Tuberculosis Prevention, Control, and Elimination
Tuberculosis Prevention, Control, and Elimination
Introduction
Tuberculosis (TB) is a preventable and, in most cases, curable disease. Nonetheless, it remains a formidable public health challenge, killing more than 1.25 million people in 2023.[1] The world's most prevalent human infection, it has affected humans across millennia and killed more people than any other disease throughout history. Long considered an infection without a known cause or treatment, disease understanding has rapidly progressed. Robert Koch discovered the Mycobacterium tuberculosis bacterium (Mtb) in 1882, and, just over 100 years later, its complete genome sequence was mapped.[2] Today, continued advancements in diagnostics, treatment, implementation science, epidemiological techniques, global cooperation, and research, amongst others, offer optimism for the prevention, control, and elimination of TB.
The causative agent of TB, Mycobacterium tuberculosis (Mtb), is a solely human pathogen, spread primarily via inhaling aerosolized droplets from an infected person. Adolescents and adults account for the majority of transmissions. Although tuberculosis most commonly infects the lungs, it is a multisystemic infection that can present with highly variable clinical findings. Of those infected with Mtb, 5% to 10% of untreated, healthy, immunocompetent individuals develop TB disease. The emergence of multiple drug-resistant TB (MDR-TB) raises the specter of untreatable illness and increasing years of life lost, particularly in low-income nations already grappling with underfunded healthcare systems.
In 1993, the World Health Organization (WHO) declared TB a global emergency when an HIV-driven increase in incidence reversed decades of progress toward global TB control.[3] In 2015, the WHO set an ambitious goal to reduce TB incidence by 90% by 2035.[WHO. End TB 2022.] However, the world is unlikely to reach its 2035 target, particularly after the devastating impacts of the COVID-19 pandemic on TB rates and recent withdrawals of political will and funding. A renewed, concerted public health effort is required to reach global and national TB elimination and eradication goals.[WHO. End TB 2022.][WHO. GTBR 2024.]
TB elimination refers to preventing disease in a specific geographic area; eradication is the permanent global elimination of disease cases and transmission. Suspected or confirmed TB disease is a condition reportable to local or state public health officials.
TB prevention, control, and elimination efforts face significant clinical- and systems-level challenges. Clinically, no effective vaccine exists across the age spectrum. TB infection (TBI) is asymptomatic and may last decades before the onset of TB disease. TB disease often presents with nonspecific symptoms that go unnoticed, facilitating high transmission rates. The Mantoux or tuberculin skin test (TST), developed in 1909, was the only available option to screen for TBI until recently, despite its known limitations in sensitivity, specificity, and the inability to differentiate TBI from TB disease. Historically, long and complex antibiotic treatment regimes, with a heavy pill burden and frequent side effects, present patient adherence difficulties. The emergence of multidrug-resistant (MDR) and extensively drug-resistant (XDR) TB further complicates treatment, necessitating specialized care and robust public health and health infrastructure.
Systems factors compound the clinical challenges. Poverty, overcrowding, malnutrition, war, and lack of public health infrastructure and universal access to healthcare perpetuate TB transmission, while global inequities hinder the distribution of newer diagnostic and treatment tools. Huge disparities in TB risk continue within and across countries. While decreased income inequality has reduced the rates of TB in low-incidence countries, TB is increasingly a disease of the marginalized in these areas.[4] Globally, families affected by TB are often left impoverished. Long latency indicates a sustained need for control efforts over an extended period. Healthcare providers will require ongoing training to maintain a high index of suspicion for TB and to exercise strong clinical judgment in differentiating TBI from TB disease appropriately. As case numbers wane, diagnostic tools, treatments, surveillance approaches, policies, and legislation must adapt. With competing health and social priorities, continued political support, funding, global collaboration, accountability, and equitable access to healthcare resources will be essential for TB control efforts.
Today, advancements in science provide hope for eliminating and eventually eradicating TB. Rapid interferon gamma-release assays (IGRAs) and nucleic acid amplification tests (NAATs) create a new global diagnostic landscape. AI-assisted technologies offer a cost-effective approach to screening for undiagnosed TB disease in high-prevalence populations. The recent addition of new or repurposed antibiotics and shorter treatment regimes offers patients increased opportunities for successful treatment completion. In resource-rich settings, whole genome sequencing (WGS) adds to the tools for antimicrobial resistance (AMR) detection and understanding TB transmission dynamics. Researchers are developing promising candidates for TB vaccines that can prevent the progression to TB disease. In the United States (US), the TB Centers of Excellence provide readily available expert advice to clinicians. Civil societies, patients, stakeholders, and advocacy groups play critical roles in improving patients' access to care and maintaining pressure on governments to clearly define and develop global and national goals, strategies, roles, funding, and mechanisms for accountability.
This activity focuses on the activities necessary for TB prevention, control, and elimination. Critically, the multiple roles and activities clinicians, the interprofessional team, and others can undertake to strengthen patient-centered and public health care, surveillance and reporting, program development, health and healthcare research, and advocacy, among others, contribute to ending TB as a human pathogen. As WHO Director-General Tedros Adhanom Ghebreyesus stated, "We have an opportunity that no generation in the history of humanity has had: the opportunity to write the final chapter in the story of tuberculosis."[5] Until TB is eradicated, it remains a risk everywhere.
This paper uses the terminology' TB infection' and 'TB disease' instead of 'latent TB' and 'active TB', respectively, as recommended by Menzies.[6] The WHO defines TBI as "a state of persistent immune response to stimulation by Mtb antigens with no evidence of clinically manifest TB disease." [7] The term' latent TB' is inconsistent with the underlying pathology of TBI, in which viable mycobacteria are held in fluctuating degrees of containment by host defenses. Guidelines from organizations such as the WHO and national health departments (eg, Canadian Tuberculosis Standards) increasingly use this terminology.[WHO. End TB 2022.]
Refer to StatPearls' related activities "Tuberculosis Overview" and "Latent Tuberculosis" for further information on the treatment and management of TBI and TB disease.
Function
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Function
Tuberculosis
Mtb is a solely human pathogen, spread primarily as an airborne aerosol from an individual in the infectious stage of the disease. Approximately 30% of individuals exposed to Mtb become infected.[8] Adaptive (T-cell) immunity (or innate immunity) contains living Mtb bacteria in 90% to 95% of individuals, resulting in TBI. The individual develops primary TB disease if the immune system is unable to contain the initial infection. Additionally, without preventive treatment, 5% to 10% of healthy, immunocompetent people with TBI will eventually develop TB disease. The highest incidence of progression to active disease occurs in the first 2 years after infection, although it can occur decades after the initial infection.[9][WHO. World TB Report 2024.] Reactivation TB is the most common form of TB disease, representing 80%-90% of cases.[2][10][11]
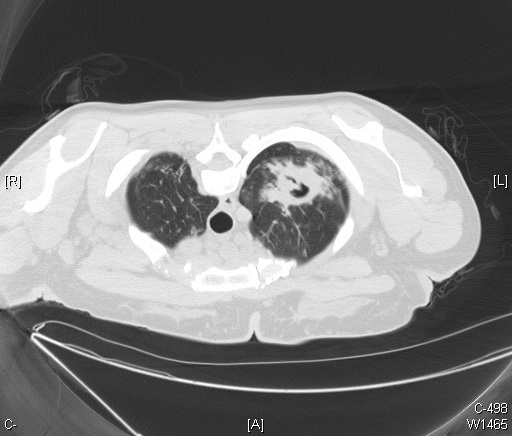
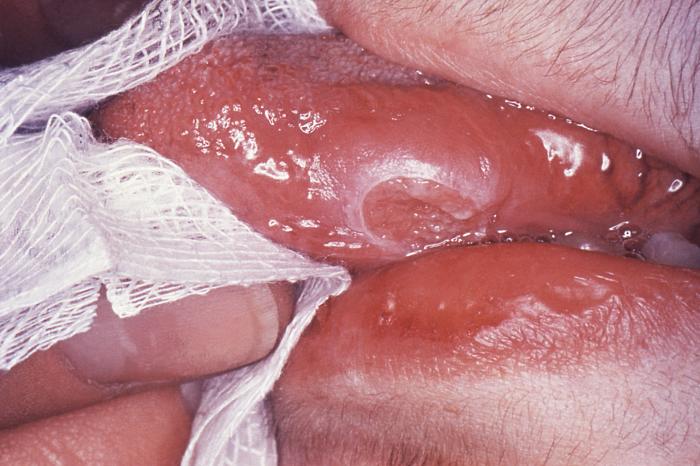
Primarily affecting the lungs, active tuberculosis is often a multi-organ disease that commonly involves the skin and the gastrointestinal, musculoskeletal, lymphoreticular, and reproductive systems.[11] Clinical symptoms include weakness, weight loss, fever, lack of appetite, and night sweats. Pulmonary TB is the most common form, presenting commonly with cough, hemoptysis, and chest pain; extrapulmonary TB presents with myriad symptoms, depending on the location of infection. Untreated TB disease kills 1 out of 2 affected people.[9]
National TB seroprevalence surveys show that as many as 50% of people with bacteriologically confirmed TB disease present without classic symptoms on questioning.[WHO. World TB Report 2024.] As these patients are not ill, they do not present for care; the diagnosis is made only through symptom-agnostic active case finding. The full significance of subclinical disease in relation to transmission, morbidity, and mortality remains unknown, with numerous clinical and epidemiological questions unanswered.
Tuberculosis and HIV coinfection merit specific mention due to unique characteristics and public health and clinical challenges.[12][13][14] People living with HIV (PLHIV) are 19 times more likely to develop active TB disease than people without HIV - increased rates of exposure, increased susceptibility to Mtb following initial or re-exposure, and high rates of reactivation appear to contribute.[15][16] Antiretroviral therapy (ART) does not completely restore Mtb immunity to baseline.[14] PLHIV are more likely to develop progressive primary TB disease within the first year of Mtb infection and are also at greater risk of developing disseminated TB than people without HIV. Based on postmortem studies, disseminated TB is often undiagnosed.[13][17] The risks of developing active TB disease increase as CD4 T lymphocyte counts decline.[13] To reduce complications and transmission, promptly identifying and treating HIV-TB coinfected individuals is of paramount importance.[17]
Childhood TB occurs most often in those under the age of 5, with the development of infection usually occurring within 1 year of infection. Childhood TB is thus an indicator of ongoing community TB transmission.
TB Burden of Disease
Global
The importance of tuberculosis as a threat to human health and development cannot be overstated. TB killed an estimated 1.25 million people (95% UI: 1.13–1.37 million) worldwide in 2023, more than any other infectious disease.[1] In 2020 alone, approximately 4000 people died every day around the globe.[18] TB causes almost double the number of global deaths as HIV.[1] TB is also the leading cause of mortality in people living with HIV. More than 80% of global TB-associated mortality occurs in low- and middle-income countries (LMICs), particularly in the Southeast Asian (45%), African (24%), and Western Pacific (17%) WHO regions.[1][19]
About 10.8 million people globally are currently living with TB disease, a slight increase from 2022.[1] Of these, 55% are men, 33% are women, and 12% are children.[WHO. Global TB Report 2024.] Additionally, an estimated 25% of the world's population has TBI.[1]
Between 2000 and 2017, the estimated global incidence rate for TB disease decreased by 1.5% yearly, with 7.1 million cases estimated in 2019. A significant decline in newly reported cases occurred in 2020 due to a decrease in TB diagnoses and treatment during the COVID-19 pandemic, followed by a notable increase in 2021, marking the first rise in 20 years. Further increases occurred in 2022 to 7.5 million cases and in 2023 to 8.2 million, the highest number of people newly diagnosed with TB since global TB monitoring began in 1995.[WHO. Global TB Report 2024] These increases likely represent a rise in cases and a backlog of diagnoses that were delayed during the pandemic.[1] Newly diagnosed TB cases in only 5 countries account for 56% of the world's total, with 26% in India, 10% in Indonesia, 6.8% in China, and 5.3% in Pakistan.[WHO. Global TB Report 2024.] Only 44% of the estimated 400,000 people ill with multidrug-resistant TB or rifampicin-resistant TB (MDR/RR-TB) were diagnosed and appropriately treated in 2023.
Despite these dismal statistics, TB prevention efforts have saved approximately 75 million lives this century.[Global TB Report 2022] The global number of annual deaths in 2023 decreased from 1.32 million in 2022, 1.4 million in 2020 and 2021, and below the 2019 pre-pandemic level of 1.34 million.[WHO. Global TB Report 2024] The increase in the annual TB incidence rate slowed to 0.2% between 2022 and 2023, down from 2.2% in each of the previous 2 years. The WHO Global TB report expressed optimism that the global TB incidence rate would slow or decrease in 2024.[WHO. Global TB Report 2024.] The gap between the number of people diagnosed with TB compared to the estimated total number of cases improved from 4 million in 2020 and 2021 to 2.7 million in 2023, which is below the 2018 gap of 3.2 million. The WHO reports an 88% treatment success rate for patients with drug-susceptible TB and an improvement to 68% for those with MDR/RR-TB. The number of TB deaths in people living with HIV has decreased from more than 500,000 in 2010 to approximately 161,000 deaths in 2023, primarily due to increased global access to antiretroviral therapy.[1]
Individuals, communities, health systems, and governments bear heavy costs associated with TB. Thirty countries have results available from surveys assessing household costs of TB between 2015 and August 2024; approximately 50% of people with TB in 2023 faced total costs during diagnosis and treatment that exceeded 20% of the annual household income, an amount the End TB strategy defines as catastrophic.[WHO. Global TB Report 2024.] Costs include direct medical expenditures, nonmedical expenditures, and indirect costs such as lost income. Catastrophic costs across countries varied widely, from 13% of households in El Salvador to 92% of Solomon Islands households.[WHO. Global TB Report 2024] Among those with drug-resistant TB, approximately 82% experienced catastrophic costs. Estimated costs ranged from US$ 76 in the Gambia to US$ 3700 in Mongolia (in constant 2024 US$).[WHO. Global TB Report 2024] Long-term complications also significantly contribute to the burden on healthcare systems. A 2022 analysis estimates that missing the WHO End TB target of a 90% reduction in TB incidence from 2015 to 2035 in Ireland, a low-incidence country, would result in 989 additional cases, 557.3 disability-adjusted life years, 35 deaths, and cost €71 million.[4]
United States
The United States also saw a recent increase in the rate of TB disease from a baseline among the lowest in the world.[20] From 1953 to 2019, TB case rates decreased more than 18-fold, from 52.6 to 2.7 cases per 100,000 population.[3] In 2023, the Centers for Disease Control (CDC) reported 9,615 new cases of TB (approximately 2.9 cases/100,000 persons), up from 8,300 cases in 2022.[21] This 16% increase marks the third consecutive year of increases after 27 years of decreases. The increases were observed across all age groups and states, as well as among both US- and non-US-born persons. The largest relative increase in case numbers and rate was in children aged 5 to 14 (42% and 45%, respectively), although the numbers are small (68 cases).[21] Based on the National Health and Nutrition Examination Survey (NHANES), 5% of US-born persons and 15.9% of persons born outside the United States have TBI, as determined by IGRA testing alone.[10]
TB among persons born outside the United States comprised 76% of US cases in 2022, representing a 16% increase; of these, people from Mexico (18.0%), the Philippines (12.5%), India (10.4%), Vietnam (8.2%), and China (5.1%) were most commonly affected.[21] While TB remains a minimal risk among US-born persons, higher rates among Native Hawaiians, Pacific Islanders, American Indians, Alaska Natives, and Black individuals reflect persistent health disparities experienced by these populations. Approximately 85% of reported cases were associated with the reactivation of TBI rather than recent transmission. HIV coinfection existed in 5% of the 83% of persons diagnosed with TB in the US in 2023 with a documented HIV status.[21]
Refer to the section on Accountability under "Improving Healthcare Team Outcomes" below for further information on the costs required to fund TB prevention, diagnostic, and treatment services.
Core TB Prevention, Control, and Elimination Activities and Functions
In the United States and other low-incidence countries, Cole et al identify 3 overarching activities that must occur to prevent, control, and eliminate TB.[3]
- Identify and complete treatment to render TB noninfectious for individuals with active TB disease.
- Find and screen persons who have had contact with TB patients. Identify those:
- who have active TB disease themselves;
- who have been infected with Mtb; or
- children and other persons at high risk who require 'window prophylaxis' (the preventive treatment of presumed TBI during the window period after exposure, before which TST or IGRA would typically become positive).
- Regularly screen and test people and populations at high risk for TBI to detect those who can most benefit from treatment to prevent active TB disease. Regular screening and testing for TB risk factors are essential for TB elimination because:
- Patients may start new immunosuppressive drugs and therapies for diverse illnesses
- Patients may have immigrated from areas where TB is endemic
- Clinicians may have diminished knowledge and reduced recognition of TB due to decreased incidence.
To effectively implement these activities, the CDC's Advisory Council for the Elimination of Tuberculosis recommends the following 12 essential components of a TB control program:[3]
- Identify the unique role and responsibilities of the public health department in tuberculosis (TB) treatment and prevention.
- Develop an overall TB control strategy, including written policies and procedures to provide guidance and oversight to facilities and practitioners involved in TB control.
- Maintain a surveillance system for timely and accurate reporting of persons with suspected or confirmed TB disease.
- Conduct routine data collection and analysis of trends within the program's jurisdiction, and apply the results to policy, planning, and prevention efforts.
- Evaluate programs, both internally and externally, to guide improvement.
- Maintain access to laboratory and radiology tests recommended for TB disease, drug resistance, and TB infection.
- Identify, manage, and treat contacts and other selected individuals infected with Mycobacterium tuberculosis.
- Manage persons with suspected or confirmed TB disease as soon as possible, begin a treatment regimen, and provide case management throughout treatment.
- Provide a thorough and timely investigation, whether investigating a source case or conducting a contact investigation.
- Ensure training and education to TB program staff, other health departments, clinicians, patients and families, community groups, and the general public.
- Work with high-risk stakeholders and populations to maximize efforts and minimize expenses. TB elimination cannot be achieved solely through public health programs.
- Participate in local, national, and international research, as program capacity permits.
While these activities may appear straightforward, the reality is that the prevention, control, and eventual elimination of TB is highly complex at local, state, country, and global levels. TB control progress requires global coordination because, until TB is eliminated globally, it can reemerge in previously eliminated areas.
Goals for TB Prevention, Control, and Elimination
Before the United Nations declared TB a global emergency in 1993, many countries saw stagnant or increasing TB incidence rates. For example, the United States experienced plateaus and increases from 1984 to 1992 due to the emergence of HIV, immigration from higher-prevalence countries, inadequate infection control in congregate settings, and reductions in funding.[3] Over time, the national and global actors increased coordination of TB control efforts, leading to the current goals and strategies.
The National Academy of Sciences' Institute of Medicine (IOM) first set US goals for TB in its 2000 Ending Neglect report.[3] Since then, increased funding has led to continual declines in TB except post-COVID. The national goal is for 95% of patients with TB disease to complete treatment within 12 months in those for whom treatment lasting 12 months or fewer is indicated. The most recent data for which completion data is available is for patients diagnosed in 2021; this shows the lowest percentage of patients completing therapy since 2008.[CDC. TB About the Data.]
The WHO developed its End TB Strategy in 2014 with a vision of a world free of TB, with zero deaths, disease, and suffering due to TB. Progress in achieving the global 2020 and 2025 milestones towards reducing the absolute number of global TB deaths by 95% and the TB incidence rate by 90% by 2035 is far from optimal (see Table 1).[WHO. Global TB Report 2024] The WHO added the removal of financial and economic barriers to diagnosis and treatment as an essential component to achieving reductions in TB morbidity and mortality. The strategy also sets high-level goals for low-incidence countries to achieve TB pre-elimination by 2035 and elimination as a public health problem by 2050.
Table 1. End TB Strategy 2025 Milestones and Progress
| Indicator | Milestone | Progress (end 2023) |
| TB incidence rate | 50% reduction by 2025 cf 2015 | 8.3% reduction |
| # of TB deaths | 75% reduction by 2025 cf 2015 | 23% reduction |
| % of TB-affected households facing catastrophic costs | Zero by 2025 | 49% reduction |
The table is adapted from the WHO Global TB Report 2024.
Progress in reducing the number of TB deaths is highly variable across countries. Progress is highest in the WHO African and European regions, at 42% and 38%, respectively. Notably, 79 countries, mainly located in these regions, have reduced mortality rates by more than 20% since 2015.[WHO. Global TB Report 2024] Thirteen countries have achieved at least a 50% reduction in TB incidence between 2015 and 2023, surpassing the End TB Strategy 2025 goals. On the other hand, the TB incidence was at least 5% higher in 2023 than in 2015 in 39 countries, including Canada, Mexico, Ukraine, and much of Oceania and South America.[WHO. Global TB Report 2024]
The WHO developed a multisector framework for TB in 2019 to ensure accountability for these goals. The United Nations also established Sustainable Development Goals (SDGs) in 2015, including a goal to decrease the incidence of TB. Other SDGs are essential to achieving global TB goals.[WHO. End TB 2022.]
(Refer to the section on Enhancing Healthcare Team Outcomes below for further information on expanding access to universal health care and other social determinants of health.)
Table 2 outlines the global milestones established at the 2023 meeting and the 2024 progress toward these goals.[WHO. Global TB Report 2024]
Table 2. 2023 UNHLM Milestones and Progress
| Indicator | Milestone | Progress (end 2023) |
| People diagnosed with TB initially tested with a WHO-recommended rapid test | 100% by 2027 | 48% |
| TB disease treatment coverage | 90% each year by 2027 | 75% |
| TB preventive treatment coverage for PLHIV | 90% each year by 2027 | 56% |
| TB preventive treatment coverage for household contacts | 90% each year by 2027 | 21% |
| Annual funding for quality prevention, diagnosis, and care for TB |
US$ 22 billion by 2027 ($35 billion by 2030) |
5.7 billion |
| Annual funding for TB research | US$ 5 billion research funding by 2027 | US$ $1 billion |
| Safe and effective TB vaccines | Vaccine rollout initiated, preferably within 5 years | 6 in phase III trials (Aug 2024) |
Table 2 is adapted from the WHO Global TB Report 2024.
Achievement of the 90% target for treatment of TB among those with the disease is equivalent to up to 45 million people treated from 2023-2027, of which 4.5 million are children and up to 1.5 million have drug-resistant TB.[WHO. Global TB Report 2024] Achieving the same treatment target for TBI is equivalent to providing preventive treatment to up to 45 million people from 2023 to 2027, of which 30 million people are household contacts of people with TB, and 15 million people are living with HIV. WHO member nations identify national targets and strategies, focusing within the global framework, depending on local epidemiology, resources, existing healthcare infrastructure, cultural factors, and other relevant considerations.
Issues of Concern
Tuberculosis Risk Factors
Risk of acquiring tuberculosis infection
The risk of acquiring TBI depends on TB epidemiology, exposure history, and comorbid conditions. While the United States and many other industrialized nations have low TB incidence, higher rates persist in certain settings and populations, such as correctional facilities, homeless shelters, indigenous peoples, immigrants, and people living with HIV.[10]
Most global TB transmission occurs in high-incidence countries in Africa, Asia, Latin America, Eastern Europe, and the Pacific Islands. High- and medium-incidence countries are defined as those with an estimated TB incidence greater than 100 and between 1 and 100 per 100,000 person-years.
Certain occupations increase the risk of TB exposure, even in low-incidence countries such as the United States. Individuals whose work requires frequent travel to high TB-incidence countries and those who work in large group settings where TB is common (eg, jails, prisons, homeless shelters, long-term care facilities) have higher TB exposure.[CDC. TB About the Data.]
While healthcare personnel no longer experience higher rates of TB than the general population, TB prevention remains vital in this setting.[22] Refer to the "Other Issues" section below for additional information on TB prevention in healthcare settings and among healthcare personnel.
Risk factors for developing tuberculosis disease
Recency of infection and immunocompromise are key risk factors for progressing to TB disease. Close contacts of a person with active TB are most likely to develop TB disease in the first 2 years after infection. Immunocompromise may be due to a wide variety of factors, such as HIV infection, genetic diseases, or medications that induce immunosuppression, including organ transplant antirejection drugs, prolonged corticosteroids, cytoreductive chemotherapy, and tumor necrosis factor-alpha antagonists. HIV infection more frequently and rapidly results in the progression of TBI to TB disease than any other risk factor, with a higher likelihood of disseminated and extrapulmonary disease.[23]
The WHO estimates that 750,000 TB deaths were due to alcohol use disorders, 700,000 to current or former smoking (affecting men more frequently than women), more than 600,000 to HIV, and 390,000 to diabetes in 2023.[WHO. GTBR 2024.] Other diseases and conditions that moderately increase TB progression risk include excessive alcohol use, malnutrition, malabsorption, gastrectomy or intestinal bypass, chronic kidney disease, leukemia, lymphoma, low body mass (≤20 kg/m2), silicosis, and older or younger age.[15][1][24]
Structural risk factors for tuberculosis transmission
Structural factors, including socioeconomic conditions, contribute significantly to TB morbidity and mortality. In a 2012 study, Ploubidis et al attributed 50% of the variability in TB incidence in low-prevalence countries to income inequality.[25] An estimated 1 million TB cases worldwide in 2023 were attributed to undernutrition.[26] Living in crowded, poor, and poorly ventilated settings all increase the risk of becoming infected with TB.[26] In the United States, the United States Preventive Services Task Force (USPSTF) notes that TB disproportionately affects people who are Black, Asian, Hispanic/Latino, Native American, Alaska Native, Native Hawaiian, or Pacific Islanders. Incidence varies across the United States, depending on geography and accommodations.[10] Some make the incorrect assumption that Indigenous people are intrinsically susceptible to TB. High TB rates in indigenous populations stem from colonization and forced displacement, resulting in high exposure rates, poor access to healthcare, and crowded living conditions, among other TB determinants.[27]
Armed conflicts, increasing food insecurity, and political and economic instability also contribute to recent global rate increases. In a 2024 trend analysis, Ayenew et al conclude the WHO End TB goals are unachievable without addressing undernutrition, forced displacement, and homelessness.[28] The 2023 United Nations High-Level Meeting (UNHLM) recognized undernutrition, stigma, and discrimination as critical drivers of TB disease and poor outcomes.
Risk factors for complications
Complications place additional burdens on many who are already at risk and can ill afford further health, social, economic, or other burdens, particularly in Low- or Middle-Income Countries (LMIC). Risk factors include socioeconomic factors such as poverty, malnutrition, and wars; immunosuppression due to HIV/AIDS, chronic immunosuppressive therapy (steroids, monoclonal antibodies against tumor necrotic factor), or a poorly developed immune system (children, older adults, primary immunodeficiency disorders), or underlying occupational disease (eg, silicosis from mining, construction, pottery, ceramics, and glassmaking, stonework, and others).
Screening and Diagnostic Tools
Recent advances in molecular methods are transforming the diagnosis of active TB and the detection of drug resistance. However, limitations in the sensitivity, specificity, and predictive capacity of widely available tools for screening and diagnosing latent TB remain major barriers to global TB prevention, treatment, and control.[29] This section provides a brief overview of issues of concern and potential opportunities related to screening and diagnostic testing.
Refer to the "Screening for Latent TB" and "Ruling Out Active TB" in the Clinical Significance section below for an outline of the clinical use and interpretation of screening and diagnostic tests. See StatPearls' companion articles, "Tuberculosis Overview" and "Latent TB," for an in-depth description.
Immune-based testing
Clinicians commonly use the TST, which has been used continuously since its development in 1909, and interferon-gamma release assays (IGRAs) to identify TBI. The TST detects M tuberculosis and other nontuberculous mycobacteria, whereas the IGRA is more specific to M tuberculosis. In contrast to tuberculin, newer skin tests based on Mtb antigens (known as TBSTs) provide the specificity and overall test accuracy of IGRAs. These tests cannot distinguish between TBI from TB disease or predict which individuals will progress from infection to active disease.
Due to cross-reactivity with the Bacille Calmette-Guérin (BCG) vaccine and, less often, nontuberculous mycobacteria (NTM), the TST has low specificity. The test is low-cost, simple to perform, and requires few resources. However, test interpretation requires sophisticated judgment based on context, such as previous BCG vaccine and the probability of prior exposure. The results must be read 48 to 72 hours following intradermal placement of purified protein derivative (PPD) by trained personnel. Test sensitivity varies with the cutoff of any induration that results. A systematic review by the United States Preventive Services Task Force (USPSTF) reports a sensitivity of 60%, 81%, and 80%, respectively, for 15 mm, 10 mm, and 5 mm cutoff thresholds.[10] The corresponding specificities are 99%, 98%, and 95%.
The IGRA measures the CD4 T-cell response to specific Mtb antigen; it does not cross-react with BCG and most NTM strains. Many sources recommend IGRA over TST due to improved specificity. However, according to pooled estimates from the USPSTF systematic review, IGRA specificity is comparable to TST, depending on the platform.[10] IGRA is 81% to 90% sensitive, which is greater than that of TSTs.
IGRA-based tests have the advantage of requiring only a single venous blood sample, with no follow-up visit necessary. Results are available in 8 to 30 hours. IGRA may be preferred for individuals with a history of BCG vaccination or those who cannot return for a follow-up visit.[8] However, IGRA use may be cost-prohibitive in LMIC countries and is more resource-intensive, requiring the availability of a laboratory and technical personnel.
The choice of a TST or IGRA for detecting latent TB comes down to resources and ease of use at the point of care. The TST remains the only test available in many parts of the world where TB is highly prevalent.[30][31] Newly developed Mtb-specific-antigen skin tests (TBSTs) are simple and require few resources. They require a follow-up visit like other TSTs and have similar diagnostic accuracies to IGRAs.[32]
Microscopy and culture
Microscopic identification of the organism in sputum or tissue samples confirms more than 50% of active TB diagnoses worldwide.[5] Microscopy has low sensitivity as the TB bacillus is slow-growing and frequently sparse within clinical samples. Sensitivity depends on the pretest probability, with higher sensitivities in higher TB prevalence areas. A lack of standardization in staining methods contributes to variable results.[33]
Culture, the gold standard for diagnosing TB disease before the advent of molecular testing, has a sensitivity of 80% to 90% and a specificity of 93%, depending on the specimen type.[34] The slow-growing and paucibacillary nature of the organism contributes to poor test performance. Further, a lack of technological capacity leads to test unavailability in many areas, particularly in LMIC. The prolonged time for result availability further limits utility, taking 2 weeks or more when using broth media and as long as 8 weeks for visible growth on solid media. Conventional drug susceptibility testing (DST) relies on culture and phenotypic testing, and can take weeks to complete.
Molecular methods
Combined with a history and physical, molecular tests using NAATs and lateral flow biomarker detection confirm TB disease in those with signs and symptoms of pulmonary TB or can increase certainty in ruling out active TB in asymptomatic individuals following a positive TST, IGRA, or other test. Furthermore, their increased ability to rapidly and accurately detect antimicrobial-resistant (AMR) Mtb increases treatment effectiveness by minimizing patient exposure to multiple antibiotics and decreasing transmission. The tests use a variety of technologies with varying specifications and recommended uses, divided into the following 3 categories:
- Initial diagnostic testing of TB with drug-resistance testing
- Initial diagnostic testing of TB without drug-resistance testing
- Follow-on testing for the detection of drug resistance after TB confirmation.[15]
As they can only detect known and common mutations, these tests do not entirely replace culture and traditional phenotypic drug resistance testing in surveillance and research.
In high-resource settings, genetic sequencing tests based on the whole genome (WGS) or targeted areas of the genome (targeted next-generation sequencing or tNGS) provide new opportunities for genotyping, cluster identification, and AMR detection.[CDC. WGS. 2024]
- Whole-genome multilocus sequence typing (wgMLST) is used for TB genotype clustering using WGS data. Each of the 2672 loci is analyzed and assigned a number; those with at least 99.7% matching loci form a genotype cluster. Detecting genotype clusters helps define and alert to possible TB clusters or outbreaks.
- Whole-genome single-nucleotide polymorphism (wgSNP) comparison identifies single-nucleotide differences or polymorphisms (SNPs) across isolates within genotype clusters, using WGS data. The number of differing SNPs between isolates provides clues about the recency of transmission in TB cases. When mapped onto phylogenetic trees, wgSNP comparisons determine transmission chains.
- WGS and tNGS identify genetic mutations associated with first- and second-line antibiotics, as well as new and repurposed drugs, such as bedaquiline, clofazimine, and linezolid.[15] By focusing on specific mutations associated with resistance, tNGS is a rapid test that may improve the detection limit for resistance-associated markers.[CDC. MDDR. 2024]
The optimal utilization of tests in different settings continues to evolve, requiring consideration of multiple variables such as the following:
- Prevalence of TB and drug resistance
- Patient population - eg, adults, children, or people living with HIV
- Testing context - eg, stand-alone or follow-on testing, centralized or decentralized services
- Pretest probability, sensitivity, and specificity
- Ease of use
- Laboratory capacity
- Cost-effectiveness - eg, higher for Xpert® and Xpert Ultra® in PLHIV and settings with higher TB prevalence, lower rates of empirical treatment, and lower pretreatment loss to follow-up.[15]
Antimicrobials and Antimicrobial Resistance
AMR is a leading cause of death due to TB and poses a threat to global public health control efforts.[18] The problem arises due to multiple factors. Most TB antimicrobials have been used for decades. TB bacteria progressively develop resistance to newly introduced antibiotics at a faster rate than the development of new ones. TB treatment historically requires complicated and prolonged antimicrobial regimes, often resulting in inadequate treatment.
Drug-resistant TB falls into several categories. Isoniazid (INH)-resistant, rifampicin-susceptible TB is the most common form of AMR TB. Rifampicin-resistant TB (RR-TB) may also be resistant to other TB drugs. MDR-TB strains are resistant to RIF and INH. Extensively drug-resistant (XDR-TB) strains are those that carry resistance to RIF, INH, a fluoroquinolone, and at least 1 of the second-line injectable drugs, such as capreomycin, kanamycin, and amikacin. Pre-XDR TB is MDR TB with the addition of fluoroquinolone resistance.[29]
INH-resistant, rifampicin-susceptible TB comprised about 10.6% of all TB worldwide in 2019. Of the 75 million TB infections diagnosed in 2022, approximately 410,000 were MDR/RR-TB.[15] Globally, approximately 13% of new cases and 17% of previously treated cases of TB were RIF-susceptible and isoniazid (INH)-resistant.[15]
The WHO conditionally recommends tNGS to detect resistance to rifampicin, isoniazid, fluoroquinolones, pyrazinamide, and ethambutol rather than phenotypic testing based on culture. If rifampicin resistance is known, the WHO recommends tNGS sequencing for a broader range of antibiotics.[15] As of August 2024, the WHO recommends new treatment regimes for people with MDR/RR-TB based on the recent focus on research and innovation. One option is a 6-month regimen of bedaquiline, delamanid, and linezolid, with the addition of levofloxacin, clofazimine, or both. The others are 9-month regimes for MDR-RR-TB without resistance to fluoroquinolones.[WHO. GTBR 2024]
The CDC has a Molecular Detection of Drug Resistance (MDDR) service accessible by phone at 404-639-2455 or by email at TBLab@cdc.gov.
Tuberculosis Vaccines
As of 2024, no effective vaccine exists for TB across the age spectrum, creating a significant barrier to TB eradication. The only licensed TB vaccine, BCG, is a live attenuated strain of M bovis that was first used in 1921.[35] It is primarily used to prevent miliary TB and Mtb meningitis in infants in countries with high TB incidence.[26]
Studies report highly variable efficacy across types of TB and populations.[26] Neonatal vaccination provides a 90% reduction in severe disease and a 59% protection against pulmonary TB. When the vaccine is given in childhood, randomized control trials (RCTs) demonstrate a protection of 92% against severe disease and 74% against pulmonary TB. Recent studies have shown that repeat BCG vaccination (Danish SSI strain) of uninfected adolescents vaccinated at birth provides about 45% efficacy in preventing TBI.[36] The BCG vaccine is not effective when given after exposure. Unfortunately, BCG vaccine protection wanes after early childhood and is poor when administered to adolescents or adults.[26] The vaccine also protects against leprosy.
The WHO first recommended universal BCG vaccination for infants and children under age 5 living in countries or settings with a high TB incidence in 1974.[26] It is ideally administered to all healthy newborn infants at birth or as soon as possible if not.[26] Countries with low or declining rates of TB can consider selective vaccination of neonates with risk factors for exposure, such as a parent with previous TB or frequent visits to a country with high TB incidence. Clinicians can consider vaccination of older individuals in specific situations. The BCG vaccine can cause lifelong false-positive TSTs, a significant drawback to its use. However, it remains the most widely used vaccine worldwide,[37] saving thousands of young lives each year. See StatPearls companion article "Bacillus Calmette-Guérin" for further information on this vaccine.
TB prevention, control, and elimination require a diversity of vaccines that are effective pre- and postexposure across the age spectrum. As of September 2023, 16 candidate adjuvanted subunit, viral vector, inactivated mycobacterial, and live mycobacterial vaccines were in clinical trials.[5][36][5] As of August 2024, 15 vaccine candidates were in clinical trials, with 4 in Phase I, 5 in Phase II, and 6 in Phase III.[1] Equitable distribution of newly available vaccines will be a key challenge.
A 2022 WHO-commissioned study presents a compelling business case for developing an effective TB vaccine in adults and adolescents.[WHO. Investment Case for New TB Vaccines] A vaccine that is 50% effective could prevent up to 8.5 million deaths, 76 million new cases, and the need for 42 million courses of treatment over a 25-year period. It would save affected households approximately US$ $4.5 billion and generate US$ $7 in economic returns to society for every US$ $1 invested, with increased productivity and avoided healthcare costs as the biggest drivers of savings.[38] Even if protection were to last only 5 years, such a vaccine would have a greater impact on TB by 2050 than an infant vaccine with 80% efficacy lasting a lifetime, as adults and adolescents account for the majority of transmission.[36]
However, the development of the TB vaccine faces many hurdles. Research is slow, risky, and expensive compared to other infectious diseases due to Mtb's slow division time and the absence of validated correlates of immune protection. Only US$ $1 billion of funding for research into vaccine and diagnostic tools for TB, compared to the US$ $5 billion deemed necessary to reach clinical TB control targets.[WHO. GTBR 2024] Parallel to vaccine development, implementation research, public engagement, and increasing trust in public health authorities are necessary to address the growing vaccine hesitancy being exported from the global north to the global south. Vaccine hesitancy is a particular concern for mRNA vaccines.[36] Additionally, LMICs lack the capacity to manufacture sufficient vaccines. The 2023 UNHLM launched a cross-sectoral TB Vaccine Accelerator Council to overcome barriers to the equitable development, licensing, manufacturing, and distribution of TB vaccines at scale.[38]
The COVID-19 Pandemic and Other Competing Health Priorities
During the pandemic, COVID-19 temporarily displaced tuberculosis as the world's most prevalent disease. Vast amounts of research, innovation, healthcare, financial, and political resources were directed toward controlling COVID-19, leading to disruptions in control of other communicable disease priorities such as HIV, TB, and childhood immunizations.
TB prevention, diagnosis, and treatment services funding dropped from close to 6 billion US dollars to less than 5 billion US dollars from 2019 to 2020 in LMIC, representing (n=132) 99% of the world's notified TB cases.[WHO. Global TB Report 2024] Decreased interest, funding, and healthcare service disruptions are estimated to have caused close to 700,000 excess deaths from TB in the 4 years from 2020 to 2023 compared to the number of deaths that would have occurred had prepandemic trends continued.[WHO. Global TB Report 2024] TB is once again the leading cause of death from infection after temporarily being unseated by COVID-19.
In addition to the many lives lost, the world also lost years of progress toward TB eradication, due to reductions in the number of people diagnosed with TB and treated in 2020 and 2021. Decreased migration also led to a lower incidence of newly identified infections in 2020 in some jurisdictions, including the United States. Worsening social determinants of tuberculosis, such as poverty and undernutrition, further contribute to losses in progress triggered by the pandemic.[18] The increased incidences of diagnosed TBI and TB disease since 2021 or 2022 likely reflect increased resources redirected back to TB and a backlog of TB diagnoses delayed due to these disruptions. While countries with significant reductions in identified TB cases, such as Indonesia and the Philippines, achieved impressive recoveries in 2022 and 2023, reestablishing prepandemic downward trends will be difficult.
Due to pandemic disruptions, many countries also experienced difficulties with case reporting and national surveillance surveys. Only 3 high TB-burden or watchlist countries had available and shared mortality data from vital registries between 2020 and 2023: the Russian Federation, China, and Brazil.[WHO. GTBR 2024] Likewise, only 3 countries completed a national TB prevalence survey from 2019 to 2023: Cambodia, India, and Timor-Leste. Analysts for the Global TB Report (GTBR) calibrated TB incidence and death estimates for 2020 to 2023 to the prepandemic period using country- and region-specific models for 49 countries that experienced disruptions. Accurate burden estimates require further surveys and up-to-date data following the COVID-19 pandemic.
Despite these dismal realities, COVID-19 also brought about the promise of a global effort to foster cooperation, financing, and rapid technological development.[35] The rapid development of the COVID-19 vaccines is one example of what is possible when health initiatives receive political and financial prioritization.
Clinical Significance
This section provides a brief overview of screening for TBI, confirming the diagnosis of TBI, screening for TB disease or active case finding, and contact investigation and outbreak management. Recommendations for screening, treatment, contact investigation, and outbreak management exist at international, national, and local levels. Patient-focused and public health clinicians should follow local guidelines, which reflect the local TB epidemiology, programming, and resources.
Recent advances have significantly altered the therapeutic landscape for the treatment of TBI and TB disease, essential components of TB control and elimination. These topics are well covered in various StatPearls companion articles. Adding new or repurposed antibiotics and shorter treatment regimes offers patients increased opportunities for successful treatment completion. By re-analyzing the results of failed non-inferiority trials examining the effectiveness of shortened TB treatment regimens using fluoroquinolones, researchers identified subgroups of patients who may benefit from fewer than 6 months of treatment. Rifamycins (eg, rifampicin) are well-tolerated and shorten treatment by optimizing anti-TB drug pharmacokinetics. People with drug-sensitive TB can receive a 4-month regimen of INH, pyrazinamide, moxifloxacin, and rifapentine, which has a longer half-life than rifampicin. Studies show children with drug-sensitive, non-severe, smear-negative TB can be treated as effectively with 16 weeks of antimicrobials instead of the usual 6-month regimen. As of August 2024, the WHO recommends a new 6-month treatment regimen consisting of bedaquiline, delamanid, and linezolid, with levofloxacin, clofazimine, or both for treating MDR/RR-TB.[WHO. GTBR 2024]
See StatPearls' companion articles, "Tuberculosis Screening," "Tuberculosis Overview," and "Latent TB Infection," for further information on administering TB tests, interpreting TB test results, populations for screening, the history, physical exam, and diagnostic testing to rule out TB disease and treatment of TBI and disease.
Identifying Populations for TB Preventive Treatment
Screening for TBI and treating identified infections is essential due to the high proportion of TB disease resulting from reactivation after TBI. In the United States, the National Society of TB Clinicians (NSTC) prioritizes the following persons for screening and treatment:
- Birth or residence in a country with a high or medium TB incidence rate, regardless of arrival year in the United States, or travel for at least 1 month in a country with a high or medium TB incidence rate
- Close contact with someone with infectious TB
- Immunosuppression, current or planned
- Other conditions or social circumstances that meet state or local criteria, including homelessness, incarceration, or occupational TB risk.(NSTC. LTBI.)
The WHO identifies similar groups, specifically the following:
- People with HIV
- Household contacts of people with TB (regardless of HIV status)
- Other people at risk.[7]
The latter category includes people initiating anti-tumor-necrosis-factor treatment, on dialysis, preparing for an organ or hematologic transplant, or with silicosis.
Neither the NSTC nor the WHO recommends routine TB screening for those with moderate risk factors for the progression of TBI to TB disease, such as smoking, diabetes, or chronic kidney disease, unless other epidemiological risk factors exist.[7][NSTC., LTBI] However, patients with these conditions found to have TBI should receive TB preventive therapy unless medical or pharmaceutical contraindications exist.
In the United States, the NSTC recommends screening all children and adults for TB risk factors at least once as part of routine primary care, with ongoing assessment for new risk factors over time.[NSTC. LTBI] People with existing or new TB risk factors should be tested, preferably with the same screening test, and treatment offered to those with TBI.
The American Thoracic Society, USPSTF, United States Centers for Disease Control (CDC), World Health Organization, and many other countries' guidelines recommend TST and IGRA as initial tests to screen for TBI.[39][40][8][40] IGRA is generally preferred in adults and older children in well-resourced settings, although TST is acceptable in most populations. IGRA requires only a single visit, but it is more expensive and requires specialized laboratory expertise and equipment. IGRA sensitivity is reduced in people with HIV and children under 2. TST and TBST are less expensive and can be performed in the field; however, these tests require a cold chain and training for test placement, reading, and interpretation.[7]
Healthcare providers screening patients for TBI must ensure adequate follow-up of all positive results and provide TB preventive treatment (TPT), preferably as part of a programmatically managed TPT system that promotes connection to care until treatment completion.[NSTC. LTBI]
Confirming TB Infection
Before diagnosing and treating a patient for TBI, clinicians must rule out TB disease in all those who screen positive to avert inadequate treatment of underlying TB disease. Ruling out TB disease requires a careful medical history and symptom review, targeted physical exam, and chest x-ray. A diagnosis of TBI is confirmed when a patient with a positive screening result has no symptoms of TB, and the chest x-ray and other diagnostic tests are negative.[7][NSTC. LTBI]
The medical history should include previous positive results from TB testing, previous and current medical conditions (with a focus on immunosuppressive conditions), current or anticipated medications (such as immunosuppressive therapy), history of TB exposure and risk factors, and the likely time of exposure (eg, recent contacts). The patient's age and pregnancy or breastfeeding status are other key factors in TBI workup and treatment decisions.
The clinician should actively assess for symptoms of TB, including prolonged cough, hemoptysis, fever or chills, night sweats, and unintentional weight loss. As TB may present paucisymptomatically, clinicians should probe for even mild symptoms. A targeted physical exam should include the oropharynx, neck, lungs, and lymphatic system, with a focus on checking for enlarged lymph nodes. Other TB symptoms may warrant examination of other organ systems.
A chest x-ray is a required part of the clinical evaluation for all patients with TBI. Clinicians must maintain a high index of suspicion in reading the chest x-ray, particularly in those with symptoms or recent exposure to TB. All patients should have a posterior-anterior chest x-ray. Additionally, children and patients with symptoms or who are immunocompromised should have a lateral chest x-ray. A normal PA and lateral chest x-ray does not rule out TB disease in those with symptoms, particularly in patients with immune compromise. Patients may require additional radiographic views or studies such as CT scans. Pregnant persons in the first trimester can usually wait until the second trimester for the chest x-ray unless HIV, immunosuppression, recent exposure, or documented conversion in the last 2 years.
As recommended in the 2024 WHO guidance document on TB diagnostics, patients in most parts of the world with signs or symptoms should undergo a rapid test for TB diagnosis, as outlined in Table 3 below.[7] The WHO recommends a variety of rapid NAATs and lateral flow biomarker detection tests for initial Mtb detection and DST over the use of microscopy, culture, and phenotypic testing, as outlined in Table 3 below. However, due to the high sensitivity of these tests and the potential for cross-reactivity, false positives can be a significant concern in countries with low incidence. Some low-incidence countries recommend the tests for all symptomatic patients, while others rely on conventional or other molecular methods for diagnosis. The United States performs WGS on all TB sputum and tissue samples for genotyping and drug resistance detection.[CDC. TBWGS]
Table 3. 2021 WHO Recommendations for TB Testing in Symptomatic Individuals
| Indication for Testing | Type of Test Recommended |
| Initial diagnostic testing of TB with drug-resistance testing |
|
| Initial diagnostic testing of TB without drug-resistance testing |
|
| Follow-up testing for the detection of drug resistance after TB confirmation |
|
Table reference: [7]
Screening for TB Disease
Increased diagnosis and care initiation for people with TB disease through systematic screening for TB disease is a central component of the WHO pillar of ensuring early diagnosis for people with TB. Waiting for patients to present to healthcare facilities once they develop symptoms misses early disease and paucisymptomatic disease. Screening for TB disease is also known as active case finding. In 2019, an estimated 2.9 million people died from TB worldwide without ever being diagnosed with TB.[41]
The WHO updated its 2013 guidelines and operational handbook in 2021 to reflect new developments, data, and modeling in TB disease screening. The guideline development group updated terminology while largely retaining existing recommendations from 2013 for TB disease screening in targeted populations. In addition to systematic symptom screening, the guideline development group added 8 new recommendations concerning tools for TB disease screening. Table 4 outlines the WHO's 2021 recommendations; the type of recommendation (existing, updated, or new), strength of recommendation (strong or conditional), and certainty of evidence (strong, moderate, low, very low) are in brackets.[41]
Table 4. 2021 WHO Recommendations for TB Disease Screening
| Screening for TB in Targeted Populations | Tools for Screening for TB |
|
1. Systematic screening for TB disease may be conducted among the general population in areas with an estimated TB prevalence of 0.5% or higher. (updated, conditional, low) |
9. Among individuals aged ≥15 years in populations where TB screening is recommended, systematic screening for TB disease may be conducted using a symptom screen, chest x-ray, or molecular WHO-recommended rapid diagnostic tests, alone or in combination (new, conditional, very low). |
|
2. Systematic screening for TB disease may be conducted among subpopulations with structural risk factors for TB, including urban poor communities, homeless communities, communities in remote or isolated areas, Indigenous populations, migrants, refugees, internally displaced persons, and other vulnerable or marginalized groups with limited access to healthcare (existing, conditional, very low). |
10. Among individuals aged ≥15 years in populations where TB screening is recommended, computer-aided detection software programs may be used instead of human readers for interpreting digital chest X-rays for screening and triage for TB disease (new, conditional, low). |
| 3. People living with HIV should be systematically screened for TB disease at each visit to a health facility (existing, strong, very low). | 11. Among adults and adolescents living with HIV, systematic screening for TB disease should be conducted using the WHO-recommended 4-symptom screen of current cough, fever, weight loss, or night sweats; those who report ≥1 of the symptoms may have TB and should be evaluated for TB and other diseases (existing, strong, moderate). |
| 4. Household contacts and other close contacts of individuals with TB disease should be systematically screened for TB disease (updated, strong, moderate). | 12. Among adults and adolescents living with HIV, C-reactive protein using a cutoff of >5mg/L may be used to screen for TB disease (new, conditional, low). |
| 5. Systematic screening for TB disease should be conducted in prisons and penitentiary institutions (updated, strong, very low). | 13. Among adults and adolescents living with HIV, chest x-ray may be used to screen for TB disease (new, conditional, moderate). |
| 6. Current and former workers in silica-exposed workplaces should be systematically screened for TB disease (existing, strong, low). | 14. Among adults and adolescents living with HIV, molecular WHO-recommended rapid diagnostic tests may be used to screen for TB disease(new, conditional, moderate). |
| 7. In settings where the TB prevalence in the general population is 100/100,000 population or higher, systematic screening for TB disease may be conducted among individuals with a risk factor for TB who are either seeking healthcare or are already in care (existing, conditional, or very low). | 15. Adult and adolescent inpatients with HIV in medical wards where the TB prevalence is >10% should be tested systematically for TB disease with a molecular WHO-recommended rapid diagnostic test (new, strong, moderate). |
| 8. People with an untreated fibrotic lesion seen on chest x-ray may be systematically screened for TB disease (existing, conditional, very low). | 16. Among individuals aged <15 who are close contacts of someone with TB, systematic screening for TB disease should be conducted using a symptom screen, including any 1 of cough, fever, or poor weight gain; or chest radiography; or both (new, strong, moderate to low). |
| 17. Among children aged <10 who are living with HIV, systematic screening for TB disease should be conducted using a symptom screen including any 1 of current cough, fever, poor weight gain, or close contact of a person with infectious TB (new, strong, low). |
Table 4 reference: [41]
Contact and Outbreak Investigations
TB contact investigations enable the early identification of TB infection and disease, providing opportunities to offer TB preventive or curative therapy, thereby reducing Mtb transmission and the incidence and severity of TB disease.[42]
The 2005 guideline from the NTCA and the CDC is the foundational document for investigating contacts of persons with infectious tuberculosis in the United States.[43] Other low-incidence, high-income countries pursuing elimination have similar guidelines appropriate to the local risk assessment, epidemiology, resources, and other factors.
Contact investigations are complicated, requiring many interdependent decisions and time-consuming interventions. The likelihood of exposure and infection determines the risk of disease transmission, influenced by various factors, including the index patient, contact, environmental, and bacteriological factors. These factors include the infective burden of Mtb, the duration and proximity of exposure, local air circulation, the virulence of the Mtb strain, and a contact's immune-impairing medical conditions, previous exposures and infections, and their intrinsic infection-fighting capacity. Clinicians can only approximate many of these factors at the time of decision-making. Thus, decisions are often based on incomplete information, compounded by suboptimal investigative tools, treatment options, and limited resources. With no established minimum safe exposure time to airborne Mtb, public health resources must focus on identifying exposed persons who are most at risk of infection or disease.
The CDC and NTCA identify 6 key steps in conducting an investigation:
- Deciding to initiate a contact investigation
- Investigating the index patient and sites of transmission
- Assigning priorities to contacts
- Diagnostic and public health evaluation of contacts
- Medical treatment for contacts with TBI
- When to expand a contact investigation.[43]
In deciding to initiate a contact investigation, patients who are positive for acid-fast bacilli on a sputum smear or have pulmonary cavities receive the highest priority, as do those who may be difficult to contact (eg, leaving the country). Only pulmonary, laryngeal, and pleural TB disease is typically transmissible; transmission from other sites occurs only in rare iatrogenic circumstances. Transmission is unusual in children younger than 10. Special circumstances, such as unusually close exposures or exposures among groups particularly vulnerable to TB disease, may warrant an investigation that public health officials would otherwise not conduct in childhood TB cases. Investigating the index patient and sites of transmission includes reviewing the medical record, interviewing the index patient, estimating the infectious period, visiting sites frequented by the patient, and obtaining a list of contacts. Developing rapport with the patient is vital in initiating a strong, ongoing therapeutic relationship. The infectious period is usually difficult to determine accurately, as symptom onset can be insidious. Experts recommend at least 3 months before the symptom onset or earlier if evidence of prolonged illness exists (eg, symptoms or advanced disease). The patient's clinicians often express concern about sharing information in the medical record with public health authorities. However, this is allowed under exemptions in the Privacy Rule of the Health Insurance Portability and Accountability Act (HIPAA) of 1996 and is essential to adequate public health action.[ASPE. HIPAA 1996]
Public health investigators use contact and exposure characteristics to prioritize contact follow-up. Symptoms, age less than 5, and immune status, particularly HIV, are the most important contact characteristics. Exposures in households, in congregate settings, or due to cough-inducing medical procedures are the highest priority. As larger spaces allow increased air diffusion, the airspace size (eg, the size of a car vs a stadium) is a key exposure factor. People sitting in the same or adjacent rows to an individual with infectious TB for at least 8 hours have a much higher risk of becoming infected with TB, demonstrating the importance of proximity to infection risk. Due to the complexity of factors determining the likelihood of transmission, the CDC and WHO do not define a set period for contact.
Case conferences are an effective way to develop the investigation plan. The infectious period, level of infectiousness, contacts, and contact cohorts for initial screening, care plan for contacts (eg, window period prophylaxis, symptomatic contacts), TB screening activities, and roles and responsibilities must be defined or confirmed. Drug-resistant TB, out-of-jurisdiction, Indigenous or incarcerated contacts, large or complex exposure sites, and evidence of transmission warrant specific discussion to ensure the implementation of appropriate measures. Public health investigators expand the initial contact cohort if they identify contacts with TBI or TB disease. In cases where primary TB is suspected, authorities conduct a 'source-case investigation,' identifying the source of infection and those potentially exposed to the index case.
Public communications, particularly if there is media interest, data management, confidentiality and consent, staffing and training, cultural competency, and social network analyses, are other critical aspects of TB contact and outbreak investigations.[BCCDC. CDC Manual TB.]
Other Issues
Tuberculosis in Healthcare Settings
This section provides a brief overview of high-level infection prevention and control measures and principles, along with specific considerations for TB screening in healthcare settings. A full description of TB in healthcare settings is beyond the scope of this article. Readers seeking further information can consult the 2005 CDC Guidelines for Preventing the Transmission of Mycobacterium tuberculosis in Healthcare Settings or local, state, or national guidelines specific to their location.[44]
Clinicians should follow relevant local, state, and federal regulations when providing occupational health services to healthcare workers. The implementation of control measures must respect the confidentiality and civil rights of patients and healthcare workers who may become infected with Mtb or develop TB disease.
Patients with pulmonary or laryngeal TB expel droplet nuclei containing Mtb when they cough, shout, or sneeze. Clinicians should maintain a high index of suspicion for pulmonary TB in patients with pleural TB. Air currents maintain the droplets in the air for a prolonged time, potentially carrying them throughout the room or building. The droplet can cause infection once it reaches another individual's alveoli. TB is not acquired from environmental surfaces; however, documented cases and pseudo-outbreaks have occurred due to inadequate cleaning and disinfection of bronchoscopes and other invasive medical equipment.[44] Other equipment, such as blood pressure cuffs or crutches, does not transmit TB. Mycobacteria demonstrate the highest intrinsic resistance to disinfection compared to other vegetative bacteria, viruses, and fungi.[44]
Healthcare workers in the United States historically experienced higher rates of TB infection and disease. However, after dramatic nosocomial outbreaks in the 1980s, healthcare organizations across high-income countries invested substantially in comprehensive TB infection prevention and control programs.[45] United States national surveillance data from 1995 to 2007 show similar TB incidence rates between healthcare workers and the general population.[22] A 2018 retrospective cohort study in a low-incidence setting demonstrated a very low TST conversion rate (0.3%) among healthcare workers, with only 7% having workplace exposure.[46]
Healthcare settings requiring TB infection prevention and control programs include inpatient and outpatient settings, laboratories, long-term care facilities, medical clinics in prisons or jails, emergency health services, and home-based healthcare settings.[44] All healthcare workers with potential exposure to TB should be included in occupational TB programming, including volunteer, part-time, temporary, and contract workers. Beyond traditional healthcare providers such as, for example, nurses, physicians, and respiratory therapists, a wide variety of other staff may be at risk for TB, including spiritual care professionals and food-service, housekeeping, or maintenance staff, among others.
Infection prevention programs roughly divide prevention measures into 3 hierarchical levels, of which administrative measures are the most important, followed by environmental controls.[44]
- Administrative measures - include, for example, having a designated person responsible for TB control, conducting a risk assessment of the setting, and education and training.
- Environmental controls - reducing the concentration of infectious droplet nuclei by controlling the source of infection through general and local exhaust ventilation and controlling the airflow to prevent contamination of adjacent areas by high-efficiency particulate air (HEPA) filtration or UV germicidal irradiation (kills Mtb bacteria with sufficient dose and time).
- Respiratory protective equipment - using personal protective equipment in known or potential high-risk exposures for TB, such as aerosol-generating procedures or transporting a TB patient.
Initiate TB airborne precautions immediately for all patients with known or suspected TB. Patients are placed in a negative pressure room until TB disease is considered unlikely, and the patient has either an alternate diagnosis explaining the symptoms or 3 negative sputum smears for AFB.[44]
TB epidemiology in many low-incidence settings no longer warrants intensive annual screening for healthcare personnel.[45] The 2019 CDC and National TB Controllers Association guidelines recommend that all workers and volunteers receive an individualized risk assessment, symptom screening, and baseline TB test before starting work in a healthcare setting.[22] The guidelines recommend against annual TB screening unless there is ongoing transmission or a known exposure. The CDC and National TB Controllers Association strongly recommend preventive TB treatment for personnel with TBI, preferably with a short-term treatment regimen. However, healthcare workers have low rates of preventive treatment uptake and completion.[46] Every effort should be made to support healthcare workers in their decision-making and adherence to treatment. The guidelines additionally recommend annual symptom screening for those with untreated TBI and annual TB education for all healthcare practitioners.[22]
Mycobacterium tuberculosis Elimination
The WHO aims to eliminate TB as a public health problem in low-incidence countries in North America, Western Europe, and the Western Pacific by 2050, defined as less than 1 case per 1,000,000 people in each nation.[WHO. End TB 2022.] The WHO has set an interim goal of pre-elimination by 2035, aiming for fewer than 10 cases per 1,000,000 people. In high-incidence countries, the End TB strategy goal is no more than 10 cases per 100,000 people by 2035, with an interim goal of fewer than 20 cases per 100,000 people by 2030. These correspond to 90% and 80% reductions in TB compared to 2015.[WHO. End TB 2022]
Despite the numerous challenges and barriers inherent to the disease, as well as other clinical, social, political, or environmental factors, achieving these goals is possible. However, countries will need to add strategies to reach TB pre-elimination and elimination goals, such as the following:[3]
- Making TB infection a reportable condition, while maintaining reportability for suspected and confirmed TB disease
- Providing directly observed therapy (DOT) to selected persons with TBI, and not only to patients with TB disease
- Broadening screening for TBI by enlisting partners such as private medical practitioners, student health services, employers, hospitalist physicians, and staff from other non-TB health department programs, while standardizing the recording of results.
- Analyzing case and infection data at least annually to provide guidance and allow targeting of subsequent testing and treatment efforts
- Maintaining public and health professional awareness of TB disease incidence and TBI prevalence through news releases, online posts, case reports, case series, epidemiologic analyses of local TB data, and presentations to state, local, and tribal medical societies and hospital staff
- Build collaborative TB initiatives with partners and collaborators in other local, neighboring, state, tribal, and federal agencies.[3]
Of note, the USPSTF bases its recommendation to screen for TB on the benefits of preventing progression to active TB in individuals, determining that screening is of moderate benefit. The task force did not consider the significant public health benefits of TB elimination when developing these individual recommendations.[8]
Enhancing Healthcare Team Outcomes
Improving TB outcomes for patients and populations requires intensive efforts across a broad spectrum of healthcare, political, research, and civil society actors. With delayed diagnoses driving transmission and high rates of preexisting or disease-induced financial destitution in many places, few diseases better exemplify the need for social protection, poverty alleviation, and universal healthcare coverage. Political will, adequate resources, regulatory frameworks for case notification, vital registration, rational use of medicines, and infection control are needed.[WHO. End TB 2022] For effective control, services must continue uninterrupted during times of war and political chaos. Health authorities and professionals must closely integrate biomedical and public health interventions to ensure early diagnosis of TB with universal drug-susceptibility testing and systematic screening of contacts and high-risk groups. All patients require treatment with support for adherence, for example, financial support and integrated services for patients coinfected with TB and HIV. Patients require management of comorbidities and TB-associated impairment. Persons at high risk of developing TB also need treatment and support, and vaccination against TB as appropriate to the local context.[WHO. End TB 2022] Intensified research and innovation efforts contribute to the discovery and implementation of new tools, interventions, and strategies. The paragraphs address key aspects of TB prevention, control, and elimination.
Interprofessional Care
Effective communication and collaboration between acute care and public health practitioners form the clinical backbone for TB prevention, control, and elimination. Public health, respiratory, infectious disease, laboratory, primary care, and other clinicians work closely together to improve TB services and care for individual patients. Primary care practitioners are critical to identifying patients at risk of TB and in need of screening due to social or medical risk factors. Infection control nurses and physicians initially reporting a TB case are essential collaborators for public health practitioners gathering background information regarding the patient and the circumstances of the illness during a contact investigation. Collaborative efforts for prevention and control of HIV and other comorbidities are critical to TB prevention and control.[WHO. End TB 2022] Laboratory and radiology specialists provide invaluable insights for accurate diagnosis and effective treatment. Healthcare providers also play a key role in advocacy and supporting civil society efforts by contributing to organizations that monitor, serve populations disproportionately affected by inequities, champion healthy public policies, or support research and innovation.
Civil society should be considered an integral component of the healthcare team for TB prevention, control, and elimination efforts. Bringing people and communities affected by TB together with clinical and program directors improves all aspects of TB care. Civil society involvement can foster trust in healthcare providers, expand the reach of educational programming, facilitate patient-centered research, enhance access to and quality of care for diagnosis and treatment, and inform surveillance and monitoring structures. The UN recognizes the importance of partnerships between public health programs, other healthcare providers, people living with TB, and communities at high risk of TB in improving TB care under its Sustainable Development Goals. Further, these recommend integrating TB programs into broader health systems, for example, integrating management and treatment of HIV and TB.
The TB Care Cascade
The TB care cascade is a framework for conceptualizing and evaluating clinical TB screening efforts. Individual and population-level screening are only effective if patients complete TPT. However, programs lose many patients along the care continuum, with fewer than half of patients identified with TBI completing treatment. Losses occur at each step in the pathway, from screening to initial connection with medical care, medical evaluation, informed patient consent for treatment, initiating and maintaining treatment, and finally, treatment completion. The majority of losses occur before patients start TPT.[6] Multi-drug antibiotic regimes, prolonged courses of administration, adverse drug effects, and emergence of drug resistance create barriers to successful TPT completion, even in places with robust public health infrastructure. Identifying and correcting cascade losses requires an interdisciplinary approach, based on programmatic evidence. Patients not identified as requiring screening or in whom the diagnosis is unsuspected are other lost opportunities for TB prevention, control, and elimination.
Even where screening links patients to care and treatment is offered, patients may decline. In 2010, Horsburgh et al found that 17.1% of 720 subjects receiving TST and offered treatment in the United States and Canada declined, even at clinics that provide both services.[47] People in contact with a patient with infectious TB were less likely to decline (OR 0.19, 95% CI 1.75-12.9), while healthcare employees were more likely (OR 4.74, 95% CI .07-.5). For those that started treatment, risk factors for noncompletion included starting the 9-month isoniazid regime, living in a congregate setting such as a nursing home, shelter, or jail, injection drug use, age older than 15 years, and employment at a health facility (OR 1.37).[47]
Research and innovation can support treatment adherence. With relatively little operator training and input, rapid tests can provide timely, highly sensitive, and specific information, immediately impacting direct patient care and public health actions.[15] Shorter treatment courses can potentially increase adherence to TB prevention and treatment regimes, for example, a regimen of isoniazid and rifapentine once per week for 12 weeks for prevention.[1]
Observational studies demonstrate that well-organized programs can achieve TPT completion rates of 50% among those potentially eligible.[6] Nurse case management, using incentives and enablers, and patient-centered treatment administration are important components. Local nurse-management services in patients' communities can support primary care providers in linking patients to TB management systems.
Ethical Considerations
Disproportionately affecting people with little power and few resources, ethical considerations are of critical importance in individual patient care and developing policies and programs for the care, control, prevention, and elimination of TB.[WHO. End TB 2022] A wide variety of ethical frameworks exist for implementing public health interventions, distinct from biomedical ones.[48][49][50] Norms such as the balance of harms and benefits of interventions apply to both; additional public health norms include the impacts on autonomy and equity, as well as the expected efficiency of proposed policies and programs.[50]
When considering quarantining an individual to ensure treatment compliance, the patient's healthcare team must first be sure that the measure is the least restrictive and least coercive available.[49] Public health colleagues working with clinicians must exhaust all options to encourage voluntary treatment compliance. In unusual cases where public health authorities issue a quarantine order, they should assist individuals in complying by addressing the burdens placed upon them, such as providing affordable medications, income assistance, and other necessary measures, whenever possible. Social workers can help identify available supports.
Human rights-based approaches are also helpful for developing TB programs and policies. Health, access to high-quality care, and social protections are human rights. Progress on these rights, among others, is necessary to reduce the risk of TBI, progression to TB disease, TB transmission, and the development of complications and disability from TB. Human rights-based approaches require the principles of nondiscrimination, equity, participation, inclusion, and accountability. Currently, the heavy burdens from the costs of medications, lost income, stigma, and social isolation are inadequately addressed.
The End TB Strategy
Effective TB prevention, control, and elimination require coordinated, mutually supportive, and broadly accepted actions at national and global levels. The most recent UN End TB Strategy, updated in 2022, refocuses on essential components to reverse the unfavorable TB trends worsened by the COVID-19 pandemic.(WHO. End TB 2022.) The strategy identifies 4 underlying principles that highlight the complexity of TB prevention, control, and elimination:
- Government stewardship and accountability with monitoring and evaluation
- Strong coalition with civil society organizations and communities
- Protection and promotion of human rights, ethics, and equity
- Adaptation of the strategy at the country level, with global collaboration.[WHO. End TB 2022]
The WHO identified the following pillars and components necessary to achieve these goals:
- Integrated, patient-centered care and preventionA. Early diagnosis of TB, including universal drug-susceptibility testing, and systematic screening of contacts andhigh-risk groupsB. Treatment of and patient support for all people with TB, including drug-resistant TBC. Collaborative TB/HIV activities, and management of comorbiditiesD. Preventive treatment of persons at high risk, and vaccination against TB
- Bold policies and supportive systemsE. Political commitment with adequate resources for TB care and preventionF. Engagement of communities, civil society organizations, and public and private care providersG. Universal health coverage policy, and regulatory frameworks for case notification, vital registration, quality and rational use of medicines, and infection controlH. Social protection, poverty alleviation, and actions on other determinants of TB
- Intensified research and innovationI. Discovery, development, and rapid uptake of new tools, interventions, and strategiesJ. Research to optimize implementation and impact, and promote innovations.[WHO. End TB 2022]
Surveillance and Reporting
Surveillance and reporting are crucial for monitoring improvements in prevention, treatment, and control services at the patient, program, and population levels. Confirmed and/or probable TB globally reportable conditions to public health authorities by physicians and/or laboratory clinicians; however, resources do not always allow complete reporting. Reporting to public health allows contact, outbreak, and source investigations. Local and regional-level program directors use line-listed data for program planning and quality improvement. Surveillance is used to monitor progress toward national or international targets, ensure accountability, and adapt goals, targets, and strategies as needed.
National and world health authorities typically calculate TB disease incidence using case notification data complemented by population-based surveys and TB inventory studies where robust reporting infrastructures are absent. Between 2010 and 2019, 29 countries, accounting for two-thirds of the global TB incidence, implemented population-based studies, and 10 countries, representing 17% of the global TB incidence, conducted TB inventory studies.[WHO. GTBR 2024] TB mortality estimates use cause-of-death data from national or sample vital registration or mortality surveys.
Efforts to improve surveillance and reporting are underway. For example, Indonesia utilized national TB inventory studies to demonstrate a significant reduction in underreporting of new TB diagnoses from 2017 to 2023, with estimates based on capture-recapture methods aligning consistently with model-based estimates in 2023. Other countries are interested in or planning national TB prevalence surveys in the upcoming years.[WHO. GTBR 2024.] Donelle et al promote the widespread use of digital, case-based, real-time TB surveillance to increase the resilience of surveillance systems during crises such as the COVID-19 pandemic.[51] However, many countries face technical, financial, political, human resource, and administrative barriers to implementation.
The CDC established the National TB Molecular Surveillance Center in 2018. It houses whole genome sequencing (WGS) data from all new TB cases reported in the United States since 2018, as well as retrospectively genotyped isolates collected before 2012 from select clusters.[CDC. TBWGS]
Political Will, Accountability, and Funding
Political will, accountability, and funding remain key barriers to adequate TB prevention, control, and elimination. Every 5 years, UN high-level meetings (UNHLM) monitor progress towards the End TB Strategy, reset specific screening, diagnostic, treatment, and other goals, update actions, focus strategies, and reinvigorate commitment for TB control. Participating governments, health leaders, and civil society gathered in 2018 and 2023, with the next meeting set for 2028. (Refer to "Goals for TB Prevention, Control, and Elimination" in the Function section for more information on progress towards measurable targets within the multisector accountability framework.)
Beyond measurable targets, the recent UNHLM further demonstrated progress towards its targets by the following:
- Strengthening the language of the need to roll out a new, effective TB vaccine
- Recognizing the gendered impact of TB
- Emphasizing multisectoral approaches to address the social determinants of health
- Commitment to 100% of people with TB having access to a health and social benefits package.
UNHLMs for pandemic prevention, preparedness, and response, as well as universal health coverage, were held concurrently with the UNHLM for TB, allowing for the alignment of complementary political agendas. For the first time at a health-related UNHLM, nations recognized "the right to enjoy and share the benefits of scientific progress."[18]
However, comments and criticisms following the 2023 UNHLM indicate that, while there is room for optimism, continued work is needed to improve political, funding, and accountability structures, legal and regulatory frameworks, multisectoral engagement, and healthier living environments. The UNHLM political declaration reflected a compromise agreement, but political will, funding, and robust accountability mechanisms remain lacking.[5] Two examples include the attendance of only 2 heads of government at the UNHLM, most concerningly by leaders from the highest-burden countries,[5][18] and replacing the well-defined and measurable "catastrophic costs" metric, a key target of the WHO End TB strategy, by "financial hardship" with no specific indicator or threshold.
In 2023, domestic funding continues to decrease across Brazil, Russia, Indonesia, China, and South Africa (BRICS countries), with steadily low international donor funding available. While international donor and domestic funding recovered in the 28 highest TB-burden countries outside of BRICS, all other LMIC face continued suppressed domestic funding and relatively steady, low international donor funding. Inadequate funding for TB prevention, diagnosis, and treatment services has continued after the sharp drop in the first year of the COVID-19 pandemic.[WHO. GTBR 2024] However, Millington notes that "the pandemic cannot be blamed for the chronic neglect of TB",[18] as less than half of the funding envisaged for prevention, treatment, research, and innovation since 2018 was realized and global funding levels are only 26% of the 22 billion envisioned by 2027.[18]
An additional concern is that while many low-burden countries recognize that investing resources in global TB control and eventual elimination is an important strategy for national TB control, many have not demonstrated the political will, funding, and accountability to address pockets of populations highly burdened by TB within their borders adequately, for example TB in homeless populations or indigenous communities.
Addressing Structural Factors that Lead to TB Transmission
Many factors necessary for TB control lie outside the direct control of the interprofessional healthcare team but serve as avenues for advocacy at local, national, and global levels. The target of a 75% reduction in TB deaths in 2025 compared to 2015 requires reducing the Case Fatality Rate (CFR) to 6.5% by 2025.[WHO. GTBR 2024] This reduction is feasible only if everyone with TB can promptly access diagnostic and treatment services. As such, the goal of universal access to healthcare (SDG 3.8) is critical to the End TB Strategy. This goal states, "Achieve universal health coverage, including financial risk protection, access to quality essential healthcare services and access to safe, effective, quality and affordable essential medicines and vaccines for all" by 2030. The United Nations monitors this SDG using universal healthcare coverage, as measured by the Service Coverage Index (SCI, ranging from 0 to 100), and catastrophic health expenditures, defined by the World Bank as spending greater than 10% of total household expenditure or income on health.[WHO. GTBR 2024]
The service coverage index increased steadily worldwide and across all regions from 2000 to 2019. Still, it stalled between 2019 and 2021 in most regions and countries, the most recent year for which data is available.[WHO. GTBR 2024] The proportion of the global population experiencing catastrophic expenditure on health also rose, from 11.4% in 2010 to 13.5% in 2019, representing 794 million and 1.04 billion people worldwide. For 7 high TB-burden countries, more than 15% of the population faced high expenditures in the years the SCI was assessed.
The SDGs, which aim to eliminate poverty and hunger, reduce gender and other inequalities, and promote broader health and well-being goals, are also crucial for achieving the objectives outlined in the Global TB Strategy. Reporting on these is beyond the scope of this paper. In many cases, the ability to achieve the SDGs lies outside the control of individual nations and is rather within the purview of the global community. Where the scope lies within countries, some possess the political will and accountability but lack funding.
A critique of the 2023 UNHLM is that the pledge to address social determinants of health lacked targets and clarity, particularly concerning undernutrition, stigma, and discrimination.[18] TB stigma is the perceived risk of Mtb transmission from people who are infected, exacerbated by TB's association with HIV, poverty, social class, and certain behaviors.[1] Nations can strengthen legal and social policies to reduce stigma, discrimination, inequalities, and human rights violations.
Many countries implement measures beyond the SDGs to reduce TB burdens and improve treatment outcomes for people with TB and their households, thereby increasing the probability of attaining national TB goals. In 2024, 122 countries reported specific national policies related to social protection for people with TB and their households, including free access to TB diagnosis (118 countries) and treatment (122 countries), treatment adherence enablers (87 countries), cash transfers (35 countries), food security support (64 countries), and loss of income compensation (47 countries).[WHO. GTBR 2024]
Research and Innovation
In 2020, UN member states adopted the WHO's global TB research and innovation strategy.[52] While technological breakthroughs are required to reach 2027 targets, current TB research funding (US$ $1 billion) is one-fifth of what is needed.[1]
TB vaccines, rapid diagnostics, and TB drugs are priorities for research. TB vaccines are necessary to prevent infection, prevent progression to disease, and improve treatment outcomes, thereby accelerating reductions in TB incidence and mortality and achieving targets. The COVID-19 pandemic serves as a model for successful vaccine development and deployment, facilitated and accelerated by economic investments and regulatory frameworks. Among the vaccines currently in development, the M72 vaccine is the most advanced, with Phase 3 trials that began in early 2024.[35] The vaccine showed 49.7% vaccine efficacy in preventing active TB among IGRA-positive, HIV-negative, 18- to 50-year-old adults living in Kenya, South Africa, and Zambia over 3 years in phase 2b trials.[35] With a recruitment period of 2 years and a patient follow-up of 4 years, preliminary results will not be available for some time.[5] An mRNA-based vaccine is now in the clinical pipeline. mRNA vaccines present an important additional tool for vaccine research, with a strong potential to accelerate TB vaccine development.[36]
Research has significantly expanded the diagnostic classes, tests, products, and methods being explored for accurate diagnosis at the point of care, as well as the availability of more effective and shorter courses of TB therapy. Over 50 tests for TB infection and disease are under development in August 2024. In contrast to the 8 TB treatment drugs in clinical trials in 2015, 29 are in trials as of August 2024. Of these, 18 are new chemical entities, and 8 are repurposed medications, including levofloxacin, linezolid, high-dose rifampicin, and clofazimine. The WHO has recently approved 3 drugs in trials for clinical use: bedaquiline, delamanid, and pretomanid. At least 30 trials are investigating drugs to prevent TB disease, including preventing MDR-TB using delamanid, optimizing treatment administration in very young children, and new models for TB treatment delivery.
Machine learning, which can potentially improve diagnostic accuracy, facilitate early diagnosis, and inform treatment strategies, reduce costs, and decrease morbidity and mortality, is another area of research—for example, computer-assisted X-ray screening to detect TB in individuals with a high likelihood of disease.
Reducing barriers to care requires implementation research to better integrate TB programs into broader health systems. For example, due to the large number of people not screened and the capacity limits of current US and Canadian TB programs, a recent editorial by Menzies proposes that primary care broadly implement TB screening for high-risk populations.[6] The author asserts that this would improve screening rates for TB risk factors and infections, as well as enhance TB preventive treatment acceptance and adherence, due to the presence of a stable, long-term relationship between the patient and practitioner. A program providing routine TB screening to eligible immigrants upon registration at primary care clinics in the United Kingdom found that clinicians diagnosed TB earlier, lowering the long-term risk of TB, despite relatively low rates of TPT initiation (26.2%).[10]
The WHO is implementing several initiatives to foster and support research, innovation, and translation into practice. A TB trial platform aims to enhance trial efficiency compared to traditional methods and promote collaboration among clinical trial sponsors, funders, civil society, and countries. The WHO's TB vaccine accelerator council, established in 2023, accelerates the translation of scientific breakthroughs into effective and accessible TB vaccines.[35] Its second meeting in 2024 established goals and milestones for the 2024 to 2025 term. A TB sequencing portal, developed with partner organizations, advances knowledge for sequencing and phenotyping of Mtb. A February 2024 consultation identified emerging areas for translating TB research into global TB policy guidelines.[WHO. GTBR 2024]
Media
(Click Image to Enlarge)
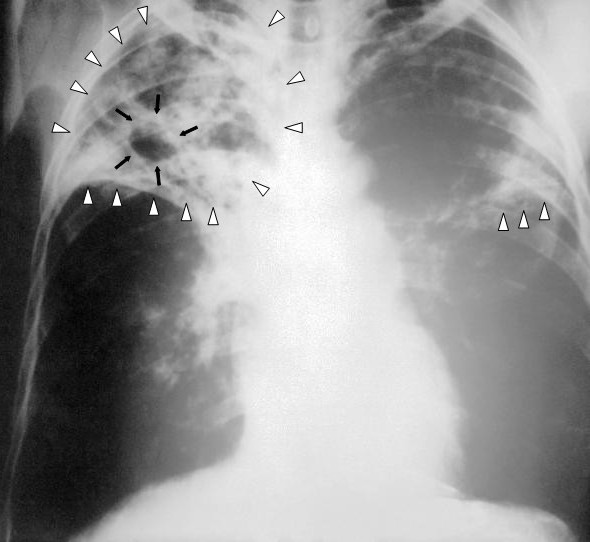
Pulmonary Tuberculosis. This image shows an anteroposterior chest x-ray of a patient diagnosed with advanced bilateral pulmonary tuberculosis. The x-ray reveals the presence of bilateral pulmonary infiltrate (white triangles), and "caving formation" (black arrows) present in the right apical region. The findings suggest far-advanced tuberculosis.
Centers for Disease Control and Prevention
(Click Image to Enlarge)
Mantoux Tuberculin Skin Test. This technician is in the process of correctly placing a Mantoux tuberculin skin test in this recipient’s forearm, which will cause a 6 mm to 10 mm wheal, or a raised area of skin surface, to form at the injection site. The Mantoux tuberculin skin test evaluates people for latent tuberculosis infection. In the United States, this skin test consists of an intradermal injection of exactly one-tenth of a milliliter of tuberculin containing 5 tuberculin units. Correct placement of this intradermal injection involves inserting the needle bevel slowly at a 5° to 15° angle. The needle bevel is advanced through the epidermis, the superficial layer of skin, approximately 3 mm so that the entire bevel is covered and lies just under the skin surface. A tense, pale wheal that is 6 mm to 10 mm in diameter appears over the needle bevel.
Greg Knobloch, Public Domain, via Wikimedia Commons
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
References
Goletti D, Meintjes G, Andrade BB, Zumla A, Shan Lee S. Insights from the 2024 WHO Global Tuberculosis Report - More Comprehensive Action, Innovation, and Investments required for achieving WHO End TB goals. International journal of infectious diseases : IJID : official publication of the International Society for Infectious Diseases. 2025 Jan:150():107325. doi: 10.1016/j.ijid.2024.107325. Epub 2024 Dec 2 [PubMed PMID: 39631498]
Cole ST, Brosch R, Parkhill J, Garnier T, Churcher C, Harris D, Gordon SV, Eiglmeier K, Gas S, Barry CE 3rd, Tekaia F, Badcock K, Basham D, Brown D, Chillingworth T, Connor R, Davies R, Devlin K, Feltwell T, Gentles S, Hamlin N, Holroyd S, Hornsby T, Jagels K, Krogh A, McLean J, Moule S, Murphy L, Oliver K, Osborne J, Quail MA, Rajandream MA, Rogers J, Rutter S, Seeger K, Skelton J, Squares R, Squares S, Sulston JE, Taylor K, Whitehead S, Barrell BG. Deciphering the biology of Mycobacterium tuberculosis from the complete genome sequence. Nature. 1998 Jun 11:393(6685):537-44 [PubMed PMID: 9634230]
Cole B, Nilsen DM, Will L, Etkind SC, Burgos M, Chorba T. Essential Components of a Public Health Tuberculosis Prevention, Control, and Elimination Program: Recommendations of the Advisory Council for the Elimination of Tuberculosis and the National Tuberculosis Controllers Association. MMWR. Recommendations and reports : Morbidity and mortality weekly report. Recommendations and reports. 2020 Jul 31:69(7):1-27. doi: 10.15585/mmwr.rr6907a1. Epub 2020 Jul 31 [PubMed PMID: 32730235]
O'Connell J, McNally C, Stanistreet D, de Barra E, McConkey SJ. Ending tuberculosis: the cost of missing the World Health Organization target in a low-incidence country. Irish journal of medical science. 2023 Aug:192(4):1547-1553. doi: 10.1007/s11845-022-03150-3. Epub 2022 Sep 19 [PubMed PMID: 36121600]
Burki T. 2023 UN High-Level Meeting on tuberculosis. The Lancet. Microbe. 2024 Feb:5(2):e107. doi: 10.1016/S2666-5247(22)00262-2. Epub 2023 Oct 10 [PubMed PMID: 37832572]
Menzies D. Screening for Latent Tuberculosis Infection. JAMA network open. 2023 May 1:6(5):e2312114. doi: 10.1001/jamanetworkopen.2023.12114. Epub 2023 May 1 [PubMed PMID: 37129898]
. WHO consolidated guidelines on tuberculosis: Module 1: Prevention – Tuberculosis preventive treatment, second edition. 2024:(): [PubMed PMID: 39298638]
US Preventive Services Task Force, Mangione CM, Barry MJ, Nicholson WK, Cabana M, Chelmow D, Coker TR, Davis EM, Donahue KE, Jaén CR, Li L, Ogedegbe G, Rao G, Ruiz JM, Stevermer J, Underwood SM, Wong JB. Screening for Latent Tuberculosis Infection in Adults: US Preventive Services Task Force Recommendation Statement. JAMA. 2023 May 2:329(17):1487-1494. doi: 10.1001/jama.2023.4899. Epub [PubMed PMID: 37129649]
Young DB, Gideon HP, Wilkinson RJ. Eliminating latent tuberculosis. Trends in microbiology. 2009 May:17(5):183-8. doi: 10.1016/j.tim.2009.02.005. Epub 2009 Apr 16 [PubMed PMID: 19375916]
Jonas DE, Riley SR, Lee LC, Coffey CP, Wang SH, Asher GN, Berry AM, Williams N, Balio C, Voisin CE, Kahwati LC. Screening for Latent Tuberculosis Infection in Adults: Updated Evidence Report and Systematic Review for the US Preventive Services Task Force. JAMA. 2023 May 2:329(17):1495-1509. doi: 10.1001/jama.2023.3954. Epub [PubMed PMID: 37129650]
Level 1 (high-level) evidenceMacPherson P, Lebina L, Motsomi K, Bosch Z, Milovanovic M, Ratsela A, Lala S, Variava E, Golub JE, Webb EL, Martinson NA. Prevalence and risk factors for latent tuberculosis infection among household contacts of index cases in two South African provinces: Analysis of baseline data from a cluster-randomised trial. PloS one. 2020:15(3):e0230376. doi: 10.1371/journal.pone.0230376. Epub 2020 Mar 17 [PubMed PMID: 32182274]
Level 3 (low-level) evidenceBruchfeld J, Correia-Neves M, Källenius G. Tuberculosis and HIV Coinfection. Cold Spring Harbor perspectives in medicine. 2015 Feb 26:5(7):a017871. doi: 10.1101/cshperspect.a017871. Epub 2015 Feb 26 [PubMed PMID: 25722472]
Level 3 (low-level) evidenceLawn SD, Wood R, Wilkinson RJ. Changing concepts of "latent tuberculosis infection" in patients living with HIV infection. Clinical & developmental immunology. 2011:2011():. pii: 980594. doi: 10.1155/2011/980594. Epub 2010 Sep 26 [PubMed PMID: 20936108]
Hamada Y, Getahun H, Tadesse BT, Ford N. HIV-associated tuberculosis. International journal of STD & AIDS. 2021 Aug:32(9):780-790. doi: 10.1177/0956462421992257. Epub 2021 Feb 20 [PubMed PMID: 33612015]
. WHO consolidated guidelines on tuberculosis: Module 3: diagnosis – rapid diagnostics for tuberculosis detection. 2021:(): [PubMed PMID: 34314130]
Kwan CK, Ernst JD. HIV and tuberculosis: a deadly human syndemic. Clinical microbiology reviews. 2011 Apr:24(2):351-76. doi: 10.1128/CMR.00042-10. Epub [PubMed PMID: 21482729]
Getahun H, Kittikraisak W, Heilig CM, Corbett EL, Ayles H, Cain KP, Grant AD, Churchyard GJ, Kimerling M, Shah S, Lawn SD, Wood R, Maartens G, Granich R, Date AA, Varma JK. Development of a standardized screening rule for tuberculosis in people living with HIV in resource-constrained settings: individual participant data meta-analysis of observational studies. PLoS medicine. 2011 Jan 18:8(1):e1000391. doi: 10.1371/journal.pmed.1000391. Epub 2011 Jan 18 [PubMed PMID: 21267059]
Level 1 (high-level) evidenceMillington KA, White RG, Lipman M, McQuaid CF, Hauser J, Wooding V, Potter J, Abubakar I, Wingfield T. The 2023 UN high-level meeting on tuberculosis: renewing hope, momentum, and commitment to end tuberculosis. The Lancet. Respiratory medicine. 2024 Jan:12(1):10-13. doi: 10.1016/S2213-2600(23)00409-5. Epub 2023 Nov 14 [PubMed PMID: 37972624]
. WHO consolidated guidelines on tuberculosis: Module 4: Treatment - Drug-resistant tuberculosis treatment. 2020:(): [PubMed PMID: 32603040]
Woodruff R, Pratt R, Kolasa M. Progress Toward Tuberculosis Elimination and Tuberculosis Program Performance - National Tuberculosis Indicators Project, 2016-2022. Morbidity and mortality weekly report. Surveillance summaries (Washington, D.C. : 2002). 2024 Jun 6:73(4):1-18. doi: 10.15585/mmwr.ss7304a1. Epub 2024 Jun 6 [PubMed PMID: 38833409]
Williams PM, Pratt RH, Walker WL, Price SF, Stewart RJ, Feng PI. Tuberculosis - United States, 2023. MMWR. Morbidity and mortality weekly report. 2024 Mar 28:73(12):265-270. doi: 10.15585/mmwr.mm7312a4. Epub 2024 Mar 28 [PubMed PMID: 38547024]
Sosa LE, Njie GJ, Lobato MN, Bamrah Morris S, Buchta W, Casey ML, Goswami ND, Gruden M, Hurst BJ, Khan AR, Kuhar DT, Lewinsohn DM, Mathew TA, Mazurek GH, Reves R, Paulos L, Thanassi W, Will L, Belknap R. Tuberculosis Screening, Testing, and Treatment of U.S. Health Care Personnel: Recommendations from the National Tuberculosis Controllers Association and CDC, 2019. MMWR. Morbidity and mortality weekly report. 2019 May 17:68(19):439-443. doi: 10.15585/mmwr.mm6819a3. Epub 2019 May 17 [PubMed PMID: 31099768]
Level 3 (low-level) evidenceBagcchi S. WHO's Global Tuberculosis Report 2022. The Lancet. Microbe. 2023 Jan:4(1):e20. doi: 10.1016/S2666-5247(22)00359-7. Epub 2022 Dec 12 [PubMed PMID: 36521512]
Shah M, Dorman SE. Latent Tuberculosis Infection. The New England journal of medicine. 2021 Dec 9:385(24):2271-2280. doi: 10.1056/NEJMcp2108501. Epub [PubMed PMID: 34879450]
Ploubidis GB, Palmer MJ, Blackmore C, Lim TA, Manissero D, Sandgren A, Semenza JC. Social determinants of tuberculosis in Europe: a prospective ecological study. The European respiratory journal. 2012 Oct:40(4):925-30 [PubMed PMID: 22267772]
Level 2 (mid-level) evidenceWorld Health Organization. BCG vaccine: WHO position paper, February 2018 - Recommendations. Vaccine. 2018 Jun 7:36(24):3408-3410. doi: 10.1016/j.vaccine.2018.03.009. Epub 2018 Mar 30 [PubMed PMID: 29609965]
Cilloni L, Dowdy DW. Tuberculosis in US Indigenous Communities: A Need for Public Health Prioritization. American journal of public health. 2024 Feb:114(2):149-151. doi: 10.2105/AJPH.2023.307544. Epub [PubMed PMID: 38335488]
Ayenew B, Belay DM, Gashaw Y, Gimja W, Gardie Y. WHO's end of TB targets: unachievable by 2035 without addressing under nutrition, forced displacement, and homelessness: trend analysis from 2015 to 2022. BMC public health. 2024 Apr 4:24(1):961. doi: 10.1186/s12889-024-18400-5. Epub 2024 Apr 4 [PubMed PMID: 38575958]
Motta I, Boeree M, Chesov D, Dheda K, Günther G, Horsburgh CR Jr, Kherabi Y, Lange C, Lienhardt C, McIlleron HM, Paton NI, Stagg HR, Thwaites G, Udwadia Z, Van Crevel R, Velásquez GE, Wilkinson RJ, Guglielmetti L, Study group on Mycobacteria (ESGMYC) of the European Society of Clinical Microbiology and Infectious Diseases (ESCMID). Recent advances in the treatment of tuberculosis. Clinical microbiology and infection : the official publication of the European Society of Clinical Microbiology and Infectious Diseases. 2024 Sep:30(9):1107-1114. doi: 10.1016/j.cmi.2023.07.013. Epub 2023 Jul 22 [PubMed PMID: 37482332]
Level 3 (low-level) evidenceLalvani A, Whitworth HS. Progress in interferon-gamma release assay development and applications: an unfolding story of translational research. Annals of translational medicine. 2019 Jul:7(Suppl 3):S128. doi: 10.21037/atm.2019.05.76. Epub [PubMed PMID: 31576335]
MacLean E, Kohli M, Weber SF, Suresh A, Schumacher SG, Denkinger CM, Pai M. Advances in Molecular Diagnosis of Tuberculosis. Journal of clinical microbiology. 2020 Sep 22:58(10):. doi: 10.1128/JCM.01582-19. Epub 2020 Sep 22 [PubMed PMID: 32759357]
Level 3 (low-level) evidenceCarranza C, Pedraza-Sanchez S, de Oyarzabal-Mendez E, Torres M. Diagnosis for Latent Tuberculosis Infection: New Alternatives. Frontiers in immunology. 2020:11():2006. doi: 10.3389/fimmu.2020.02006. Epub 2020 Sep 10 [PubMed PMID: 33013856]
Wu RI, Mark EJ, Hunt JL. Staining for acid-fast bacilli in surgical pathology: practice patterns and variations. Human pathology. 2012 Nov:43(11):1845-51. doi: 10.1016/j.humpath.2012.01.006. Epub 2012 Apr 26 [PubMed PMID: 22542129]
Brodie D, Schluger NW. The diagnosis of tuberculosis. Clinics in chest medicine. 2005 Jun:26(2):247-71, vi [PubMed PMID: 15837109]
The Lancet Infectious Diseases. A new agenda to control tuberculosis. The Lancet. Infectious diseases. 2023 Nov:23(11):1207. doi: 10.1016/S1473-3099(23)00636-9. Epub [PubMed PMID: 37890907]
Looney MM, Hatherill M, Musvosvi M, Flynn J, Kagina BM, Frick M, Kafuko Z, Schmidt A, Southern J, Wilder-Smith A, Tippoo P, Paradkar V, Popadić D, Scriba TJ, Hanekom W, Giersing B. Conference report: WHO meeting summary on mRNA-based tuberculosis vaccine development. Vaccine. 2023 Nov 22:41(48):7060-7066. doi: 10.1016/j.vaccine.2023.10.026. Epub 2023 Oct 21 [PubMed PMID: 37872013]
Andersen P, Doherty TM. The success and failure of BCG - implications for a novel tuberculosis vaccine. Nature reviews. Microbiology. 2005 Aug:3(8):656-62 [PubMed PMID: 16012514]
Ghebreyesus TA, Lima NT. The TB Vaccine Accelerator Council: harnessing the power of vaccines to end the tuberculosis epidemic. The Lancet. Infectious diseases. 2023 Nov:23(11):1222-1223. doi: 10.1016/S1473-3099(23)00589-3. Epub 2023 Sep 21 [PubMed PMID: 37742697]
Lewinsohn DM, Leonard MK, LoBue PA, Cohn DL, Daley CL, Desmond E, Keane J, Lewinsohn DA, Loeffler AM, Mazurek GH, O'Brien RJ, Pai M, Richeldi L, Salfinger M, Shinnick TM, Sterling TR, Warshauer DM, Woods GL. Official American Thoracic Society/Infectious Diseases Society of America/Centers for Disease Control and Prevention Clinical Practice Guidelines: Diagnosis of Tuberculosis in Adults and Children. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2017 Jan 15:64(2):111-115. doi: 10.1093/cid/ciw778. Epub [PubMed PMID: 28052967]
Level 1 (high-level) evidence. WHO consolidated guidelines on tuberculosis: Module 3: Diagnosis – Tests for tuberculosis infection. 2022:(): [PubMed PMID: 36441853]
. WHO consolidated guidelines on tuberculosis: Module 2: screening – systematic screening for tuberculosis disease. 2021:(): [PubMed PMID: 33822560]
Level 1 (high-level) evidence. Recommendations for Investigating Contacts of Persons with Infectious Tuberculosis in Low- and Middle-Income Countries. 2012:(): [PubMed PMID: 24404639]
National Tuberculosis Controllers Association, Centers for Disease Control and Prevention (CDC). Guidelines for the investigation of contacts of persons with infectious tuberculosis. Recommendations from the National Tuberculosis Controllers Association and CDC. MMWR. Recommendations and reports : Morbidity and mortality weekly report. Recommendations and reports. 2005 Dec 16:54(RR-15):1-47 [PubMed PMID: 16357823]
Jensen PA, Lambert LA, Iademarco MF, Ridzon R, CDC. Guidelines for preventing the transmission of Mycobacterium tuberculosis in health-care settings, 2005. MMWR. Recommendations and reports : Morbidity and mortality weekly report. Recommendations and reports. 2005 Dec 30:54(RR-17):1-141 [PubMed PMID: 16382216]
Dobler CC, Farah WH, Alsawas M, Mohammed K, Breeher LE, Murad MH, Molella RG. Tuberculin Skin Test Conversions and Occupational Exposure Risk in US Healthcare Workers. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2018 Feb 10:66(5):706-711. doi: 10.1093/cid/cix861. Epub [PubMed PMID: 29028965]
Chia SZG, How KBM, Chlebicki MP, Ling ML, Gan WH. A retrospective review of tuberculosis exposure among health care workers in a tertiary hospital. American journal of infection control. 2020 Jun:48(6):650-655. doi: 10.1016/j.ajic.2019.10.014. Epub 2019 Dec 2 [PubMed PMID: 31806237]
Level 2 (mid-level) evidenceHorsburgh CR Jr, Goldberg S, Bethel J, Chen S, Colson PW, Hirsch-Moverman Y, Hughes S, Shrestha-Kuwahara R, Sterling TR, Wall K, Weinfurter P, Tuberculosis Epidemiologic Studies Consortium. Latent TB infection treatment acceptance and completion in the United States and Canada. Chest. 2010 Feb:137(2):401-9. doi: 10.1378/chest.09-0394. Epub 2009 Sep 30 [PubMed PMID: 19793865]
Kass NE. An ethics framework for public health. American journal of public health. 2001 Nov:91(11):1776-82 [PubMed PMID: 11684600]
Upshur RE. Principles for the justification of public health intervention. Canadian journal of public health = Revue canadienne de sante publique. 2002 Mar-Apr:93(2):101-3 [PubMed PMID: 11968179]
Marckmann G, Schmidt H, Sofaer N, Strech D. Putting public health ethics into practice: a systematic framework. Frontiers in public health. 2015:3():23. doi: 10.3389/fpubh.2015.00023. Epub 2015 Feb 6 [PubMed PMID: 25705615]
Level 1 (high-level) evidenceDonelle L, Comer L, Hiebert B, Hall J, Shelley JJ, Smith MJ, Kothari A, Burkell J, Stranges S, Cooke T, Shelley JM, Gilliland J, Ngole M, Facca D. Use of digital technologies for public health surveillance during the COVID-19 pandemic: A scoping review. Digital health. 2023 Jan-Dec:9():20552076231173220. doi: 10.1177/20552076231173220. Epub 2023 May 17 [PubMed PMID: 37214658]
Level 2 (mid-level) evidenceGebreselassie N, Kasaeva T, Zignol M. A global strategy for tuberculosis research and innovation. The European respiratory journal. 2020 Nov:56(5):. pii: 2003539. doi: 10.1183/13993003.03539-2020. Epub 2020 Nov 12 [PubMed PMID: 33184100]