Introduction
The airway comprises the passages of the respiratory tract that conduct air from the external environment to the alveoli. This conduit extends from the nares and oral opening to the terminal bronchioles, where gas exchange occurs within the alveolar respiratory units. The airway is divided into upper and lower portions, each with distinct anatomical structures and specialized functions.[1][2][3] In addition to conducting airflow, the airway filters, humidifies, and warms inspired air, provides defense against pathogens through mucosal and lymphoid tissues, and contributes to phonation and olfaction.[4]
Airway anatomy underpins clinical practice in anesthesia, emergency care, trauma, and respiratory disease, where patency is fundamental to oxygenation and survival. Anatomical detail also frames surgical practice, shaping approaches to endotracheal intubation, cricothyrotomy, and tracheostomy, and influencing outcomes in procedures involving the thyroid or tonsils, where adjacent structures or tissue hypertrophy may compromise the passage of air. Thorough knowledge of airway structure and function enables accurate assessment, supports safe device placement, guides management of developmental and acquired variations, and allows rapid, effective intervention in urgent situations.
Structure and Function
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Structure and Function
Gross Anatomy
The airway is divided into upper and lower portions, reflecting fundamental differences in structure, function, and clinical relevance along its course (see Image. Respiratory System). The discussion that follows introduces the key structures in these regions.
Upper airway
The upper airway extends from the nares and oral cavity to the level of the larynx and is lined predominantly by mucous membrane. The region's central conduit, the pharynx, occupies the space between the skull base and the esophagus and is divided into 3 continuous segments (see Image. Tonsils and Throat). The nasopharynx extends posteriorly from the nares and nasal cavity, with its superior limit at the cranial base and its inferior border defined by the palate, which separates it from the oropharynx. The oropharynx lies between the palate and the hyoid bone and is demarcated anteriorly from the oral cavity by the tonsillar arch. Inferior to this component, the hypopharynx provides continuity with both the esophagus and the larynx, beginning below the level of the hyoid bone.
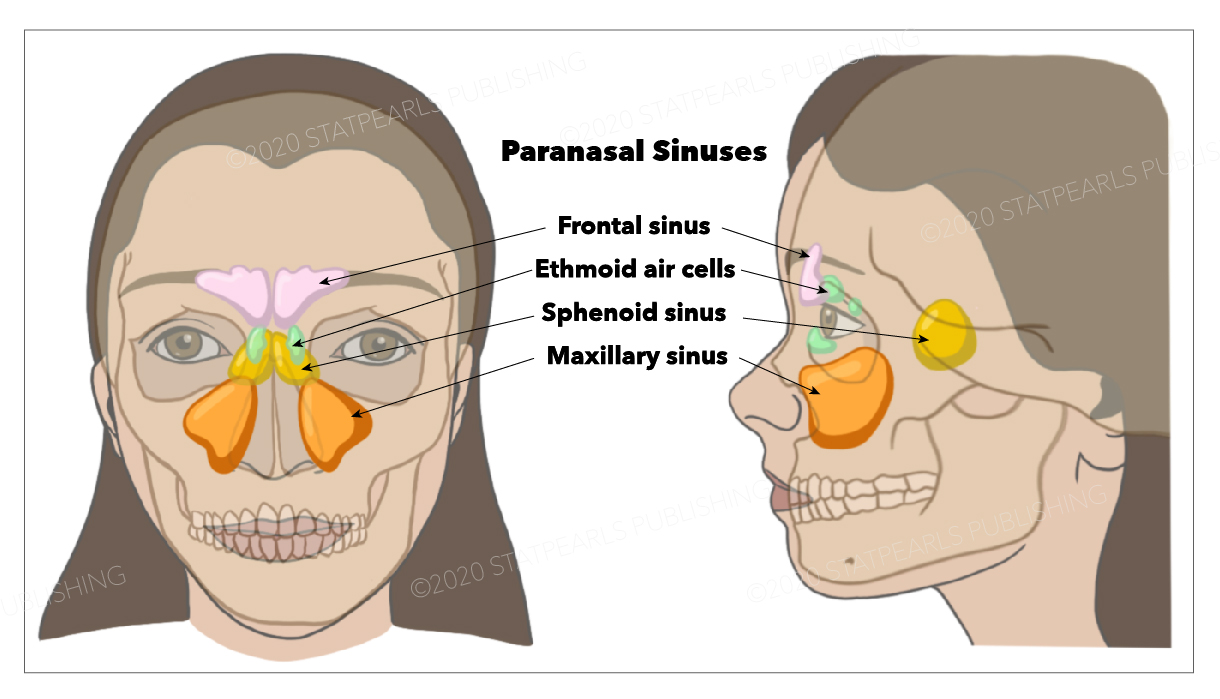
Additional components of the upper airway include the nasal cavity and turbinates, which generate airflow turbulence to facilitate filtration and humidification (see Image. Nasal Cavity). The paranasal sinuses—frontal, maxillary, ethmoid, and sphenoid—support resonance of the voice and further condition inhaled air (see Image. Paranasal Sinuses). The larynx, situated between the pharynx and trachea, consists of 9 cartilages: 3 unpaired (thyroid, cricoid, and epiglottis) and 3 paired (arytenoid, corniculate, and cuneiform). Within the laryngeal framework, the epiglottis and vocal folds form the glottic opening (see Image. Anatomy of the Larynx). Intrinsic muscles such as the posterior cricoarytenoid, the only abductor, and the cricothyroid, a tensor, maintain airway protection and enable phonation.
Lower airway
The lower airway begins at the trachea, a tubular passage lined by ciliated pseudostratified columnar epithelium and supported by C-shaped hyaline cartilage rings. This structure descends into the thorax and bifurcates at the sternal angle, giving rise to the main bronchi. The right main bronchus is wider, shorter, and more vertical than the left, making it more prone to aspiration. Each main bronchus branches into lobar bronchi, 2 on the left and 3 on the right, and subsequently into segmental bronchi that supply discrete bronchopulmonary segments.
Distal to the segmental branches, the bronchioles emerge as airways lacking cartilage and measuring approximately 1 mm in diameter. Conducting bronchioles function solely to facilitate airflow. Terminal bronchioles represent the final generation without respiratory surfaces. Respiratory bronchioles contain scattered alveoli that lead into alveolar ducts (see Image. Respiratory Zone).[5]
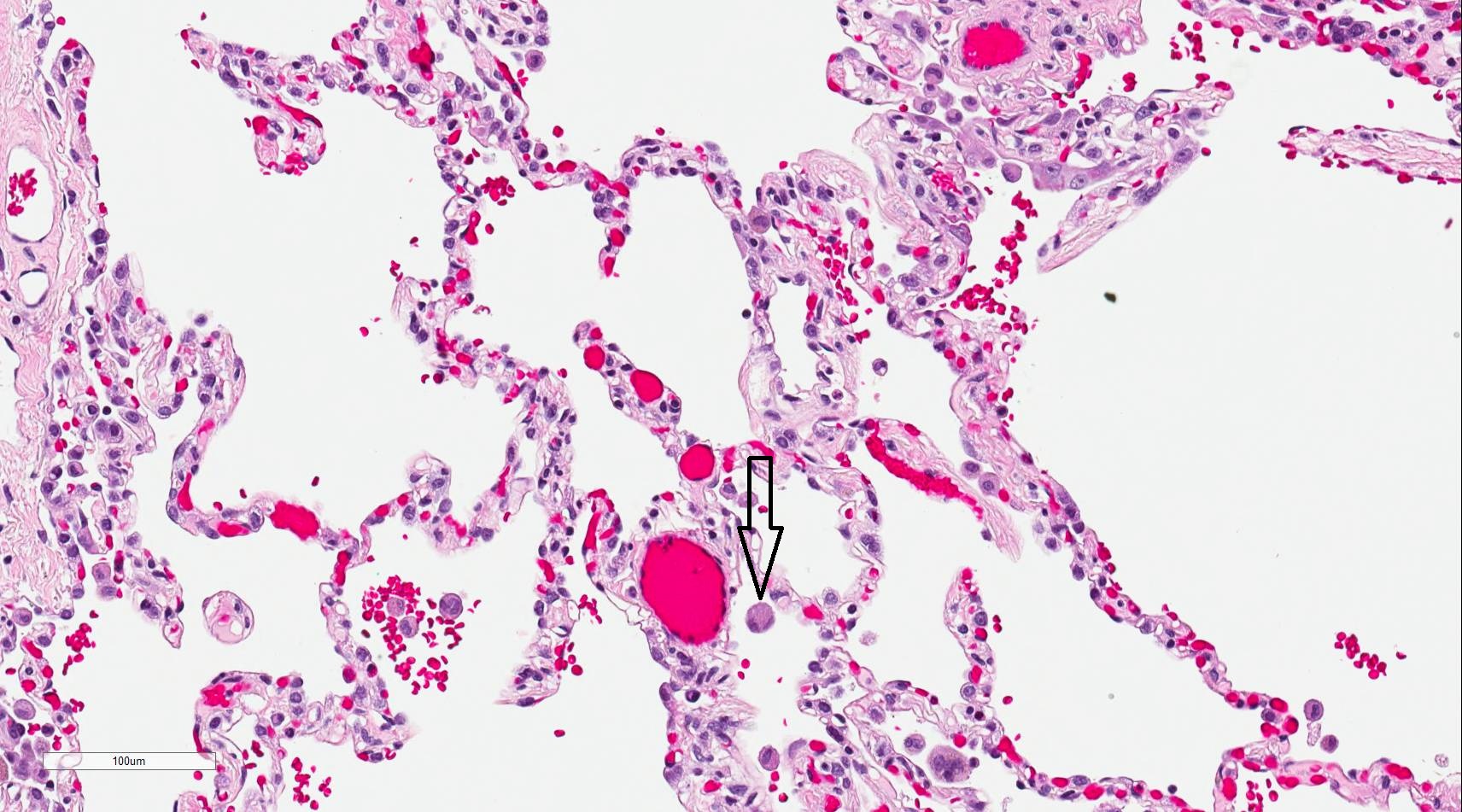
The alveolar region forms the terminal gas-exchange unit. Type I pneumocytes create the thin air-blood barrier, while type II pneumocytes regenerate the epithelial lining and produce pulmonary surfactant (see Image. Gas Exchange at the Air-Blood Barrier). Club cells contribute protective proteins such as CC16, and alveolar macrophages provide innate immune defense.[6] Alveolar ducts open into alveolar sacs, clusters of interconnected alveoli linked by pores of Kohn that equalize pressure. Together with the surrounding capillary network, these sacs establish the functional surface for gas exchange.[7]
The airway receives dual vascular supply from the pulmonary and bronchial circulations. Parasympathetic fibers from the vagus nerve mediate bronchoconstriction and stimulate mucus secretion, whereas sympathetic input from thoracic nerves produces bronchodilation.[8] Obstruction at the level of the pharynx is a frequent contributor to obstructive sleep apnea (OSA). Injury to the recurrent laryngeal nerve can compromise airway protection through impaired vocal fold function.[9]
Histology
The airways conduct ventilation from the external environment to the distal respiratory surfaces, where gas exchange sustains respiratory function.[10] Along this course, the epithelial lining undergoes characteristic transitions. The nasal cavity, trachea, and bronchi are lined by ciliated pseudostratified columnar epithelium containing goblet cells, whereas the bronchioles are lined by simple columnar or cuboidal epithelium with club (Clara) cells. At the alveolar level, type I pneumocytes establish the thin air–blood barrier, and type II pneumocytes secrete surfactant and regenerate the epithelial lining.[11] Additional specialized cells, including basal progenitor cells, brush cells, and neuroendocrine cells, contribute to epithelial renewal, chemosensory detection, and regulation of airway tone.[12]
Function
In addition to conducting airflow, the airway contributes to homeostasis by serving as a barrier and regulatory interface with the external environment. The mucous lining provides a moisture barrier, thereby humidifying inspired air and limiting water loss during ventilation. Rich vascularization, together with structural features such as the nasal turbinates, warms and conditions inspired air, thereby functioning as a thermal barrier. Protection against infection is mediated by mucosa-associated lymphoid tissue, while alveolar macrophages, dendritic cells, and club cells provide local immune defense at the level of the air-blood barrier (see Image. Alveolar Macrophage.[13]
Filtration and clearance mechanisms include nasal hairs and turbinates, which trap particulates, and the mucociliary escalator of the tracheobronchial tree, which transports inhaled material toward the oropharynx.[14] Beyond these defensive and regulatory roles, the airway also supports olfaction, phonation, protective reflexes such as coughing and sneezing, and modulation of airway resistance.
Embryology
The airway originates from the primitive foregut and pharyngeal arches during early embryogenesis. By the 4th week of gestation, the laryngotracheal groove appears and elongates into the laryngotracheal diverticulum, which becomes separated from the esophagus by the formation of the tracheoesophageal septum.[15] The laryngeal cartilages and intrinsic muscles arise predominantly from the 4th and 6th pharyngeal arches. The endoderm forms the respiratory epithelium. The surrounding mesoderm differentiates into cartilage, smooth muscle, and connective tissue.[16]
Airway and lung development progress through distinct but overlapping stages. During the embryonic stage, which spans weeks 4 to 7 of gestation, the laryngotracheal diverticulum arises from the foregut and gives rise to the primary bronchi. The pseudoglandular stage extends from weeks 5 to 16, characterized by extensive branching morphogenesis that produces the terminal bronchioles. However, respiratory bronchioles and alveoli are not yet present.
The canalicular stage, occurring from weeks 16 to 26, is marked by the differentiation of respiratory bronchioles, increased vascularization, and the initial appearance of type I and type II pneumocytes. Between weeks 26 and 36, the saccular stage begins, during which terminal sacs develop and type II pneumocytes initiate surfactant production, a critical step for potential extrauterine survival.
The alveolar stage extends from approximately 36 weeks of gestation to about 8 years of age, with continued maturation and multiplication of alveoli that progressively expand the gas-exchange surface area.[17] Alveolarization remains incomplete at birth. Ongoing alveolar development during early childhood is essential for achieving functional lung capacity.
Blood Supply and Lymphatics
The airway receives a dual vascular supply from the pulmonary and bronchial circulations. The pulmonary arteries transport deoxygenated blood to the alveoli for gas exchange, whereas the bronchial arteries deliver systemic oxygenated blood to the conducting airways and associated supporting structures. Venous return from the pulmonary circulation occurs through the pulmonary veins, while bronchial venous drainage exhibits variability, emptying into the azygos, hemiazygos, or intercostal veins, with partial contribution to the pulmonary veins.
Blood Supply
The pulmonary circulation arises from the right and left pulmonary arteries, which accompany the bronchial tree and deliver deoxygenated blood to the alveolar capillaries for gas exchange. Oxygenated blood subsequently returns to the left atrium through the pulmonary veins.[18] In contrast, the bronchial circulation provides systemic oxygenated blood derived from the thoracic aorta, including 2 left bronchial arteries and a right bronchial artery that typically originates from either the superior intercostal artery or a common trunk. This system supplies the bronchial walls, connective tissues, and visceral pleura.
Venous Drainage
Venous drainage of the airway varies by region. In the proximal airways, bronchial veins located near the hilum drain predominantly into the azygos vein on the right and into the hemiazygos or intercostal veins on the left.[19] In the distal airways, venous return occurs primarily through the pulmonary veins, creating a small physiologic right-to-left shunt as deoxygenated bronchial blood mixes with oxygenated pulmonary venous blood.[20]
Lymphatic Drainage
The lymphatic system of the airway consists of superficial and deep plexuses that provide an organized pathway for lymphatic drainage. Lymph flows sequentially from intrapulmonary nodes to the bronchopulmonary (hilar) nodes, then progresses to the tracheobronchial and paratracheal nodes before entering the bronchomediastinal trunks. These trunks ultimately empty into either the right lymphatic duct or the thoracic duct. Such pathways hold particular clinical importance in the dissemination of infection and malignancy and are central to lung cancer staging.[21]
Nerves
The airway is innervated by a complex network of cranial and autonomic nerves that collectively regulate sensation, motor activity, and airway tone. The pharynx receives contributions from cranial nerves VII (facial), IX (glossopharyngeal), X (vagus), and XII (hypoglossal), which coordinate swallowing and maintain airway patency through combined motor and sensory control. The larynx is supplied by branches of the vagus nerve, specifically the superior and recurrent laryngeal nerves, which provide motor innervation to the intrinsic laryngeal muscles and sensory input to the mucosa.[22] Recent studies have elucidated that the cranial root of cranial nerve XI (spinal accessory nerve) contributes motor fibers to the vagus nerve complex, supplying the pharyngeal and laryngeal muscles involved in swallowing and airway control.[23]
Parasympathetic fibers from the vagus nerve induce bronchoconstriction and stimulate mucus secretion, while afferent vagal pathways mediate protective reflexes such as coughing through rapidly adapting receptors and C fibers.[24] Sympathetic innervation arises from thoracic sympathetic ganglia and produces bronchodilation with decreased glandular secretion. Sensory regulation is further supported by stretch receptors in the bronchial smooth muscle and interalveolar tissues, which contribute to reflex modulation of respiration, and by juxtacapillary (J) receptors, which detect interstitial edema and trigger rapid shallow breathing.[25][26]
Muscles
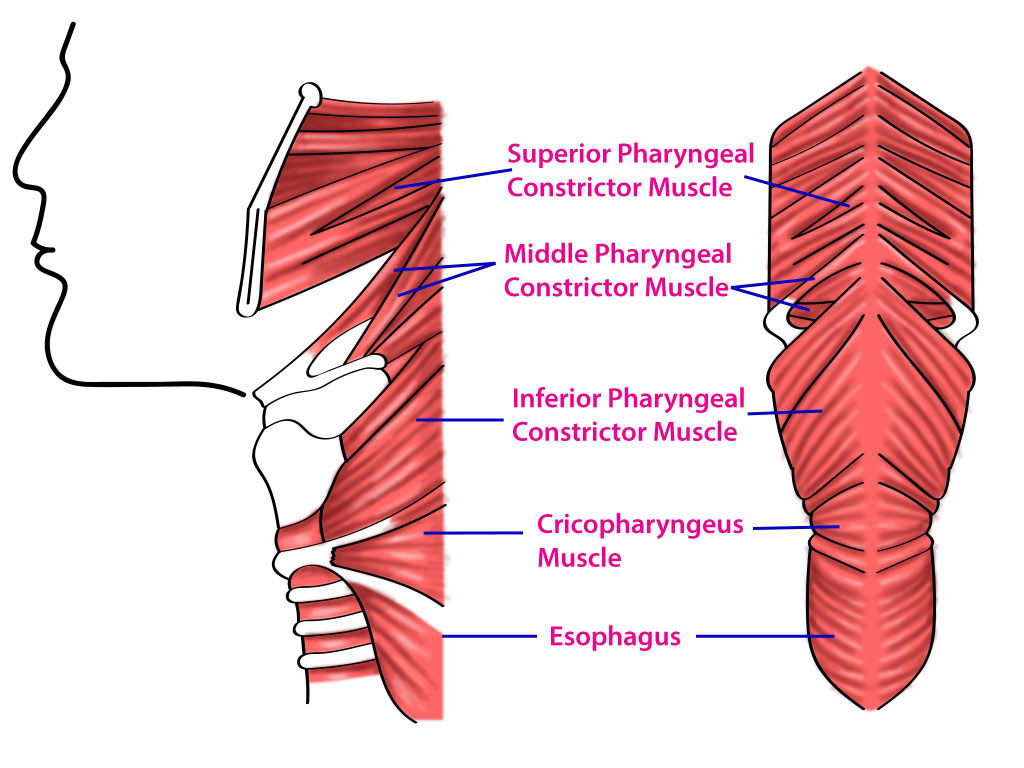
The muscles of the airway provide both structural stability and dynamic regulation of airflow and swallowing. The pharyngeal musculature consists of striated fibers under combined somatic and visceral control. The superior, middle, and inferior constrictor muscles coordinate the act of swallowing while also contributing to airway patency (see Image. Pharyngeal Constrictors).[27] The intrinsic muscles of the larynx, including the posterior cricoarytenoid, lateral cricoarytenoid, cricothyroid, and thyroarytenoid, regulate the glottic aperture, adjust the tension of the vocal folds, and ensure airway protection during deglutition (see Image. Laryngeal Muscles). Of these intrinsic muscles, the posterior cricoarytenoid is the sole abductor of the vocal cords.[28]
Extrinsic laryngeal muscles, composed of the suprahyoid and infrahyoid groups, reposition the larynx within the neck to facilitate both swallowing and phonation. In the lower airway, smooth muscle fibers are distributed throughout the conducting bronchioles and operate under autonomic control. Parasympathetic stimulation induces bronchoconstriction and mucus secretion, whereas sympathetic activation promotes bronchodilation.[29]
Physiologic Variants
Anatomic and developmental variations of the airway carry important clinical implications. The most common congenital anomaly is the tracheoesophageal fistula, frequently associated with esophageal atresia, which arises from incomplete separation of the trachea and esophagus during embryogenesis.[30] Tracheomalacia, whether congenital or acquired, results from inadequate cartilaginous support and produces excessive collapsibility of the tracheal walls.[31][32]
Bronchial anomalies may also occur, including an accessory tracheal bronchus, sometimes termed a “pig bronchus,” which originates directly from the trachea and can predispose affected individuals to recurrent pulmonary infections.[33] Developmental variation in the paranasal sinuses, such as hypoplasia or aplasia of the frontal and sphenoid sinuses, modifies upper airway anatomy but typically does not compromise function. Laryngeal differences, including a bifid or high-riding epiglottis in children, are also observed and may complicate airway management during intubation.[34]
Surgical Considerations
Knowledge of airway anatomy is essential in both trauma and surgical practice, where the preservation of airway patency is fundamental to survival. During trauma and resuscitation, airway assessment represents the first priority, as obstruction or loss of patency can quickly result in hypoxia and death.[35] The upper airway may be secured with airway devices or bypassed through endotracheal intubation, a procedure that requires precise recognition of anatomic landmarks such as the vocal cords and cricoid cartilage.
When intubation proves unsuccessful, a surgical airway may be established by cricothyrotomy, which provides rapid access through the cricothyroid membrane. However, the procedure is contraindicated in young children, in whom the cricoid constitutes the narrowest portion of the airway.[36] Tracheostomy offers a long-term solution for surgical airway access, usually created between the 2nd and 4th tracheal rings, and demands careful consideration of neighboring structures, including the thyroid isthmus and great vessels.
Surgical interventions in the neck, such as thyroidectomy or management of trauma, may precipitate airway compromise through hemorrhage, edema, or recurrent laryngeal nerve injury, potentially causing vocal cord paralysis.[37] Pathological enlargement of lymphoid tissue, as seen in tonsillar hypertrophy, can obstruct the upper airway and frequently necessitates tonsillectomy, particularly in pediatric patients with OSA.[38][39]
Clinical Significance
Airway Assessment
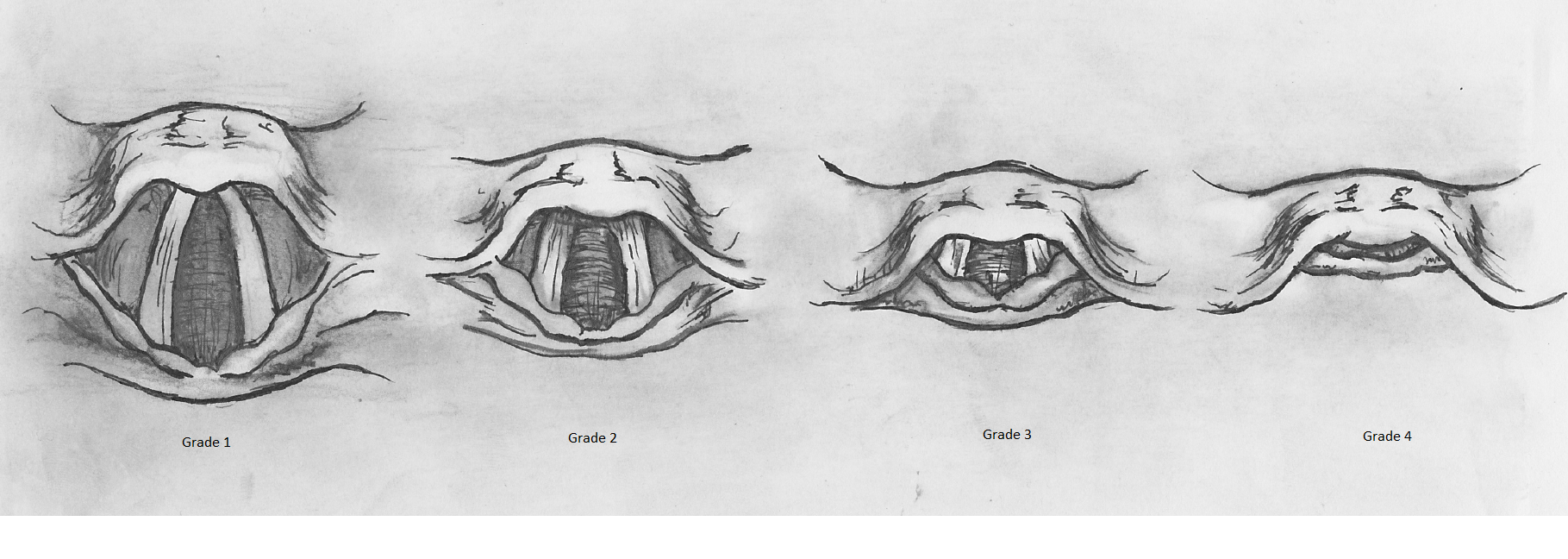
Structured evaluation of airway anatomy and visualization during laryngoscopy are central to predicting and overcoming difficulties in securing the airway. The Mallampati score and the “3-3-2” rule provide predictive frameworks for assessing potential difficulty in intubation, focusing on oral cavity dimensions, the hyoid-mental distance, and the hyoid-thyroid cartilage distance (see Image. Mallampati Score Classification).[40][41] The Cormack-Lehane classification further guides airway management in anesthesia by describing the laryngeal view obtained during direct laryngoscopy (see Image. Cormack-Lehane Grading System).[42]
When intubation is unsuccessful, emergency access may be achieved surgically, with the trachea serving as a key landmark for tracheostomy or cricothyrotomy, except where it is covered by the thyroid isthmus.[43] In pediatric patients, cricothyrotomy is contraindicated because the cricoid cartilage constitutes the narrowest portion of the airway, a developmental feature that increases the risk of obstruction and requires alternative strategies for airway management.[44]
Patient Positioning for Endotracheal Intubation
Optimal visualization of the glottis during endotracheal intubation requires alignment of the oral, pharyngeal, and laryngeal axes. Flexion of the cervical spine combined with extension of the head at the atlantooccipital joint, known as the sniffing position, reduces the angle between the line of sight and the glottic opening, facilitating passage of the endotracheal tube (see Image. Sniffing Position for Airway Alignment).[45] Placement of a pillow beneath the head and neck can assist in maintaining this alignment, particularly in adults or patients with limited neck mobility.
Airway Anatomy in Disease and Diagnosis
Structural and functional characteristics of the airway have direct implications in the pathogenesis and recognition of several common clinical conditions. For example, the right main bronchus, being wider, shorter, and more vertically oriented than the left, predisposes to aspiration, making it the most frequent site for foreign body lodgment or aspiration pneumonia.[46] Upper airway collapse during sleep contributes to OSA, a syndrome that underscores the role of pharyngeal structure in maintaining airway patency.[47] Narrowing of the lower airways due to edema, mucus accumulation, or bronchospasm manifests clinically as wheezing on auscultation, reflecting the functional importance of airway caliber in airflow resistance.[48]
Other Issues
Multiple intrinsic and extrinsic factors influence airway structure and performance, increasing susceptibility to obstruction, infection, and respiratory compromise. For instance, calcification of the laryngeal cartilages, loss of elastic recoil in lung tissue, and impaired mucociliary clearance occur with advancing age. These changes increase the risk of airway obstruction and predispose older adults to respiratory infection.[49] Differences in airway caliber and lung volume between men and women alter ventilatory mechanics. These anatomic and physiologic variations contribute to sex-specific susceptibility to certain respiratory conditions.[50]
Obesity contributes to structural and functional alterations of the airway, particularly through pharyngeal fat accumulation and diminished upper airway muscle tone. These factors predispose affected individuals to OSA and complicate airway management in clinical settings.[51] Chronic tobacco exposure leads to epithelial metaplasia, impaired ciliary clearance, and excessive mucus production. Together, these pathologic changes drive the development of chronic bronchitis and chronic obstructive pulmonary disease, underscoring the airway’s vulnerability to environmental insults.[52][53]
Congenital anomalies, including laryngeal webs, subglottic stenosis, and tracheal agenesis, demonstrate how disruptions in development can profoundly impair airway function. Although uncommon, these conditions emphasize the airway’s susceptibility to malformation and its critical role in maintaining respiratory viability.
Media
(Click Image to Enlarge)
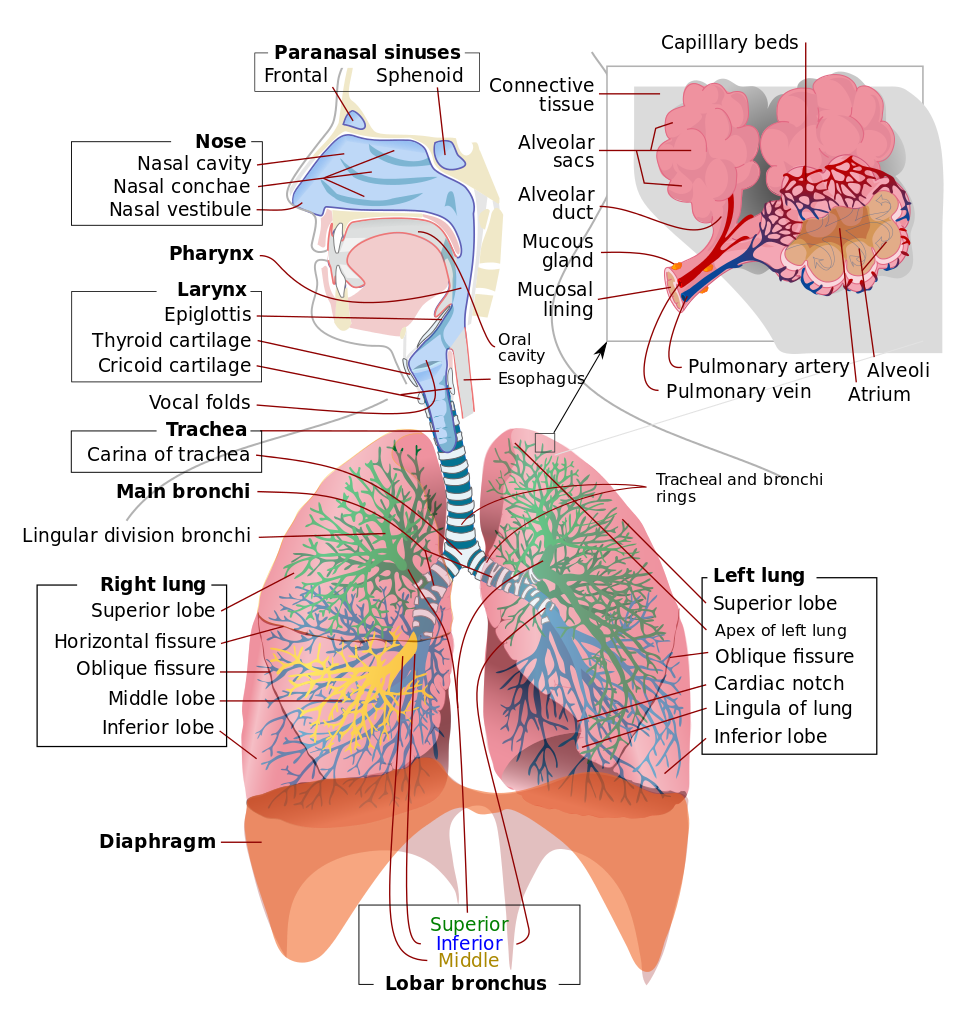
Respiratory System. This illustration depicts the upper respiratory tract, comprising the paranasal sinuses, nose, nasal cavity, nasal conchae, nasal vestibule, pharynx, larynx, epiglottis, and vocal folds. Of the paranasal sinuses, the frontal and sphenoid sinuses are included. The illustration also shows the lower respiratory region, comprised of the thyroid cartilage, cricoid cartilage, trachea, and tracheal carina, main bronchi, tracheal and bronchial rings, lobar bronchi (superior, middle, and inferior), and right and left lungs. The insert shows alveolar anatomy, which includes the alveolar sacs, alveolar duct, mucous gland, mucosal lining, pulmonary artery and vein, capillary beds, and alveolar atrium.
LadyofHats, Public Domain, via Wikimedia Commons
(Click Image to Enlarge)
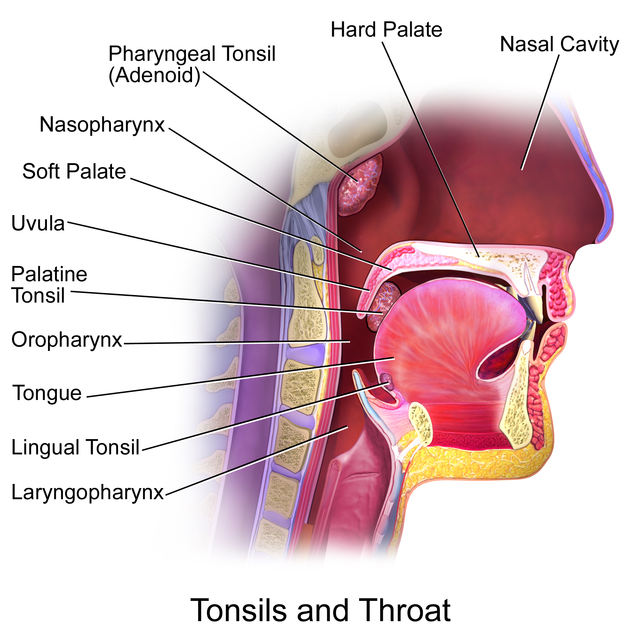
Tonsils and Throat. Midsagittal section of the upper aerodigestive tract showing the anatomical location of the palatine, pharyngeal (adenoid), and lingual tonsils relative to the nasopharynx, oropharynx, and laryngopharynx. These lymphoid structures form the Waldeyer ring and are positioned to sample inhaled and ingested antigens
Blausen.com staff. Medical Gallery of Blausen Medical 2014. WikiJournal of Medicine. doi: 10.15347/wjm/2014.010. ISSN 2002-4436. [CC BY 3.0 (https://creativecommons.org/licenses/by/3.0)] via Wikimedia Commons.
(Click Image to Enlarge)
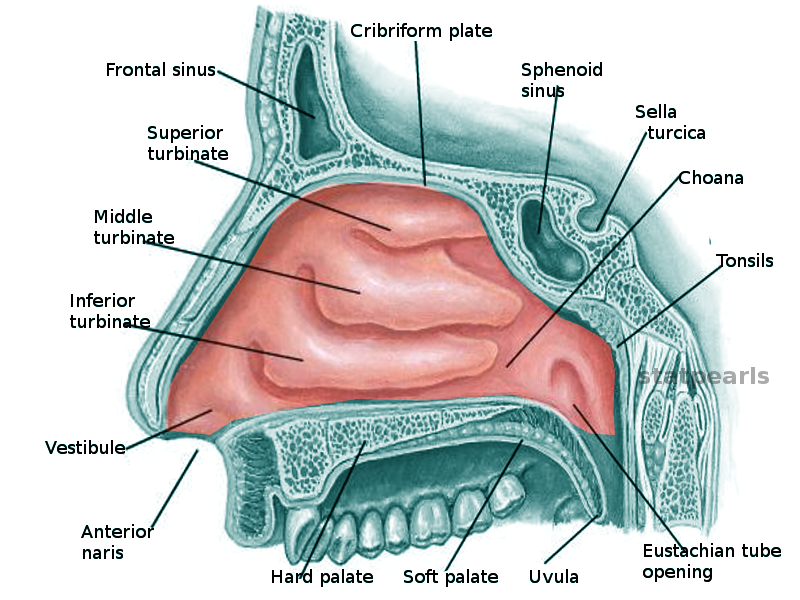
Nasal Cavity. This illustration shows the nasal cavity and the anatomic relationships between the structures in the region, which include the superior, middle, and inferior turbinates, vestibule, anterior naris, frontal sinus, cribriform plate, sphenoid sinus, sella turcica, choana, tonsils, Eustachian tube opening, uvula, and hard and soft palate.
Contributed by O Chaigasame, MD
(Click Image to Enlarge)
(Click Image to Enlarge)
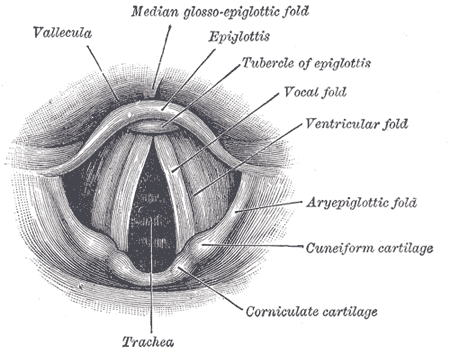
Anatomy of the Larynx. Laryngoscopic view showing the interior structures of the larynx, including the vallecula, epiglottis, tubercle of the epiglottis, vocal fold, ventricular fold, aryepiglottic fold, cuneiform cartilage, corniculate cartilage, and trachea.
Henry Vandyke Carter, Public Domain, via Wikimedia Commons
(Click Image to Enlarge)
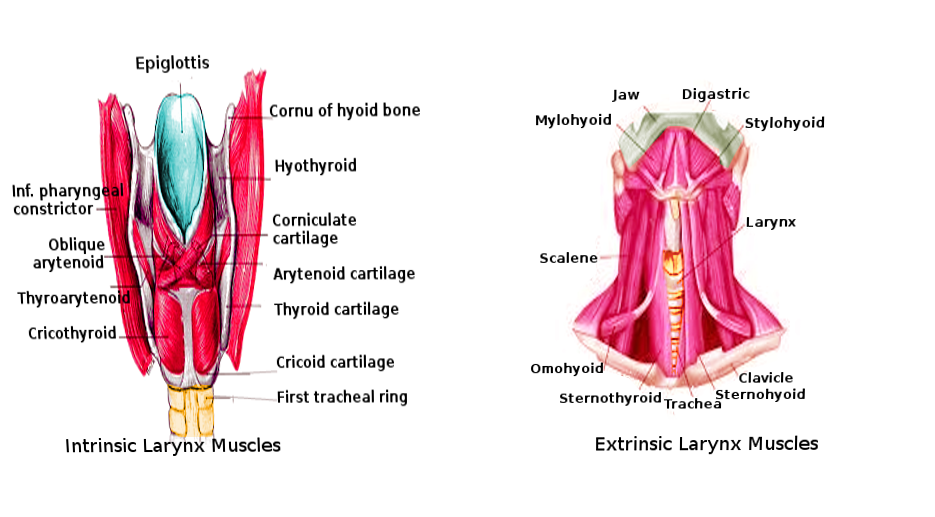
Laryngeal Muscles. These images show the anatomic relationships between the intrinsic and extrinsic laryngeal muscles. Structures in the left image include the epiglottis, cornu of the hyoid bone, hyothyroid, laryngeal cartilages (corniculate, arytenoid, thyroid, and cricoid), 1st tracheal ring, inferior pharyngeal constrictor, and laryngeal muscles (oblique arytenoid, thyroarytenoid, and cricothyroid). Structures in the right image include the jaw, digastric, stylohyoid, larynx, clavicle, sternohyoid, trachea, sternothyroid, omohyoid, scalene, and mylohyoid.
Contributed by O Chaigasame, MD
(Click Image to Enlarge)
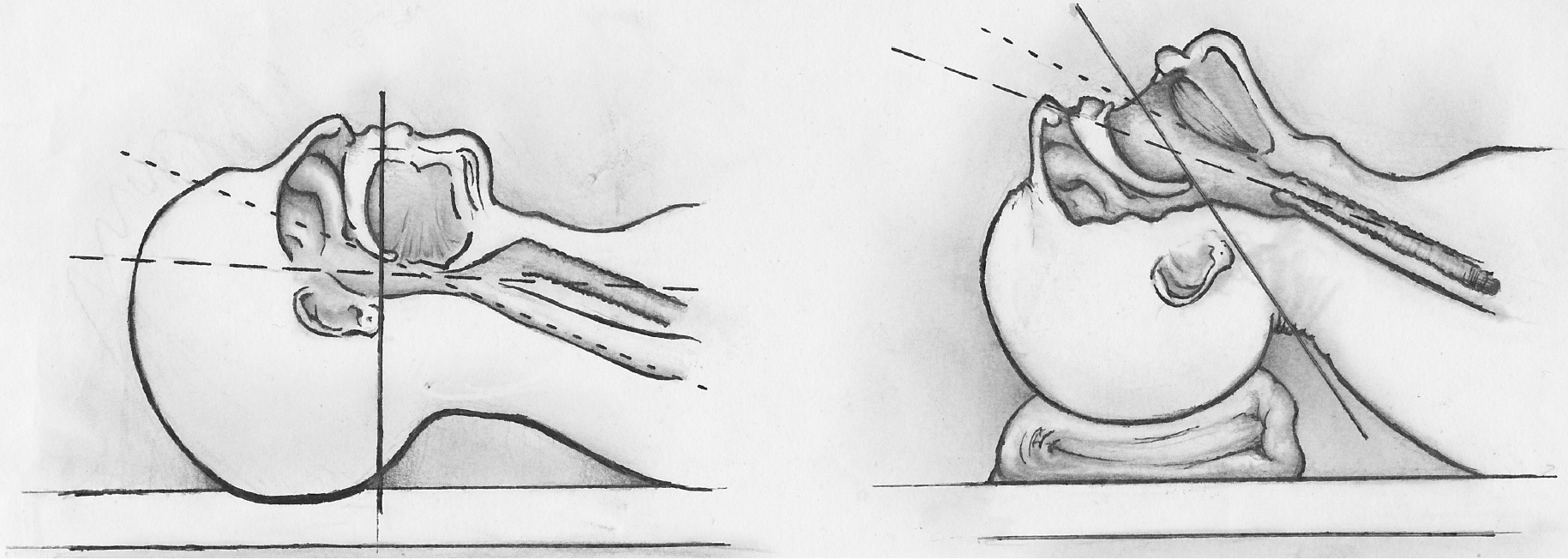
Sniffing Position for Airway Alignment. The image compares neutral head positioning (left) with the sniffing position (right), showing sagittal sections of the airway. In the sniffing position, neck flexion and head extension align the oral, pharyngeal, and laryngeal axes to facilitate intubation.
Contributed by S Hunt, DO
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
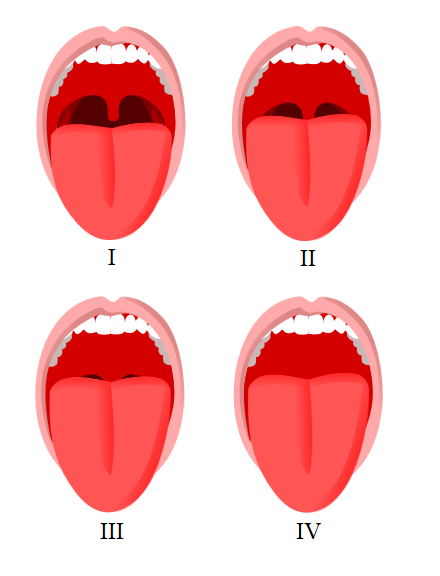
Mallampati Score Classification. This image shows the visual differences in oropharyngeal anatomy used to assign Mallampati classes during airway assessment.
Jmarchn, Public Domain, via Wikimedia Commons
(Click Image to Enlarge)
(Click Image to Enlarge)
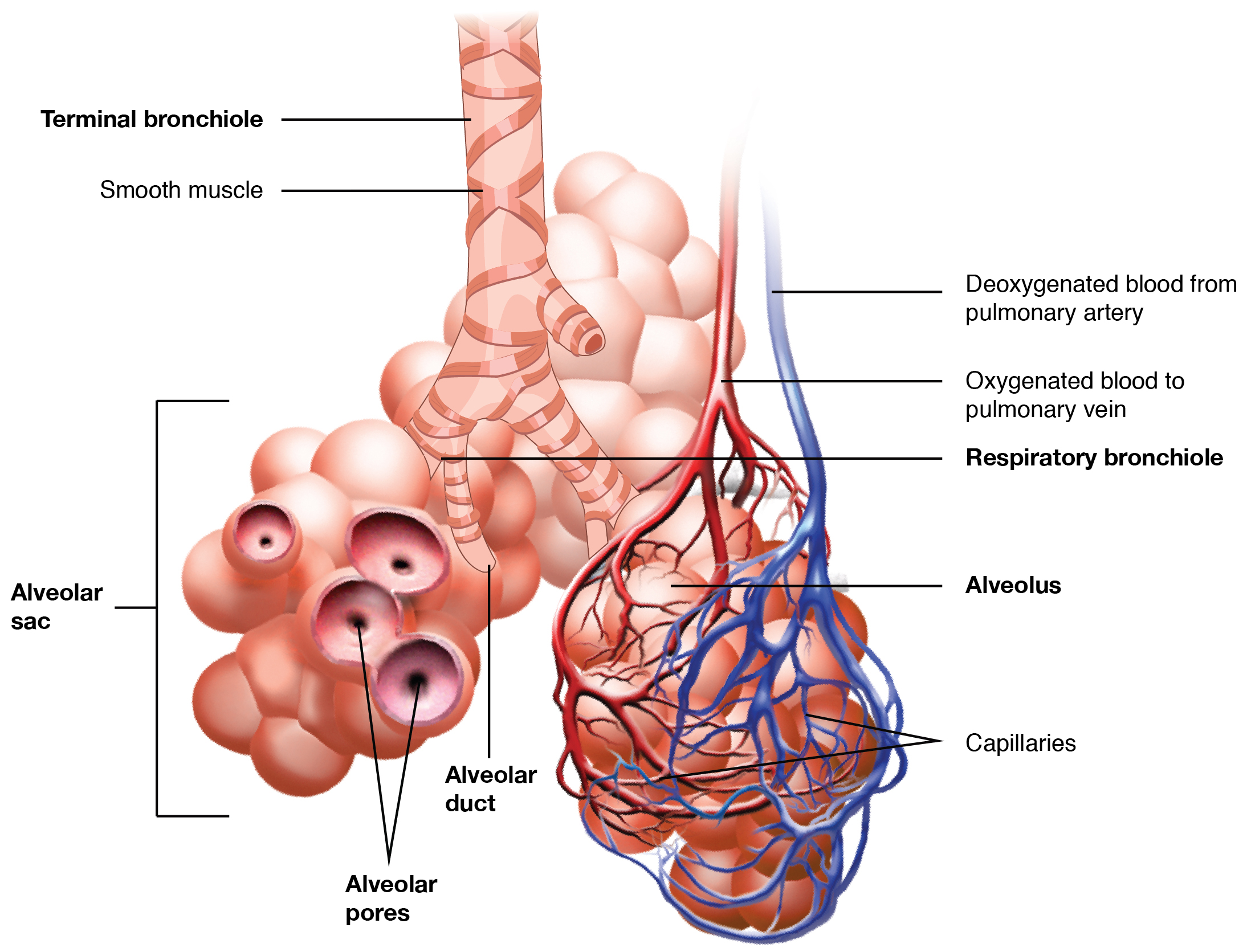
Respiratory Zone. This image depicts the transition from conducting airways to the respiratory zone, illustrating key structures responsible for pulmonary gas exchange.
OpenStax College, Public Domain, via Wikimedia Commons
(Click Image to Enlarge)
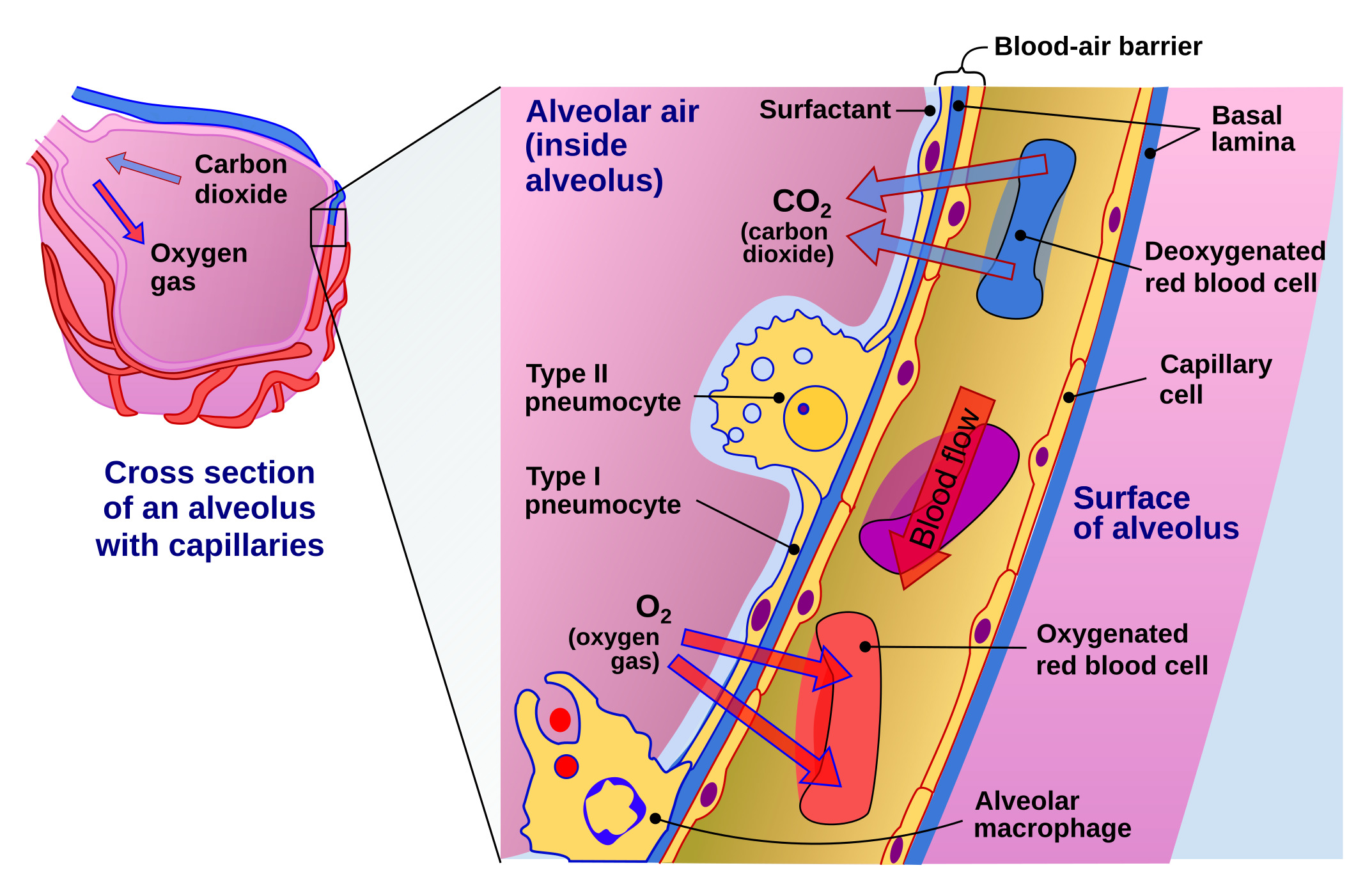
Gas Exchange at the Air-Blood Barrier. This illustration shows the alveolar wall and capillary cells forming the air-blood barrier. Oxygen and carbon dioxide diffuse across this barrier during respiration.
Delmalani18, Public Domain, via Wikimedia Commons
References
Wani TM, Bissonnette B, Engelhardt T, Buchh B, Arnous H, AlGhamdi F, Tobias JD. The pediatric airway: Historical concepts, new findings, and what matters. International journal of pediatric otorhinolaryngology. 2019 Jun:121():29-33. doi: 10.1016/j.ijporl.2019.02.041. Epub 2019 Mar 3 [PubMed PMID: 30861424]
Benner A, Sharma P, Sharma S. Anatomy, Head and Neck: Cervical, Respiratory, Larynx, and Cricoarytenoid. StatPearls. 2025 Jan:(): [PubMed PMID: 30855891]
Saran M, Georgakopoulos B, Bordoni B. Anatomy, Head and Neck, Larynx Vocal Cords. StatPearls. 2025 Jan:(): [PubMed PMID: 30570963]
De Rose V, Molloy K, Gohy S, Pilette C, Greene CM. Airway Epithelium Dysfunction in Cystic Fibrosis and COPD. Mediators of inflammation. 2018:2018():1309746. doi: 10.1155/2018/1309746. Epub 2018 Apr 8 [PubMed PMID: 29849481]
Wang Y, Wang L, Ma S, Cheng L, Yu G. Repair and regeneration of the alveolar epithelium in lung injury. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2024 Apr 30:38(8):e23612. doi: 10.1096/fj.202400088R. Epub [PubMed PMID: 38648494]
Hussell T, Bell TJ. Alveolar macrophages: plasticity in a tissue-specific context. Nature reviews. Immunology. 2014 Feb:14(2):81-93. doi: 10.1038/nri3600. Epub 2014 Jan 21 [PubMed PMID: 24445666]
Oldham MJ, Moss OR. Pores of Kohn: forgotten alveolar structures and potential source of aerosols in exhaled breath. Journal of breath research. 2019 Mar 27:13(2):021003. doi: 10.1088/1752-7163/ab0524. Epub 2019 Mar 27 [PubMed PMID: 30731449]
Estime SR, Kuza CM. Trauma Airway Management: Induction Agents, Rapid Versus Slower Sequence Intubations, and Special Considerations. Anesthesiology clinics. 2019 Mar:37(1):33-50. doi: 10.1016/j.anclin.2018.09.002. Epub 2018 Dec 19 [PubMed PMID: 30711232]
Clark CM, Kugler K, Carr MM. Common causes of congenital stridor in infants. JAAPA : official journal of the American Academy of Physician Assistants. 2018 Nov:31(11):36-40. doi: 10.1097/01.JAA.0000546480.64441.af. Epub [PubMed PMID: 30358678]
Knight DA, Holgate ST. The airway epithelium: structural and functional properties in health and disease. Respirology (Carlton, Vic.). 2003 Dec:8(4):432-46 [PubMed PMID: 14708552]
Whitsett JA, Kalin TV, Xu Y, Kalinichenko VV. Building and Regenerating the Lung Cell by Cell. Physiological reviews. 2019 Jan 1:99(1):513-554. doi: 10.1152/physrev.00001.2018. Epub [PubMed PMID: 30427276]
Crystal RG, Randell SH, Engelhardt JF, Voynow J, Sunday ME. Airway epithelial cells: current concepts and challenges. Proceedings of the American Thoracic Society. 2008 Sep 15:5(7):772-7. doi: 10.1513/pats.200805-041HR. Epub [PubMed PMID: 18757316]
Hiemstra PS, McCray PB Jr, Bals R. The innate immune function of airway epithelial cells in inflammatory lung disease. The European respiratory journal. 2015 Apr:45(4):1150-62. doi: 10.1183/09031936.00141514. Epub 2015 Feb 19 [PubMed PMID: 25700381]
Randell SH, Boucher RC, University of North Carolina Virtual Lung Group. Effective mucus clearance is essential for respiratory health. American journal of respiratory cell and molecular biology. 2006 Jul:35(1):20-8 [PubMed PMID: 16528010]
Schittny JC. Development of the lung. Cell and tissue research. 2017 Mar:367(3):427-444. doi: 10.1007/s00441-016-2545-0. Epub 2017 Jan 31 [PubMed PMID: 28144783]
Morrisey EE, Hogan BL. Preparing for the first breath: genetic and cellular mechanisms in lung development. Developmental cell. 2010 Jan 19:18(1):8-23. doi: 10.1016/j.devcel.2009.12.010. Epub [PubMed PMID: 20152174]
Proud D, Leigh R. Epithelial cells and airway diseases. Immunological reviews. 2011 Jul:242(1):186-204. doi: 10.1111/j.1600-065X.2011.01033.x. Epub [PubMed PMID: 21682746]
Hsia CC, Hyde DM, Ochs M, Weibel ER, ATS/ERS Joint Task Force on Quantitative Assessment of Lung Structure. An official research policy statement of the American Thoracic Society/European Respiratory Society: standards for quantitative assessment of lung structure. American journal of respiratory and critical care medicine. 2010 Feb 15:181(4):394-418. doi: 10.1164/rccm.200809-1522ST. Epub [PubMed PMID: 20130146]
McCullagh A, Rosenthal M, Wanner A, Hurtado A, Padley S, Bush A. The bronchial circulation--worth a closer look: a review of the relationship between the bronchial vasculature and airway inflammation. Pediatric pulmonology. 2010 Jan:45(1):1-13. doi: 10.1002/ppul.21135. Epub [PubMed PMID: 20025051]
Hsia CC, Hyde DM, Weibel ER. Lung Structure and the Intrinsic Challenges of Gas Exchange. Comprehensive Physiology. 2016 Mar 15:6(2):827-95. doi: 10.1002/cphy.c150028. Epub 2016 Mar 15 [PubMed PMID: 27065169]
Egashira R, Yamada D, Jacob J. Anatomy of Pulmonary Lymphatics and Lymphoid Tissues. Seminars in ultrasound, CT, and MR. 2025 Sep:46(4):266-271. doi: 10.1053/j.sult.2025.07.002. Epub 2025 Jul 22 [PubMed PMID: 40706642]
Brinkman JE, Toro F, Sharma S. Physiology, Respiratory Drive. StatPearls. 2025 Jan:(): [PubMed PMID: 29494021]
Roberts SO, Cardozo A. A detailed review of the spinal accessory nerve and its anatomical variations with cadaveric illustration. Anatomical science international. 2024 Jun:99(3):239-253. doi: 10.1007/s12565-024-00770-w. Epub 2024 May 2 [PubMed PMID: 38696101]
Mazzone SB, Undem BJ. Vagal Afferent Innervation of the Airways in Health and Disease. Physiological reviews. 2016 Jul:96(3):975-1024. doi: 10.1152/physrev.00039.2015. Epub [PubMed PMID: 27279650]
Castro JM, Resende RR, Mirotti L, Florsheim E, Albuquerque LL, Lino-dos-Santos-Franco A, Gomes E, de Lima WT, de Franco M, Ribeiro OG, Russo M. Role of m2 muscarinic receptor in the airway response to methacholine of mice selected for minimal or maximal acute inflammatory response. BioMed research international. 2013:2013():805627. doi: 10.1155/2013/805627. Epub 2013 Apr 18 [PubMed PMID: 23691511]
Tindle J, Tadi P. Neuroanatomy, Parasympathetic Nervous System. StatPearls. 2025 Jan:(): [PubMed PMID: 31985934]
Albahout KS, Lopez RA. Anatomy, Head and Neck, Pharynx. StatPearls. 2025 Jan:(): [PubMed PMID: 31334991]
Suárez-Quintanilla J, Fernández Cabrera A, Sharma S. Anatomy, Head and Neck: Larynx. StatPearls. 2025 Jan:(): [PubMed PMID: 30855790]
Barnes PJ. Neural control of human airways in health and disease. The American review of respiratory disease. 1986 Dec:134(6):1289-314 [PubMed PMID: 3538958]
Baldwin DL, Yadav D. Esophageal Atresia. StatPearls. 2025 Jan:(): [PubMed PMID: 32809683]
Wallis C, Alexopoulou E, Antón-Pacheco JL, Bhatt JM, Bush A, Chang AB, Charatsi AM, Coleman C, Depiazzi J, Douros K, Eber E, Everard M, Kantar A, Masters IB, Midulla F, Nenna R, Roebuck D, Snijders D, Priftis K. ERS statement on tracheomalacia and bronchomalacia in children. The European respiratory journal. 2019 Sep:54(3):. pii: 1900382. doi: 10.1183/13993003.00382-2019. Epub 2019 Sep 28 [PubMed PMID: 31320455]
Boogaard R, Huijsmans SH, Pijnenburg MW, Tiddens HA, de Jongste JC, Merkus PJ. Tracheomalacia and bronchomalacia in children: incidence and patient characteristics. Chest. 2005 Nov:128(5):3391-7 [PubMed PMID: 16304290]
Chaddha U, Chang CF, Lee C. Congenital Tracheobronchial Branching Anomalies. Annals of the American Thoracic Society. 2018 Aug:15(8):995-997. doi: 10.1513/AnnalsATS.201802-088CC. Epub [PubMed PMID: 30067096]
Rutter MJ. Congenital laryngeal anomalies. Brazilian journal of otorhinolaryngology. 2014 Nov-Dec:80(6):533-9. doi: 10.1016/j.bjorl.2014.08.001. Epub 2014 Aug 21 [PubMed PMID: 25457074]
Langeron O, Birenbaum A, Amour J. Airway management in trauma. Minerva anestesiologica. 2009 May:75(5):307-11 [PubMed PMID: 19412149]
Kovacs G, Sowers N. Airway Management in Trauma. Emergency medicine clinics of North America. 2018 Feb:36(1):61-84. doi: 10.1016/j.emc.2017.08.006. Epub [PubMed PMID: 29132582]
Biello A, Kinberg EC, Menon G, Wirtz ED. Thyroidectomy. StatPearls. 2025 Jan:(): [PubMed PMID: 33085426]
Randall DA. Current Indications for Tonsillectomy and Adenoidectomy. Journal of the American Board of Family Medicine : JABFM. 2020 Nov-Dec:33(6):1025-1030. doi: 10.3122/jabfm.2020.06.200038. Epub [PubMed PMID: 33219085]
Olszewska E, Woodson BT. Palatal anatomy for sleep apnea surgery. Laryngoscope investigative otolaryngology. 2019 Feb:4(1):181-187. doi: 10.1002/lio2.238. Epub 2019 Jan 10 [PubMed PMID: 30828637]
Rosero EB, Corbett J, Mau T, Joshi GP. Intraoperative Airway Management Considerations for Adult Patients Presenting With Tracheostomy: A Narrative Review. Anesthesia and analgesia. 2021 Apr 1:132(4):1003-1011. doi: 10.1213/ANE.0000000000005330. Epub [PubMed PMID: 33369928]
Level 3 (low-level) evidenceDetsky ME, Jivraj N, Adhikari NK, Friedrich JO, Pinto R, Simel DL, Wijeysundera DN, Scales DC. Will This Patient Be Difficult to Intubate?: The Rational Clinical Examination Systematic Review. JAMA. 2019 Feb 5:321(5):493-503. doi: 10.1001/jama.2018.21413. Epub [PubMed PMID: 30721300]
Level 1 (high-level) evidenceJunco K, Chandran SK. Anatomy, Head and Neck: Larynx Muscles. StatPearls. 2025 Jan:(): [PubMed PMID: 31424815]
Mieczkowski B, Seavey BF. Anatomy, Head and Neck, Trachea. StatPearls. 2025 Jan:(): [PubMed PMID: 28846296]
Harless J, Ramaiah R, Bhananker SM. Pediatric airway management. International journal of critical illness and injury science. 2014 Jan:4(1):65-70. doi: 10.4103/2229-5151.128015. Epub [PubMed PMID: 24741500]
Alvarado AC, Panakos P. Endotracheal Tube Intubation Techniques. StatPearls. 2024 Jan:(): [PubMed PMID: 32809565]
Marini JJ, Gattinoni L. Management of COVID-19 Respiratory Distress. JAMA. 2020 Jun 9:323(22):2329-2330. doi: 10.1001/jama.2020.6825. Epub [PubMed PMID: 32329799]
Khan RM, Sharma PK, Kaul N. Airway management in trauma. Indian journal of anaesthesia. 2011 Sep:55(5):463-9. doi: 10.4103/0019-5049.89870. Epub [PubMed PMID: 22174462]
Undem BJ, Taylor-Clark T. Mechanisms underlying the neuronal-based symptoms of allergy. The Journal of allergy and clinical immunology. 2014 Jun:133(6):1521-34. doi: 10.1016/j.jaci.2013.11.027. Epub 2014 Jan 13 [PubMed PMID: 24433703]
Thurlbeck WM. Postnatal human lung growth. Thorax. 1982 Aug:37(8):564-71 [PubMed PMID: 7179184]
LoMauro A, Aliverti A. Sex differences in respiratory function. Breathe (Sheffield, England). 2018 Jun:14(2):131-140. doi: 10.1183/20734735.000318. Epub [PubMed PMID: 29875832]
Bellizzi MG, Pace A, Iannella G, Maniaci A, Paternò DS, Tutino S, Sorbello M, Ronsivalle SM, Magliulo G, Greco A, De Virgilio A, Mancini P, Croce E, Molinari G, Lucidi D, Lechien JR, Moffa A, Caranti A, La Via L. Airway Management in Obstructive Sleep Apnea: A Comprehensive Review of Assessment Strategies, Techniques, and Technological Advances. Healthcare (Basel, Switzerland). 2025 Jul 26:13(15):. doi: 10.3390/healthcare13151823. Epub 2025 Jul 26 [PubMed PMID: 40805856]
Level 3 (low-level) evidenceKim V, Criner GJ. Chronic bronchitis and chronic obstructive pulmonary disease. American journal of respiratory and critical care medicine. 2013 Feb 1:187(3):228-37. doi: 10.1164/rccm.201210-1843CI. Epub 2012 Nov 29 [PubMed PMID: 23204254]
Thomson NC. Challenges in the management of asthma associated with smoking-induced airway diseases. Expert opinion on pharmacotherapy. 2018 Oct:19(14):1565-1579. doi: 10.1080/14656566.2018.1515912. Epub 2018 Sep 8 [PubMed PMID: 30196731]
Level 3 (low-level) evidence