Introduction
Hemangioblastomas are rare, benign, highly vascular WHO grade I tumors that primarily involve the central nervous system (CNS). These tumors most commonly develop in the cerebellum, followed by the spinal cord and brainstem, and can be sporadic or associated with von Hippel-Lindau (VHL) disease, an autosomal dominant genetic disorder that predisposes individuals to the formation of various tumors.[1][2] Approximately 45% of people with VHL disease develop CNS hemangioblastomas, with an estimated 20% of those with CNS hemangioblastomas having concurrent VHL disease.[3][4] Hemangioblastomas are uncommon, representing 1% to 2.5% of all brain tumors and 2% to 10% of spinal cord tumors, and typically present between the ages of 30 and 60 years with a slight male predominance.[5][6]
The clinical presentation of hemangioblastomas varies depending on their location in the CNS and can significantly impact quality of life. Cerebellar tumors will often cause headaches, nausea, vomiting, and signs of increased intracranial pressure, whereas brainstem tumors can cause motor and sensory deficits, ataxia, and potentially fatal intracerebral hemorrhage. Spinal hemangioblastomas may present with localized pain, motor weakness, sensory disturbances, and dysfunction of the bowel or bladder.[6]
The diagnosis is typically made using magnetic resonance imaging (MRI), which usually reveals a cystic mass with a contrast-enhancing mural nodule. The primary treatment for these tumors is surgical resection, with preoperative embolization being used occasionally due to the vascularity of the tumor. Stereotactic radiosurgery and novel agents targeting vascular endothelial growth factor pathways are potential treatments for multiple or surgically inaccessible tumors.[7][8] The prognosis following surgery is usually favorable, with recurrence rates of less than 25%, although routine clinical follow-up is essential, particularly in patients with VHL disease.[9]
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
Hemangioblastoma occurs sporadically (75%) or in association with VHL disease. A germline mutation of the VHL gene causes this autosomal dominant disease. However, somatic mutations of the VHL gene, amongst other genomic aberrations, have been identified in sporadic cases.[10] The VHL gene, a tumor-suppressor gene located on chromosome 3p25-26, encodes the 213-amino-acid VHL protein (pVHL). The identified VHL gene alterations include missense, splice-site, and frameshift (deletions > insertions) mutations, in addition to epigenetic suppression of the VHL gene through promoter methylation.[10][11][12] VHL gene mutation or deletion is more common in VHL-associated hemangioblastomas, whereas promoter methylation has only been detected in sporadic cases. In addition, loss of heterozygosity in chromosomes 6 and 10, even in the absence of VHL gene mutation, has been identified in sporadic cases, suggesting that alternative pathways for sporadic disease development can occur.[10]
Epidemiology
Hemangioblastomas constitute approximately 1% to 2.5% of all intracranial tumors and about 2% to 10% of primary spinal cord tumors. The overall incidence rate of CNS hemangioblastomas is approximately 0.141 per 100,000 person-years. These tumors are rare, with only 1 person per million estimated to develop a hemangioblastoma annually. These tumors are more commonly diagnosed in adults between the ages of 30 and 65, with a slight male predominance. The male-to-female ratio ranges from 1.3:1 to 2.6:1.[9][13]
Anatomic Distribution
Although these tumors can occur anywhere in the CNS, most hemangioblastomas develop in the posterior fossa of the brain in the cerebellum. Approximately 45% to 50% of these tumors are located in the cerebellum, 30% to 40% in the spinal cord, and 5% to 10% in the brainstem.[14] Supratentorial hemangioblastomas are rare, with most located in the sellar/parasellar region.[15] Sporadic hemangioblastomas are usually solitary, with multiple tumors within the neuraxis, raising the suspicion of the presence of VHL disease. In patients with sporadic hemangioblastomas, isolated cerebellar lesions are those most commonly found at presentation. However, individuals with VHL disease may have multiple lesions in the spinal cord, cerebellum, and brainstem.[9]
Pathophysiology
The pVHL plays an essential role in cell cycle regulation and is involved in the degradation of hypoxia-inducible factors. Amongst several downstream targets, hypoxia-inducible factors are known to activate the transcription of erythropoietin, vascular endothelial growth factor, and platelet-derived growth factor in hypoxic conditions. Loss of pVHL function can mimic hypoxia, and as a consequence, cells are unable to degrade hypoxia-inducible factors despite the presence of oxygen.[16] This occurrence leads to the upregulation of downstream targets, contributing to the characteristic hypervascularity of hemangioblastomas and their overproduction of erythropoietin. The 2-hit theory of biallelic inactivation of the VHL gene is thought to initiate tumorigenesis.
In VHL syndrome, affected organs reveal microscopic developmental changes and the formation of preneoplastic lesions. Precursor cells are developmentally arrested in hemangioblasts that have biallelic VHL inactivation. However, these cells can initiate reactive angiogenesis through unknown triggering mechanisms, resulting in a subset of cells that may grow faster and progress to form a hemangioblastoma. During growth, hemangioblastoma cells imitate the morphology of embryonic hemangioblasts, the precursors of hematopoietic and endothelial cells.[17]
Histopathology
The hallmark histological feature of hemangioblastomas is their biphasic tissue composition, comprising stromal cells and capillary networks. Stromal cells that are large and polygonal compose the neoplastic element that drives tumor growth and neovascularization. These cells have clear cytoplasm that is filled with lipid vacuoles, giving them a foamy appearance, along with round or oval nuclei and a prominent nucleolus.[13] The vascular component of hemangioblastomas has a dense capillary network and sinusoids. These blood vessels have a single layer of endothelial cells that are often dilated, contributing to the highly vascular nature of the tumor (see Image. Hemangioblastoma). The vascular structure of the tumor helps it to grow by supplying nutrients and oxygen. Additionally, these capillaries have pericytes and smooth muscle cells, which provide structural support.[13]
Immunohistochemical staining is essential for differentiating hemangioblastomas from other CNS tumors. Stromal cells in hemangioblastomas show several specific markers that include alpha-inhibin, S100 protein, and vimentin. The presence of alpha-inhibin helps to distinguish hemangioblastomas from metastatic clear cell renal cell carcinoma, which can have a similar microscopic appearance. In addition, stromal cells do not express epithelial markers (eg, cytokeratin), which are typically identified in clear cell renal cell carcinoma. The endothelial markers CD31 and CD34 demonstrate the vascular network of the tumor.[18]
History and Physical
Clinical History
The clinical evaluation for hemangioblastoma begins with a comprehensive history and physical examination, which may lead the physician to suspect an intracranial pathologic process. In patients with hemangioblastoma, the clinical presentation depends on the location of the tumor and the areas of the CNS involved. Clinical symptoms often develop when these slow-growing tumors have eventually exerted enough mass effect on the CNS to disrupt the function of the adjacent area. The most common location for these tumors is in the posterior fossa, which can obstruct cerebrospinal fluid flow through the fourth ventricle or the cerebral aqueduct, resulting in increased intracranial pressure, which leads to vomiting, ataxia, and occasionally fatal intracerebral hemorrhage. Spinal hemangioblastomas, in contrast, can cause localized pain, motor weakness, sensory disturbances, and bowel or bladder dysfunction.
A detailed family history is recommended to determine whether there may be a genetic linkage. The personal or family history may reveal VHL-related tumors (eg, retinal angiomas) that are classified as benign vascular hamartomas, clear cell renal cell carcinomas, pheochromocytomas, and pancreatic neuroendocrine neoplasms.[19] Additionally, a history of VHL-related tumor removal surgery in the patient or a family member is significant.[3][4]
Physical Examination
In patients with hemangioblastomas, clinical findings can include signs of increased intracranial pressure and cerebral mass effect. Additional clinical findings on physical examination depend on the tumor's location within the CNS.[4] Neurological examination is essential for assessing the impact of the tumor on various CNS functions. The following findings are associated with hemangioblastomas located in these CNS areas:
- Ataxia: Difficulty with coordination and balance that leads to gait disturbances and difficulty performing fine motor tasks can indicate a cerebellar tumor location.
- Dysmetria: Dysmetria is tested using finger-to-nose or heel-to-shin tests and is primarily present with tumors located in the cerebellum.
- Nystagmus: Cerebellar or brainstem involvement may be indicated by the presence of nystagmus.[4]
- Back pain: Localized or radicular back pain may radiate along the distribution of the affected nerve roots.
- Motor weakness and myelopathy: Weakness commonly arises in the muscles innervated by the affected spinal level.
- Sensory disturbances: Loss of sensation or paresthesias in the dermatomes typically corresponds to the involved spinal levels.[7]
Additionally, a systemic physical examination is warranted in patients with VHL disease to identify other syndrome manifestations, including:
Evaluation
Diagnostic Imaging Studies
Clinical findings can guide physicians in selecting the optimal imaging study for the diagnostic evaluation of a suspected tumor.
Magnetic resonance imaging
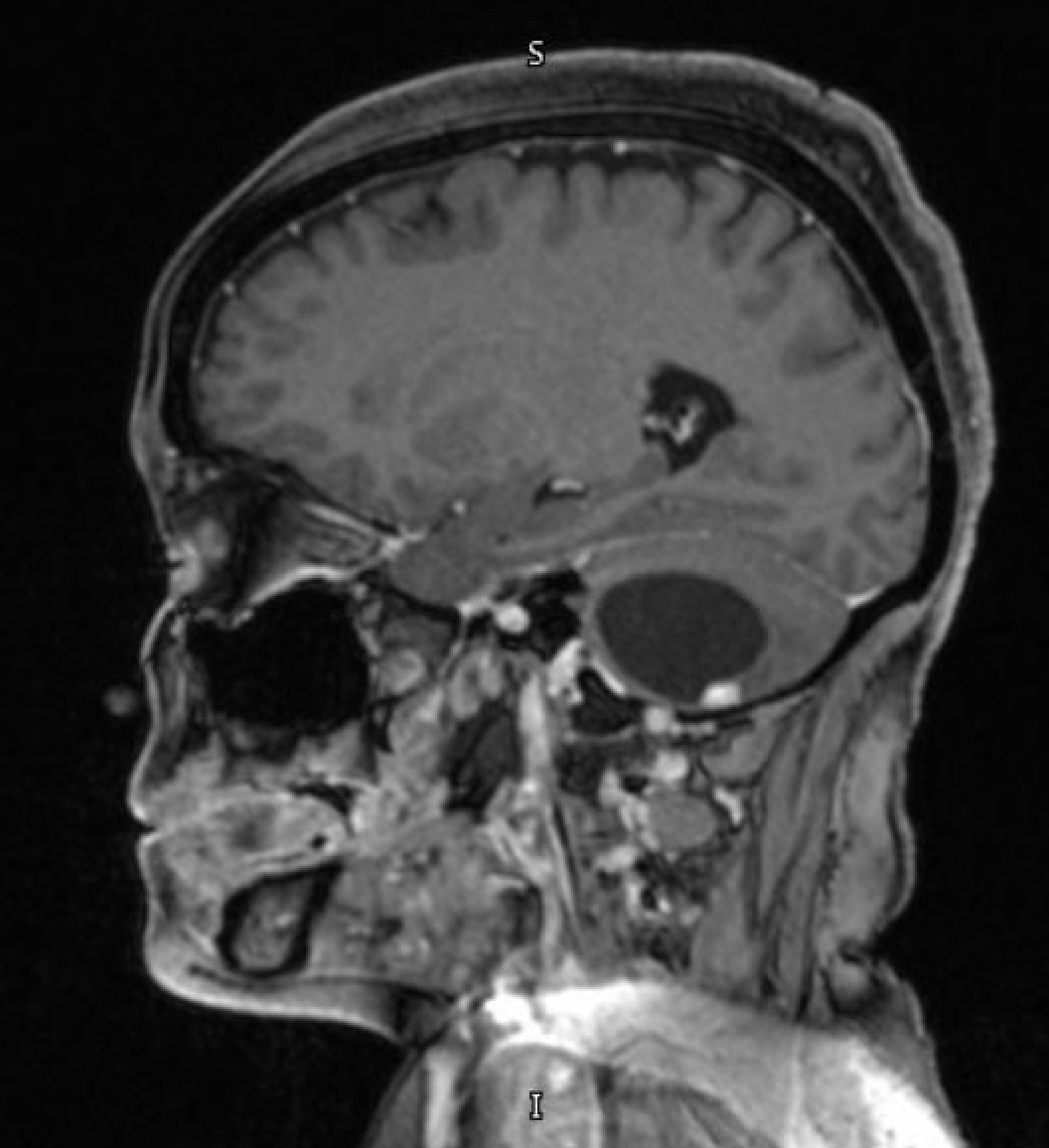
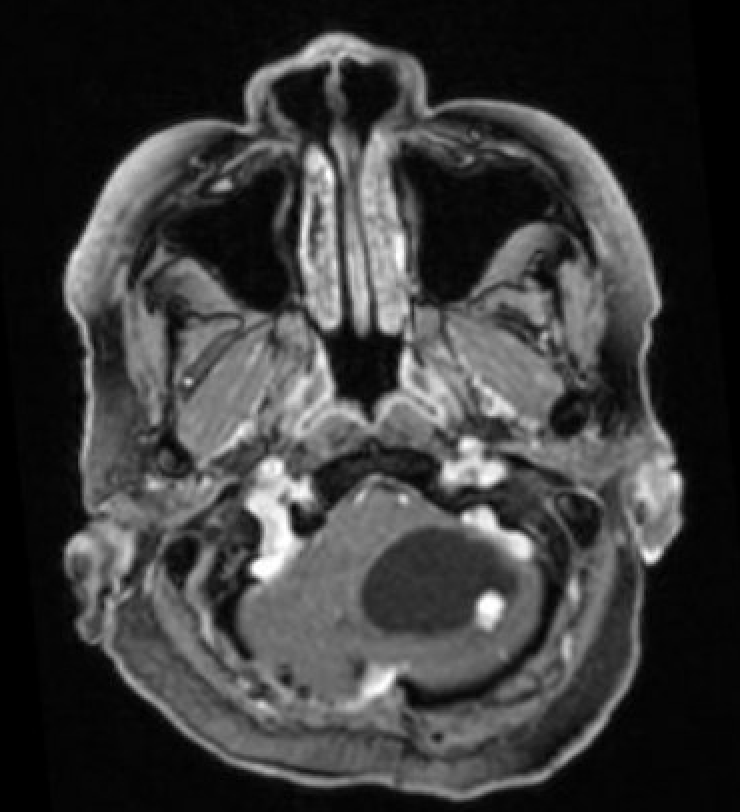
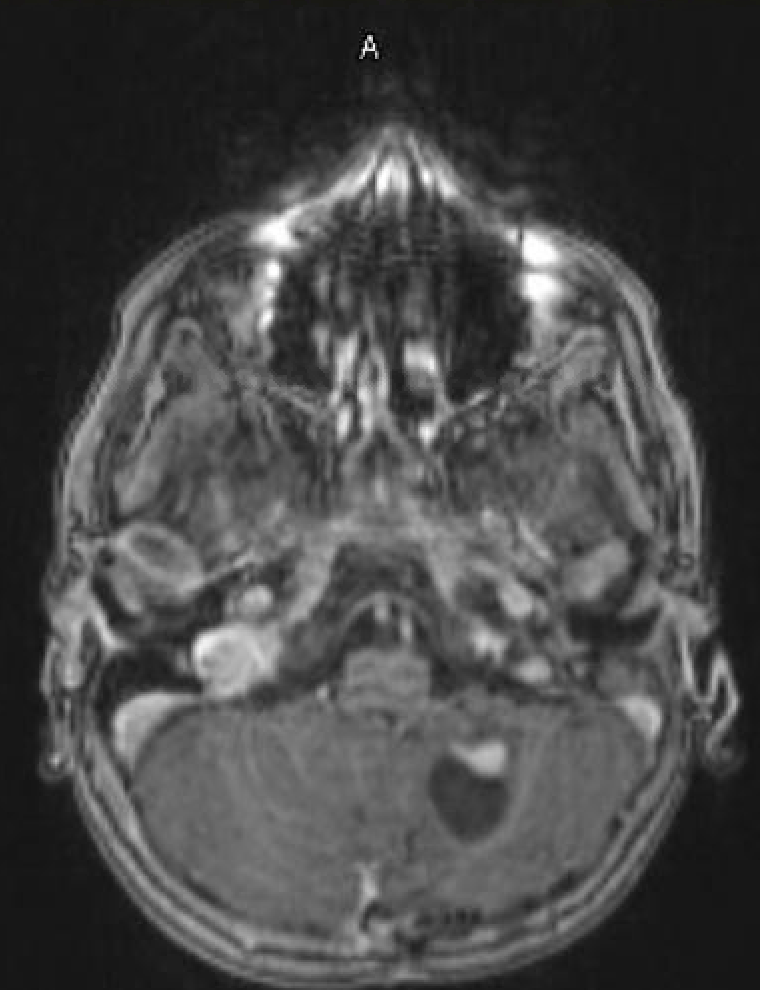
Magnetic resonance imaging (MRI) is the preferred modality for diagnosing CNS hemangioblastomas due to its detailed visualization of the soft tissue of CNS structures. A characteristic MRI finding of hemangioblastomas is a mural nodule attached to a cyst wall. This feature typically appears as a well-circumscribed lesion that enhances with contrast administration and has an associated cystic component.[6][20] The following findings are frequently observed in the MRI of hemangiomas:
- T1: The mural nodule appears hypointense or isointense compared to the brain. The cyst content shows a signal similar to that of cerebrospinal fluid.
- T1 with contrast: The mural nodule shows vivid enhancement with gadolinium contrast administration; however, the cyst wall does not usually enhance (see Images. Axial MRI Head With Contrast and Sagittal MRI Head With Contrast).
- T2: The mural nodule appears hyperintense. Flow voids are seen in 60% to 70% of cases and are due to enlarged blood vessels, often located at the periphery of the cyst. The fluid-filled cyst has a signal intensity similar to that of cerebrospinal fluid.
- MR perfusion: High relative cerebral blood volume ratios are better observed with this study.
Computed tomography imaging
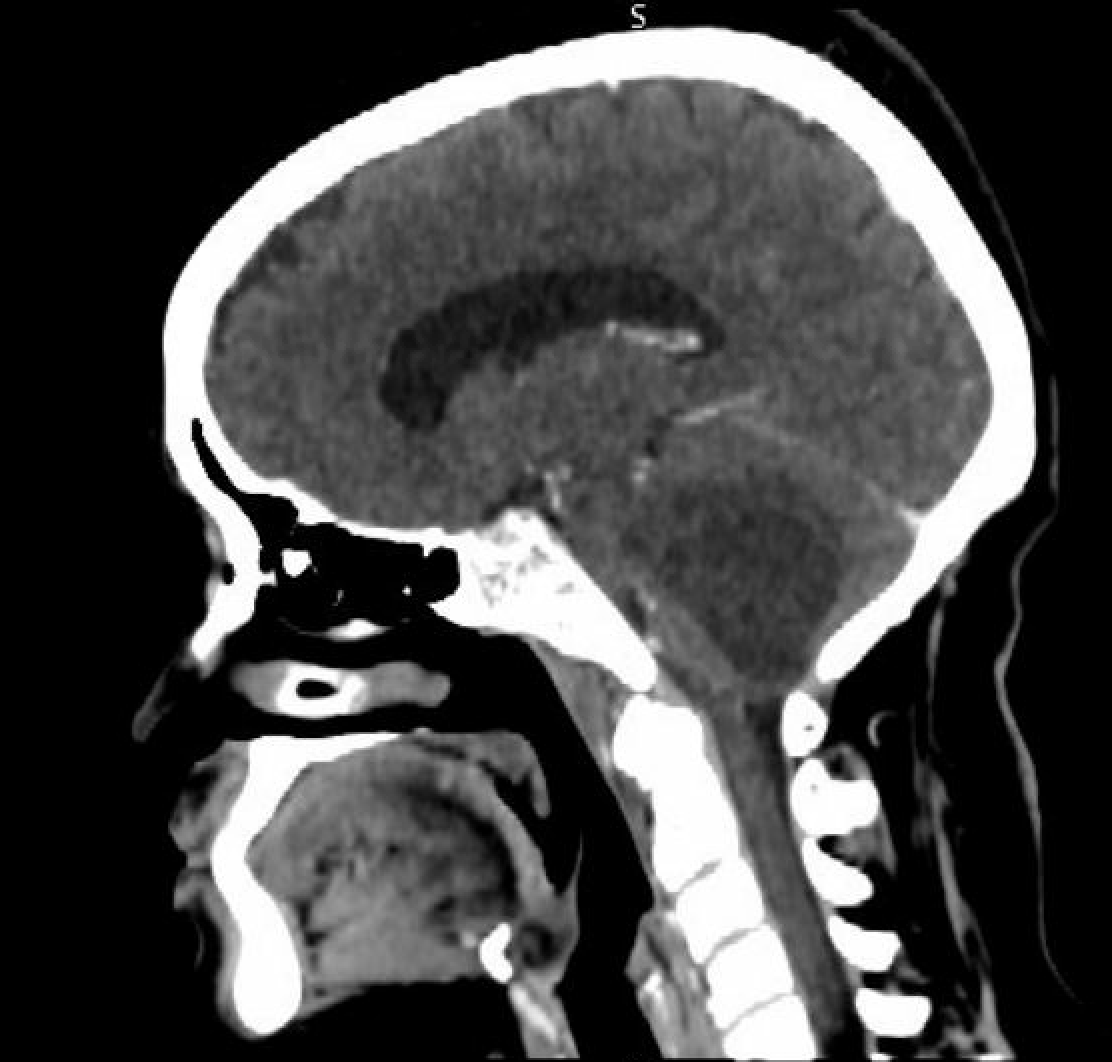
In computed tomography (CT) studies, the mural nodule in hemangioblastomas appears isodense compared to the brain on noncontrast images, surrounded by hypodense cystic fluid. The mural nodule also demonstrates intense homogeneous enhancement (see Image. Computed tomography With Contrast). The cyst walls usually do not enhance. Calcification is not present in these tumors.[6]
Angiography
Studies using angiography demonstrate enlarged feeding arteries and dilated draining veins in hemangioblastomas. A dense tumor blush is commonly observed, illustrating the prominent blood supply of the tumor.[6]
Laboratory Studies
Laboratory studies are primarily performed for the preoperative evaluation of the patient and include a complete blood count, liver function tests, renal function tests, and coagulation profiles.[4] In cases where the hemangioblastoma is associated with von Hippel-Lindau disease, genetic testing for the VHL gene mutation is recommended.[4]
Treatment / Management
Surgical Treatment
Surgical resection is the treatment of choice for CNS hemangioblastoma, especially for symptomatic tumors associated with mass effect. The goal of the surgery is complete tumor removal while minimizing injury to surrounding neural tissue. Due to the increased vascularity of these tumors, preoperative planning is essential to minimize intraoperative hemorrhage. Preoperative embolization can be considered to decrease the vascularity of the tumor and facilitate a safe surgical resection.[21][22][23] Because most tumors arise in the posterior fossa, the usual surgical approach is a suboccipital craniotomy, which provides an excellent corridor through which to resect this lesion.[2] Microsurgical techniques are used to resect the tumor with the help of a microscope. Intraoperative neurophysiological monitoring can help preserve neurological function during spinal and cranial surgery. Image-guided navigation systems may also be used to improve the accuracy of tumor localization and resection.[2] In patients with von Hippel-Lindau disease and multiple hemangioblastomas located throughout the CNS, only those lesions that are felt to be associated with neurological impairment are considered for surgical resection. (A1)
Medical Management
Medical management of CNS hemangioblastoma is typically supportive, as these tumors are benign lesions that do not invade surrounding neural tissue or metastasize. Chemotherapy has no defined role for these tumors. Novel therapeutic agents that include antiangiogenic factors have demonstrated promising results in managing these tumors, especially in patients with VHL gene mutations.[4] Bevacizumab is a monoclonal antibody that targets vascular endothelial growth factor and has been investigated for its ability to inhibit tumor growth by reducing angiogenesis.[24] Other tyrosine kinase inhibitors (eg, sunitinib and sorafenib) have also been considered for their antiangiogenic properties.[4][8](B3)
Radiation Therapy
Stereotactic radiosurgery (SRS) is a noninvasive treatment option that delivers a high dose of radiation precisely to the tumor while sparing surrounding normal neural tissue. SRS is beneficial for small, surgically inaccessible hemangioblastomas or residual tumors that remain after surgery. The Gamma Knife and CyberKnife are common treatment modalities used for stereotactic radiosurgery (SRS).[25](B2)
Tumor Surveillance
Routine follow-up with MRI is essential for detecting tumor recurrence or progression. The follow-up imaging duration varies based on the initial tumor characteristics, including the extent of resection achieved and the presence of VHL mutation. MRI scans are typically performed every 6 to 12 months postoperatively for the first few years, and then yearly if no recurrence is detected.[9]
A similar surveillance strategy is recommended for VHL disease. A follow-up assessment is essential due to the risk of tumor recurrence or the development of new lesions. Follow-up usually includes repeat imaging and ophthalmologic examination for retinal hemangioblastomas. Genetic counseling and testing are recommended for patients and their families to identify individuals at risk and implement early surveillance strategies.[4][12]
Differential Diagnosis
Metastatic Carcinoma
Brain metastases from various primary tumors can easily mimic hemangioblastomas in the posterior fossa of the brain. Metastatic lesions are the most common tumors found in the posterior fossa of the brain. Renal cell carcinoma (RCC) closely resembles hemangioblastomas, both radiographically and histologically. Renal cell carcinoma metastases can present as well-circumscribed hypervascular lesions with a similar clear cell morphology to that of hemangioblastomas. Immunohistochemical staining is required to differentiate between both pathologies. Hemangioblastomas typically express alpha-inhibin and S100 protein, whereas RCC metastases express epithelial markers, eg, cytokeratin and epithelial membrane antigen.[26][27]
Pilocytic Astrocytoma
A pilocytic astrocytoma is a common benign tumor usually found in children and young adults. Radiographically, this tumor can present with cystic and solid components similar to those of hemangioblastomas. Both tumor types are well-circumscribed and will enhance after contrast administration. On histopathological examination, pilocytic astrocytomas demonstrate Rosenthal fibers and eosinophilic granular bodies, both of which are absent in hemangioblastomas. Immunohistochemical staining can further help to differentiate hemangioblastomas from pilocytic astrocytomas, which usually express GFAP.[28] (see Image. Pilocytic Astrocytoma).
Ependymoma
Ependymomas arise from the lining of the fourth ventricle. Due to having a similar location and the occasional cystic appearance, ependymomas can appear similar to hemangioblastomas. Ependymomas are differentiated from hemangioblastomas by their characteristic perivascular pseudorosettes and ependymal rosettes. In addition, ependymomas express GFAP and EMA, which are absent in hemangioblastomas.[28][29]
Vascular Malformations
Vascular lesions, eg, arteriovenous malformations and cavernous malformations, can mimic hemangioblastomas on imaging due to their vascular nature. Arteriovenous malformations demonstrate a vascular nidus with direct arteriovenous shunting on imaging, while cavernous malformations consist of dilated and thin-walled capillaries without intervening brain tissue. These imaging features can help distinguish vascular lesions from the organized capillary network and stromal cells of hemangioblastomas in most cases. Cerebral angiography can further aid in defining these vascular malformations.[30]
Other Cystic Lesions
Other CNS cystic lesions, eg, arachnoid cysts and neuroenteric cysts, should be considered in the differential diagnosis when they are present in the posterior fossa. Arachnoid cysts are benign fluid-filled sacs that arise from the arachnoid membrane and are usually asymptomatic unless they are large and cause neurological symptoms related to mass effect. Unlike hemangioblastomas, arachnoid cysts do not enhance with contrast on MRI. Neuroenteric cysts are rare congenital lesions that also appear cystic but have distinct histological features that include a lining comprised of gastrointestinal or respiratory epithelium.[31]
Treatment Planning
Radiation Therapy Planning
Treatment planning is a crucial process that involves evaluating the patient's clinical presentation and their corresponding radiographic images to achieve optimal clinical outcomes. Therapy planning takes into account several factors, including tumor size, location, vascularity, and the patient's overall health. Because these tumors are very vascular, the treatment plan must be precise and individualized to optimize the therapeutic outcome for the patient and minimize potential treatment-related complications.
Simulation Imaging
Simulation imaging is a process that utilizes imaging techniques to plan the safe and accurate administration of radiation therapy to a well-defined treatment area. A CT scan is performed with the patient in the treatment position, which provides detailed anatomical information and allows for the tumor and any critical surrounding structures to be defined. MRI is often combined with CT images to enhance the soft tissue discrimination and further improve the accuracy of tumor delineation. This combination is particularly crucial for CNS tumors, where precise targeting is essential to prevent damage to healthy brain tissue.[32]
Radiation Therapy
After the simulation is complete and the tumor borders are accurately defined, treating clinicians assess the target volumes designated to receive the radiation therapy. The gross tumor volume encompasses the visible tumor as depicted on imaging studies. The clinical target volume encompasses the gross tumor and any area that may have potential microscopic disease spread. The planning target volume adds a margin around the clinical target volume to compensate for patient movement or other variations and uncertainties in radiation delivery. This practice will ensure that the prescribed dose covers the target area despite these variables.[32] This form of radiation treatment may be used to treat pancreatic or renal tumors.
Radiation therapy for hemangioblastomas primarily involves stereotactic radiosurgery (SRS). Devices capable of delivering SRS include the Gamma Knife, CyberKnife, and linear accelerator. SRS is primarily used for intracranial disease. The Gamma knife represents the most widely published technology for treating hemangioblastoma. Many authors have described different target volumes for SRS administration that vary from center to center. The median marginal doses for SRS range from 12 to 29.9 Gy, with a maximum dose of 13.3 to 40 Gy. Median isodose lines range from 50% to 90%. Follow-up periods following treatment range from 12 to 102 months. These parameters demonstrate satisfactory tumor control rates, with a pooled 5-year progression-free survival rate of approximately 88.4%.[33][34][35][36]
Stereotactic Radiosurgery
SRS is recommended for incompletely resected and recurrent tumors and is also appropriate for patients with significant medical comorbidities or with tumors in locations that are not amenable to surgical resection. Patients with VHL disease who have had multiple prior surgical resections are usually good candidates for SRS. Common adverse effects include hydrocephalus, radiation necrosis, peritumoral edema, headache, nausea, and vomiting. Tumor control rates are 92% at 3 years, 89% at 5 years, and 79% at 10 years. Overall survival rates are 94% at 3 years, 90% at 5 years, and 74% at 10 years.[35][36][37]
Various studies report a range of radiation doses for hemangioblastoma treatment with SRS. The radiation dose ranges from 14 to 35 Gy, with a median dose of approximately 18 to 21 Gy. Dose selection will depend on factors such as tumor size and location, as well as patient-specific factors, including age and overall health. Smaller tumors receive higher doses per treatment. Doses administered near critical structures, eg, the brainstem or optic nerves, require careful planning to minimize potential adverse effects. SRS is a single-fraction treatment where the entire radiation dose is delivered in a single session. The treatment takes only a few minutes, but the planning and preparation process can take much longer. The long-term adverse effects of SRS are still under investigation. These patients need routine follow-up MRIs to monitor for tumor control and potential adverse effects from the radiation therapy.[38][39]
Prognosis
The prognosis for patients with hemangioblastoma depends on several factors, eg, the location of the tumor, its size at the time of presentation, and whether the occurrence is sporadic or associated with VHL disease. Postoperatively, the success of the surgery and the extent of resection will also help to determine the prognosis. In sporadic hemangioblastomas that are not associated with any genetic syndrome, the prognosis is generally excellent after complete surgical resection.
These tumors grow slowly, and complete surgical resection often leads to long-term disease-free survival. Recurrence rates are low if the tumor is completely removed. Studies have shown that patients with sporadic hemangioblastomas have a significantly high long-term survival rate, with a 5-year survival rate exceeding 90%.[1][4][9] The prognosis for patients with VHL is more complicated because patients can have other tumors, eg, renal cell carcinoma, pheochromocytoma, and pancreatic tumors. When such patients develop CNS involvement, their overall outcome tends to be worse.[3][4]
Complications
Hemangioblastomas in the cerebellum or brainstem can obstruct the cerebrospinal fluid pathways, causing hydrocephalus that will require additional surgical interventions, eg, having a ventriculoperitoneal shunt placed or a third ventriculostomy performed. Tumors in specific locations can cause unique neurological deficits. Cerebellar tumors can cause cerebellar signs, in contrast to spinal cord tumors, which can be associated with motor weakness and sensory disturbances. Brainstem tumors can affect the cranial nerves, causing facial weakness, dysphagia, and diplopia.[1][2][4][14]
Surgical resection of hemangioblastomas carries the risk of significant intraoperative hemorrhage due to their profound vascular supply. Preoperative embolization can reduce but does not totally eliminate the risk of bleeding. A complete surgical resection is difficult to achieve, particularly in critical areas, eg, the brainstem and spinal cord, where postoperative complications, eg, new or worsened neurological deficits, can result. Surgery in the posterior fossa also carries the risk of a postoperative cerebrospinal fluid leak, which can result in meningitis if not recognized promptly and managed accordingly. Appropriate closure techniques and vigilant postoperative monitoring are essential for good clinical outcomes.[2][20]
Patients with VHL disease are at a high risk of having multiple lesions and tumor recurrence, requiring routine MRI surveillance at regular intervals. Tumor recurrence or the development of new tumors might necessitate repeat surgery, increasing the cumulative risk of postoperative complications. In general, only symptomatic hemangioblastomas are removed in patients with VHL disease. This genetic disease is also associated with other neoplasms, including pheochromocytomas, renal cell carcinomas, and pancreatic neuroendocrine tumors that can lead to systemic complications (eg, hypertension and renal dysfunction). An interprofessional approach is essential for managing these issues. The chronic nature of the disease and ongoing surveillance can significantly impact the patient's quality of life; therefore, psychological support and rehabilitation services are essential.[5][4][17]
Deterrence and Patient Education
Like any other disease, patient education is essential, especially in patients with von Hippel-Lindau disease. These patients must understand that they have a condition with a genetic predisposition that makes them prone to develop other tumors in other parts of the body. Regularly scheduled MRIs and imaging studies are essential to monitor these tumors. Early detection and treatment are essential. The patient should have appropriate information regarding their disease and the genetic knowledge of their condition to help them recognize their symptoms early and plan their future accordingly, such as having genetic counseling before starting a family.
Enhancing Healthcare Team Outcomes
The management of hemangioblastoma demands a well-coordinated, interprofessional approach involving physicians, advanced practitioners, nurses, pharmacists, and other healthcare professionals. Neurosurgeons, in particular, play a pivotal role and should work closely with colleagues across specialties through regular meetings to design and adjust individualized treatment and follow-up plans. These collaborative efforts promote timely and accurate diagnosis, which is especially important given the complexity and rarity of hemangioblastomas. A thorough review of each patient’s medical and family history is essential to detect underlying genetic conditions, eg, von Hippel-Lindau disease. Effective communication among team members supports the seamless delivery of care, while ongoing discussions with patients and their families about treatment options encourage shared decision-making, which is central to patient-centered care.
To maintain high-quality care, all healthcare professionals involved must engage in continuous education and remain updated on evolving treatment modalities and clinical guidelines. Physicians and advanced practitioners bear the responsibility of delivering evidence-based interventions, while nurses and pharmacists support the care process through medication management, symptom monitoring, and patient education. Interprofessional communication also plays a key role in ensuring that safety protocols are followed and that care is coordinated across inpatient and outpatient settings. Implementing continuous quality improvement initiatives allows teams to track patient outcomes, identify areas for improvement, and refine strategies that enhance safety and effectiveness. This collective commitment to collaboration, education, and quality care ultimately improves outcomes and elevates the overall performance of the healthcare team.
Media
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
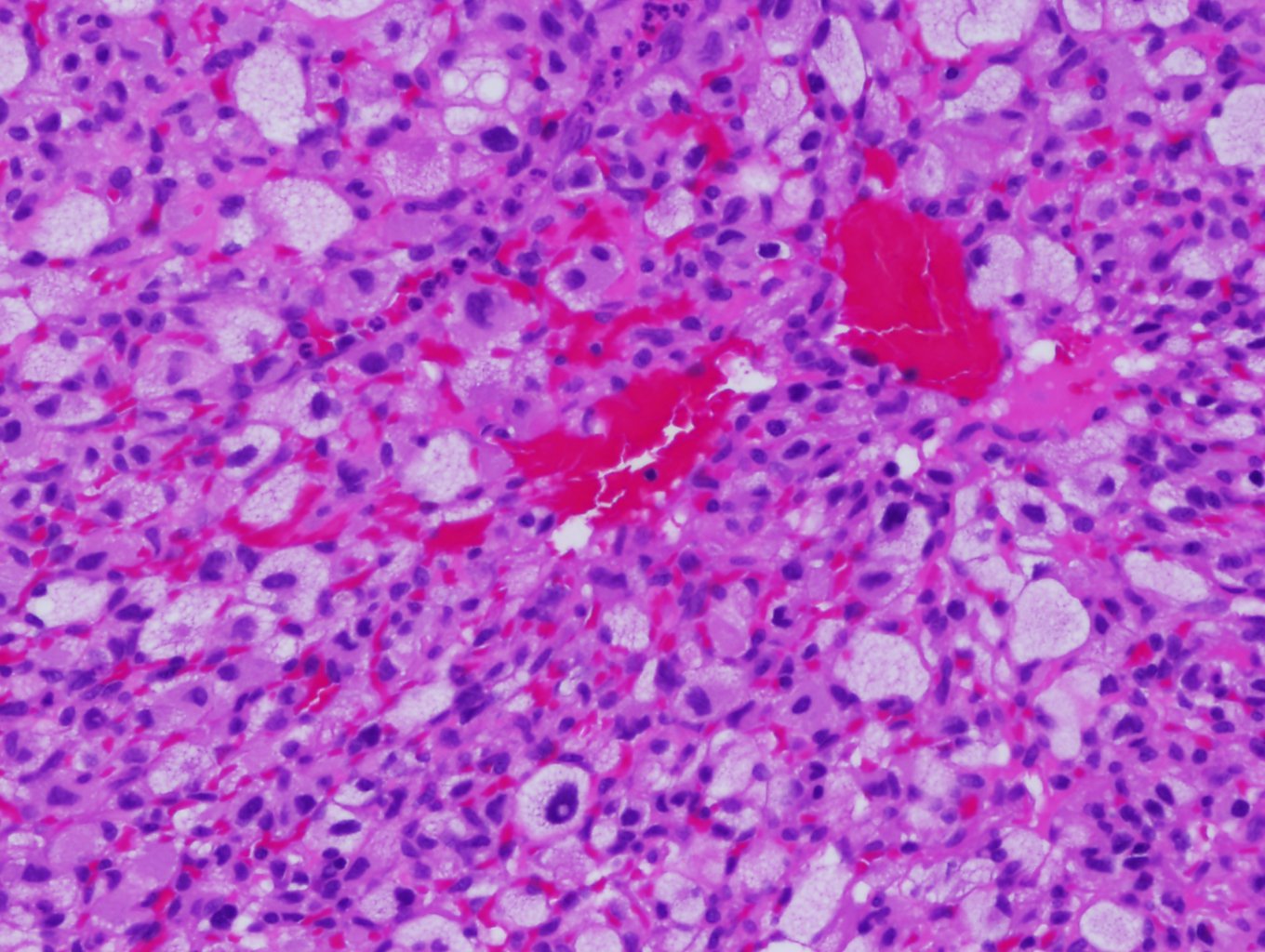
Hemangioblastoma. Hemangioblastomas have a hallmark histological feature: their biphasic tissue composition comprising stromal cells and capillary networks. The vascular component of hemangioblastomas has dense capillary networks and sinusoids. These blood vessels have a single layer of endothelial cells that are often dilated, contributing to the tumor's highly vascular nature.
Contributed by D Neelon, MD
References
Conway JE, Chou D, Clatterbuck RE, Brem H, Long DM, Rigamonti D. Hemangioblastomas of the central nervous system in von Hippel-Lindau syndrome and sporadic disease. Neurosurgery. 2001 Jan:48(1):55-62; discussion 62-3 [PubMed PMID: 11152361]
Bründl E, Schödel P, Ullrich OW, Brawanski A, Schebesch KM. Surgical resection of sporadic and hereditary hemangioblastoma: Our 10-year experience and a literature review. Surgical neurology international. 2014:5():138. doi: 10.4103/2152-7806.141469. Epub 2014 Sep 22 [PubMed PMID: 25317353]
Friedrich CA. Von Hippel-Lindau syndrome. A pleomorphic condition. Cancer. 1999 Dec 1:86(11 Suppl):2478-82 [PubMed PMID: 10630173]
Ganeshan D, Menias CO, Pickhardt PJ, Sandrasegaran K, Lubner MG, Ramalingam P, Bhalla S. Tumors in von Hippel-Lindau Syndrome: From Head to Toe-Comprehensive State-of-the-Art Review. Radiographics : a review publication of the Radiological Society of North America, Inc. 2018 May-Jun:38(3):849-866. doi: 10.1148/rg.2018170156. Epub 2018 Mar 30 [PubMed PMID: 29601266]
Farrukh HM. Cerebellar hemangioblastoma presenting as secondary erythrocytosis and aspiration pneumonia. The Western journal of medicine. 1996 Feb:164(2):169-71 [PubMed PMID: 8775737]
Ho VB, Smirniotopoulos JG, Murphy FM, Rushing EJ. Radiologic-pathologic correlation: hemangioblastoma. AJNR. American journal of neuroradiology. 1992 Sep-Oct:13(5):1343-52 [PubMed PMID: 1414827]
Cvek J, Knybel L, Reguli S, Lipina R, Hanzlikova P, Šilhán P, Resova K, Blazek T, Palicka M, Feltl D. Stereotactic radiotherapy for spinal hemangioblastoma - disease control and volume analysis in long-term follow up. Reports of practical oncology and radiotherapy : journal of Greatpoland Cancer Center in Poznan and Polish Society of Radiation Oncology. 2022:27(1):134-141. doi: 10.5603/RPOR.a2022.0003. Epub 2022 Mar 22 [PubMed PMID: 35402025]
Mak G, Algird A, Greenspoon J, Provias J, Hirte H. Cervicomedullary hemangioblastoma treated with bevacizumab. Neuro-oncology advances. 2020 Jan-Dec:2(1):vdaa076. doi: 10.1093/noajnl/vdaa076. Epub 2020 Sep 3 [PubMed PMID: 32908970]
Level 3 (low-level) evidenceYin X, Duan H, Yi Z, Li C, Lu R, Li L. Incidence, Prognostic Factors and Survival for Hemangioblastoma of the Central Nervous System: Analysis Based on the Surveillance, Epidemiology, and End Results Database. Frontiers in oncology. 2020:10():570103. doi: 10.3389/fonc.2020.570103. Epub 2020 Sep 9 [PubMed PMID: 33014882]
Takayanagi S, Mukasa A, Tanaka S, Nomura M, Omata M, Yanagisawa S, Yamamoto S, Ichimura K, Nakatomi H, Ueki K, Aburatani H, Saito N. Differences in genetic and epigenetic alterations between von Hippel-Lindau disease-related and sporadic hemangioblastomas of the central nervous system. Neuro-oncology. 2017 Sep 1:19(9):1228-1236. doi: 10.1093/neuonc/nox034. Epub [PubMed PMID: 28379443]
Takami H, Graffeo CS, Perry A, Brown DA, Meyer FB, Burns TC, Parney IF. Presentation, imaging, patterns of care, growth, and outcome in sporadic and von Hippel-Lindau-associated central nervous system hemangioblastomas. Journal of neuro-oncology. 2022 Sep:159(2):221-231. doi: 10.1007/s11060-022-04021-8. Epub 2022 Jul 28 [PubMed PMID: 35902552]
Maher ER, Neumann HP, Richard S. von Hippel-Lindau disease: a clinical and scientific review. European journal of human genetics : EJHG. 2011 Jun:19(6):617-23. doi: 10.1038/ejhg.2010.175. Epub 2011 Mar 9 [PubMed PMID: 21386872]
Kim H, Park IS, Jo KW. Meningeal supratentorial hemangioblastoma in a patient with von hippel-lindau disease mimicking angioblastic menigioma. Journal of Korean Neurosurgical Society. 2013 Nov:54(5):415-9. doi: 10.3340/jkns.2013.54.5.415. Epub 2013 Nov 30 [PubMed PMID: 24379949]
Vetrano IG, Gioppo A, Faragò G, Pinzi V, Pollo B, Broggi M, Schiariti M, Ferroli P, Acerbi F. Hemangioblastomas and Other Vascular Origating Tumors of Brain or Spinal Cord. Advances in experimental medicine and biology. 2023:1405():377-403. doi: 10.1007/978-3-031-23705-8_14. Epub [PubMed PMID: 37452946]
Level 3 (low-level) evidenceJankovic D, Vuong K, Splavski B, Rotim K, Arnautovic KI. Supratentorial Hemangioblastoma in Adults: A Systematic Review and Comparison of Infratentorial and Spinal Cord Locations. World neurosurgery. 2023 May:173():48-62. doi: 10.1016/j.wneu.2023.02.071. Epub 2023 Feb 22 [PubMed PMID: 36822402]
Level 1 (high-level) evidenceWiesener MS, Eckardt KU. Erythropoietin, tumours and the von Hippel-Lindau gene: towards identification of mechanisms and dysfunction of oxygen sensing. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 2002 Mar:17(3):356-9 [PubMed PMID: 11865075]
Klingler JH, Gläsker S, Bausch B, Urbach H, Krauss T, Jilg CA, Steiert C, Puzik A, Neumann-Haefelin E, Kotsis F, Agostini H, Neumann HPH, Beck J. Hemangioblastoma and von Hippel-Lindau disease: genetic background, spectrum of disease, and neurosurgical treatment. Child's nervous system : ChNS : official journal of the International Society for Pediatric Neurosurgery. 2020 Oct:36(10):2537-2552. doi: 10.1007/s00381-020-04712-5. Epub 2020 Jun 7 [PubMed PMID: 32507909]
Yoda RA, Cimino PJ. Neuropathologic features of central nervous system hemangioblastoma. Journal of pathology and translational medicine. 2022 May:56(3):115-125. doi: 10.4132/jptm.2022.04.13. Epub 2022 May 3 [PubMed PMID: 35501672]
Louise M Binderup M, Smerdel M, Borgwadt L, Beck Nielsen SS, Madsen MG, Møller HU, Kiilgaard JF, Friis-Hansen L, Harbud V, Cortnum S, Owen H, Gimsing S, Friis Juhl HA, Munthe S, Geilswijk M, Rasmussen ÅK, Møldrup U, Graumann O, Donskov F, Grønbæk H, Stausbøl-Grøn B, Schaffalitzky de Muckadell O, Knigge U, Dam G, Wadt KA, Bøgeskov L, Bagi P, Lund L, Stochholm K, Ousager LB, Sunde L. von Hippel-Lindau disease: Updated guideline for diagnosis and surveillance. European journal of medical genetics. 2022 Aug:65(8):104538. doi: 10.1016/j.ejmg.2022.104538. Epub 2022 Jun 13 [PubMed PMID: 35709961]
Bonneville F, Sarrazin JL, Marsot-Dupuch K, Iffenecker C, Cordoliani YS, Doyon D, Bonneville JF. Unusual lesions of the cerebellopontine angle: a segmental approach. Radiographics : a review publication of the Radiological Society of North America, Inc. 2001 Mar-Apr:21(2):419-38 [PubMed PMID: 11259705]
Palavani LB, Andreão FF, de Abreu LV, Batista S, Borges J, Oliveira LB, Bertani R, Filho JAA. Assessing the efficacy and safety of hemangioblastoma embolization: A comprehensive systematic review and meta-analysis. Journal of clinical neuroscience : official journal of the Neurosurgical Society of Australasia. 2023 Nov:117():104-113. doi: 10.1016/j.jocn.2023.09.021. Epub 2023 Oct 1 [PubMed PMID: 37788533]
Level 1 (high-level) evidenceWang H, Zhang L, Wang H, Nan Y, Ma Q. Spinal hemangioblastoma: surgical procedures, outcomes and review of the literature. Acta neurologica Belgica. 2021 Aug:121(4):973-981. doi: 10.1007/s13760-020-01420-4. Epub 2020 Jul 7 [PubMed PMID: 32638270]
Jankovic D, Hanissian A, Rotim K, Splavski B, Arnautovic KI. Novel Clinical Insights into Spinal Hemangioblastoma in Adults: A Systematic Review. World neurosurgery. 2022 Feb:158():1-10. doi: 10.1016/j.wneu.2021.10.105. Epub 2021 Oct 21 [PubMed PMID: 34687932]
Level 1 (high-level) evidenceSokol Z, Hoeft A, Kung D, Belman N, Oselkin M. Intra-arterial Bevacizumab for Posterior Fossa Hemangioblastoma. Cureus. 2022 Dec:14(12):e32624. doi: 10.7759/cureus.32624. Epub 2022 Dec 17 [PubMed PMID: 36654589]
Kano H, Shuto T, Iwai Y, Sheehan J, Yamamoto M, McBride HL, Sato M, Serizawa T, Yomo S, Moriki A, Kohda Y, Young B, Suzuki S, Kenai H, Duma C, Kikuchi Y, Mathieu D, Akabane A, Nagano O, Kondziolka D, Lunsford LD. Stereotactic radiosurgery for intracranial hemangioblastomas: a retrospective international outcome study. Journal of neurosurgery. 2015 Jun:122(6):1469-78. doi: 10.3171/2014.10.JNS131602. Epub 2015 Mar 27 [PubMed PMID: 25816088]
Level 2 (mid-level) evidenceLee C, Park JW, Suh JH, Nam KH, Moon KC. Histologic variations and immunohistochemical features of metastatic clear cell renal cell carcinoma. Korean journal of pathology. 2013 Oct:47(5):426-32. doi: 10.4132/KoreanJPathol.2013.47.5.426. Epub 2013 Oct 25 [PubMed PMID: 24255630]
Kojima F, Musangile FY, Matsuzaki I, Yorita K, Kuroda N, Nagashima Y, Murata SI. Current Knowledge and Prospects for Renal Hemangioblastoma and Renal Cell Carcinoma with Hemangioblastoma-like Features. Biomedicines. 2023 May 17:11(5):. doi: 10.3390/biomedicines11051467. Epub 2023 May 17 [PubMed PMID: 37239138]
Suri VS, Tatke M, Singh D, Sharma A. Histological spectrum of ependymomas and correlation of p53 and Ki-67 expression with ependymoma grade and subtype. Indian journal of cancer. 2004 Apr-Jun:41(2):66-71 [PubMed PMID: 15318011]
Louis DN, Perry A, Reifenberger G, von Deimling A, Figarella-Branger D, Cavenee WK, Ohgaki H, Wiestler OD, Kleihues P, Ellison DW. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: a summary. Acta neuropathologica. 2016 Jun:131(6):803-20. doi: 10.1007/s00401-016-1545-1. Epub 2016 May 9 [PubMed PMID: 27157931]
Mulligan PR, Prajapati HJ, Martin LG, Patel TH. Vascular anomalies: classification, imaging characteristics and implications for interventional radiology treatment approaches. The British journal of radiology. 2014 Mar:87(1035):20130392. doi: 10.1259/bjr.20130392. Epub [PubMed PMID: 24588666]
Epelman M, Daneman A, Blaser SI, Ortiz-Neira C, Konen O, Jarrín J, Navarro OM. Differential diagnosis of intracranial cystic lesions at head US: correlation with CT and MR imaging. Radiographics : a review publication of the Radiological Society of North America, Inc. 2006 Jan-Feb:26(1):173-96 [PubMed PMID: 16418251]
Pereira GC, Traughber M, Muzic RF Jr. The role of imaging in radiation therapy planning: past, present, and future. BioMed research international. 2014:2014():231090. doi: 10.1155/2014/231090. Epub 2014 Apr 10 [PubMed PMID: 24812609]
Hanakita S, Koga T, Shin M, Takayanagi S, Mukasa A, Tago M, Igaki H, Saito N. The long-term outcomes of radiosurgery for intracranial hemangioblastomas. Neuro-oncology. 2014 Mar:16(3):429-33. doi: 10.1093/neuonc/not201. Epub 2013 Dec 12 [PubMed PMID: 24335701]
Johnson S, Niranjan A, Kano H, Lunsford LD. Leksell Radiosurgery for the 3 H Tumors: Hemangiomas, Hemangioblastomas, and Hemangiopericytomas. Progress in neurological surgery. 2019:34():223-231. doi: 10.1159/000493068. Epub 2019 May 16 [PubMed PMID: 31096251]
Yoo KH, Park DJ, Marianayagam NJ, Gu X, Pollom EL, Soltys SG, Chang SD, Meola A. Stereotactic Radiosurgery for Cranial and Spinal Hemangioblastomas: A Single-Institution Retrospective Series. Neurosurgery. 2024 Mar 1:94(3):630-642. doi: 10.1227/neu.0000000000002728. Epub 2023 Oct 17 [PubMed PMID: 37967154]
Level 2 (mid-level) evidencePan J, Ho AL, D'Astous M, Sussman ES, Thompson PA, Tayag AT, Pangilinan L, Soltys SG, Gibbs IC, Chang SD. Image-guided stereotactic radiosurgery for treatment of spinal hemangioblastoma. Neurosurgical focus. 2017 Jan:42(1):E12. doi: 10.3171/2016.10.FOCUS16361. Epub [PubMed PMID: 28041328]
Kano H, Niranjan A, Mongia S, Kondziolka D, Flickinger JC, Lunsford LD. The role of stereotactic radiosurgery for intracranial hemangioblastomas. Neurosurgery. 2008 Sep:63(3):443-50; discussion 450-1. doi: 10.1227/01.NEU.0000313120.81565.D7. Epub [PubMed PMID: 18812955]
Pan J, Jabarkheel R, Huang Y, Ho A, Chang SD. Stereotactic radiosurgery for central nervous system hemangioblastoma: systematic review and meta-analysis. Journal of neuro-oncology. 2018 Mar:137(1):11-22. doi: 10.1007/s11060-017-2697-0. Epub 2017 Dec 4 [PubMed PMID: 29204841]
Level 1 (high-level) evidenceAsthagiri AR, Mehta GU, Zach L, Li X, Butman JA, Camphausen KA, Lonser RR. Prospective evaluation of radiosurgery for hemangioblastomas in von Hippel-Lindau disease. Neuro-oncology. 2010 Jan:12(1):80-6. doi: 10.1093/neuonc/nop018. Epub 2009 Dec 23 [PubMed PMID: 20150370]