 Acute Generalized Exanthematous Pustulosis
Acute Generalized Exanthematous Pustulosis
Introduction
Acute generalized exanthematous pustulosis (AGEP) is a rare cutaneous adverse reaction characterized by the rapid development and dissemination of sterile, nonfollicular pustules on an erythematous base (see Image. Acute Generalized Exanthematous Pustulosis, Left Lower Limb).[1] Initially considered a variant of pustular psoriasis, AGEP was established as a distinct clinical entity in 1980.[2]
Most cases arise as adverse drug reactions, although infectious insults and physical triggers have also been implicated. While often self-limited, AGEP can result in systemic complications. Identification and discontinuation of the offending agent remain the cornerstone of therapy.[3][4]
AGEP is generally classified among severe cutaneous adverse reactions (SCARs) as a type IV hypersensitivity disorder. However, the full pathophysiologic mechanisms of this condition remain incompletely defined.[5]
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
The majority of reported AGEP cases result from adverse drug reactions, with several confirmed through patch testing. Systemic antibiotics are the most frequently implicated, particularly β-lactams and macrolides. Other reported triggers include hydroxychloroquine, antifungal, antiviral, and antiparasitic agents, as well as antineoplastic and antirheumatic drugs, analgesics, anticonvulsants, and intravenous contrast media. Corticosteroids have also been identified as causative agents in rare cases.
AGEP has additionally been linked to infections, spider bites, contact allergens, herbal preparations, and psoralen–UVA therapy. In some patients, no specific trigger is identified, or sufficient evidence to establish causality is lacking.[6]
Medications most often implicated as suspected causes of AGEP include, but are not limited to, the following:
- Antibiotics: β-Lactams, macrolides, quinolones, tetracyclines, antituberculosis agents, sulfonamides
- Other anti-infectives: Antifungals, antimalarials, antiprotozoals, antivirals, antihelmintic agents
- Analgesics: Nonsteroidal anti-inflammatory drugs, opioids, acetaminophen, muscle relaxants
- Nervous system medications: Anticonvulsants, antidepressants, mood stabilizers, antipsychotics
- Cardiovascular medications: Calcium channel blockers, β-blockers, angiotensin-converting enzyme inhibitors, renin-angiotensin-aldosterone system modulators, diuretics, lipid-lowering agents, antiplatelet drugs
- Respiratory medications: Leukotriene receptor antagonists
- Antineoplastic agents: Kinase inhibitors, monoclonal antibodies, immunomodulators, chemotherapeutic drugs
- Other agents: Metformin, proton-pump inhibitors, antihistamines, contrast media, corticosteroids, topical drugs, mercury, herbal preparations, vitamins, vaccines, anti-anemic drugs, anesthetics [7][8]
Drugs are most frequently implicated in AGEP, as immune-mediated reactions to drugs are more consistent and reproducible than responses to other reported triggers. Early recognition and withdrawal of the causative drug remain the most effective strategies to hasten resolution and prevent complications.
Epidemiology
AGEP is a rare condition with an estimated incidence of 1 to 5 cases per million annually. Underreporting is likely, given the clinical overlap with other drug-induced eruptions and the self-limited course that may resolve before dermatologic evaluation.[9][10]
The age distribution is broad, as demonstrated in a 2022 case series of 297 patients ranging from 1 to 93 years. Most cases occurred in individuals older than 25, particularly between 40 and 64 years. Similar to other drug-induced SCARs, AGEP shows a female predominance, with women accounting for 65% to 80% of reported cases.[11]
The timing of onset and resolution varies by causative medication and its pharmacokinetics. Differences in drug metabolism between sexes may partly explain the higher frequency in women.[12][13] More than 85% to 90% of cases arise after drug exposure, and geographic patterns reflect medication availability, such as the high prevalence of pristinamycin-associated AGEP in France.[14][15]
Genetic associations have been reported, including human leukocyte antigens B51 (HLA-B51), DR11 (HLA-B-DR11), and DQ3 (HLA-DQ3), which may facilitate T-cell activation. Mutations in the interleukin-36 (IL-36) receptor antagonist gene have also been described, resulting in enhanced IL-36 signaling.
Pathophysiology
The pathogenesis of AGEP is incompletely characterized. The condition is generally considered a T-cell-mediated type IV hypersensitivity reaction associated with neutrophilic inflammation. Following exposure to a triggering factor, such as a new medication, host proteins bind drug metabolites to form epitopes that are captured by antigen-presenting cells. Drug-specific CD4+ and CD8+ T cells are then activated and migrate to the skin, where the release of perforin, granzyme B, and Fas ligand induces keratinocyte apoptosis. This process produces vesicles that evolve into sterile pustules as neutrophils are recruited via CXCL8 and IL-8. Interferon-γ amplifies neutrophil recruitment through additional CXCL8 release, while granulocyte-macrophage colony-stimulating factor prolongs neutrophil survival by preventing apoptosis.
Innate immune pathways have also been implicated, supported by elevated IL-17 and IL-22 levels in the peripheral blood of individuals with AGEP. T-helper 17 (Th17) cells produce IL-17 and IL-22, which stimulate keratinocytes to release IL-8, thereby sustaining neutrophil recruitment and pustule formation.[16]
Mutations in the IL-36 receptor antagonist gene leading to dysregulated IL-36 signaling further implicate the IL-36 cytokine cascade in disease progression. Increased production of IL-4 and IL-5 has also been reported, potentially explaining the peripheral eosinophilia and eosinophilic infiltrates observed in AGEP. Additional studies are required to clarify the immunopathogenesis of this condition.
Histopathology
Histopathologic features of AGEP include nonfollicular spongiform pustules within the epidermis, particularly within and beneath the stratum corneum, producing a subcorneal dermatosis. Edema of the papillary dermis with neutrophilic and eosinophilic perivascular infiltrates is typical. Additional findings may include necrotic keratinocytes, confluence of intraepidermal and subcorneal pustules, and mixed dermal and interstitial inflammatory infiltrates. When differentiating AGEP from pustular psoriasis, the presence of necrotic keratinocytes, epidermal spongiosis, and mixed neutrophilic and eosinophilic infiltrates favors AGEP, while dilated blood vessels within dermal papillae are characteristic of psoriasis and absent in AGEP.
Although rarely performed in routine biopsy analysis, gene expression profiling of AGEP skin has demonstrated increased IL36G expression compared to healthy controls. Immunohistochemical studies further show strong IL-36α and IL-36γ expression within pustules and in the peripustular epidermis of AGEP lesions.
History and Physical
The clinical appearance of AGEP is characterized by numerous tiny nonfollicular sterile pustules on a background of erythema that develop and spread rapidly, typically associated with pruritus or burning sensations and accompanied by objective or subjective fever. Although often described as “pinhead-sized,” pustules may coalesce, become more prominent, and lead to superficial epidermal detachment with gentle pressure resembling a positive Nikolsky sign.
Symptoms usually begin in flexural or intertriginous areas before generalizing to the trunk, extremities, and face within hours to days, while typically sparing the palms and soles. Mucosal involvement is uncommon but, when present, is generally limited to the oral and buccal mucosa.[17]
Pustules desquamate in a collarette-like pattern during the following 1 to 2 weeks. Additional findings may include lymphadenopathy or atypical features such as purpura, vesicles, bullae, or involvement localized to the face, neck, and chest. Historical clues supporting AGEP include rapid onset, usually within 10 days or less of exposure to the causative drug, and resolution within approximately 15 days after discontinuation. The time from onset to resolution may be prolonged in certain cases, such as AGEP induced by hydroxychloroquine or terbinafine.[18]
Evaluation
In addition to a detailed history, physical examination, and medication reconciliation, diagnostic evaluation should include a punch biopsy containing a pustule and laboratory testing to assess systemic involvement. Recommended studies include complete blood count, comprehensive metabolic panel with renal and hepatic function testing, and C-reactive protein.
The presence of systemic symptoms and hemodynamic instability determines the urgency of evaluation and the appropriate setting, whether outpatient or inpatient. Referral to a specialized burn unit may be warranted when the clinical presentation overlaps with other severe cutaneous adverse reactions, such as Stevens–Johnson syndrome or toxic epidermal necrolysis.[19]
The EuroSCAR criteria, published in 2001 by Sidoroff et al and often referred to as the "AGEP validation score," integrate clinical findings, laboratory data, and histopathology to stratify diagnostic likelihood. Scores of 0 or below exclude AGEP, scores of 1 to 4 remain indeterminate, scores of 5 to 7 indicate a probable diagnosis, and scores of 8 to 12 confirm the diagnosis. Outlined below are the EuroSCAR criteria used to establish diagnostic certainty of AGEP.
- Exanthem features
- Pustules: Pustules not appreciable or presence cannot be determined (0); pustules present and equivocal (+1); pustules present in a significant number, nonfollicular, and minuscule or "pinhead" smaller than 5 mm, possibly confluent (+2).
- Erythema: Erythema not appreciable or presence cannot be determined (0); erythema present and equivocal (+1); erythema present and widespread as a base upon which the pustules are located (+2).
- Distribution: Distribution pattern cannot be determined (0); distribution pattern equivocal (+1); distribution begins in intertriginous regions or face with rapid spread to trunk and limbs (+2).
- Postpustular desquamation: Absent or cannot be determined (0); present (+1).
- Other features
- Mucosal involvement: Present (-2); absent (0).
- Onset: Greater than 10 days (-2); 10 days or fewer (0).
- Resolution: Greater than 15 days (-4); 15 days or fewer (0).
- Temperature: Less than 38 °C (100.4 °F) (0); fever greater than or equal to 38 °C (100.4 °F) (+1).
- White blood cell count: Less than 7,000 cells/mm³ (0); greater than or equal to 7,000 cells/mm³ (+1).
- Histologic features
- Histology: Consistent with another diagnosis (-10); not representative or unavailable (0).
- Peripheral neutrophil exocytosis: Present (+1).
- Pustules without spongiform changes: Nonspongiform pustules, whether subcorneal, intraepidermal, or nonspecific, with papillary edema (+2); spongiform pustules, whether subcorneal, intraepidermal, or nonspecific, without papillary edema (+2).
- Spongiform pustules with papillary edema: Present (+3).
In addition to clinical evaluation and biopsy, confirming the culprit medication may be appropriate in cases of polypharmacy. Diagnostic procedures that provoke symptoms should not be attempted until at least 6 weeks after complete resolution. Patch testing is the preferred method, with intradermal or prick testing considered if patch results are negative and the anticipated benefit outweighs the risk. The estimated sensitivity of patch testing for AGEP is up to 58%. Therefore, a negative result does not exclude a causative agent.[20]
Ex vivo assays, including the lymphocyte transformation test and enzyme-linked immunospot assay (ELISpot), have potential clinical value but remain limited in their use. These approaches avoid the need to reproduce symptoms and may be better tolerated by patients. However, these diagnostic modalities are constrained by limited availability, the requirement for viable patient-derived T lymphocytes, and widely variable sensitivity for delayed hypersensitivity reactions. Reported sensitivity ranges from 27% to 74% for the lymphocyte transformation test and 35% to 85% for the enzyme-linked immunospot assay.
Treatment / Management
Treatment requires discontinuation of the offending agent and provision of supportive care, including topical ceramides and prevention of secondary infection through appropriate skin hygiene. Additional supportive measures include topical corticosteroids, antipyretics, and antihistamines.[21]
Systemic therapies reported in inpatient cases include corticosteroids, cyclosporine, acitretin, dapsone, infliximab, and intravenous immune globulin. Systemic corticosteroids or cyclosporine may be considered in severe or refractory AGEP, with possible escalation to infliximab or intravenous immune globulin. These approaches, however, are not routinely indicated. Evidence-based guidelines for AGEP unresponsive to supportive care are lacking, as current recommendations rely on case reports rather than controlled studies. For patients with a history of AGEP, empiric avoidance of the suspected agent and thorough documentation of the adverse drug reaction in the medical record are appropriate.
Differential Diagnosis
Since AGEP progresses through pustular and desquamative phases, the differential diagnosis includes pustular psoriasis, subcorneal pustular dermatosis (Sneddon-Wilkinson disease), varicelliform eruption, pustular vasculitis, staphylococcal scalded skin syndrome, pustular erythema multiforme, bullous impetigo, bullous tinea, pemphigus foliaceus, pemphigus erythematosus, pemphigus vulgaris, and other severe cutaneous adverse reactions and drug eruptions such as Stevens-Johnson syndrome, toxic epidermal necrolysis, and drug reaction with eosinophilia and systemic symptoms. Among these skin conditions, pustular psoriasis is generally the most challenging to distinguish from AGEP.
Prognosis
The prognosis for AGEP is generally favorable, with symptoms improving or resolving within 15 days of discontinuing the offending drug. Recovery timelines may vary depending on the pharmacokinetics of the causative agent, with hydroxychloroquine-induced AGEP often lasting longer due to the drug’s prolonged half-life. Identifying an appropriate therapeutic alternative is critical to overall patient outcomes when AGEP is triggered by a medication essential for long-term management of conditions such as cardiovascular disease, inflammatory arthritis, or epilepsy.
A previously reported case-fatality rate of 2% to 5% is difficult to attribute to AGEP alone, as comorbidities confound interpretation. Recent literature describes deaths in patients with AGEP. However, most cases involved individuals with preexisting critical illness, some of whom had resolution of AGEP before death. Fatal outcomes were more often attributed to sepsis or multiorgan failure.
Complications
Reported complications of AGEP include bacterial superinfection of desquamating skin, hepatomegaly, lymphadenopathy, liver injury, kidney injury, hypocalcemia, pleural effusions, respiratory distress, agranulocytosis, and multiorgan involvement. Reported frequencies are likely influenced by reporting bias and reliance on retrospective hospital data.[22]
In a review of 340 AGEP cases in the U.S., approximately 8% of patients experienced acute kidney injury, defined as a serum creatinine 1.5 times baseline, and 8.5% had liver enzyme elevations at least twice the upper limit of normal. In severe or atypical cases refractory to supportive care and discontinuation of the offending agent, AGEP may mimic septic shock or overlap clinically with toxic epidermal necrolysis or drug reaction with eosinophilia and systemic symptoms, with multiorgan involvement necessitating additional interventions. Overall, complications are uncommon, and most cases resolve following cessation of the causative medication.
Deterrence and Patient Education
Patients may be reassured that AGEP often resolves with discontinuation of the causative agent, the passage of time, and symptom-guided therapy. The previously cited case-fatality rate of 2% to 5% cannot be definitively attributed to AGEP alone without consideration of comorbidities. Topical corticosteroids, prevention of secondary skin infection, and application of moisturizers to rehydrate desquamating skin are often sufficient treatment measures.
If the causative agent is uncertain, patch or intradermal testing may be considered at least 6 weeks after complete resolution. Empiric avoidance of the suspected trigger and appropriate documentation of the adverse drug reaction in the patient’s medical record, without confirmatory testing, is also reasonable.
Enhancing Healthcare Team Outcomes
Although AGEP is typically self-limited and resolves following discontinuation and avoidance of the triggering agent, the rapid progression of symptoms, associated discomfort, and risk of secondary infection without appropriate skin care can cause significant morbidity. Delayed or incorrect diagnosis may prolong exposure to the causative drug or lead to unnecessary systemic corticosteroid therapy, despite limited evidence that corticosteroids accelerate recovery or reduce disease duration.
In contrast, patients with mild or localized AGEP caused by an essential medication for which no suitable alternative exists may be considered for continued therapy. In such cases, informed decision-making requires further data to elucidate the pathogenesis, define the risks of reexposure, and establish evidence-based treatment recommendations.
Collaboration among an interprofessional team of dermatologists, internists, critical care physicians, immunologists, nurses, and other healthcare specialists in both clinical and academic settings is essential to advance patient-centered care. Increased clinical recognition and systematic reporting of AGEP in inpatient and outpatient populations, together with controlled trials in severe or refractory cases and detailed immunologic investigations, are required to clarify its pathogenesis and inform evidence-based management. Expanding the knowledge base will improve diagnostic accuracy, strengthen patient counseling, and support the development of effective therapeutic strategies.
Media
(Click Image to Enlarge)
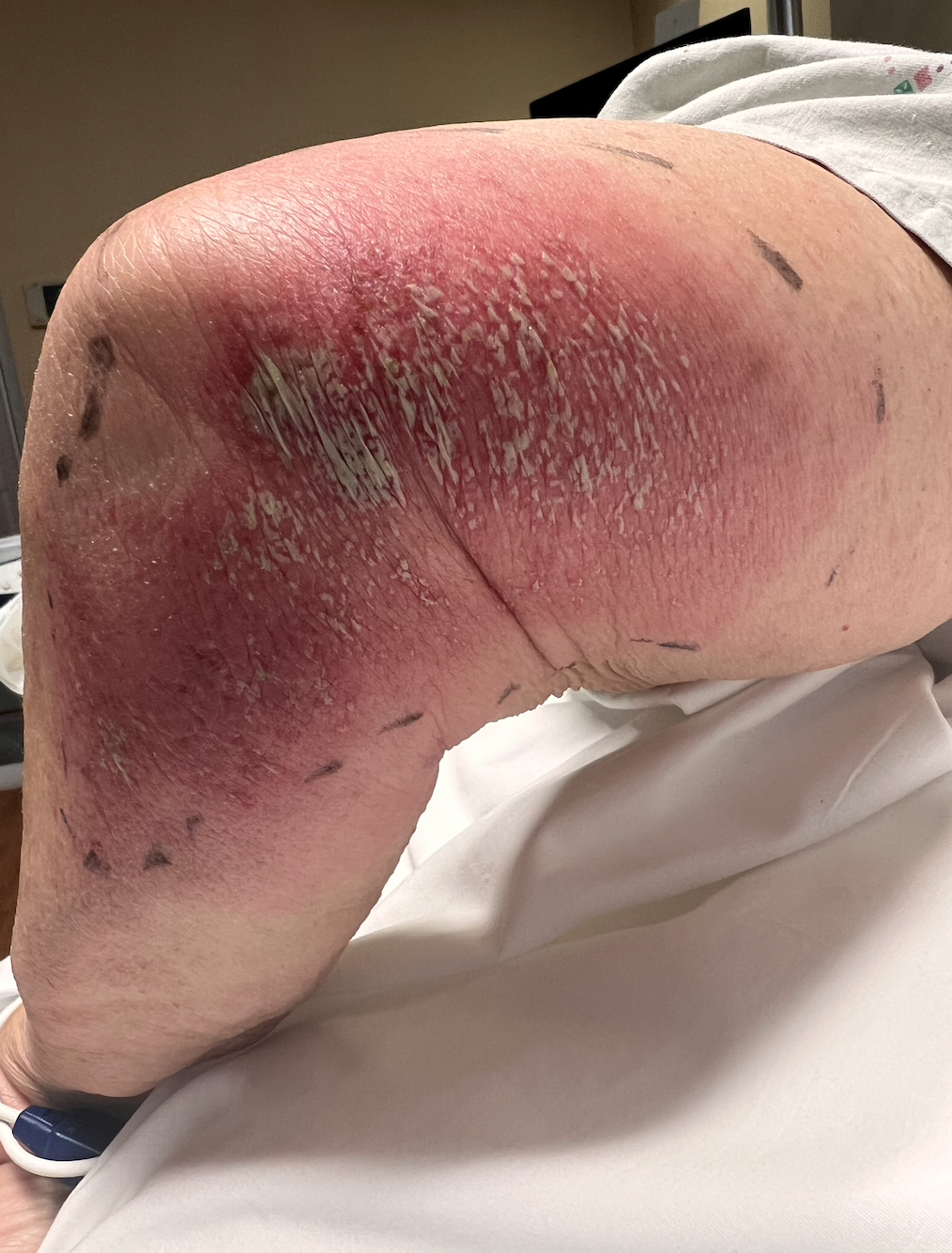
Acute Generalized Exanthematous Pustulosis, Left Lower Limb. This image shows a patient's left thigh, knee, and leg with extensive erythema, swelling, and marked desquamation. Clusters of superficial pustules and scaling are dispersed across the inflamed skin. Markings outline the borders of the rash for assessment. The findings are consistent with an acute, severe pustular cutaneous eruption, such as acute generalized exanthematous pustulosis.
Contributed by Venu Ganipisetti, MD
References
Szatkowski J, Schwartz RA. Acute generalized exanthematous pustulosis (AGEP): A review and update. Journal of the American Academy of Dermatology. 2015 Nov:73(5):843-8. doi: 10.1016/j.jaad.2015.07.017. Epub 2015 Sep 6 [PubMed PMID: 26354880]
Beylot C, Bioulac P, Doutre MS. [Acute generalized exanthematic pustuloses (four cases) (author's transl)]. Annales de dermatologie et de venereologie. 1980 Jan-Feb:107(1-2):37-48 [PubMed PMID: 6989310]
Level 3 (low-level) evidenceVallejo-Yagüe E, Martinez-De la Torre A, Mohamad OS, Sabu S, Burden AM. Drug Triggers and Clinic of Acute Generalized Exanthematous Pustulosis (AGEP): A Literature Case Series of 297 Patients. Journal of clinical medicine. 2022 Jan 13:11(2):. doi: 10.3390/jcm11020397. Epub 2022 Jan 13 [PubMed PMID: 35054090]
Level 2 (mid-level) evidenceCreadore A, Desai S, Alloo A, Dewan AK, Bakhtiar M, Cruz-Diaz C, Femia A, Fox L, Katz KL, Micheletti R, Nelson CA, Ortega-Loayza AG, Patrinely JR Jr, Plovanich M, Rosenbach M, Shaigany S, Shields BE, Saleh JZ, Sharif-Sidi Z, Shinkai K, Smith J, Su C, Wanat KA, Wieser JK, Wright S, Noe MH, Mostaghimi A. Clinical Characteristics, Disease Course, and Outcomes of Patients With Acute Generalized Exanthematous Pustulosis in the US. JAMA dermatology. 2022 Feb 1:158(2):176-183. doi: 10.1001/jamadermatol.2021.5390. Epub [PubMed PMID: 34985493]
Hadavand MA, Kaffenberger B, Cartron AM, Trinidad JCL. Clinical presentation and management of atypical and recalcitrant acute generalized exanthematous pustulosis. Journal of the American Academy of Dermatology. 2022 Sep:87(3):632-639. doi: 10.1016/j.jaad.2020.09.024. Epub 2020 Sep 11 [PubMed PMID: 32926975]
de Groot AC. Results of patch testing in acute generalized exanthematous pustulosis (AGEP): A literature review. Contact dermatitis. 2022 Aug:87(2):119-141. doi: 10.1111/cod.14075. Epub 2022 Mar 14 [PubMed PMID: 35187690]
Meier-Schiesser B, Feldmeyer L, Jankovic D, Mellett M, Satoh TK, Yerly D, Navarini A, Abe R, Yawalkar N, Chung WH, French LE, Contassot E. Culprit Drugs Induce Specific IL-36 Overexpression in Acute Generalized Exanthematous Pustulosis. The Journal of investigative dermatology. 2019 Apr:139(4):848-858. doi: 10.1016/j.jid.2018.10.023. Epub 2018 Nov 2 [PubMed PMID: 30395846]
Sidoroff A, Halevy S, Bavinck JN, Vaillant L, Roujeau JC. Acute generalized exanthematous pustulosis (AGEP)--a clinical reaction pattern. Journal of cutaneous pathology. 2001 Mar:28(3):113-9 [PubMed PMID: 11168761]
Sussman M, Napodano A, Huang S, Are A, Hsu S, Motaparthi K. Pustular Psoriasis and Acute Generalized Exanthematous Pustulosis. Medicina (Kaunas, Lithuania). 2021 Sep 23:57(10):. doi: 10.3390/medicina57101004. Epub 2021 Sep 23 [PubMed PMID: 34684041]
Feldmeyer L, Heidemeyer K, Yawalkar N. Acute Generalized Exanthematous Pustulosis: Pathogenesis, Genetic Background, Clinical Variants and Therapy. International journal of molecular sciences. 2016 Jul 27:17(8):. doi: 10.3390/ijms17081214. Epub 2016 Jul 27 [PubMed PMID: 27472323]
De A, Das S, Sarda A, Pal D, Biswas P. Acute Generalised Exanthematous Pustulosis: An Update. Indian journal of dermatology. 2018 Jan-Feb:63(1):22-29. doi: 10.4103/ijd.IJD_581_17. Epub [PubMed PMID: 29527022]
Chaabouni R, Bahloul E, Ennouri M, Atheymen R, Sellami K, Marrakchi S, Charfi S, Boudaya S, Amouri M, Bougacha N, Turki H. Hydroxychloroquine-induced acute generalized exanthematous pustulosis: a series of seven patients and review of the literature. International journal of dermatology. 2021 Jun:60(6):742-748. doi: 10.1111/ijd.15419. Epub 2021 Feb 17 [PubMed PMID: 33598928]
Zucker I, Prendergast BJ. Sex differences in pharmacokinetics predict adverse drug reactions in women. Biology of sex differences. 2020 Jun 5:11(1):32. doi: 10.1186/s13293-020-00308-5. Epub 2020 Jun 5 [PubMed PMID: 32503637]
Mockenhaupt M. Epidemiology of cutaneous adverse drug reactions. Allergologie select. 2017:1(1):96-108. doi: 10.5414/ALX01508E. Epub 2017 Aug 4 [PubMed PMID: 30402608]
Sidoroff A, Dunant A, Viboud C, Halevy S, Bavinck JN, Naldi L, Mockenhaupt M, Fagot JP, Roujeau JC. Risk factors for acute generalized exanthematous pustulosis (AGEP)-results of a multinational case-control study (EuroSCAR). The British journal of dermatology. 2007 Nov:157(5):989-96 [PubMed PMID: 17854366]
Level 2 (mid-level) evidenceKabashima R, Sugita K, Sawada Y, Hino R, Nakamura M, Tokura Y. Increased circulating Th17 frequencies and serum IL-22 levels in patients with acute generalized exanthematous pustulosis. Journal of the European Academy of Dermatology and Venereology : JEADV. 2011 Apr:25(4):485-8. doi: 10.1111/j.1468-3083.2010.03771.x. Epub [PubMed PMID: 20569282]
Zhang J, Lei Z, Xu C, Zhao J, Kang X. Current Perspectives on Severe Drug Eruption. Clinical reviews in allergy & immunology. 2021 Dec:61(3):282-298. doi: 10.1007/s12016-021-08859-0. Epub 2021 Jul 17 [PubMed PMID: 34273058]
Level 3 (low-level) evidencede Oliveira GV, Maia MLP, Leão FAA, Sad EF, Miotto IZ, Silva MR, Ramos-E-Silva M. What to expect when AGEP is induced by terbinafine? Case report and critical review of the literature. Mycoses. 2022 Oct:65(10):918-925. doi: 10.1111/myc.13506. Epub 2022 Aug 25 [PubMed PMID: 35876217]
Level 3 (low-level) evidenceChowdhury TA, Talib KA, Patricia J, Nye KD, Moosa SA. Rare and Complicated Overlap of Stevens-Johnson Syndrome and Acute Generalized Exanthematous Pustulosis. Cureus. 2021 Jun:13(6):e15921. doi: 10.7759/cureus.15921. Epub 2021 Jun 25 [PubMed PMID: 34336425]
Barbaud A, Castagna J, Soria A. Skin tests in the work-up of cutaneous adverse drug reactions: A review and update. Contact dermatitis. 2022 May:86(5):344-356. doi: 10.1111/cod.14063. Epub 2022 Mar 9 [PubMed PMID: 35122269]
Copaescu AM, Ben-Shoshan M, Trubiano JA. Tools to improve the diagnosis and management of T-cell mediated adverse drug reactions. Frontiers in medicine. 2022:9():923991. doi: 10.3389/fmed.2022.923991. Epub 2022 Oct 13 [PubMed PMID: 36313986]
Hotz C, Valeyrie-Allanore L, Haddad C, Bouvresse S, Ortonne N, Duong TA, Ingen-Housz-Oro S, Roujeau JC, Wolkenstein P, Chosidow O. Systemic involvement of acute generalized exanthematous pustulosis: a retrospective study on 58 patients. The British journal of dermatology. 2013 Dec:169(6):1223-32. doi: 10.1111/bjd.12502. Epub [PubMed PMID: 23855377]
Level 2 (mid-level) evidence