Introduction
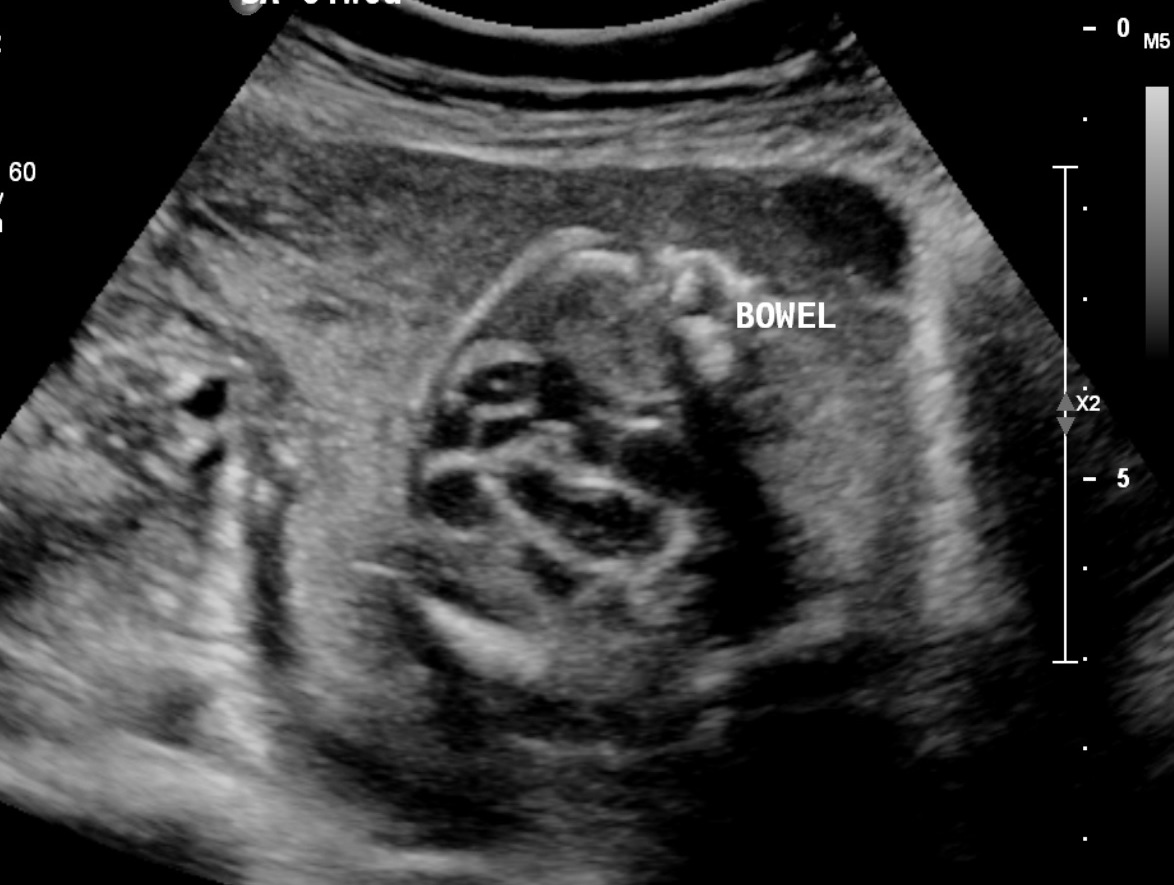
Fetal echogenic bowel (FEB) is an increased echogenicity of fetal bowel on prenatal ultrasound, and is considered a soft marker of aneuploidy (see Image. Fetal Echogenic Bowel, Ultrasound). FEB is detected on the fetal ultrasound as a hyperechoic and well-defined area in the lower abdomen of the fetus. An associated focus of shadowing may suggest calcification. In the third trimester, this can be a normal finding, representing meconium in the fetus's bowel. However, its identification in the second trimester can be associated with several fetal disorders.
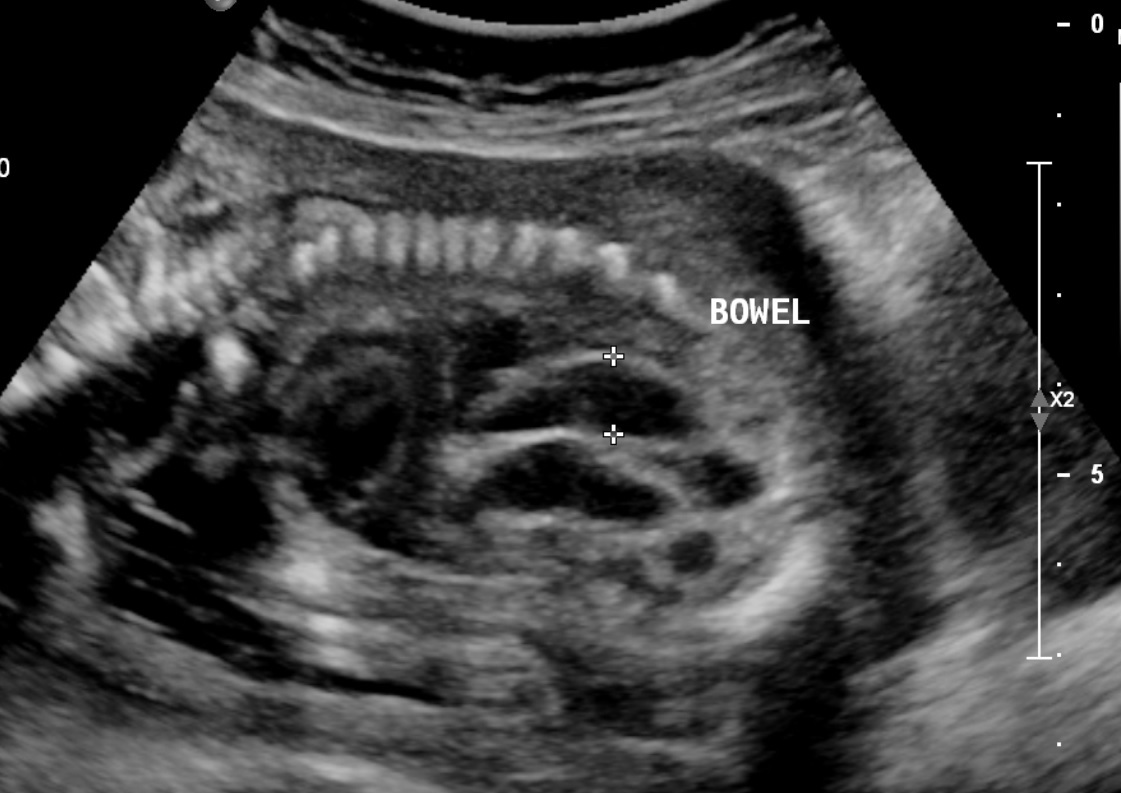
Typically, the echogenicity is compared with the surrounding fetal iliac bone or femur. The echogenicity can also be compared with the fetal lung or liver (see Image. Fetal Bowel Echogenicity, Ultrasound).[1][2][3] The finding of FEB can occur as focal, multifocal, or diffuse. There may be single or multiple loops of the bowel, and it can manifest as solid echogenicity within the lumen or as echogenicity of the walls only.[4] The most widely accepted grading system for FEB compares the echogenicity of the fetal bowel with that of the fetal iliac crest. This classification was developed by Slotnick et al and is outlined as follows:
- Grade 1: mildly increased; less echogenic compared to the bone
- Grade 2: moderately increased; the same echogenicity as the bone
- Grade 3: pronounced increase; more echogenic than the bone [1]
The grading system is dependent on the subjective assessment of the sonographer and thus can be associated with significant inter- and intra-observer variability. Additionally, the transducer frequency and grading can affect this scoring. With the advancement of technology and the availability of high-frequency transducers, echogenic findings can be exaggerated, leading to an overdiagnosis of FEB.[5] Hence, the recommendation is to use a lower frequency transducer (<5 MHz) at a lower gain setting for optimal results.[6]
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
FEB was initially described as a normal variant and a transient finding during prenatal ultrasound. Most fetuses with an isolated echogenic bowel have normal short- and long-term neonatal outcomes, and the majority show regression of this finding in subsequent scans.[7][8][9]
However, echogenic bowel can be associated with chromosomal abnormalities (eg, trisomies 21, 13, 18), cystic fibrosis, congenital infection (eg, TORCH infections), intrauterine growth restriction, gastrointestinal malformations (eg, gastric obstruction, atresia, perforation, meconium ileus), placental insufficiency, intraamniotic bleeding as well as intrauterine and neonatal death.[4][10][11][12] The incidence of these associations varies considerably between studies.[13]
An echogenic bowel is more closely associated with multi-system malformations in fetuses with underlying chromosomal abnormalities. The most common associated systemic involvement in these cases is cardiovascular anomalies (eg, tetralogy of Fallot, coarctation of the aorta, pulmonary artery hypoplasia, tricuspid atresia, cardiomegaly), urinary tract abnormalities (eg, posterior urethral valves, megabladder, bilateral renal dysplasia), craniocerebral anomalies (eg, hydrocephalus), and gastrointestinal malformations.[4]
Isolated echogenic bowel has been reported in a few cases with the use of natural iron-rich mineral water during pregnancy.[14] Additionally, other studies have noted differences in intestinal microbiota after delivery between healthy fetuses and those with hyperechogenic bowel.[15] However, these associations still require further investigation.
Epidemiology
The incidence of echogenic bowel varies from 0.2% to 1.8% in prenatal sonograms performed in the second trimester.[16] The variable incidence between different studies could be attributed to the high-risk population in some samples. Additionally, this could also be attributed to the fact that the detection of echogenic bowel is prone to significant inter-observer variability since the diagnostic criteria are based on a subjective assessment, which is prone to over- or underestimation of the finding based on the level of expertise of the sonographer as well as the frequency and the gain settings of the ultrasound machine.[3][17]
Pathophysiology
The pathophysiology of FEB is related to the underlying etiology. The fetus begins to swallow amniotic fluid by the end of the first trimester, which transits through the intestines. When this passage is slow or obstructed, the contents in the bowel can become progressively thick, leading to a bright appearance during the ultrasound. The primary mechanism may involve meconium stasis or hypercellular meconium, which can occur for several reasons.[17]
Meconium Stasis
Meconium stasis can occur reasons, include the following:
- Hypoperistalsis
- Distal obstruction of the bowel
- Meconium ileus
- Fetal intestinal ischemia
- Fetal intestinal perforation
Infants with abnormal karyotypes may have decreased levels of microvillar enzymes, leading to hypoperistalsis, further impeding the passage of meconium in utero. This meconium can be inspissated, especially in the second trimester when the bowel lumens are small. As the pregnancy progresses, the amount of fetal swallowing of amniotic fluid increases, along with the volume of the small bowel, resulting in the resolution of meconium stasis and, consequently, the resolution of the hyperechogenic appearance of the bowel.[18]
The pathophysiology of the association between echogenic bowel and TORCH infection is also thought to result from direct viral-induced cytotoxic effect and ischemia of the endothelial cells in the fetal bowel, leading to hypoperistalsis, ileus, or fetal intestinal perforation.[19] Proximal bowel obstruction can interfere with normal amniotic fluid swallowing and lead to decreased fluid content in the meconium, which subsequently leads to the formation of meconium plugs, which can be identified as increased echogenicity.[20]
Hypercellular Meconium
Hypercellular meconium can occur for the following reasons:
- Abnormal enzyme secretion
- Proximal obstruction of the bowel
- Feto-placental bleeding into the amniotic fluid
- Intrauterine transfusions
Conditions such as cystic fibrosis can lead to abnormalities in enzyme secretion, resulting in the abnormal consistency of meconium. This is usually detected as diffusely increased echogenicity of the bowel along with bowel dilatation and calcification.[21] Swallowed blood products due to maternal-fetal hemorrhage or intrauterine transfusions can also lead to hypercellular meconium and the finding of echogenic bowel.[20] Other possibilities, such as compromised placental and fetal perfusion leading to the redistribution of blood flow and, subsequently, gut ischemia, have been postulated.[22]
History and Physical
History and physical examination can help differentiate various pathologies associated with FEB. Prenatal history should focus on additional pregnancy risk factors (including advanced maternal age, gestational diabetes, fetal growth restriction, and polyhydramnios), other major and minor fetal anomalies, results of prior imaging and screening tests, past medical history, and comorbidities.[16] The postnatal examination should be comprehensive, with special attention to congenital anomalies. A thorough abdominal exam can provide clues, such as palpable intestinal loops indicating bowel obstruction. The patency of the neonatal gastrointestinal tract should also be confirmed.
Evaluation
A pragmatic approach should be adopted while investigating pregnancies with the finding of FEB in the second trimester.[11] Patients should be stratified by FEB grade and the presence of any additional risk factors or findings. Further evaluation should be offered, and an interdisciplinary team, including a genetic counselor, neonatologist, and maternal-fetal medicine (MFM) specialist, should be involved.
Comprehensive Ultrasound Evaluation
A detailed ultrasound evaluation should be performed to assess for additional markers or features suggesting chromosome abnormalities (eg, nuchal translucency, cardiac anomalies).[6] If there is a normal 4-chamber view and the outflow tracts are visualized as normal, then a fetal echocardiogram is not necessary. However, if these views are abnormal or not obtainable, a fetal echocardiogram should be performed.[23] Assessment of amniotic fluid is also helpful in detecting maternal-fetal hemorrhage as a cause of the echogenic bowel. Fetal magnetic resonance imaging (MRI) could also be considered in cases of FEB associated with bowel dilatation.[13]
Genetic Screening Recommendations
The Society for Maternal-Fetal Medicine (SMFM) recommends counseling to estimate the probability of aneuploidy, and, for patients without previous aneuploidy screening, further genetic screening, including cell-free fetal DNA testing or a quadruple screen (if cell-free DNA is unavailable or cost-prohibitive).[6][24]
Invasive Diagnostic Testing
Amniocentesis should be offered to all patients, regardless of screening results. In cases with additional findings, including the presence of other soft sonographic markers, major anomalies, or a positive aneuploidy screen, amniocentesis should be offered for definitive diagnosis, and chromosomal microarray can be considered.[9] Additionally, if amniocentesis is performed in patients with FEB, amniotic fluid should be sent for fetal karyotype and polymerase chain reaction for congenital infections.
It is also important to note that echogenic bowel is present as an isolated finding in 4% to 25% of fetuses with aneuploidy, making invasive diagnostic testing reasonable to offer all patients with FEB.[6][25][26] However, SMFM recommends no additional testing for aneuploidy specifically due to an isolated FEB with negative genetic screening results.[6]
Cystic Fibrosis Screening
Cystic fibrosis screening should be performed if it has not already been done as part of routine screening. If both parents are found to be carriers, the patient should be referred for genetic counseling, and diagnostic testing should be offered.[6]
Serologic Testing for TORCH Infections
A thorough history should be performed to screen patients for maternal infection with potential causative agents, including cytomegalovirus (CMV), toxoplasmosis, rubella, varicella, herpes, and parvovirus. Routine testing for other potential congenital infections is generally not useful, as the chance of a positive result is low without a history of exposure or other clinical risk factors.[6]
Although symptomatic maternal infection is uncommon, CMV immunoglobulin G (IgG) and IgM titers should be ordered, with IgG avidity testing as needed. If results suggest primary CMV infection (eg, positive IgM with low avidity IgG), amniocentesis with polymerase chain reaction (PCR) for CMV should be considered to confirm the diagnosis.[6][27]
Treatment / Management
Diagnosis of echogenic bowel in the second trimester warrants thorough antenatal and postnatal follow-up due to the increased risk of fetal abnormalities. (see Image. Diagnostic Flowchart of a Fetus With Hyperechogenic Bowel.) The management of FEB depends on the degree of echogenicity and the presence of associated findings. Early identification and appropriate follow-up can help optimize outcomes for both the fetus and the expectant parents.
Prenatal Management
Serial sonographic screening throughout the pregnancy is recommended for reassessment and to monitor for associated conditions, including fetal growth restriction, bowel dilatation, and polyhydramnios, which are themselves associated with adverse outcomes.[20] SMFM recommends a third-trimester ultrasound for reassessment of FEB and evaluation of fetal growth.[6]
A large retrospective cohort study of singleton gestations found that, after excluding cases of echogenic bowel due to aneuploidy and CMV infection, the risk of intrauterine fetal demise was 7.3% in the group with isolated echogenic bowel on mid-trimester ultrasound compared to 0.9% in the nonechogenic bowel group.[28] In this study, all fetal deaths in the echogenic bowel group occurred before 30 weeks, at an average gestation of 23.7 weeks. For this reason, the authors suggest that early initiation of serial ultrasounds may be warranted. (B2)
Postnatal Management
The association of gastrointestinal abnormalities with other comorbidities in infants with a diagnosis of FEB highlights the need for these children to be delivered at centers with higher-level neonatal intensive care capabilities and pediatric subspecialists readily available. Pediatric care providers attending the birth should be informed of the antenatal findings of FEB to ensure appropriate postnatal evaluation.
Postnatally, a comprehensive clinical examination must be performed, with particular attention to the abdomen to detect palpable loops of bowel, which could indicate potential obstruction. Passage of meconium and normal feeding patterns should be ensured. Any delay in meconium passage beyond 24 to 48 hours of life or decreased stool output during the first week of life may indicate meconium ileus and should prompt additional consideration for cystic fibrosis while awaiting newborn screening results. Careful monitoring is indicated to prevent complications.
Abdominal x-rays, upper gastrointestinal series, barium, or gastrograffin enema may be required to evaluate for intestinal obstruction.[29] When meconium ileus is suspected, water-soluble contrast enemas can be both diagnostic and therapeutic.(B3)
Genetic and infectious disease evaluation should be pursued based on prenatal risk stratification. If it is an isolated finding, and cystic fibrosis and chromosomal conditions have been excluded, there is no need for postnatal follow-up or any further treatment. However, when antenatal testing is incomplete or suggests abnormalities, additional testing for congenital infections may be warranted based on clinical presentation and prenatal findings. For infants requiring surgical intervention or intensive monitoring, a multidisciplinary team approach involving neonatologists, pediatric surgeons, and subspecialists in genetics and pulmonology ensures optimal outcomes.
Differential Diagnosis
The prenatal ultrasound finding of echogenic bowel is considered a soft marker, and when detected in the second trimester, it should prompt further evaluation to detect additional abnormalities. As discussed above, it may be associated with multiple etiologies. The following differential diagnoses should be considered while evaluating FEB.
- Increased cellularity or thick meconium: intestinal obstruction, intestinal atresia, cystic fibrosis, chromosomal abnormality
- Fetal swallowing of blood: fetoplacental hemorrhage, bleeding into the amniotic fluid, placental abruption
- Inflammation of the bowel: TORCH infection
- Decreased blood flow in the bowel: fetal growth restriction, feto-placental insufficiency
- False-positive finding: frequency and gain settings of the ultrasound machine altered images, expertise of the sonographer
The diagnosis can often be clarified by identifying additional clues that suggest the underlying etiology. For example, additional abnormalities, such as a congenital heart defect or a thick nuchal cord, increase the likelihood of an aneuploidy. Features of fetal ascites or hydrops are more suggestive of an infectious etiology, whereas a thickened, dilated bowel wall associated with signs of obstruction or calcification may indicate cystic fibrosis.[29]
Prognosis
The prognosis of FEB depends upon the underlying pathophysiology.[30] Isolated echogenic bowel can be a benign finding with normal neonatal outcomes. However, outcomes can be impacted when it is associated with other ultrasonographic markers or pregnancy complications. Pregnancies complicated by FEB and associated with other anomalies, fetal growth restriction, elevated alpha-fetoprotein, or progressive worsening in grading are associated with less favorable to poor outcomes.[16]
Complications
Complications associated with echogenic bowel are related to the underlying pathophysiology. In pregnancies associated with intrauterine growth restriction or elevated alpha-fetoprotein, complications may arise from underlying feto-placental bleeding or congenital anomalies. The association between echogenic bowel and intrauterine fetal death may be related to underlying uteroplacental insufficiency.[31]
Consultations
Management of FEB requires a multidisciplinary and interprofessional approach, involving different specialties to ensure tailored, comprehensive care. Collaborative evaluation ensures a comprehensive approach to care and informed decision-making for the patient. Specialties that may be consulted as part of the diagnostic and management process include the following:
- Maternal-fetal medicine
- Neonatology
- Pediatric surgery
- Clinical genetics
- Pediatric radiology
- Pediatric gastroenterology
- Pediatric infectious disease
Deterrence and Patient Education
Deterrence and patient education for FEB focus on reducing anxiety through clear communication and appropriate counseling. Expectant parents with a fetus found to have FEB should be informed that while FEB can be associated with conditions such as chromosomal abnormalities, infections, or cystic fibrosis, it is often a benign and self-limited finding, particularly when isolated. Clinicians should emphasize the importance of follow-up imaging, targeted screening, and possible genetic testing when indicated. Educating patients about the range of possible outcomes, the rationale behind recommended evaluations, and the collaborative care approach helps support shared decision-making and reduces unnecessary concern, while ensuring appropriate and timely clinical management.
Enhancing Healthcare Team Outcomes
FEB is an uncommon but significant finding in second-trimester ultrasound and requires further sonographic screening. A well-structured grading system, suggested by Slotnick et al, can be used when reporting the findings to reduce diagnostic subjectivity.[1] Effective management of FEB requires coordinated multidisciplinary and interprofessional collaboration to address the diverse diagnostic and therapeutic considerations this prenatal finding presents. The complexity of potential underlying etiologies, ranging from chromosomal abnormalities and cystic fibrosis to congenital infections and normal variants, necessitates a systematic team approach.
MFM specialists often serve as the primary coordinators of care, providing comprehensive risk assessment and developing individualized management plans. They interpret ultrasound findings within the clinical context, order additional diagnostic testing, and manage high-risk pregnancies through delivery. MFM specialists facilitate communication between subspecialties and ensure appropriate timing of interventions.
Radiologists and sonographers provide critical diagnostic imaging expertise, performing detailed anatomical surveys to identify associated anomalies and conducting serial evaluations to monitor progression. Their standardized reporting and quality imaging are essential for accurate diagnosis and ongoing assessment.
Genetic counselors and clinical geneticists play pivotal roles in risk stratification and family counseling. They coordinate chromosomal analysis, assess family history for inherited conditions, arrange cystic fibrosis carrier testing, and provide comprehensive counseling regarding recurrence risks and reproductive options. Their expertise ensures informed decision-making throughout the pregnancy.
Perinatal nurses serve as essential care coordinators, providing patient education, emotional support, and ensuring seamless communication between appointments and subspecialty consultations. They monitor maternal well-being and facilitate adherence to recommended surveillance protocols.
Neonatologists contribute expertise regarding potential postnatal complications and delivery planning. Early consultation enables the preparation of the delivery room and the development of immediate postnatal management strategies, particularly when associated anomalies are suspected.
Pediatric gastroenterologists may also be consulted when specific gastrointestinal pathology is suspected, providing expertise in conditions such as meconium ileus or other bowel disorders that may require specialized postnatal intervention.
Optimal outcomes depend on regular multidisciplinary team meetings, standardized communication protocols, and clear delineation of responsibilities. Each team member must understand their role within the broader care framework while maintaining flexibility to adapt as new information emerges. This collaborative approach ensures comprehensive evaluation, appropriate counseling, and coordinated care planning that addresses both immediate pregnancy management and long-term family needs, ultimately optimizing outcomes for both mother and fetus.[6]
Media
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
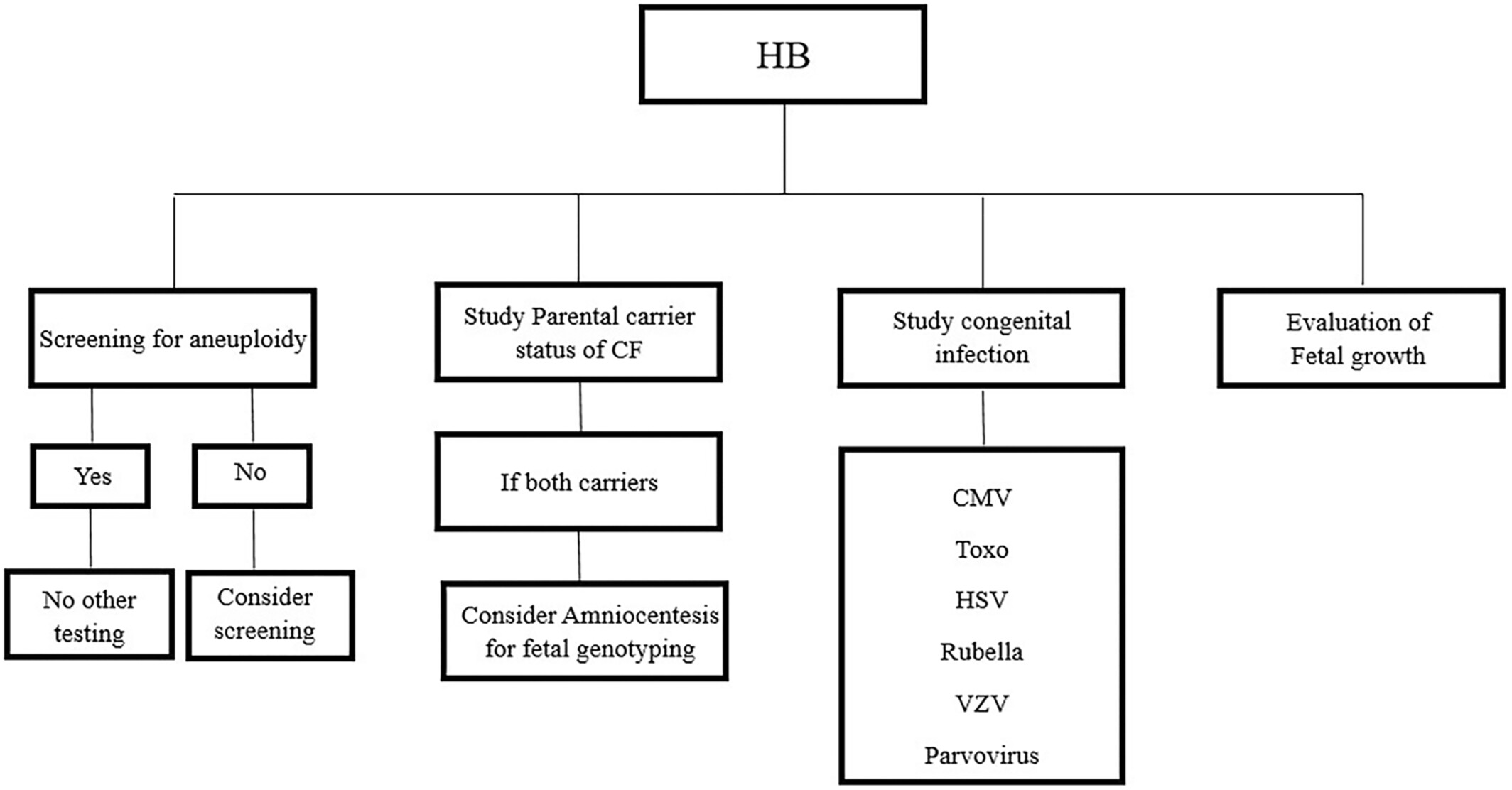
Diagnostic Flowchart of a Fetus With Hyperechogenic Bowel.
Vena F, Mazza A, Bartolone, et al. Hyperechogenic fetal bowel: current evidence-based prenatal diagnosis and management. J Clin Ultrasound. 2023;51(7):1172-1178. doi: 10.1002/jcu.23528.
References
Slotnick RN, Abuhamad AZ. Prognostic implications of fetal echogenic bowel. Lancet (London, England). 1996 Jan 13:347(8994):85-7 [PubMed PMID: 8538346]
Nyberg DA, Dubinsky T, Resta RG, Mahony BS, Hickok DE, Luthy DA. Echogenic fetal bowel during the second trimester: clinical importance. Radiology. 1993 Aug:188(2):527-31 [PubMed PMID: 8327709]
Sevens TJ, Chudleigh T. Fetal echogenic bowel: Is there a national consensus on identification and reporting? Ultrasound (Leeds, England). 2024 Feb:32(1):11-18. doi: 10.1177/1742271X231164951. Epub 2023 Apr 20 [PubMed PMID: 38314020]
Level 3 (low-level) evidenceVena F, Mazza A, Bartolone M, Vasta A, D'Alberti E, Di Mascio D, D'Ambrosio V, Volpe G, Signore F, Pizzuti A, Giancotti A. Hyperechogenic fetal bowel: Current evidence-based prenatal diagnosis and management. Journal of clinical ultrasound : JCU. 2023 Sep:51(7):1172-1178. doi: 10.1002/jcu.23528. Epub 2023 Aug 8 [PubMed PMID: 37553773]
Vincoff NS, Callen PW, Smith-Bindman R, Goldstein RB. Effect of ultrasound transducer frequency on the appearance of the fetal bowel. Journal of ultrasound in medicine : official journal of the American Institute of Ultrasound in Medicine. 1999 Dec:18(12):799-803; quiz 805-6 [PubMed PMID: 10591442]
Society for Maternal-Fetal Medicine (SMFM). Electronic address: pubs@smfm.org, Prabhu M, Kuller JA, Biggio JR. Society for Maternal-Fetal Medicine Consult Series #57: Evaluation and management of isolated soft ultrasound markers for aneuploidy in the second trimester: (Replaces Consults #10, Single umbilical artery, October 2010; #16, Isolated echogenic bowel diagnosed on second-trimester ultrasound, August 2011; #17, Evaluation and management of isolated renal pelviectasis on second-trimester ultrasound, December 2011; #25, Isolated fetal choroid plexus cysts, April 2013; #27, Isolated echogenic intracardiac focus, August 2013). American journal of obstetrics and gynecology. 2021 Oct:225(4):B2-B15. doi: 10.1016/j.ajog.2021.06.079. Epub 2021 Jun 23 [PubMed PMID: 34171388]
Ameratunga DM, Said JM, Reidy K, Palma-Dias R. Perinatal outcomes following the ultrasound diagnosis of echogenic bowel: an Australian perspective. Fetal diagnosis and therapy. 2012:31(3):179-84. doi: 10.1159/000336123. Epub 2012 Feb 25 [PubMed PMID: 22378220]
Level 2 (mid-level) evidencePatel Y, Boyd PA, Chamberlain P, Lakhoo K. Follow-up of children with isolated fetal echogenic bowel with particular reference to bowel-related symptoms. Prenatal diagnosis. 2004 Jan:24(1):35-7 [PubMed PMID: 14755407]
Level 2 (mid-level) evidenceD'Amico A, Buca D, Rizzo G, Khalil A, Silvi C, Makatsariya A, Nappi L, Liberati M, D'Antonio F. Outcome of fetal echogenic bowel: A systematic review and meta-analysis. Prenatal diagnosis. 2021 Mar:41(4):391-399. doi: 10.1002/pd.5638. Epub 2021 Feb 21 [PubMed PMID: 31981377]
Level 1 (high-level) evidenceGholami F, Mehrabi S, Nazari N, Shateranni F, Asemi Z. Echogenic bowel on the second-trimester ultrasound as an indicator of jejunal atresia: a case report. Annals of medicine and surgery (2012). 2024 Nov:86(11):6843-6845. doi: 10.1097/MS9.0000000000002608. Epub 2024 Sep 24 [PubMed PMID: 39525759]
Level 3 (low-level) evidenceSimon-Bouy B, Satre V, Ferec C, Malinge MC, Girodon E, Denamur E, Leporrier N, Lewin P, Forestier F, Muller F, French Collaborative Group. Hyperechogenic fetal bowel: a large French collaborative study of 682 cases. American journal of medical genetics. Part A. 2003 Sep 1:121A(3):209-13 [PubMed PMID: 12923859]
Level 3 (low-level) evidenceAl-Kouatly HB, Chasen ST, Streltzoff J, Chervenak FA. The clinical significance of fetal echogenic bowel. American journal of obstetrics and gynecology. 2001 Nov:185(5):1035-8 [PubMed PMID: 11717628]
Carcopino X, Chaumoitre K, Shojai R, Akkawi R, Panuel M, Boubli L, D'ercole C. Foetal magnetic resonance imaging and echogenic bowel. Prenatal diagnosis. 2007 Mar:27(3):272-8 [PubMed PMID: 17278175]
Lavie A, Reicher L, Zohav E, Ram M, Malovitz S. Isolated fetal echogenic bowel and iron-rich mineral water supplement: a case series and review of the literature. Journal of obstetrics and gynaecology : the journal of the Institute of Obstetrics and Gynaecology. 2022 Jul:42(5):1149-1154. doi: 10.1080/01443615.2021.2024800. Epub 2022 Feb 10 [PubMed PMID: 35142237]
Level 2 (mid-level) evidenceZhao Y, Lyu G. Fetal echogenic bowel may be related to intestinal microbiota: A prospective cohort study. Journal of clinical ultrasound : JCU. 2024 Nov-Dec:52(9):1338-1345. doi: 10.1002/jcu.23794. Epub 2024 Aug 30 [PubMed PMID: 39212092]
Buiter HD, Holswilder-Olde Scholtenhuis MA, Bouman K, van Baren R, Bilardo CM, Bos AF. Outcome of infants presenting with echogenic bowel in the second trimester of pregnancy. Archives of disease in childhood. Fetal and neonatal edition. 2013 May:98(3):F256-9. doi: 10.1136/archdischild-2012-302017. Epub 2012 Sep 18 [PubMed PMID: 22990134]
Level 2 (mid-level) evidenceHarrison KL, Martinez D, Mason G. The subjective assessment of echogenic fetal bowel. Ultrasound in obstetrics & gynecology : the official journal of the International Society of Ultrasound in Obstetrics and Gynecology. 2000 Nov:16(6):524-9 [PubMed PMID: 11169345]
Sepulveda W, Sebire NJ. Fetal echogenic bowel: a complex scenario. Ultrasound in obstetrics & gynecology : the official journal of the International Society of Ultrasound in Obstetrics and Gynecology. 2000 Nov:16(6):510-4 [PubMed PMID: 11169342]
Gabrielli L, Bonasoni MP, Chiereghin A, Piccirilli G, Borgatti EC, Simonazzi G, Salfi NCM, Tamagnini I, Lazzarotto T. Pathophysiology of Hyperechogenic Bowel in Congenitally Human Cytomegalovirus Infected Fetuses. Microorganisms. 2020 May 22:8(5):. doi: 10.3390/microorganisms8050779. Epub 2020 May 22 [PubMed PMID: 32455864]
Laird A, Shekleton P, Nataraja RM, Kimber C, Pacilli M. Incidence of gastro-intestinal anomalies and surgical outcome of fetuses diagnosed with echogenic bowel and bowel dilatation. Prenatal diagnosis. 2019 Nov:39(12):1115-1119. doi: 10.1002/pd.5552. Epub 2019 Sep 10 [PubMed PMID: 31461799]
Berlin BM, Norton ME, Sugarman EA, Tsipis JE, Allitto BA. Cystic fibrosis and chromosome abnormalities associated with echogenic fetal bowel. Obstetrics and gynecology. 1999 Jul:94(1):135-8 [PubMed PMID: 10389734]
Al-Kouatly HB, Chasen ST, Karam AK, Ahner R, Chervenak FA. Factors associated with fetal demise in fetal echogenic bowel. American journal of obstetrics and gynecology. 2001 Nov:185(5):1039-43 [PubMed PMID: 11717629]
Level 2 (mid-level) evidence. AIUM Practice Parameter for the Performance of Fetal Echocardiography. Journal of ultrasound in medicine : official journal of the American Institute of Ultrasound in Medicine. 2020 Jan:39(1):E5-E16. doi: 10.1002/jum.15188. Epub [PubMed PMID: 31846540]
Bromley B, Doubilet P, Frigoletto FD Jr, Krauss C, Estroff JA, Benacerraf BR. Is fetal hyperechoic bowel on second-trimester sonogram an indication for amniocentesis? Obstetrics and gynecology. 1994 May:83(5 Pt 1):647-51 [PubMed PMID: 8164918]
Strocker AM, Snijders RJ, Carlson DE, Greene N, Gregory KD, Walla CA, Platt LD. Fetal echogenic bowel: parameters to be considered in differential diagnosis. Ultrasound in obstetrics & gynecology : the official journal of the International Society of Ultrasound in Obstetrics and Gynecology. 2000 Nov:16(6):519-23 [PubMed PMID: 11169344]
MacGregor SN, Tamura R, Sabbagha R, Brenhofer JK, Kambich MP, Pergament E. Isolated hyperechoic fetal bowel: significance and implications for management. American journal of obstetrics and gynecology. 1995 Oct:173(4):1254-8 [PubMed PMID: 7485332]
Society for Maternal-Fetal Medicine (SMFM), Hughes BL, Gyamfi-Bannerman C. Diagnosis and antenatal management of congenital cytomegalovirus infection. American journal of obstetrics and gynecology. 2016 Jun:214(6):B5-B11. doi: 10.1016/j.ajog.2016.02.042. Epub 2016 Feb 20 [PubMed PMID: 26902990]
Goetzinger KR, Cahill AG, Macones GA, Odibo AO. Echogenic bowel on second-trimester ultrasonography: evaluating the risk of adverse pregnancy outcome. Obstetrics and gynecology. 2011 Jun:117(6):1341-1348. doi: 10.1097/AOG.0b013e31821aa739. Epub [PubMed PMID: 21606744]
Level 2 (mid-level) evidenceIrish MS, Ragi JM, Karamanoukian H, Borowitz DS, Schmidt D, Glick PL. Prenatal diagnosis of the fetus with cystic fibrosis and meconium ileus. Pediatric surgery international. 1997 Jul:12(5-6):434-6 [PubMed PMID: 9244121]
Level 3 (low-level) evidenceJackson CR, Orford J, Minutillo C, Dickinson JE. Dilated and echogenic fetal bowel and postnatal outcomes: a surgical perspective. Case series and literature review. European journal of pediatric surgery : official journal of Austrian Association of Pediatric Surgery ... [et al] = Zeitschrift fur Kinderchirurgie. 2010 May:20(3):191-3. doi: 10.1055/s-0030-1247523. Epub 2010 Feb 19 [PubMed PMID: 20175047]
Level 2 (mid-level) evidenceMailath-Pokorny M, Klein K, Klebermass-Schrehof K, Hachemian N, Bettelheim D. Are fetuses with isolated echogenic bowel at higher risk for an adverse pregnancy outcome? Experiences from a tertiary referral center. Prenatal diagnosis. 2012 Dec:32(13):1295-9. doi: 10.1002/pd.3999. Epub 2012 Oct 25 [PubMed PMID: 23097266]
Level 2 (mid-level) evidence