Introduction
Body plethysmography, performed using a large plastic box, is a well-established technique for assessing lung function. This technique traces its origins to concepts developed in the late 19th century, with technical implementation beginning in the 1950s.[1] Over time, it has evolved into an advanced technique that provides detailed insights into lung physiology. Body plethysmography is a vital pulmonary function test that measures lung volumes and airway resistance, offering a comprehensive insight into breathing mechanics. Unlike simple spirometry, which only captures airflow and lung capacity, body plethysmography assesses total lung volume—including residual volume (RV) and total lung capacity (TLC)—airway resistance, and intrathoracic gas volume (ITGV), without requiring forced breathing maneuvers. This technique provides crucial information for diagnosing and managing various respiratory conditions, such as asthma, chronic obstructive pulmonary disease (COPD), restrictive lung impairments, and other disorders affecting lung function.[2][3]
The procedure is typically performed inside an airtight chamber known as a plethysmograph, where patients breathe through a mouthpiece connected to a pneumotachograph. As the patient breathes, changes in pressure within the chamber are recorded and used to calculate lung volumes and airway resistance. This method's accuracy stems from its ability to assess trapped air and detect small airway changes that are not always evident in other testing methods. Consequently, body plethysmography is considered the gold standard for measuring lung volumes.[4]
Body plethysmography plays a crucial role in the early detection of lung abnormalities, monitoring disease progression, and evaluating treatment responses. The ability of body plethysmography to distinguish between obstructive and restrictive lung diseases helps healthcare providers tailor treatment strategies more effectively. As the prevalence of respiratory diseases continues to rise globally, accurate and detailed pulmonary function testing, such as body plethysmography, becomes increasingly important in pulmonology.
Rationale for Body Plethysmography
Body plethysmography is essential for diagnosing conditions where lung volumes and airway resistance are altered. This technique allows for the following:
- Detection of trapped air in diseases such as COPD
- Differentiation between obstructive and restrictive lung diseases
- Accurate measurement of TLC
- Assessment of airway resistance and conductance
Table 1. Comparison of Lung Volumes Measured by Spirometry and Body Plethysmography
| Lung Function Parameters | Spirometry | Body Plethysmography |
| Tidal volume (TV) | + | + |
| Inspiratory reserve volume (IRV) | + | + |
| Expiratory reserve volume (ERV) | + | + |
| Residual volume (RV) | − | + |
| Functional residual capacity (FRC) | − | + |
| Total lung capacity (TLC) | − | + |
Specimen Collection
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Specimen Collection
Principle
Plethysmography is based on the fundamental principle known as Boyle-Mariotte's law. This law states that under isothermal conditions, the product of a gas's pressure (P) and volume (V)—(P×V)—remains constant and is the basis of the theory. Due to the close contact with capillary blood, the gas in the lungs is isothermal (see Image. Volume-Time Display).[5] One of the notable advantages of this technique is its ability to rapidly obtain multiple measurements of RV and TLC. Because the alveolar gas composition must be restored to the control state before these tests can be repeated—a process that frequently takes 10 to 20 minutes in individuals with COPD—this is not feasible with the washout and dilution procedures. All of the gas in the lung, including that in poorly ventilated places, is measured through plethysmography. Therefore, in patients with COPD, plethysmography typically yields higher and more accurate measurements of functional residual capacity (FRC), RV, and TLC compared to gas dilution methods. TLC values obtained with plethysmography are often 2 to 3 liters higher than those measured by gas methods.[6]
The body plethysmograph was first introduced for clinical use by DuBois et al in 1956.[2][3] The plethysmograph is a closed chamber of about 500 to 800 L capacity. There are 2 types of boxes—the more commonly used constant-volume type and the flow type.
A research participant sits in a sealed, airtight chamber that is designed to measure changes in pressure, flow, or volume during body plethysmography. The body plethysmograph is most frequently used to quantify airway resistance (R) and thoracic gas volume (VTG). The VTG is typically equal to the FRC and measured at the tidal end-expiratory position.The reciprocal of airway resistance (R) is commonly used to calculate airway conductance (G). Specific airway conductance (sGaw) refers to conductance per unit of lung volume and essentially measures the ease of airflow, independent of lung volume. A higher sGaw indicates better airflow.[7] The body plethysmograph can also be used to perform spirometry, bronchial challenge, and pulmonary compliance testing.
Procedures
Measurement of Thoracic Gas Volume
Panting method: Respiratory movements producing inspiration and expiration are associated with decompression and compression of the air inside the lungs. Suppose the initial lung volume is V and the airway pressure is P. In that case, inspiration increases ITGV by V, which is associated with a fall in the pressure by P. According to Boyle's law, when temperature is constant, the product of volume and pressure is also constant.[8]
Thus, PV = (P − ΔP) (V + ΔV)
Expanding this equation:
PV = PV + PΔV − ΔPV − ΔPΔV
Due to its modest size, ΔPΔV can be disregarded.
Rearranging the terms gives:
V = (ΔV/ΔP) (P)
The term V is the thoracic gas volume (VTG). The airway pressure is atmospherically corrected for water vapor.
Thus, VTG = (ΔV/ΔP) (PB − PH2O)
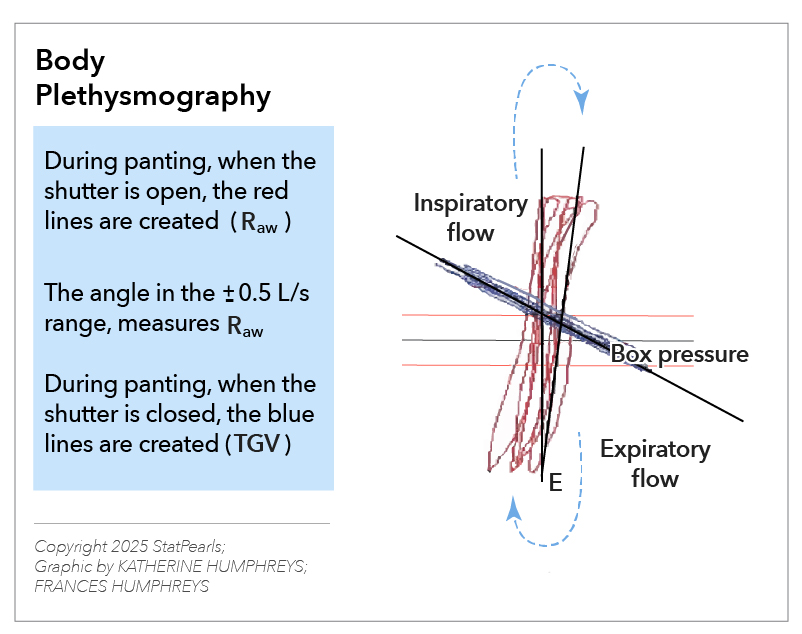
In a body plethysmograph, when the participant breathes (pants) against a closed shutter in front of the mouth and the mouth pressure is measured, the fall in pressure during inspiration (P) can be easily estimated. The expansion of the chest produces an increase in the box pressure as it is a closed chamber. The box pressure is calibrated in terms of volume. The increase in volume (V) can be easily measured by recording the change in box pressure. Thus, to measure VTG, measurements of the mouth pressure and the box pressure are required when the participant inspires against a closed shutter (see Image. Body Plethysmography).
Measurement of Airway Resistance
To measure airway resistance (Raw), the participant breathes through a flow-measuring device (pneumotach). The measured airflow is labeled as V0. The change in airflow can be related to the change in pressure, as resistance = pressure/airflow.[9]
Thus, Raw = ΔP/ΔV2
Multiplying both the numerator and denominator by ΔV,
Raw = (ΔP/V) (ΔV/ΔV2)
Although ΔP/ΔV can be measured from the slope of 2 measurements plotted on the X and Y axes, ΔV/ΔV2 requires displaying the box volume change against the airflow.
Specific airway resistance (sRaw) is the ratio of the shift volume, or box pressure, to flow rate, stated in appropriate units. Closed curves are produced by plotting shift volume on the horizontal axis against airflow on the vertical axis. The reciprocal slope of these breathing loops represents a certain amount of airway resistance. In healthy individuals, the curves are approximately straight lines. In contrast, patients with respiratory illnesses display unique patterns, aiding in differential diagnosis and providing insights into the particular condition (see Image. Measurement of Airway Resistance).
The relationship between lung volume and pressure is crucial for understanding lung mechanics. At closing mouth pressure, this relationship is linear, meaning that as intrathoracic pressure changes, lung volume changes proportionally. This principle, based on Boyle's law, is central to plethysmographic calculations.
Calculations
The slopes (measured as the tangent of the angle 0 of the slope) of V0/V and P/V obtained with the shutter open and closed, respectively, multiplied by the respective calibration factors, provide the values for these terms. The atmospheric pressure is required to calculate (PB PH20).[5][6][7]
For example, if the calibration (Cal) scale for airflow is 0.5 L/s/cm and for box pressure is 5 mL/cm, then the cal factor for V0/V is 100/s. Similarly, for P/V, the cal factor is 1 cm H20/mL (taking the mouth pressure cal scale as 5 cm H20/cm).
A correction is necessary for the participant's volume if the calibrations are performed with an empty box because the effective volume of the box is reduced by the participant's body volume. Thus, either the box pressure can be calibrated with the participant inside, holding their breath, or a correction may be applied as below:
Corrected ΔV = ΔV × (body volume − body weight)/box volume
This method requires the participant to pant to measure P and also assumes that by breathing against a closed shutter, the mouth pressure reflects the airway pressure. This assumption may not be true in patients with airway obstruction. Panting itself can be challenging, requiring training and cooperation from the participant.
To address these limitations, Krell et al and Agrawal and Kumar described a modification of the body plethysmography that does not require panting (Quiet-breathing method). Using this method, Gaw can be calculated directly without measuring VTG.[10][11]
Measurement of Specific Airway Conductance
Multiplying the equations above,
VTG × Raw = (ΔV/ΔP) (PB – PH2O) × (ΔP/ΔV) (ΔV/ΔV°) = (PB – PH2O) (ΔV/ΔV°)
As measurement of P is no longer required, panting against a closed shutter becomes unnecessary. The product of VTG and Raw represents airway resistance normalized to lung volume, also known as specific airway resistance (sRaw).
Thus sRaw = (PB – PH2o) (ΔV/ΔV°)
sGaw is the inverse of sRaw. The VTG is expressed in liters (body temperature and pressure saturated) and is the volume of gas in the lung when the mouth shutter is closed. Raw is reported in cm H2O/L/s. sGaw is expressed in s/cm H2O (that is, s·cm H2O−1) and is the reciprocal of Raw (1/Raw) divided by the lung volume at which the resistance measurement is made.
Table 2. Calculations and Definitions of Thoracic Gas Volume, Airway Resistance, and Specific Airway Conductance
| Parameter | Formula | Definition |
| Thoracic gas volume (VTG) | VTG = (ΔV/ΔP) × (PB − PH2O) | Volume of gas in lungs at shutter closure |
| Airway resistance (Raw) | Raw = ΔP/ΔV | Resistance to airflow in the airway |
| Specific airway conductance (sGaw) | sGaw = 1/Raw | Measure of airway patency normalized for lung volume |
Indications
Indications for Body Plethysmography
Determination of VTG, sRaw, and sGaw through body plethysmography is indicated in the following situations:
- Diagnosis of restrictive lung disease.
- Measurement of lung volume to differentiate between obstructive and restrictive processes.
- Assessment of obstructive lung conditions that can result in artificially low results when measured by helium dilution or nitrogen washout, such as cystic fibrosis and bullous emphysema.
- Calculation of trapped gas (FRC by Helium Dilution versus FRC by Plethysmography).
- Measurement of lung volume when multiple trials are required and the participant is incapable of performing multibreath tests.
- Evaluation of airflow resistance.
- Evaluation of bronchodilator responsiveness, as indicated by alterations in sRaw and sGaw.
- Determining whether methacholine or histamine causes bronchial hyperactivity.
- Monitoring the disease's progression and reaction to therapy.[5][12]
Contraindications to Body Plethysmography
- There are no absolute contraindications to body plethysmography.
- Relative contraindications to body plethysmography are as follows:
- Body casts, mental disarray, poor motor coordination, or other issues that make it difficult for the patient to enter the body box or carry out the necessary movements, such as breathing against a closed shutter.
- Claustrophobia can be exacerbated by entering the body box.
- Existence of equipment or other conditions that could interfere with pressure changes, such as a transtracheal oxygen catheter, a chest tube, or a ruptured eardrum, or that cannot fit into the plethysmograph, such as continuous intravenous infusions with pumps or other equipment.
- Oxygen treatment is ongoing and cannot be stopped momentarily.[5][12][13]
Potential Diagnosis
Table 3. Changes Observed in Lung Volumes and Resistance on Body Plethysmography in Major Pulmonary Pathologies [14][15]
| Pulmonary Conditions |
FRC |
RV |
TLC |
Raw |
sRaw |
|
Obstructive airway diseases |
Normal or increased |
Normal or increased |
Normal |
Increased |
Increased |
|
Hyperinflation |
Increased |
Increased |
Normal or increased |
Normal |
Increased |
|
Restrictive disorders |
Decreased |
Decreased or normal |
Decreased |
Normal |
Normal |
Abbreviations: FRC, functional residual capacity; RV, residual volume; TLC, total lung capacity; Raw, airway resistance; sRaw, specific airway conductance.
Normal and Critical Findings
Reporting Findings of Body Plethysmography
The reported VTG should adhere to the following guidelines:
- Should be averaged from a minimum of 3 to 5 separate, acceptable panting maneuvers.
- Should be calculated using values that agree within 5% of the mean. Widely varying values should be averaged and reported as a variable.
- Should indicate whether the thoracic volume was at FRC or some other level.
- Should be compared with other lung volume measurements, such as helium dilution or nitrogen washout, if these tests are performed.
- Should be corrected for patient weight for some systems.[16][17]
During the same testing session, lung volumes, including the slow vital capacity (VC) maneuver, its subdivisions, inspiratory capacity (IC), and expiratory reserve volume (ERV), should be measured. Before disconnecting from the measuring system, VC, IC, and ERV should be measured with each VTG trial.
- The largest volume of VC or FVC obtained should be used to calculate derived lung volumes, such as TLC, RV, and RV/TLC.
- The mean values should be reported for IC and ERV from acceptable VTG maneuvers.
- Various methods exist to calculate TLC; the most common is TLC = mean FRC + mean IC.
- RV = TLC − largest VC.
The reported Raw and sGaw should adhere to the following guidelines:
- Should be calculated from the ratio of closed and open shutter tangents for each maneuver.
- Should be averaged from 3 to 5 separate, acceptable maneuvers; reproducibility should be based on sGaw, and the suggested limit for variance is within 10% of the mean, such as if the measured results are ≤0.17, accept ±0.01, or if the measured results are ≥0.20, use ±0.02.
- The open-shutter tangent (V/Pbox) should be measured between flows of +0.5 and −0.5 L/s. For loops that display hysteresis, the inspiratory limbs may be used.
- sGaw should be calculated using the VTG at which the shutter was closed for each maneuver.
The normal range of predicted values of lung volumes is as follows:
- RV and TLC normal range: 80% to 120% of predicted.
- RV/TLC and IC/TLC normal range: 80% to 120% of predicted.
- Raw: 130% predicted.
- Gaw: 80% predicted.[18]
Interfering Factors
Acceptability Criteria
The validity of the results depends on both the proper functioning of the equipment and the individual's ability to perform acceptable and reproducible maneuvers.[5]
VTG maneuvers are acceptable in these situations:
- The displayed tracing demonstrates proper panting technique, with the loop generated against a closed shutter appearing closed or nearly closed. To avoid pressure changes brought on by the lips, the patient should use their hands to support their cheeks without raising the shoulders or supporting the elbows.
- All recorded pressure changes must remain within the calibrated range of each transducer, and the complete trace should be visible. Pressure fluctuations that are too large or too small may result in inaccurate measurements.
- Tracings should remain stable on the screen or recording device, with thermal equilibrium clearly achieved, typically after 1 to 2 minutes.
- The frequency of panting is roughly 1 Hz. The plethysmograph system may be specifically made to conduct non-panting motions.
Raw and sGaw maneuvers may be considered acceptable in these situations:
- They satisfy the aforementioned requirements (points 1-3).
- A comparatively closed loop is demonstrated by the open-shutter panting maneuver, especially between +0.5 and −0.5 L/s.
- To facilitate interpretation, the panting frequency during serial measurements in a particular patient is maintained constant (a range of 90-150 cycles/min, 1.5-2.5 Hz). The frequency should also be maintained constant for serial testing and within-testing session comparisons, such as pre- and post-bronchodilator testing.
Complications
Possible Complications
- Syncopal attacks secondary to raised intrathoracic pressures due to improper panting against a closed shutter while measuring VTG and Raw.
- Hypercapnia or hypoxia secondary to prolonged confinement in the body box chamber, if not opened periodically.
- Infections can spread through unclean equipment, such as mouthpieces, or inadvertent transmission of bodily fluids or droplet nuclei from patient to patient or patient to technician.[5]
Patient Safety and Education
Quality Control
Body plethysmography is technically demanding, requiring careful attention to various details. Quality control must be maintained.[14] Otherwise, the following errors can happen:
- Overestimation of VTG in patients with severe obstruction or induced bronchospasm unless a slow panting speed (approximately 1 cycle/s) is maintained.
- Erroneous measurement of VTG, Raw, or Gaw due to improper panting technique. Excessive pressure fluctuations or signal drift during panting may invalidate VTG, Raw, or sGaw.
- VTG or Raw measurements may be invalidated by non-panting maneuvers in plethysmographs with integrated thermal leakage.
- Slopes of VTG or Raw tangents, computer-determined, may be inaccurate. In many systems, the slopes are determined using a best-fit regression analysis. If unnecessary data points are collected because of poor panting or severe signal drift, this technique may yield results that differ greatly.
- Every slope must be visually examined and modified following a recognized laboratory protocol.
- The measured VTG may be elevated due to compression effects caused by excessive abdominal gas or the use of accessory muscles during panting.
- In many systems, the slopes are determined using a best-fit regression analysis. If unnecessary data points are collected because of poor panting or severe signal drift, this technique may yield results that differ greatly. Every slope must be visually examined and modified following a recognized laboratory protocol.
- Several transducers should be carefully calibrated, with attention given to leakage, thermal stability, and frequency response.
- When the equipment is moved to a different place or calibrated at prescribed frequencies, accuracy is questionable.[5][12][19]
Precautions
The following precautions are recommended to ensure accurate and reliable body plethysmography measurements:
- The mouth pressure, box, and volume should be checked every day.
- In addition to using the autocalibration system daily, systems should be manually calibrated at least once every 3 months.
- The linearity of the flow-sensing device should be checked at least once every week.
- A known resistor to measure airway resistance should be used, and the findings should be computed at least once every quarter.
- Volume should be checked with an isothermal bottle at least annually.
- Tests on standard participants (biological controls, or bio-QC) should be performed at least monthly and at any time accuracy is suspected.
- To establish statistical variation for comparison, standard participants should be tested more frequently at first.
Clinical Significance
Body plethysmography is a valuable tool for diagnosing, assessing, and treating pulmonary diseases. The accurate quantification of the lung volumes, airway resistance, and thoracic gas volume allows the distinction between obstructive and restrictive lung diseases. The capacity of body plethysmography to identify gas trapping and small airway dysfunction makes it particularly useful for identifying diseases such as COPD and asthma, even in their early stages. Body plethysmography is also valuable for assessing treatment response, monitoring disease progression, and guiding individualized therapeutic interventions in pulmonary disorders.
Table 4. Clinical Interpretation of Derived Indices
| Parameter | Increased in | Decreased in |
| TLC | Hyperinflation (COPD, asthma) | Restrictive lung diseases (fibrosis) |
| RV | Air trapping (COPD, emphysema) | Restrictive lung diseases |
| Raw | Airway obstruction (asthma, bronchitis) | - |
| sGaw | - | Severe airway narrowing (COPD, fibrosis) |
Abbreviations: TLC, total lung capacity; RV, residual volume; Raw, airway resistance; sGaw, Specific airway conductance; COPD, chronic obstructive pulmonary disease.
Body plethysmography is an essential tool for diagnosing and monitoring respiratory diseases. The ability of body plethysmography to measure lung volumes beyond conventional spirometry makes it indispensable in pulmonology. Integrating plethysmographic data with clinical assessment enhances disease diagnosis, management, and treatment optimization.
Media
(Click Image to Enlarge)
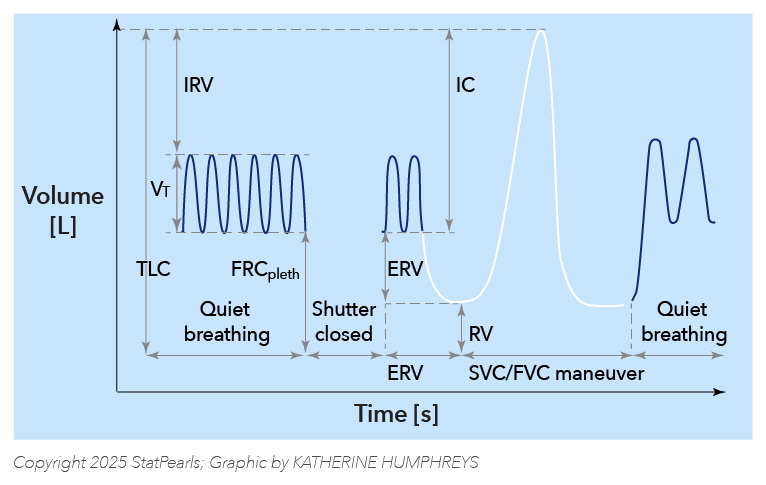
Volume-Time Display. The graph illustrates a volume-time display that shows the following sequence: quiet breathing for recording specific airway resistance loops, a period when the shutter is closed for the determination of FRCpleth, and subsequently a period during which the patient performs an expiratory reserve volume (ERV) maneuver, followed by a slow vital capacity maneuver (SVC) to determine inspiratory vital capacity (IVC) and to derive residual volume (RV) and total lung capacity (TLC). Commonly, this is followed by a forced vital capacity (FVC) maneuver that also yields the forced expiratory volume in 1 s (FEV1) and the maximum expiratory flows (MEFs) at different lung volumes.
Illustration by K Humphreys and F Humphreys
Adapted from Criée CP, Sorichter S, Smith HJ, et al. Body plethysmography—its principles and clinical use.
(Click Image to Enlarge)
(Click Image to Enlarge)
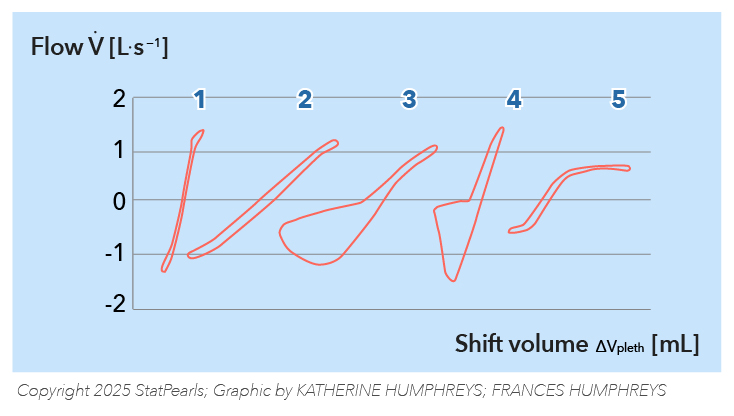
Measurement of Airway Resistance. This schematic represents the specific resistance loops (sRaw) for different conditions: (1) a normal research participant, (2) a research participant with increased large airway resistance, (3) a research participant with chronic airflow obstruction, (4) a research participant with obesity or diaphragmatic paralysis, and (5) a research participant with upper airway obstruction.
Illustration by K Humphreys and F Humphreys
Adapted from Criée CP, Sorichter S, Smith HJ, et al. Body plethysmography—its principles and clinical use.
References
West JB. The birth of clinical body plethysmography: it was a good week. The Journal of clinical investigation. 2004 Oct:114(8):1043-5 [PubMed PMID: 15489948]
DUBOIS AB, BOTELHO SY, COMROE JH Jr. A new method for measuring airway resistance in man using a body plethysmograph: values in normal subjects and in patients with respiratory disease. The Journal of clinical investigation. 1956 Mar:35(3):327-35 [PubMed PMID: 13295397]
DUBOIS AB, BOTELHO SY, BEDELL GN, MARSHALL R, COMROE JH Jr. A rapid plethysmographic method for measuring thoracic gas volume: a comparison with a nitrogen washout method for measuring functional residual capacity in normal subjects. The Journal of clinical investigation. 1956 Mar:35(3):322-6 [PubMed PMID: 13295396]
COMROE JH Jr, BOTELHO SY, DUBOIS AB. Design of a body plethysmograph for studying cardiopulmonary physiology. Journal of applied physiology. 1959 May:14(3):439-44 [PubMed PMID: 13654183]
Criée CP, Sorichter S, Smith HJ, Kardos P, Merget R, Heise D, Berdel D, Köhler D, Magnussen H, Marek W, Mitfessel H, Rasche K, Rolke M, Worth H, Jörres RA, Working Group for Body Plethysmography of the German Society for Pneumology and Respiratory Care. Body plethysmography--its principles and clinical use. Respiratory medicine. 2011 Jul:105(7):959-71. doi: 10.1016/j.rmed.2011.02.006. Epub 2011 Feb 26 [PubMed PMID: 21356587]
Barisione G, Pellegrino R. Body Plethysmography is Helpful for COPD Diagnosis, Determination of Severity, Phenotyping, and Response to Therapy. COPD. 2015:12(6):591-4. doi: 10.3109/15412555.2015.1043524. Epub 2015 Sep 29 [PubMed PMID: 26418437]
Topalovic M, Derom E, Osadnik CR, Troosters T, Decramer M, Janssens W, Belgian Pulmonary Function Study Investigators. Airways resistance and specific conductance for the diagnosis of obstructive airways diseases. Respiratory research. 2015 Jul 22:16(1):88. doi: 10.1186/s12931-015-0252-0. Epub 2015 Jul 22 [PubMed PMID: 26194099]
Habib MP, Engel LA. Influence of the panting technique on the plethysmographic measurement of thoracic gas volume. The American review of respiratory disease. 1978 Feb:117(2):265-71 [PubMed PMID: 637410]
Dubois AB. Airway resistance. American journal of respiratory and critical care medicine. 2000 Aug:162(2 Pt 1):345-6 [PubMed PMID: 10934050]
Krell WS, Agrawal KP, Hyatt RE. Quiet-breathing vs. panting methods for determination of specific airway conductance. Journal of applied physiology: respiratory, environmental and exercise physiology. 1984 Dec:57(6):1917-22 [PubMed PMID: 6511564]
Agrawal KP, Kumar A. Fall in specific airway conductance at residual volume in small airway obstruction. Respiration physiology. 1980 Apr:40(1):65-78 [PubMed PMID: 7394366]
de Mir Messa I, Sardón Prado O, Larramona H, Salcedo Posadas A, Villa Asensi JR, Grupo de Técnicas de la Sociedad Española de Neumología Pediátrica. [Body plethysmography (I): Standardisation and quality criteria]. Anales de pediatria (Barcelona, Spain : 2003). 2015 Aug:83(2):136.e1-7. doi: 10.1016/j.anpedi.2014.10.029. Epub 2015 Mar 18 [PubMed PMID: 25797588]
Level 2 (mid-level) evidenceSylvester KP, Clayton N, Cliff I, Hepple M, Kendrick A, Kirkby J, Miller M, Moore A, Rafferty GF, O'Reilly L, Shakespeare J, Smith L, Watts T, Bucknall M, Butterfield K. ARTP statement on pulmonary function testing 2020. BMJ open respiratory research. 2020 Jul:7(1):. doi: 10.1136/bmjresp-2020-000575. Epub [PubMed PMID: 32631927]
Wanger J, Clausen JL, Coates A, Pedersen OF, Brusasco V, Burgos F, Casaburi R, Crapo R, Enright P, van der Grinten CP, Gustafsson P, Hankinson J, Jensen R, Johnson D, Macintyre N, McKay R, Miller MR, Navajas D, Pellegrino R, Viegi G. Standardisation of the measurement of lung volumes. The European respiratory journal. 2005 Sep:26(3):511-22 [PubMed PMID: 16135736]
Gupta YS, Shah SS, Ahire CK, Kamble P, Khare AS, More SS. Body plethysmography in chronic obstructive pulmonary disease patients: A cross-sectional study. Lung India : official organ of Indian Chest Society. 2018 Mar-Apr:35(2):127-131. doi: 10.4103/lungindia.lungindia_238_17. Epub [PubMed PMID: 29487247]
Level 2 (mid-level) evidenceMiller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, Crapo R, Enright P, van der Grinten CP, Gustafsson P, Jensen R, Johnson DC, MacIntyre N, McKay R, Navajas D, Pedersen OF, Pellegrino R, Viegi G, Wanger J, ATS/ERS Task Force. Standardisation of spirometry. The European respiratory journal. 2005 Aug:26(2):319-38 [PubMed PMID: 16055882]
Pellegrino R, Viegi G, Brusasco V, Crapo RO, Burgos F, Casaburi R, Coates A, van der Grinten CP, Gustafsson P, Hankinson J, Jensen R, Johnson DC, MacIntyre N, McKay R, Miller MR, Navajas D, Pedersen OF, Wanger J. Interpretative strategies for lung function tests. The European respiratory journal. 2005 Nov:26(5):948-68 [PubMed PMID: 16264058]
Stocks J, Quanjer PH. Reference values for residual volume, functional residual capacity and total lung capacity. ATS Workshop on Lung Volume Measurements. Official Statement of The European Respiratory Society. The European respiratory journal. 1995 Mar:8(3):492-506 [PubMed PMID: 7789503]
Alter P, Orszag J, Wouters EFM, Vogelmeier CF, Jörres RA. Differences in the Measurement of Functional Residual Capacity Between Body Plethysmographs of Two Manufacturers. International journal of chronic obstructive pulmonary disease. 2022:17():1477-1482. doi: 10.2147/COPD.S363493. Epub 2022 Jun 24 [PubMed PMID: 35774592]