnold D, Cunningham D. International Rare Cancers Initiative Multicenter Randomized Phase II Trial of Cisplatin and Fluorouracil Versus Carboplatin and Paclitaxel in Advanced Anal Cancer: InterAAct. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2020 Aug 1:38(22):2510-2518. doi: 10.1200/JCO.19.03266. Epub 2020 Jun 12 [PubMed PMID: 32530769]
Level 1 (high-level) evidenceRao S, Jones M, Bowman J, Tian C, Spano JP. POD1UM-303/InterAACT 2: A phase III, global, randomized, double-blind study of retifanlimab or placebo plus carboplatin-paclitaxel in patients with locally advanced or metastatic squamous cell anal carcinoma. Frontiers in oncology. 2022:12():935383. doi: 10.3389/fonc.2022.935383. Epub 2022 Aug 24 [PubMed PMID: 36091159]
Level 1 (high-level) evidenceWang Y, Yu X, Zhao N, Wang J, Lin C, Izaguirre EW, Farmer M, Tian G, Somer B, Dubal N, Schwartz DL, Ballo MT, VanderWalde NA. Definitive Pelvic Radiotherapy and Survival of Patients With Newly Diagnosed Metastatic Anal Cancer. Journal of the National Comprehensive Cancer Network : JNCCN. 2019 Jan:17(1):29-37. doi: 10.6004/jnccn.2018.7085. Epub [PubMed PMID: 30659127]
Ott PA, Piha-Paul SA, Munster P, Pishvaian MJ, van Brummelen EMJ, Cohen RB, Gomez-Roca C, Ejadi S, Stein M, Chan E, Simonelli M, Morosky A, Saraf S, Emancipator K, Koshiji M, Bennouna J. Safety and antitumor activity of the anti-PD-1 antibody pembrolizumab in patients with recurrent carcinoma of the anal canal. Annals of oncology : official journal of the European Society for Medical Oncology. 2017 May 1:28(5):1036-1041. doi: 10.1093/annonc/mdx029. Epub [PubMed PMID: 28453692]
Gunderson LL, Moughan J, Ajani JA, Pedersen JE, Winter KA, Benson AB 3rd, Thomas CR Jr, Mayer RJ, Haddock MG, Rich TA, Willett CG. Anal carcinoma: impact of TN category of disease on survival, disease relapse, and colostomy failure in US Gastrointestinal Intergroup RTOG 98-11 phase 3 trial. International journal of radiation oncology, biology, physics. 2013 Nov 15:87(4):638-45. doi: 10.1016/j.ijrobp.2013.07.035. Epub 2013 Sep 10 [PubMed PMID: 24035327]
Level 1 (high-level) evidenceRoohipour R, Patil S, Goodman KA, Minsky BD, Wong WD, Guillem JG, Paty PB, Weiser MR, Neuman HB, Shia J, Schrag D, Temple LK. Squamous-cell carcinoma of the anal canal: predictors of treatment outcome. Diseases of the colon and rectum. 2008 Feb:51(2):147-53. doi: 10.1007/s10350-007-9125-z. Epub 2008 Jan 8 [PubMed PMID: 18180997]
Bikhchandani J. Posttreatment Surveillance of Anal Cancer. Surgical oncology clinics of North America. 2025 Jan:34(1):83-89. doi: 10.1016/j.soc.2024.08.003. Epub 2024 Oct 24 [PubMed PMID: 39547771]