Introduction
First described by CF Richter in his 1792 doctoral thesis, laryngeal clefting (LC), also known as laryngotracheoesophageal clefting, is a congenital malformation of the wall separating the laryngotracheal complex from the esophagus. The condition is observed in 1 out of every 10,000 to 20,000 live births. Most cases are sporadic, but they can be associated with syndromes, such as Opitz-Frias and other nonsyndromic anatomical abnormalities of the cardiovascular (16%-33%), gastrointestinal (16%-67%), genitourinary (14%-44%), respiratory (2%-9%), and craniofacial (5%-15%) systems.[1][2][3][4]
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
Normal embryological development of the upper aerodigestive tract depends on appropriate migration of mesenchyme from the fourth and sixth branchial arches, and from the endoderm of the foregut, respectively. As the foregut develops in a caudal to cranial direction, during the fourth and fifth weeks of gestation, the lateral tracheoesophageal folds move medially to fuse and form the tracheoesophageal septum, thus separating the primordial esophagus from the laryngotracheal tube.[5]
There are multiple theories proposed to explain the development of LC:
- Increased intraembryonic pressure due to lordotic curvature of the spine during heart development, causing strain and displacement of the developing esophagus
- Incomplete or anomalous re-canalization of the esophagus
- Persistence of an abnormal vessel leading to vascular insufficiency in the foregut, resulting in growth anomalies
- Abnormal stem cell differentiation leading to failure of septation [6]
Embryological models in rats have demonstrated a relationship between doxorubicin exposure, tracheoesophageal fistula, and VACTERL syndrome (vertebral anomalies, anal atresia [imperforate anus], cardiac defects, tracheoesophageal fistula with or without esophageal atresia, renal anomalies, limb abnormalities [especially radial limb defects]). Still, LC was not observed in these experiments.[7]
Epidemiology
The overall incidence of LC is estimated to be between 1:10,000 and 1:20,000 live births.[8][9] This is very likely an underestimate, since small clefts are often asymptomatic. LC is uncommon, accounting for approximately 1.5% of pediatric laryngeal pathologies.[10] A 2006 study used endoscopy to evaluate 264 consecutive pediatric patients with chronic cough or aspiration and identified 20 cases of type I LC, which accounted for only 7.6% of the cases in their patient pool.[11] Studying the epidemiology of LC in detail is challenging due to its low prevalence; however, the limited data available have shown a higher incidence in males than females (a ratio of 5:3), and there is no evidence to suggest a racial predilection.[2][12][13] Most cases of LC are sporadic, and although the condition can occur in the context of several different syndromes, no specific genetic sequence has been identified that causes isolated LC.
Pathophysiology
LC presents on a continuum with mild stridor at one end and respiratory distress at the other. The most common symptoms are coughing and stridor, which worsen with feeding, as well as recurrent aspiration-induced upper respiratory infections. The severity of presentation depends primarily on the depth of the cleft, which is most often described using the 4-tiered Benjamin-Inglis classification, developed in 1989.[14] Type I clefts can be treated conservatively with watchful waiting, but types II through IV require either endoscopic or open surgical intervention. Post-intervention outcomes are variable, and patients may continue to aspirate due to other comorbidities, even with full closure of the cleft.
History and Physical
Most patients with LC present with frequent coughing, difficulty feeding, and recurrent respiratory tract infections. The differential diagnosis for these complaints is broad; therefore, the initial encounter should include a thorough history and physical examination that evaluates for more common problems before focusing on potential rare congenital laryngeal anomalies. Taking a comprehensive history of the patient is the first step in the diagnosis process. This should begin with a discussion of the pregnancy, including prenatal care, maternal and fetal complications during gestation, duration of the pregnancy, type of delivery, need for any neonatal resuscitation or intensive care unit admission, and results of newborn screening blood and hearing tests. Specific inquiries into feeding habits, frequency of regurgitation, frequency of upper respiratory infections, weight gain, aspiration, coughing, choking, or other respiratory compromise should also be pursued.
As LC can be associated with various syndromes, the parents should be asked about the presence of similar symptoms in other family members. Direct questions about surgeries on the digestive or respiratory system early in life may provide helpful information that more open-ended queries may not. The physical examination should comprehensively evaluate the body systems that may be involved with syndromes that can include LC.[15] Important symptoms and findings within those systems are highlighted below.
- Neurological: Evaluation of muscle tone and reflexes
- Craniofacial: Malformation of external and middle ears, cleft lip or palate, hypertelorism
- Respiratory: Stridor, retractions, desaturations
- Determining if these symptoms are positional is especially important. Worsening symptoms in a supine position may indicate laryngomalacia.
- Gastrointestinal: Anal atresia
- Genitourinary: Hypospadias and other anatomical malformations
- Extremities: Polydactyly or syndactyly
Evaluation
The timing and sequence of evaluation for upper respiratory tract disorders, such as LC, vary from practice to practice but should generally be initiated as soon as possible to limit respiratory complications, particularly in higher-grade (types III and IV) clefting. Noninvasive tests can be considered first for patients presenting with mild symptoms. A chest x-ray may be used to screen for active pneumonia in patients who are acutely symptomatic, but it is not routinely necessary. The most common study performed before invasive testing is the modified barium swallow study (MBSS), also known as the video-fluoroscopic swallow study.[16] This test evaluates the swallow dynamically, providing information on the oral, pharyngeal, and upper esophageal phases of deglutition. Laryngeal penetration and aspiration are easily identified with MBSS, but attention to the entire swallow is also important, as other associated dysfunctions can indicate underlying neurological issues or additional anatomical defects.[17]
The presence of swallowing deficits has been shown to predict worse functional outcomes.[18] Fiberoptic endoscopic evaluation of swallowing (FEES) is another test that can be performed outside the operating room to help diagnose LC. The examination consists of using a flexible transnasal endoscope to observe the patient swallowing dyed materials. The most common finding described in LC is a posterior-anterior penetration pattern through the cleft.[19] This contrasts with the more common lateral-to-medial pattern seen in other swallowing disorders, such as neuromuscular dyscoordination. Endoscopic swallowing evaluation is less commonly pursued in infants, as obtaining patient cooperation is challenging, and the degree of penetration or aspiration cannot be graded quantitatively. Remember that although useful, these studies provide only a single snapshot of the patient’s swallow. Patients with intermittent aspiration and frequent upper respiratory infections but a normal MBSS or FEES should still be considered for surgical evaluation.
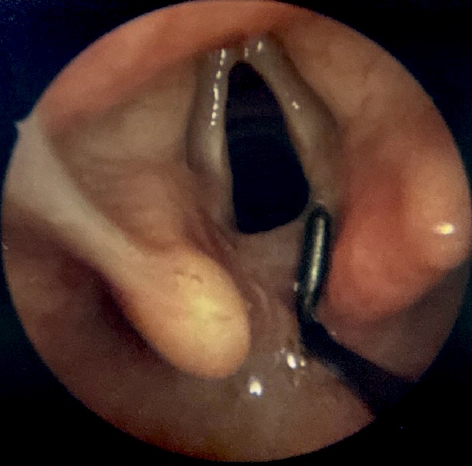
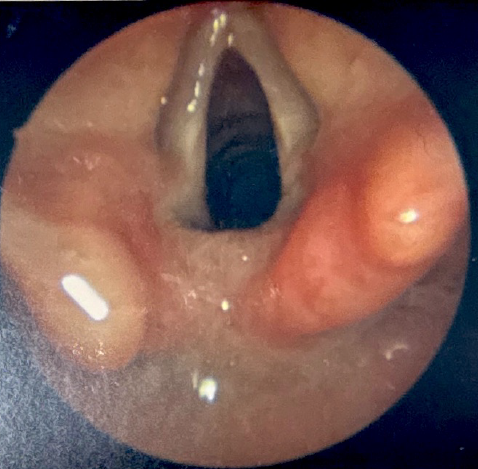
Microlaryngoscopy and bronchoscopy (MLB) with palpation of the interarytenoid space is the gold standard for LC diagnosis. This procedure is performed under general anesthesia, with the patient breathing spontaneously in the absence of positive pressure ventilation, which might otherwise mask the airway collapse associated with tracheobronchomalacia, a common comorbidity of LC.[20][21] The patient is positioned supine, and direct laryngoscopy is performed with the topical application of a weight-based 4% lidocaine solution to the larynx to reduce the risk of laryngospasm. After suspension, a surgical microscope or Hopkins rod telescope is used to visualize the larynx in greater detail. A right-angle probe or a similar instrument is subsequently used to palpate the interarytenoid space (see Image. Diagnostic Laryngoscopy of a Type I Laryngeal Cleft and Image. Diagnostic Laryngoscopy of a Type I Laryngeal Cleft Before Palpation). Type I clefts are typically not detectable without palpation, as redundant mucosa in the area usually prolapses into the defect, mimicking the appearance of normal anatomy.[22] Palpation should be continued along the entire length of the defect to assess its anatomical extent and inform management accordingly. Recording photos or videos of the examination is crucial for treatment planning and counseling patients or their parents.
Once examination under anesthesia is complete, the cleft can be classified using the Benjamin-Inglis system, which describes clefts based on their depth in relation to key anatomical landmarks.[14] Type I is purely interarytenoid, and type II extends into the posterior cricoid plate but not completely through it. Type III fully splits the posterior cricoid plate but does not extend past the thoracic inlet, and type IV extends beyond the thoracic inlet (see Image. Benjamin-Inglis Grading System for Laryngeal Clefts). Other classification systems have also been published but have not been widely adopted.[2][9][23][24][25] In 2006, Sandu and Monnier further refined the Benjamin-Inglis classification system by subdividing types III and IV.[26] Type IIIa is a full division of the posterior cricoid plate, but no further, and IIIb extends beyond the posterior cricoid plate but not to the thoracic inlet. Type IVa extends distal to the thoracic inlet but proximal to the bronchi, and type IVb reaches down into the bronchi. The surgeon must be alert for comorbidities during evaluation, especially during MLB. Findings characteristic of gastroesophageal reflux disease (GERD) and laryngomalacia are the most common.[27][28] Tracheobronchomalacia, tracheoesophageal fistula, and tracheobronchial dyskinesia may also be seen alongside type III and IV clefts.[29]
Once the diagnosis of LC is established, clinical judgement should guide investigation for further defects. LC is associated with CHARGE (coloboma, heart malformations, choanal atresia, growth and mental retardation, genitourinary malformations, ear malformations) syndrome, VACTERL association, 22q11.2 microdeletion (DiGeorge syndrome/velocardiofacial syndrome), Pallister-Hall syndrome (polydactyly, cutaneous syndactyly, hypothalamic hamartoma, imperforate anus, renal anomalies, and bifid uvula), and Opitz-Frias syndrome (hypertelorism, hypospadias, brain defects, intellectual disability, cleft lip/palate, and imperforate anus) among other, rarer conditions.[3][15] Suspicion for these syndromes should trigger evaluation of the neurological, cardiac, pulmonary, gastrointestinal, and genitourinary systems.
Treatment / Management
Conservative Management
There is a general consensus that patients with symptomatic type I LC should undergo a trial of conservative management with swallowing therapy, thickened feeds, and aggressive management of GERD. Authors estimate that anywhere from 19% to 66% of patients with type I LC experience full improvement with this approach.[30][31] Some debate remains regarding the duration of this trial, with recommendations ranging from 1 month to 1 year before considering operative intervention. Conservative management should be discontinued, however, if there is worsening aspiration, recurrent respiratory infections, or poor weight gain. Some clinicians forgo conservative management in patients with comorbidities that increase the risk of aspiration, such as neurological issues affecting swallowing coordination.[32] Typically, conservative measures do not play a role in the management of cleft types II through IV.
Interarytenoid Injection Augmentation
Interarytenoid injection augmentation (IIa) is a procedure in which temporary filler material, usually sodium carboxymethylcellulose, is injected into the opposing medial surfaces of the LC to obturate the cleft in the short term (~3 months). This technique is often used as an intermediate measure in type I clefts that have not responded to conservative management or as a first intervention in some type II clefts, and is not used in types III or IV clefts, as they require more aggressive surgical solutions. Few patients' symptoms fully resolve after IIa; it is more commonly used as a type of diagnostic or prognostic intervention to estimate the chance of improvement after formal surgical repair.[33] Approximately 60% of patients experience a significant improvement in radiological and swallowing function following IIa.[33] Depending on the injectate, results can last anywhere from one to three months. Gelfoam is reported to have the shortest duration, with calcium hydroxyapatite having the longest (6–12 months).[34]
Endoscopic Repair With Suture Closure
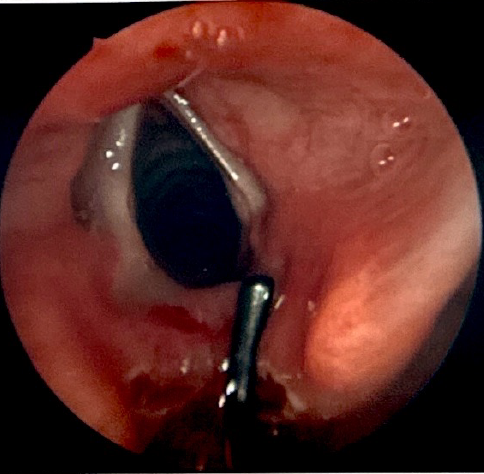
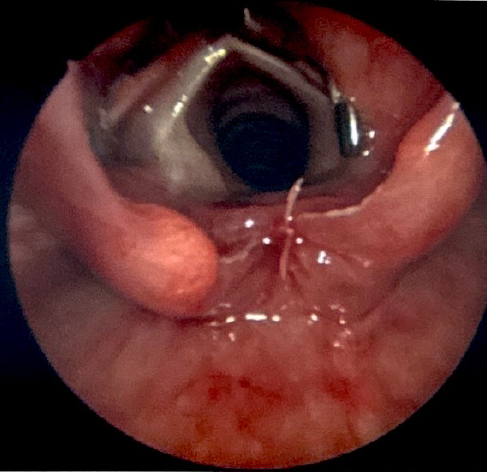
This technique is suitable for all types I and II clefts, as well as most type III clefts. There have been reports of repairing type IV clefts in this manner, but this is not a common practice. During this procedure, the patient is suspended using a laryngoscope that provides visualization of the most distal portion of the cleft. A carbon dioxide laser, thulium fiber laser, or standard laryngeal surgical instruments are used to denude the opposing mucosal surfaces of the cleft (see Image. Type I Laryngeal Cleft Undergoing Repair).[35] The defect is then closed with sutures up to the level of the arytenoid cartilages, either in a single or double layered fashion (see Image. Type I Laryngeal Cleft After Endoscopic Repair With Sutures). Surgeons often reinforce the suture closure with a surgical glue, such as fibrin.
Throughout the procedure, the patient is oxygenated intermittently as dictated by pulse oximetry via a ventilation port on the laryngoscope, an endotracheal tube in the oropharynx, jet ventilation, or intermittent intubation. Close coordination with the anesthesiologist is paramount, especially given the increased risk of airway fire associated with the laser. Outcomes of endoscopic intervention are generally good, especially in lower-grade clefts. Approximately 90% of patients demonstrate improvement in their presenting symptoms 12 months postoperatively.[30] More recently, a variation of the endoscopic approach has been described, using a surgical robot for improved access and visualization to decrease operative times for type I LC repairs.[36]
Open Surgical Repair
This technique is the most invasive and is only used in complicated type III LCs, most type IV LCs, and those that have failed endoscopic repair. There are 3 approaches to large clefts:
- Lateral pharyngotomy approach: This is most often performed if the LC has less than 2 cm of tracheal extension. The main risk is injury to the recurrent laryngeal nerves.[8]
- Anterior laryngofissure: This is the most common approach to the cervical portion of severe clefts. A transverse cervical incision is made at the level of the cricothyroid membrane, the isthmus of the thyroid is divided, and the strap muscles are separated to expose the thyroid cartilage.[15] This approach poses no risk to the recurrent laryngeal nerves and is a simpler dissection overall; however, there is a reportedly increased risk of laryngeal instability or growth disturbance postoperatively.[9][17][37]
- Transthoracic approach: This access is mandatory for type IV clefts that, by definition, extend beyond the thoracic inlet. Many experts combine thoracotomy and anterior laryngofissure to approach the intra- and extra-thoracic aspects of the defect.[38][39][40] This approach requires assistance from pediatric thoracic surgery, pediatric cardiac anesthesia, and in many cases, the extracorporeal membrane oxygenation or cardiopulmonary bypass teams to eliminate the obstruction that an endotracheal tube would place in the operating field.
Once the defect is fully exposed via the chosen approach, the sequence and technique of the repair vary from surgeon to surgeon. The method described by Geller et al is summarized here.[40] The esophageal and tracheal aspects of the defect are separated to permit independent closure. The esophageal defect is sutured closed first, typically using interrupted absorbable 4-0 to 6-0 polyglactin or nonabsorbable 6-0 polypropylene sutures, along with a knot pusher.[16][41] Next, the tracheal edges are incised to promote scar formation between the opposing raw edges, then sutured closed end to end. It may not be possible to close the cleft in separate esophageal and tracheal layers; however, every effort should be made to do so to facilitate the placement of an interposition graft, thereby decreasing the risk of suture line dehiscence.
At the level of the cricoid, a carved costal cartilage graft is placed to reinforce the laryngeal closure and prevent the development of postoperative stenosis. One or both sternohyoid muscles may be separated from the sternal or hyoid insertions and interposed between the laryngotracheal and esophageal repairs with sutures securing them to the deep cervical fascia on the contralateral side of the neck. There are reports of many other materials being used as interposition grafts, including, but not limited to, pericardium, sternocleidomastoid flaps, pleura, jejunum, and tibial periosteum.[42] Regardless of the material used, an interposition graft is essential to decrease the risk of repair dehiscence, tracheoesophageal fistula, or other severe complications, even if the tracheal and esophageal suture lines are carefully positioned to avoid overlap. The best outcomes appear to result from vascularized interpositions.[4] Once the interposition graft is in place, the cartilaginous incisions made during the approach are closed along with the overlying soft tissue.
The patient is typically left intubated for anywhere from 1 to 10 days, depending on the clinical course. Tracheostomy is usually avoided because of the risk the tube poses to the newly-sewn repair. Serial MLBs are performed, often beginning around postoperative days 10 to 14, with minor procedures, such as granulation tissue debridement, performed as needed. Strict medical control of GERD must be maintained throughout the recovery period to optimize healing. If the patient’s comorbidities permit, trials of oral intake can be initiated 14 days or more after the operation. A post-treatment MBSS is usually obtained 2 to 3 months after surgery.
Differential Diagnosis
The wide range of presenting symptoms associated with the different types of LC requires a broad differential diagnosis. Patients with minor symptoms from a low-grade LC, such as coughing, positional stridor, and minor feeding difficulties, should have conditions like GERD, laryngomalacia, and eosinophilic esophagitis ruled out before investigating the possibility of a congenital anomaly. More severe symptoms like recurrent aspiration pneumonia, choking episodes, and weight loss may be caused by tracheoesophageal fistula, vocal cord paresis, and severe laryngomalacia in addition to LC.
Prognosis
LC prognosis depends upon the severity of the presenting symptoms.
- Type I and II LC have very good prognoses. Some authors have found that more than half of patients with type I LC resolve their symptoms with medical management alone. A successful IIa with recurrence of symptoms as the injectate absorbs predicts successful endoscopic repair with a greater than 90% success rate.[43]
- The prognosis of type III and IV LC has improved as the development of endoscopic techniques has decreased morbidity, and a better understanding of postoperative management has reduced mortality from 40% to 90% in the 1980s to as low as 6% in the 2010s.[29][44] Higher grade clefts, however, are often associated with other serious congenital anomalies that may substantially increase the risk of morbidity and mortality. In 1990, Myer et al reported that 60% of deaths after LC repair occurred in patients with other comorbidities.[12]
Complications
Complications vary depending on the severity of the LC. Small type I LCs may produce no symptoms or complications and resolve as the patient grows. With larger defects, the risk for systemic complications increases, particularly of the pulmonary and gastrointestinal systems. The most common complications of untreated LCs are recurrent pneumonias, choking spells, aspiration, and malnutrition.
Each type of intervention has its unique set of complications as well:
- IIa: Recurrence of symptoms will necessarily occur with this approach; due to the bioresorbable nature of the injectate materials, the effects will wear off with time. True complications associated with this procedure are rare, with some study results reporting no complications at all.[34][45] Results from one study reported post-injection laryngeal swelling that resolved with systemic steroids.[46] Care must be taken to avoid injection into the subglottis, which would present as worsening biphasic stridor or even respiratory distress.
- Endoscopic repair: Complication rates with this procedure depend on the type of defect being repaired. Repairs of type I and II clefts are generally well tolerated, but reported complications include postoperative dehydration, laryngeal or supraglottic scarring, and oral lacerations from incautious instrumentation. Postoperative dehiscence is rare with these smaller defects.[45] Type III defects have a much higher risk of postoperative dehiscence, tracheoesophageal fistula formation, and persistent aspiration. Complication rates are estimated to be 25% to 43%.[47][48]
- Open repair: In addition to the hazards of endoscopic repair, open repair of type III and IV LCs also poses the risk of recurrent laryngeal nerve injury and tracheomalacia, correcting the latter of which may require subsequent resection of the affected segment or aortopexy. The rates of repair breakdown, tracheoesophageal fistula, and persistent symptoms are also higher after repair of high-grade LCs, with revision required in up to 50% of cases.[4][35][4][49][50][51][52]
Deterrence and Patient Education
Thus far, no specific risk factors have been identified for development of LCs. They appear to be sporadic in most cases, and no environmental exposure or genetic anomaly has been associated with isolated LC.[53] Education of the caregivers of patients with LC can, however, help to reduce complications. A detailed review of the patient's pathology, prognosis, and treatment plan will empower the caregivers to be knowledgeable, active participants in the clinical course. Caregiver education becomes critical in the postoperative period when the day-to-day care of the patient is turned over to them. A patient with LC may have a tracheostomy, gastrostomy, or other intervention that requires near constant attention to optimize outcomes. How caregivers handle these burdens and advocate for patients is a crucial contributing factor to short- and long-term outcomes.
Enhancing Healthcare Team Outcomes
The potentially complicated care of these patients necessitates the involvement of many consultants and auxiliary services.
- Even the mildest cases of LC require the care of speech-language pathologists during diagnosis, because they are the experts who perform MBSS. They will also be involved in conservative management, providing feeding therapy and titration of a thickened diet.
- Skilled nursing care is essential for both caring for patients during their postoperative hospital stay and for providing education and a point of contact for the patient's caregivers at home.
- Medical management of various comorbidities may require consultations with specialists in gastroenterology, pulmonology, neurology, nephrology, cardiology, and other relevant fields.
- Type III and IV clefts often benefit from consultation with pediatric surgery or thoracic surgery, as access to the distal portions of these defects can be challenging to obtain. Pediatric general surgeons and pediatric otolaryngologists are most familiar with the repair of tracheoesophageal fistulae, and their expertise in managing this similar pathology can prove useful. Cooperation with pediatric cardiac anesthesia, extracorporeal membrane oxygenation teams, and/or cardiopulmonary bypass teams is often necessary for repair of type IV LC.
Direct communication and collaboration with all these services, as well as others, will result in enhanced outcomes for patients with complex medical issues, particularly if emergency treatment is required after repair of a laryngeal cleft. As the closure is fragile during the early recovery period, reintubation can be especially dangerous.
Media
(Click Image to Enlarge)
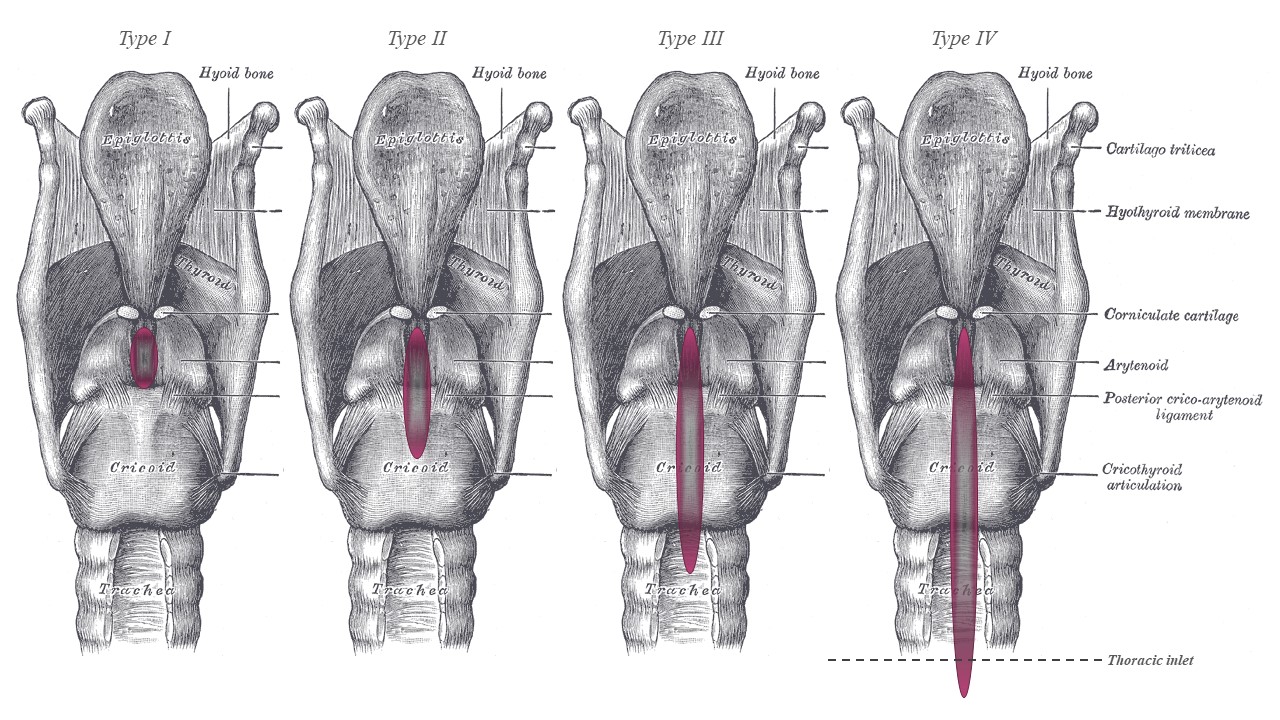
Benjamin-Inglis Grading System for Laryngeal Clefts. Type I is purely interarytenoid, and type II extends into the posterior cricoid plate but not completely through it. Type III fully splits the posterior cricoid plate but does not extend past the thoracic inlet, and type IV extends beyond the thoracic inlet.
Henry Vandyke Carter, Public Domain, via Wikimedia Commons
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
References
Roth B, Rose KG, Benz-Bohm G, Günther H. Laryngo-tracheo-oesophageal cleft. Clinical features, diagnosis and therapy. European journal of pediatrics. 1983 Mar:140(1):41-6 [PubMed PMID: 6873110]
DuBois JJ, Pokorny WJ, Harberg FJ, Smith RJ. Current management of laryngeal and laryngotracheoesophageal clefts. Journal of pediatric surgery. 1990 Aug:25(8):855-60 [PubMed PMID: 2401940]
Tyler DC. Laryngeal cleft: report of eight patients and a review of the literature. American journal of medical genetics. 1985 May:21(1):61-75 [PubMed PMID: 4003449]
Simpson BB, Ryan DP, Donahoe PK, Schnitzer JJ, Kim SH, Doody DP. Type IV laryngotracheoesophageal clefts: surgical management for long-term survival. Journal of pediatric surgery. 1996 Aug:31(8):1128-33 [PubMed PMID: 8863248]
Kluth D, Steding G, Seidl W. The embryology of foregut malformations. Journal of pediatric surgery. 1987 May:22(5):389-93 [PubMed PMID: 3585660]
Merei JM, Hutson JM. Embryogenesis of tracheo esophageal anomalies: a review. Pediatric surgery international. 2002 Sep:18(5-6):319-26 [PubMed PMID: 12415347]
Qi BQ, Merei J, Farmer P, Hasthorpe S, Myers NA, Beasley SW, Hutson JM. Cardiovascular malformations in rat fetuses with oesophageal atresia and tracheo-oesophageal fistula induced by adriamycin. Pediatric surgery international. 1997:12(8):556-64 [PubMed PMID: 9354725]
Level 2 (mid-level) evidenceEvans KL, Courteney-Harris R, Bailey CM, Evans JN, Parsons DS. Management of posterior laryngeal and laryngotracheoesophageal clefts. Archives of otolaryngology--head & neck surgery. 1995 Dec:121(12):1380-5 [PubMed PMID: 7488367]
Evans JN. Management of the cleft larynx and tracheoesophageal clefts. The Annals of otology, rhinology, and laryngology. 1985 Nov-Dec:94(6 Pt 1):627-30 [PubMed PMID: 4073743]
Moungthong G, Holinger LD. Laryngotracheoesophageal clefts. The Annals of otology, rhinology, and laryngology. 1997 Dec:106(12):1002-11 [PubMed PMID: 9415595]
Chien W, Ashland J, Haver K, Hardy SC, Curren P, Hartnick CJ. Type 1 laryngeal cleft: establishing a functional diagnostic and management algorithm. International journal of pediatric otorhinolaryngology. 2006 Dec:70(12):2073-9 [PubMed PMID: 16959329]
Myer CM 3rd, Cotton RT, Holmes DK, Jackson RK. Laryngeal and laryngotracheoesophageal clefts: role of early surgical repair. The Annals of otology, rhinology, and laryngology. 1990 Feb:99(2 Pt 1):98-104 [PubMed PMID: 2301875]
Phelan PD, Stocks JG, Williams HE, Danks DM. Familial occurrence of congenital laryngeal clefts. Archives of disease in childhood. 1973 Apr:48(4):275-8 [PubMed PMID: 4705933]
Benjamin B, Inglis A. Minor congenital laryngeal clefts: diagnosis and classification. The Annals of otology, rhinology, and laryngology. 1989 Jun:98(6):417-20 [PubMed PMID: 2729823]
Leboulanger N, Garabédian EN. Laryngo-tracheo-oesophageal clefts. Orphanet journal of rare diseases. 2011 Dec 7:6():81. doi: 10.1186/1750-1172-6-81. Epub 2011 Dec 7 [PubMed PMID: 22151899]
Rahbar R, Rouillon I, Roger G, Lin A, Nuss RC, Denoyelle F, McGill TJ, Healy GB, Garabedian EN. The presentation and management of laryngeal cleft: a 10-year experience. Archives of otolaryngology--head & neck surgery. 2006 Dec:132(12):1335-41 [PubMed PMID: 17178945]
Johnston DR, Watters K, Ferrari LR, Rahbar R. Laryngeal cleft: evaluation and management. International journal of pediatric otorhinolaryngology. 2014 Jun:78(6):905-11. doi: 10.1016/j.ijporl.2014.03.015. Epub 2014 Mar 27 [PubMed PMID: 24735606]
Strychowsky JE, Dodrill P, Moritz E, Perez J, Rahbar R. Swallowing dysfunction among patients with laryngeal cleft: More than just aspiration? International journal of pediatric otorhinolaryngology. 2016 Mar:82():38-42. doi: 10.1016/j.ijporl.2015.12.025. Epub 2016 Jan 7 [PubMed PMID: 26857313]
Boseley ME, Ashland J, Hartnick CJ. The utility of the fiberoptic endoscopic evaluation of swallowing (FEES) in diagnosing and treating children with Type I laryngeal clefts. International journal of pediatric otorhinolaryngology. 2006 Feb:70(2):339-43 [PubMed PMID: 16125795]
Mitchell DB, Koltai P, Matthew D, Bailey CM, Evans JN. Severe tracheobronchomalacia associated with laryngeal cleft. International journal of pediatric otorhinolaryngology. 1989 Dec:18(2):181-5 [PubMed PMID: 2625393]
Denneny JC 3rd. Bronchomalacia in the neonate. The Annals of otology, rhinology, and laryngology. 1985 Sep-Oct:94(5 Pt 1):466-9 [PubMed PMID: 3901858]
Ojha S, Ashland JE, Hersh C, Ramakrishna J, Maurer R, Hartnick CJ. Type 1 laryngeal cleft: a multidimensional management algorithm. JAMA otolaryngology-- head & neck surgery. 2014 Jan:140(1):34-40. doi: 10.1001/jamaoto.2013.5739. Epub [PubMed PMID: 24263209]
Jáuregui EJ, Propst EJ, Johnson K. Current management of type III and IV laryngotracheoesophageal clefts: the case for a revised cleft classification. Current opinion in otolaryngology & head and neck surgery. 2020 Dec:28(6):435-442. doi: 10.1097/MOO.0000000000000669. Epub [PubMed PMID: 33109943]
Level 3 (low-level) evidenceBLUMBERG JB, STEVENSON JK, LEMIRE RJ, BOYDEN EA. LARYNGOTRACHEOESOPHAGEAL CLEFT, THE EMBRYOLOGIC IMPLICATIONS: REVIEW OF THE LITERATURE. Surgery. 1965 Apr:57():559-66 [PubMed PMID: 14275784]
PETTERSSON G. Inhibited separation of larynx and the upper part of trachea from oesophagus in a newborn; report of a case successfully operated upon. Acta chirurgica Scandinavica. 1955 Dec 31:110(3):250-4 [PubMed PMID: 13292072]
Level 3 (low-level) evidenceSandu K, Monnier P. Endoscopic laryngotracheal cleft repair without tracheotomy or intubation. The Laryngoscope. 2006 Apr:116(4):630-4 [PubMed PMID: 16585871]
Parsons DS, Stivers FE, Giovanetto DR, Phillips SE. Type I posterior laryngeal clefts. The Laryngoscope. 1998 Mar:108(3):403-10 [PubMed PMID: 9504615]
Parsons DS, Herr T. Delayed diagnosis of a laryngotracheoesophageal cleft. International journal of pediatric otorhinolaryngology. 1997 Mar 6:39(2):169-73 [PubMed PMID: 9104626]
Shehab ZP, Bailey CM. Type IV laryngotracheoesophageal clefts -- recent 5 year experience at Great Ormond Street Hospital for Children. International journal of pediatric otorhinolaryngology. 2001 Jul 30:60(1):1-9 [PubMed PMID: 11434948]
Martha VV, Vontela S, Calder AN, Martha RR, Sataloff RT. Laryngeal cleft: A literature review. American journal of otolaryngology. 2021 Nov-Dec:42(6):103072. doi: 10.1016/j.amjoto.2021.103072. Epub 2021 Apr 20 [PubMed PMID: 33957543]
Lawlor CM, Choi SS. Triological Society Best Practice: Repair of Type 1 Laryngeal Cleft. The Laryngoscope. 2023 Jun:133(6):1279-1280. doi: 10.1002/lary.30612. Epub 2023 Feb 9 [PubMed PMID: 36757026]
Berzofsky CE, Lando T, Ettema S, Nelson J, Woodson G. Indications for Surgical Repair of Type 1 Laryngeal Cleft. The Annals of otology, rhinology, and laryngology. 2018 Apr:127(4):217-222. doi: 10.1177/0003489417752187. Epub 2018 Jan 17 [PubMed PMID: 29338291]
Miller AL, Hersh CJ, Johnson KE, Hartnick CJ. Short-term swallowing outcomes following type 1 laryngeal cleft injection. International journal of pediatric otorhinolaryngology. 2019 Jan:116():159-163. doi: 10.1016/j.ijporl.2018.10.040. Epub 2018 Nov 1 [PubMed PMID: 30554689]
Cohen MS, Zhuang L, Simons JP, Chi DH, Maguire RC, Mehta DK. Injection laryngoplasty for type 1 laryngeal cleft in children. Otolaryngology--head and neck surgery : official journal of American Academy of Otolaryngology-Head and Neck Surgery. 2011 May:144(5):789-93. doi: 10.1177/0194599810395082. Epub [PubMed PMID: 21493369]
Garabedian EN, Pezzettigotta S, Leboulanger N, Harris R, Nevoux J, Denoyelle F, Roger G. Endoscopic surgical treatment of laryngotracheal clefts: indications and limitations. Archives of otolaryngology--head & neck surgery. 2010 Jan:136(1):70-4. doi: 10.1001/archoto.2009.197. Epub [PubMed PMID: 20083782]
Worden CP, Prince AC, Kirse SN, Rutter C, Shields BH, Hackman TG, Yarbrough WG, Zanation AM, Zdanski CJ. Transoral robotic surgery for pediatric upper airway pathology: An institutional update. International journal of pediatric otorhinolaryngology. 2024 Sep:184():112073. doi: 10.1016/j.ijporl.2024.112073. Epub 2024 Aug 15 [PubMed PMID: 39154570]
Donahoe PK, Gee PE. Complete laryngotracheoesophageal cleft: management and repair. Journal of pediatric surgery. 1984 Apr:19(2):143-8 [PubMed PMID: 6726566]
Kawaguchi AL, Donahoe PK, Ryan DP. Management and long-term follow-up of patients with types III and IV laryngotracheoesophageal clefts. Journal of pediatric surgery. 2005 Jan:40(1):158-64; discussion 164-5 [PubMed PMID: 15868578]
Ryan DP, Muehrcke DD, Doody DP, Kim SH, Donahoe PK. Laryngotracheoesophageal cleft (type IV): management and repair of lesions beyond the carina. Journal of pediatric surgery. 1991 Aug:26(8):962-9; discussion 969-70 [PubMed PMID: 1919990]
Geller K, Kim Y, Koempel J, Anderson KD. Surgical management of type III and IV laryngotracheoesophageal clefts: the three-layered approach. International journal of pediatric otorhinolaryngology. 2010 Jun:74(6):652-7. doi: 10.1016/j.ijporl.2010.03.013. Epub 2010 Apr 22 [PubMed PMID: 20416955]
Nayak A, Chappity P, Pradhan S, Pradhan P, Parida P, Vinusree K. Management of Symptomatic Grade I and II Laryngeal Cleft: Experience of a Tertiary Care Center and Review of Literature. Indian journal of otolaryngology and head and neck surgery : official publication of the Association of Otolaryngologists of India. 2022 Oct:74(Suppl 2):2367-2371. doi: 10.1007/s12070-020-02184-2. Epub 2020 Oct 7 [PubMed PMID: 36452777]
Garabedian EN, Ducroz V, Roger G, Denoyelle F. Posterior laryngeal clefts: preliminary report of a new surgical procedure using tibial periosteum as an interposition graft. The Laryngoscope. 1998 Jun:108(6):899-902 [PubMed PMID: 9628507]
Jáuregui EJ, Abts MF, Dahl JP, Parikh SR, Horn DL, Pickens M, Park JS, DeMarre K, Hoang J, Johnson K. Beyond Laryngeal Clefts: Interarytenoid Injection Augmentation to Predict Success of Suture Augmentation in Children. The Laryngoscope. 2023 Jul:133(7):1749-1756. doi: 10.1002/lary.30374. Epub 2022 Sep 7 [PubMed PMID: 36069277]
Thiel G, Clement WA, Kubba H. The management of laryngeal clefts. International journal of pediatric otorhinolaryngology. 2011 Dec:75(12):1525-8. doi: 10.1016/j.ijporl.2011.08.020. Epub 2011 Sep 19 [PubMed PMID: 21937125]
Timashpolsky A, Schild SD, Ballard DP, Leventer SP, Rosenfeld RM, Plum AW. Management of Type 1 Laryngeal Clefts: A Systematic Review and Meta-analysis. Otolaryngology--head and neck surgery : official journal of American Academy of Otolaryngology-Head and Neck Surgery. 2021 Mar:164(3):489-500. doi: 10.1177/0194599820947742. Epub 2020 Aug 18 [PubMed PMID: 32807006]
Level 1 (high-level) evidenceMangat HS, El-Hakim H. Injection augmentation of type I laryngeal clefts. Otolaryngology--head and neck surgery : official journal of American Academy of Otolaryngology-Head and Neck Surgery. 2012 May:146(5):764-8. doi: 10.1177/0194599811434004. Epub 2012 Jan 18 [PubMed PMID: 22261489]
Leishman C, Monnier P, Jaquet Y. Endoscopic repair of laryngotracheoesophageal clefts: experience in 17 cases. International journal of pediatric otorhinolaryngology. 2014 Feb:78(2):227-31. doi: 10.1016/j.ijporl.2013.10.068. Epub 2013 Nov 20 [PubMed PMID: 24332198]
Level 3 (low-level) evidenceRahbar R, Chen JL, Rosen RL, Lowry KC, Simon DM, Perez JA, Buonomo C, Ferrari LR, Katz ES. Endoscopic repair of laryngeal cleft type I and type II: when and why? The Laryngoscope. 2009 Sep:119(9):1797-802. doi: 10.1002/lary.20551. Epub [PubMed PMID: 19554639]
Mathur NN, Peek GJ, Bailey CM, Elliott MJ. Strategies for managing Type IV laryngotracheoesophageal clefts at Great Ormond Street Hospital for Children. International journal of pediatric otorhinolaryngology. 2006 Nov:70(11):1901-10 [PubMed PMID: 16901551]
Pinlong E, Lesage V, Robert M, Mercier C, Ployet MJ. Type III-IV laryngotracheoesophageal cleft: report of a successfully treated case. International journal of pediatric otorhinolaryngology. 1996 Aug:36(3):253-62 [PubMed PMID: 8864808]
Level 3 (low-level) evidenceRobie DK, Pearl RH, Gonsales C, Restuccia RD, Hoffman MA. Operative strategy for recurrent laryngeal cleft: a case report and review of the literature. Journal of pediatric surgery. 1991 Aug:26(8):971-3; discussion 973-4 [PubMed PMID: 1919991]
Level 3 (low-level) evidencePinlong E, Lesage V, Robert M, Leddet I, Chamboux-Cheliakine C, Maurage C, Ployet MJ. [Need for multidisciplinary care management in the treatment of type III laryngo-tracheo-esophageal cleft]. Annales d'oto-laryngologie et de chirurgie cervico faciale : bulletin de la Societe d'oto-laryngologie des hopitaux de Paris. 1996:113(1):34-9 [PubMed PMID: 8763773]
Dombrowski ND, Li Y, Zhao CX, Agrawal PB, Rahbar R. Familial and genetic factors in laryngeal cleft: Have we learned anything? International journal of pediatric otorhinolaryngology. 2020 Nov:138():110283. doi: 10.1016/j.ijporl.2020.110283. Epub 2020 Jul 29 [PubMed PMID: 32771712]