Indications
Sodium-glucose cotransporter 2 (SGLT2) inhibitors are antihyperglycemic agents that target SGLT2 channels in the proximal convoluted tubules. These drugs lower blood glucose by preventing the reabsorption of filtered glucose from the tubular lumen. Several SGLT2 inhibitors have received approval from the U.S. Food and Drug Administration (FDA) for use in adults, including canagliflozin, dapagliflozin, empagliflozin, ertugliflozin, and bexagliflozin.
Canagliflozin, dapagliflozin, and empagliflozin are also approved for pediatric patients aged 10 years and older. In contrast, ertugliflozin and bexagliflozin are restricted to adults. Indications vary by agent, but all are recommended as adjuncts to diet and exercise for improving glycemic control in adults with type 2 diabetes mellitus (T2DM).[1] Separately, sotagliflozin, a dual SGLT1 and SGLT2 inhibitor, is FDA-approved specifically to reduce heart failure risk, without an indication for glycemic control.
Agent-Specific U.S. Food and Drug Administration Approvals for Sodium-Glucose Cotransporter 2 and Dual Sodium-Glucose Cotransporter 1/2 Inhibitors as of June 2025
SGLT2 and SGLT1/2 inhibitors are foundational drugs in modern diabetes care with additional indications in cardiovascular and renal disease risk reduction. The approved clinical uses of individual drugs within this pharmacologic class as of mid-2025 are discussed below (see Image. U.S. Food and Drug Administration-Approved Indications of Sodium-Glucose Cotransporter 2 and Sodium-Glucose Cotransporter 1/2 Inhibitors).
Canagliflozin
Canagliflozin is indicated as an adjunct to diet and exercise for improving glycemic control in adults and pediatric patients aged 10 years and older with T2DM. In addition to its antihyperglycemic effects, canagliflozin has received FDA approval for the following risk-reduction indications:
-
Major adverse cardiovascular events (MACE): Reduces the risk of cardiovascular death, nonfatal myocardial infarction, and nonfatal stroke in adults with T2DM and established cardiovascular disease (CVD).
-
Renal and cardiovascular protection in diabetic nephropathy: Reduces the risk of end-stage kidney disease (ESKD), doubling of serum creatinine, cardiovascular death, and hospitalization for heart failure (HHF) in adults with T2DM and diabetic nephropathy, defined by albuminuria exceeding 300 mg/day.
These indications are supported by robust evidence from pivotal cardiovascular and renal outcome trials. The Canagliflozin Cardiovascular Assessment Study (CANVAS) Program, which integrated data from the CANVAS and CANVAS-R trials, demonstrated a significant reduction in MACE among patients with T2DM and high cardiovascular risk. Canagliflozin was associated with a 14% relative risk reduction in the composite outcome of cardiovascular death, nonfatal myocardial infarction, or nonfatal stroke.[2]
The Canagliflozin and Renal Endpoints in Diabetes with Established Nephropathy Clinical Evaluation (CREDENCE) trial provided definitive evidence for renal protection. In patients with T2DM and chronic kidney disease (CKD) with albuminuria, canagliflozin significantly reduced the risk of the primary composite renal endpoint—ESKD, doubling of serum creatinine, or renal or cardiovascular death—by 30% compared to placebo.[3] The trial also demonstrated consistent reductions in HHF and cardiovascular mortality.
Dapagliflozin
Dapagliflozin is indicated as an adjunct to diet and exercise for improving glycemic control in adults and pediatric patients aged 10 years and older with T2DM. Beyond glycemic management, dapagliflozin has received FDA approval for the following risk-reduction indications in adults:
-
Heart failure with reduced (HFrEF) or preserved (HFpEF) ejection fraction: Reduces the risk of cardiovascular death, HHF, and urgent heart failure visits in adults with heart failure, irrespective of ejection fraction status.
-
CKD: Reduces the risk of sustained decline in estimated glomerular filtration rate (eGFR), progression to ESKD, cardiovascular death, and HHF in adults with CKD at risk of progression.
-
Heart failure in T2DM: Reduces the risk of HHF in adults with T2DM and either established CVD or multiple cardiovascular risk factors.
These indications are supported by a comprehensive clinical trial program. The Dapagliflozin Effect on Cardiovascular Events–Thrombolysis in Myocardial Infarction 58 (DECLARE-TIMI 58) trial evaluated dapagliflozin in over 17,000 patients with T2DM and either established atherosclerotic cardiovascular disease (ASCVD) or multiple cardiovascular risk factors. While the trial did not demonstrate a significant reduction in MACE, dapagliflozin significantly reduced the composite endpoint of cardiovascular death or HHF, as well as HHF alone.[4]
The Dapagliflozin and Prevention of Adverse Outcomes in Heart Failure (DAPA-HF) trial further established dapagliflozin’s efficacy in patients with HFrEF, demonstrating a significant reduction in the risk of worsening heart failure or cardiovascular death, independent of diabetes status. These benefits were extended to patients with mildly reduced (HFmrEF) and preserved (HFpEF) ejection fraction in the Dapagliflozin Evaluation to Improve the Lives of Patients With Preserved Ejection Fraction Heart Failure (DELIVER) trial, confirming dapagliflozin’s utility across the full spectrum of heart failure phenotypes.
A pooled meta-analysis of DAPA-HF and DELIVER, including over 11,000 participants, demonstrated a significant reduction in both cardiovascular and all-cause mortality, reinforcing dapagliflozin’s role in comprehensive heart failure management. Additionally, the Dapagliflozin and Prevention of Adverse Outcomes in Chronic Kidney Disease (DAPA-CKD) trial provided robust evidence for renal protection, showing that dapagliflozin significantly reduced the risk of sustained decline in eGFR, progression to ESKD, and cardiovascular or renal death in patients with CKD, irrespective of T2DM status.[5]
Empagliflozin
Empagliflozin is indicated as an adjunct to diet and exercise for improving glycemic control in adults and pediatric patients aged 10 years and older with T2DM. In addition to its glycemic benefits, empagliflozin has received FDA approval for the following therapeutic indications:
-
Cardiovascular risk reduction in heart failure: Reduces the risk of cardiovascular death and HHF in adults with heart failure, irrespective of ejection fraction status.
-
Renal and cardiovascular protection in CKD: Reduces the risk of sustained decline in eGFR, progression to ESKD, cardiovascular death, and hospitalization in adults with CKD at risk of progression.
-
Cardiovascular risk reduction in T2DM with established ASCVD: Reduces the risk of cardiovascular death in adults with T2DM and established ASCVD.
These indications are supported by robust evidence from multiple large-scale, randomized controlled trials (RCTs). In patients with T2DM and established ASCVD, empagliflozin has demonstrated significant reductions in MACE, including all-cause mortality, consistent with its role as a preferred agent in this population per guideline recommendations.
Empagliflozin’s efficacy in heart failure spans the full spectrum of ejection fraction. The Empagliflozin Outcome Trial in Patients With Chronic Heart Failure With Reduced Ejection Fraction (EMPEROR-Reduced) trial confirmed its benefit in patients with HFrEF, while the Empagliflozin Outcome Trial in Patients With Chronic Heart Failure With Preserved Ejection Fraction (EMPEROR-Preserved) trial extended these findings to those with HFpEF, independent of diabetes status. Additionally, the Empagliflozin in Patients Hospitalized for Acute Decompensated Heart Failure (EMPULSE) trial provided evidence for the initiation of empagliflozin in patients hospitalized with acute heart failure, including those postmyocardial infarction, highlighting its utility in acute care settings to improve clinical outcomes.[6]
Ertugliflozin
Ertugliflozin is indicated as an adjunct to diet and exercise for improving glycemic control in adults with T2DM. This agent is not approved for use in pediatric populations. Unlike other SGLT2 inhibitors, ertugliflozin’s current FDA-approved indications are limited to glycemic management, without additional cardiovascular or renal risk-reduction claims.
The cardiovascular safety of ertugliflozin was evaluated in the Ertugliflozin Cardiovascular Outcomes Trial (VERTIS CV), a large, randomized, placebo-controlled outcomes study involving over 8,200 patients with T2DM and established ASCVD.[7] The trial met its primary endpoint, demonstrating noninferiority to placebo for MACE, which included cardiovascular death, nonfatal myocardial infarction, and nonfatal stroke. The incidence of MACE was identical in the ertugliflozin and placebo groups (11.9%), with a hazard ratio of 0.97, confirming cardiovascular safety. However, ertugliflozin did not demonstrate superiority for MACE reduction or key secondary endpoints, including cardiovascular death or HHF.
A nominal 30% reduction in HHF was observed (2.5% vs. 3.6%), but this effect was not part of the hierarchical testing sequence and therefore not statistically confirmatory. Consequently, ertugliflozin remains approved solely for glycemic control, without formal cardiovascular or renal protective indications.
Bexagliflozin
Bexagliflozin is indicated as an adjunct to diet and exercise for improving glycemic control in adults with T2DM. This drug is not currently FDA-approved for use in pediatric patients. Bexagliflozin's approved indications are limited to glycemic management, without additional cardiovascular or renal risk-reduction claims.
Cardiovascular safety was evaluated in a dedicated cardiovascular outcomes trial (referred to as "Trial 6" in the prescribing information), which enrolled adults with T2DM and either established ASCVD, heart failure, or multiple cardiovascular risk factors. The trial met its primary safety objective of noninferiority for MACE, confirming that bexagliflozin does not increase cardiovascular risk compared with placebo. Although favorable trends were observed for outcomes such as cardiovascular death and HHF, these findings did not reach statistical significance for superiority. Consequently, while bexagliflozin demonstrates a favorable safety profile and modest cardiometabolic benefits, including improvements in glycemic control, blood pressure, and body weight, its regulatory approval remains limited to glycemic management in adults with T2DM.[8]
Sotagliflozin
Sotagliflozin is a dual SGLT1/SGLT2 inhibitor, approved in the U.S. and indicated to reduce the risk of cardiovascular death, HHF, and urgent heart failure visits in adults with heart failure, including those with preserved or reduced ejection fraction, or cardiovascular risk factors such as T2DM or CKD. The approval of sotagliflozin was supported by 2 pivotal Phase 3 clinical trials. The Effect of Sotagliflozin on Cardiovascular Events in Patients With Type 2 Diabetes Post Worsening Heart Failure (SOLOIST-WHF) trial evaluated sotagliflozin in patients with T2DM who were recently hospitalized for worsening heart failure. Initiation of therapy shortly after stabilization led to a significant reduction in the composite outcome of cardiovascular death and heart failure-related events.
The Sotagliflozin in Patients With Diabetes and Chronic Kidney Disease (SCORED) trial enrolled patients with T2DM and CKD, demonstrating that sotagliflozin reduced the risk of cardiovascular events, including hospitalizations and urgent visits for heart failure. These trials collectively established sotagliflozin’s efficacy in reducing heart failure-related morbidity and mortality across a broad spectrum of patients, including those with recent decompensation, preserved or reduced ejection fraction, and coexisting diabetes or kidney disease. Unlike other SGLT2 inhibitors, sotagliflozin’s dual mechanism of action—targeting both SGLT1 in the gastrointestinal tract and SGLT2 in the kidney—may contribute to its unique clinical profile.
Overall, SGLT2 inhibitors are now recognized as a foundational therapy in the management of heart failure, as endorsed by the 2022 American Heart Association/American College of Cardiology/Heart Failure Society of America (AHA/ACC/HFSA) guidelines and reinforced by 2023-2024 expert consensus decision pathways.[9][10] These agents are recommended across the full spectrum of heart failure phenotypes, irrespective of the presence of T2DM.
For patients with symptomatic Stage C HFrEF, defined as having a left ventricular ejection fraction (LVEF) of or below 40%, SGLT2 inhibitors are a core component of guideline-directed medical therapy and carry a Class I recommendation. The initial pharmacologic regimen for this population includes an SGLT2 inhibitor, an angiotensin receptor–neprilysin inhibitor, an evidence-based β-blocker, and a mineralocorticoid receptor antagonist.
In patients with HFpEF, defined as having LVEF of at least 50%, SGLT2 inhibitors are recommended as 1st-line therapy, with additional agents considered based on individual clinical characteristics. For patients who are at risk for heart failure (Stage A) or have preheart failure (Stage B), SGLT2 inhibitors are advised in those with T2DM and either established ASCVD or high cardiovascular risk. The guide identifies dapagliflozin, empagliflozin, and sotagliflozin as therapeutic options for patients with Stage C and D heart failure.
Also, as per the 2024 Kidney Disease: Improving Global Outcomes (KDIGO) Chronic Kidney Disease Guidelines, SGLT2 inhibitors are a foundational therapy for a broad range of patients and are strongly recommended (1A) to slow the progression of CKD.[11] This recommendation applies to adults with an eGFR of 20 ml/min/1.73 m² or higher who have either T2DM or heart failure, or who have a urine albumin-to-creatinine ratio of 200 mg/g or greater, irrespective of diabetes status. Robust evidence from landmark trials such as the CREDENCE, DAPA-CKD, and Empagliflozin in Patients With Chronic Kidney Disease (EMPA-KIDNEY) trials demonstrates that canagliflozin, dapagliflozin, and empagliflozin significantly reduce the risk of sustained eGFR decline, ESKD, cardiovascular death, and hospitalization.
A key consideration is that an initial, reversible dip in eGFR upon initiation of SGLT2 inhibitor therapy is an expected hemodynamic effect and does not warrant discontinuation. Treatment may be continued even if eGFR falls below the 20 ml/min/1.73 m² initiation threshold until kidney replacement therapy is required. However, the guideline explicitly notes that kidney transplant recipients were not a focus of these trials, highlighting the need for further research. Therefore, SGLT2 inhibitors are not recommended in this population due to insufficient safety and efficacy data.
Mechanism of Action
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Mechanism of Action
Blood Glucose Management
SGLT2 inhibitors, including canagliflozin, dapagliflozin, empagliflozin, ertugliflozin, and bexagliflozin, target SGLT2 proteins in the proximal convoluted tubules of the kidneys. These proteins normally reabsorb filtered glucose and sodium. By competitively inhibiting SGLT2, these drugs reduce glucose reabsorption by 30% to 60%, lower the renal threshold for glucose, and increase urinary glucose excretion, leading to an average hemoglobin A1c (HbA1c) reduction of 0.5% to 1.0% in patients with T2DM.[12]
Cardioprotective Effects
SGLT2 inhibitors exert cardioprotective effects through multiple physiological mechanisms, primarily by inhibiting SGLT2-mediated glucose and sodium reabsorption in the proximal convoluted tubules of the kidneys. This inhibition increases distal tubular sodium delivery, suppressing the renin-angiotensin-aldosterone system.[13] The resultant reduction in preload, driven by natriuresis and diuresis, and afterload, mediated by arterial vasodilation, contributes to improved cardiac hemodynamics.
Both clinical and preclinical evidence support the hemodynamic benefits of SGLT2 inhibitors. Empagliflozin has been shown to reduce mean arterial pressure and the ambulatory arterial stiffness index in clinical studies.[14] Dapagliflozin enhances vasodilation, improves endothelial function, and mitigates oxidative stress, thereby promoting vascular health, as demonstrated in preclinical models and clinical trials.[15][16]
Beyond hemodynamic effects, SGLT2 inhibitors influence cardiac biomarkers and myocardial metabolism.[17] In patients with T2DM, canagliflozin attenuates the rise in N-terminal pro–B-type natriuretic peptide (NT-proBNP) and high-sensitivity troponin I, both markers of cardiac stress, as reported in the CANVAS trial.
These agents also shift cardiac energy metabolism from glucose and fatty acid oxidation toward ketogenesis. Mild hyperketonemia promotes the use of β-hydroxybutyrate as an efficient myocardial fuel source, potentially improving cardiac efficiency and exerting antiarrhythmic effects through membrane potential stabilization.
Large-scale RCTs confirm the clinical relevance of these mechanisms. The DAPA-HF and EMPEROR-Reduced trials demonstrated that dapagliflozin and empagliflozin, respectively, significantly reduce HHF and cardiovascular mortality in patients with HFrEF, irrespective of T2DM status.[18][19] Both trials also reported reductions in all-cause mortality, underscoring the broad cardioprotective benefits of SGLT2 inhibitors.
According to the “thrifty substrate” hypothesis, mild and persistent hyperketonemia induced by SGLT2 inhibitor therapy promotes the use of ketone bodies as an alternative myocardial fuel. β-hydroxybutyrate is actively transported into the heart via specific transporters, including the monocarboxylate transporter (MCT) system, where it undergoes oxidation in place of fatty acids and glucose.[20] SGLT2 inhibitor treatment decreases plasma glucose levels and increases glucagon production, thereby stimulating hepatic ketogenesis. Ketone bodies provide a more efficient energy substrate for the myocardium, potentially improving cardiac energetics and function. Furthermore, β-hydroxybutyrate exerts antiarrhythmic effects through stabilization of the myocardial cell membrane potential.[21]
Nephroprotective Effects
SGLT2 inhibitors exert nephroprotective effects primarily by increasing distal sodium delivery and inhibiting tubuloglomerular feedback, resulting in afferent vasoconstriction and reduced intraglomerular pressure. Lower intraglomerular pressure decreases albuminuria and slows CKD progression, as demonstrated in the CREDENCE, DAPA-CKD, and EMPA-KIDNEY trials. Inhibition of proximal glucose and sodium reabsorption also promotes natriuresis. SGLT2 inhibitors further decrease effective circulating volume, lower blood pressure, and induce modest weight loss. Beyond hemodynamic effects, these drugs attenuate inflammatory and fibrotic pathways, reduce kidney hypoxia, and influence mitochondrial metabolism in renal tissue.[22][23]
Pharmacokinetics
SGLT2 inhibitors are well absorbed from the gastrointestinal tract. The effect of food on pharmacokinetics is not statistically significant. Therefore, ertugliflozin, dapagliflozin, and empagliflozin may be administered with or without meals. However, administration before meals is recommended to mitigate postprandial plasma glucose elevation resulting from delayed intestinal glucose absorption.
SGLT2 inhibitors demonstrate high plasma protein binding (PPB). Dapagliflozin exhibits 91% PPB, empagliflozin 86% PPB, ertugliflozin 93% PPB, and canagliflozin the highest at 99% PPB. PPB is not significantly altered in patients with renal or hepatic impairment. The volumes of distribution for canagliflozin (83.5 L), dapagliflozin (118 L), ertugliflozin (85.5 L), and empagliflozin (74 L) indicate extensive tissue distribution.
SGLT2 inhibitors undergo biotransformation predominantly via glucuronidation mediated by uridine 5′-diphosphate-glucuronosyltransferase (UGT), with minimal involvement of cytochrome P450 enzymes. UGT1A9 plays a central role in their metabolism. Canagliflozin is primarily metabolized by UGT1A9 and UGT2B4; ertugliflozin by UGT1A9 and UGT2B; dapagliflozin by UGT1A9; and empagliflozin by UGT2B7, UGT1A3, UGT1A8, and UGT1A9.[24]
Following glomerular filtration, SGLT2 inhibitors act at the luminal membrane of the early nephron, where they inhibit up to 60% of filtered glucose reabsorption. These agents have long elimination half-lives, supporting once-daily administration.[25]
Administration
SGLT2 inhibitors are administered orally, with dosing determined by the clinical indication (see Image. Dosing and Indications of Sodium-Glucose Cotransporter 2 Inhibitors). Renal function should be assessed prior to initiating dapagliflozin, and patients with volume depletion should have this condition corrected before therapy. Current guidance advises withholding SGLT2 inhibitors before surgery, prolonged fasting, or critical illness, when the risk of ketoacidosis is increased.[26] With the exception of canagliflozin, which is taken before the 1st meal of the day, all other SGLT2 inhibitors may be administered with or without food.
Canagliflozin (Invokana) is available as 100 mg and 300 mg tablets. Dapagliflozin (Farxiga) is available in 5 mg and 10 mg tablets. Empagliflozin (Jardiance) is available in 10 mg and 25 mg tablets and is taken once daily in the morning. Ertugliflozin (Steglatro) is available in 5 mg and 15 mg tablets and is also administered once daily in the morning. Bexagliflozin (Brenzavvy) is available in 20 mg tablets and is administered once daily in the morning. Sotagliflozin (Inpefa) is available in 200 mg and 400 mg tablets.
Fixed-dose combinations of SGLT2 inhibitors are marketed as brand-only formulations. These preparations include canagliflozin with metformin (Invokamet, Invokamet XR), dapagliflozin with extended-release metformin (Xigduo XR) and with saxagliptin (Qtern), and empagliflozin with linagliptin (Glyxambi) or with metformin in both immediate- and extended-release forms (Synjardy, Synjardy XR). Ertugliflozin is available in combination with metformin (Segluromet) and with sitagliptin (Steglujan). As of 2025, all of these formulations remain under patent protection and lack generic equivalents in the U.S.[27][28][29]
Type 2 Diabetes Mellitus
According to the 2022 American Diabetes Association (ADA) guidelines, the recommended initial therapy for T2DM is metformin in combination with comprehensive lifestyle modification. Based on additional risk factors, including ASCVD, heart failure, and CKD, the use of SGLT2 inhibitors may be appropriate. The maximum recommended doses for these agents are discussed below.
Canagliflozin is initiated at 100 mg once daily and may be increased to 300 mg daily. This medication is not recommended for glycemic control in patients with an eGFR less than 30 mL/min/1.73 m².
Dapagliflozin is started at 5 mg once daily, with the option to increase to 10 mg once daily to achieve glycemic targets. This agent is not recommended for glycemic control in patients with an eGFR less than 45 mL/min/1.73 m².
Empagliflozin is initiated at 10 mg once daily and may be increased to 25 mg daily for additional glycemic control in adults and pediatric patients aged 10 years and older. This drug is not recommended for glycemic control in individuals with an eGFR less than 30 mL/min/1.73 m². Importantly, empagliflozin should be withheld for at least 3 days, if possible, before major surgery or procedures associated with prolonged fasting.
Ertugliflozin is initiated at 5 mg once daily and may be increased to 15 mg daily, but is not recommended for glycemic control in patients with an eGFR less than 45 mL/min/1.73 m². Bexagliflozin is administered at a fixed dose of 20 mg once daily and is not recommended for patients with an eGFR less than 30 mL/min/1.73 m².[30]
Heart Failure with Reduced Ejection Fraction
According to the 2022 AHA/ACC/HFSA guidelines, guideline-directed medical therapy for HFrEF includes the use of SGLT2 inhibitors. In patients with chronic symptomatic HFrEF, SGLT2 inhibitors are recommended to reduce heart failure-related hospitalization and cardiovascular mortality, regardless of the presence of T2DM. The preferred agents are dapagliflozin 10 mg once daily, empagliflozin 10 mg once daily, and sotagliflozin 200 mg once daily, which may be titrated to 400 mg once daily.[31]
Heart Failure with Preserved Ejection Fraction
According to the 2023 ACC Expert Consensus, SGLT2 inhibitors are indicated for all patients with HFpEF. The recommended agents are dapagliflozin 10 mg once daily and empagliflozin 10 mg once daily.[32]
Chronic Kidney Disease
According to the 2022 joint consensus of KDIGO and the ADA, SGLT2 inhibitors with established kidney benefits are recommended for patients with T2DM, CKD, and eGFR greater than 20 mL/min/1.73 m². Once initiated, these agents may be continued even at lower eGFR levels. A reversible decline in eGFR may occur at the start of treatment, but this transient change does not generally warrant discontinuation. In patients with urinary albumin greater than 200 mg/g creatinine, SGLT2 inhibitors are advised to reduce CKD progression and cardiovascular events. Hypovolemia increases the risk of acute kidney injury (AKI). Thus, volume status should be optimized before initiating therapy. The preferred agents are canagliflozin 100 mg once daily, dapagliflozin 10 mg once daily, and empagliflozin 10 mg once daily.[33]
Use in Specific Patient Populations
SGLT2 inhibitors require careful consideration when prescribed to patients with certain conditions due to potential variations in safety and efficacy. Below is a summary of the uses and recommended precautions of this drug class in these special populations.
Risks in individuals with liver dysfunction
Dose adjustment of SGLT2 inhibitors is not required in mild or moderate hepatic impairment. In severe hepatic impairment, canagliflozin and ertugliflozin have not been studied and are, therefore, not recommended. Empagliflozin appears well-tolerated in patients with mild, moderate, or severe hepatic impairment.[34]
Patients with cirrhosis are at increased risk of infection, which may potentiate the genitourinary infections associated with SGLT2 inhibitors. Fluid overload is common in cirrhosis, but these patients often have a relative reduction in effective circulating volume and may rapidly develop AKI due to volume shifts induced by SGLT2 inhibitors. Careful risk-benefit assessment is required before initiating therapy in patients with cirrhosis.[35]
Safety concerns in patients with renal impairment
According to consensus guidelines from the ADA and KDIGO, SGLT2 inhibitors with established kidney or cardiovascular benefit are recommended for patients with T2DM, CKD, and an eGFR greater than 20 mL/min/1.73 m². Once initiated, therapy may be continued at lower levels of eGFR. Individual patient factors should be considered before initiation when discrepancies in clinical status are present. In patients with diabetic kidney disease, use of an SGLT2 inhibitor is advised when urinary albumin exceeds 200 mg/g creatinine to slow CKD progression and reduce cardiovascular events. Patients with hypovolemia remain at risk for AKI. Therefore, volume status should be optimized before starting therapy.
Pregnancy-specific guidance
SGLT2 inhibitors are contraindicated in pregnancy due to evidence of reproductive toxicity in animal studies, with the highest risk occurring during the 2nd and 3rd trimesters.[36] According to the American College of Obstetricians and Gynecologists (ACOG) guidelines for gestational diabetes mellitus, insulin is the preferred treatment in these patients. Metformin and, less commonly, glyburide, may be considered as practical alternatives in women who decline insulin or in whom safety concerns limit insulin use.[37]
Breastfeeding considerations
SGLT2 inhibitors have high PPB and are unlikely to be secreted into breastmilk in clinically significant amounts. Nonetheless, the use of SGLT2 inhibitors during breastfeeding is not recommended due to the potential risk of kidney toxicity in the growing infant.[38][39][40][41]
Precautions in older adults
SGLT2 inhibitors may increase the incidence of adverse events related to reduced intravascular volume and hypotension. According to the SGLT2 inhibitors in Older Diabetic patients (SOLD) study, these agents are safe and effective for older adults with diabetes.[42]
Adverse Effects
The most frequently reported adverse events associated with SGLT2 inhibitor therapy include female genital mycotic infections, urinary tract infections (UTIs), polyuria, nausea, and constipation.[43] The following section outlines these adverse effects and other clinically relevant complications.
Genital Mycotic Infections
Genital mycotic infections encompass vulvovaginal candidiasis, vulvovaginitis, vulval abscess, and bacterial vaginitis. Female sex and a history of recurrent genital mycotic infections, defined as 3 or more episodes per year, are the strongest risk factors. Preventive strategies include optimizing glycemic control and maintaining personal hygiene. These infections are generally mild and resolve promptly with appropriate therapy. Discontinuation of SGLT2 inhibitors is rarely required. Management typically involves oral antifungal agents, such as fluconazole, or short-course topical antifungal therapy with miconazole or clotrimazole applied for 1 to 3 days.[44]
Urosepsis and Pyelonephritis
Serious UTIs, including urosepsis and pyelonephritis, have been reported with SGLT2 inhibitor therapy. SGLT2 inhibitors induce glucosuria by inhibiting glucose reabsorption in the proximal tubule, thereby creating a favorable milieu for bacterial growth and potentially increasing the risk of UTIs. A meta-analysis of 52 RCTs demonstrated a dose-dependent relationship between dapagliflozin use and UTIs.[45] However, in a large cohort study, the risk of urosepsis with SGLT2 inhibitors was comparable to that observed with dipeptidyl peptidase 4 inhibitors in real-world practice.[46]
The absence of consistent evidence for an increased UTI risk may be partly explained by enhanced urinary flow resulting from osmotic diuresis and natriuresis. Nonetheless, clinicians should exercise caution when prescribing SGLT2 inhibitors in patients with impaired urinary flow or bladder outlet obstruction. A case report described acute pyelonephritis associated with dapagliflozin use in a patient with bladder outlet obstruction. Such cases warrant prompt clinical evaluation and appropriate treatment.[47]
Lower Limb Amputation
Risk factors for lower-limb amputation include peripheral vascular disease, neuropathy, a history of diabetic foot ulcer, and prior amputations. SGLT2 inhibitors should be discontinued in the presence of active lower-limb ulcers or infection. Canagliflozin has been most strongly associated with an increased risk of amputation compared with empagliflozin, while dapagliflozin has been linked to a higher risk of toe amputation.[48]
A pooled analysis of phase 3 RCTs also demonstrated a slight increase in toe amputations with ertugliflozin, although all affected patients had preexisting peripheral neuropathy and peripheral artery disease.[49] In 2020, the FDA removed the boxed warning regarding amputation risk with canagliflozin, citing updated safety data. Although the absolute risk appears lower than earlier reports suggested, careful patient monitoring remains essential.
Diabetic Ketoacidosis
SGLT2 inhibitors are associated with nearly a 3-fold increased risk of diabetic ketoacidosis (DKA). Patients presenting with clinical features suggestive of DKA should be promptly evaluated and treated, and the SGLT2 inhibitor should be discontinued. Clinicians should assess individual risk factors for DKA before initiating therapy and consider temporary discontinuation of SGLT2 inhibitors during clinical situations that predispose to ketoacidosis. Among SGLT2 inhibitors, the risk of DKA appears highest with canagliflozin, followed by empagliflozin and dapagliflozin.[50]
Euglycemic Diabetic Ketoacidosis
Euglycemic DKA is defined by the triad of increased anion gap metabolic acidosis, ketosis, and serum glucose below 250 mg/dL. SGLT2 inhibitors have been implicated in this condition, likely due to noninsulin-dependent glucose clearance, hyperglucagonemia, and volume depletion. Ertugliflozin and canagliflozin have been most frequently associated with euglycemic DKA, although the risk extends to other SGLT2 inhibitors as well.[51][52]
Acute Kidney Injury
AKI may occur with the initiation of SGLT2 inhibitor therapy due to intravascular volume contraction. Clinicians should evaluate and correct volume status before initiating therapy, particularly in older adults who are receiving diuretics or have renal impairment. A recent pharmacovigilance study using the FDA's Adverse Event Reporting System (FAERS) database demonstrated a significant association between canagliflozin, as well as other SGLT2 inhibitors, and AKI. Patients older than 65 years were identified as being at particularly high risk.[53]
Hypoglycemia
The risk of hypoglycemia increases when SGLT2 inhibitors are administered concomitantly with insulin secretagogues such as sulfonylureas or with insulin itself. Clinicians should reduce the dose of the insulin secretagogue or insulin when combined with SGLT2 inhibitors. Evidence suggests that the risk of hypoglycemia is higher in older adults receiving SGLT2 inhibitors, warranting cautious use.[54]
Fournier Gangrene
Fournier gangrene is a rare but life-threatening necrotizing fasciitis of the perineum that requires urgent surgical intervention. Cases have been documented in both male and female patients. Clinical manifestations include fever, pain, tenderness, and swelling in the genital or perineal region. Severe outcomes include hospitalization, multiple surgeries, and death. Risk factors include preexisting diabetes, alcohol use, hypertension, advanced age, obesity, smoking, liver failure, immunocompromised states (HIV, inflammatory bowel disease, malignancy), end-stage renal disease, and prior radiotherapy. Iatrogenic procedures in the genital area, as well as piercing, implants, and intravenous drug use, can serve as portals of entry for microorganisms.
Most infections are polymicrobial. Therefore, empiric antibiotic therapy should provide coverage for Streptococcus, methicillin-resistant Staphylococcus aureus, Pseudomonas, coliforms, Bacteroides, and Clostridium. Fungal superinfection requires antifungal coverage, such as amphotericin B or other appropriate agents. Emergent surgical debridement, broad-spectrum antibiotic therapy, and resuscitation with intravenous fluids and vasopressors are the cornerstones of management.[55]
Hypersensitivity Reactions
Erythema, rash, pruritus, and angioedema have been reported with SGLT2 inhibitor therapy. The drug should be discontinued, and patients should be monitored until symptoms resolve.[56]
Bone Fracture
SGLT2 inhibitors have been linked to an increased risk of bone fractures. Canagliflozin, in particular, has been associated with fracture events occurring as early as 12 weeks after treatment initiation.[57] In contrast, another study found no increased fracture risk with canagliflozin compared with glucagon-like peptide 1 receptor agonist therapy, although unmeasured confounding and measurement error limit the reliability of this finding. Proposed mechanisms include intravascular volume contraction leading to dizziness and falls, as well as alterations in calcium, phosphate, and vitamin D homeostasis that may reduce bone mineral density.[58]
Bladder Cancer
A pooled analysis of 22 RCTs indicated a possible association between dapagliflozin and bladder cancer. Consequently, dapagliflozin should be avoided in patients with active bladder cancer. However, the same meta-analysis demonstrated that SGLT2 inhibitors overall were not significantly associated with an increased cancer risk compared with other active glucose-lowering therapies.[59] Clinical guidance advises against the use of dapagliflozin in patients with hematuria or a prior history of bladder cancer, given the potential concern for tumor promotion in susceptible individuals.[60]
Hyperkalemia
Canagliflozin has been linked to an increased risk of hyperkalemia, particularly when administered in combination with angiotensin-converting enzyme inhibitors or angiotensin receptor blockers. This effect is most pronounced in patients with renal impairment, necessitating close electrolyte monitoring when initiating or adjusting therapy.[61][62]
Dyslipidemia
SGLT2 inhibitors can cause modest changes in lipid metabolism, including a small increase in low-density lipoprotein cholesterol and high-density lipoprotein levels. Regular lipid profile monitoring is recommended to identify clinically relevant changes and guide further management if necessary.[63]
Contraindications
The use of SGLT2 inhibitors is contraindicated in patients receiving dialysis. Hypersensitivity reactions, including anaphylaxis or angioedema to any of these agents, also constitute an absolute contraindication.
Pharmacologic Interactions and Diagnostic Interference
The risk of hypoglycemia increases when SGLT2 inhibitors are combined with an insulin secretagogue, such as a sulfonylurea, or insulin. Clinicians should therefore reduce the dose of the insulin secretagogue or insulin to minimize this risk.
SGLT2 inhibitors, including empagliflozin, decrease sodium-glucose and lithium-glucose reabsorption in the proximal convoluted tubules, which increases renal excretion of sodium, glucose, and lithium. Concurrent use of an SGLT2 inhibitor with lithium can reduce serum lithium concentrations.[64]
Canagliflozin increases the plasma maximum concentration (Cmax, 36%) and the area under the curve (AUC, 20%) of digoxin. Given the narrow therapeutic index of digoxin, therapeutic drug monitoring is recommended when the 2 agents are coadministered.[65]
UGT enzyme inducers such as rifampin, phenytoin, ritonavir, and phenobarbital reduce canagliflozin exposure (AUC), which may diminish the drug’s effectiveness. Increasing the dose of canagliflozin may be considered when used with UGT enzyme inducers.[66]
False-positive urine glucose tests may occur because SGLT2 is the primary pathway for renal glucose reabsorption. Inhibition of SGLT2 increases urinary glucose, making urine testing unreliable for monitoring glycemic control in patients with diabetes.[67]
1,5-Anhydroglucitol structurally resembles glucose. The 1,5-anhydroglucitol assay is unreliable for assessing glycemic control in patients receiving SGLT2 inhibitors.[68]
Nonselective β-blockers may mask the adrenergic symptoms of hypoglycemia. Since SGLT2 inhibitors can also induce hypoglycemia, concurrent administration with β-blockers requires caution.[69]
Monitoring
Volume status and renal function should be assessed at baseline before initiating SGLT2 inhibitors because all 4 agents can cause intravascular volume contraction, which may result in a symptomatic decrease in blood pressure and a transient alteration in serum creatinine. Therefore, renal function and blood pressure should be monitored routinely after treatment initiation. Closer monitoring is warranted in individuals who have a history of renal dysfunction, are receiving loop diuretics, or are of advanced age treated with an SGLT2 inhibitor due to a higher risk of volume depletion.
Laboratory monitoring should include a complete blood count, basic metabolic panel, lipid panel, and kidney function tests, since changes in serum creatinine, eGFR, hematocrit, hemoglobin, low-density lipoprotein cholesterol, serum bicarbonate, serum phosphate, and potassium may occur. Patients should also be regularly assessed for clinical signs and symptoms of Fournier gangrene.
Blood glucose and HbA1c should be measured at baseline and monitored routinely during therapy. Patients who present with clinical signs or symptoms suggestive of metabolic acidosis should undergo thorough evaluation for ketoacidosis, as DKA may occur even when serum glucose levels are below 250 mg/dL.
The SGLT2 inhibitor should be discontinued immediately if ketoacidosis develops. Appropriate management with insulin, intravenous fluids, and carbohydrate replacement should also be initiated. Although the risk of DKA is substantially higher in patients with type 1 diabetes mellitus (T1DM) than those with T2DM receiving SGLT2 inhibitors, severe cases requiring hospitalization have been reported in both groups. Therapy with SGLT2 inhibitors is not indicated in patients with T1DM.
Individuals receiving therapy with insulin or insulin secretagogues in combination with SGLT2 inhibitors should be monitored closely for hypoglycemic symptoms. Individuals with a history of genital mycotic infections warrant regular surveillance, as SGLT2 inhibitors increase the risk of genital mycotic infections in both male and female patients. Clinicians should monitor for clinical features of UTI, including dysuria, urinary frequency, urgency, and suprapubic discomfort, and obtain urinalysis in cases of suspected infection. As previously discussed, therapeutic drug monitoring of lithium and digoxin is required when these agents are administered concurrently with SGLT2 inhibitors.
Patients should be evaluated for risk factors for lower-limb amputation before initiating canagliflozin or ertugliflozin, including peripheral vascular disease, history of amputation, neuropathy, elevated HbA1c at baseline, and diabetic foot ulcers. Patients receiving canagliflozin or ertugliflozin should be routinely monitored for infection or ulceration of the lower extremities, as nontraumatic lower-limb amputations have been reported. The most commonly implicated etiologies necessitating amputation include lower-extremity infections, gangrene, and foot ulcers.[70]
Laboratory and Clinical Monitoring of Diabetes Treatment
Glycemic targets should be monitored routinely in individuals with diabetes. According to the ADA 2022 guidelines, the recommended goals for nonpregnant adults include an HbA1c level below 7% without significant hypoglycemia, preprandial capillary plasma glucose between 80 and 130 mg/dL, and peak postprandial capillary plasma glucose less than 180 mg/dL.[71]
Routine Evaluation in Heart Failure
Monitoring of patients with heart failure should include regular assessment of fluid intake and output, vital signs, and body weight measured at the same time each day. Clinical manifestations of hypoperfusion and congestion must also be evaluated. Laboratory parameters, including serum electrolytes, serum creatinine, and blood urea nitrogen, should be monitored closely during medication titration or adjustment, according to ACC/AHA/HFSA 2022 guidelines.
Surveillance of Kidney Function in Chronic Kidney Disease
Renal function monitoring in patients treated with SGLT2 inhibitors should include measurement of the albumin-to-creatinine ratio and eGFR. Clinicians should recognize that initiation of SGLT2 inhibitor therapy is associated with an acute eGFR reduction. This initial decline should be anticipated and is not an indication for discontinuation if the amount of reduction is less than 30%, as long-term therapy has been shown to improve renal and cardiovascular outcomes in patients with CKD.[72]
Toxicity
SGLT2 inhibitors lack a specific antidote, and dialysis does not enhance their elimination. A retrospective review of overdoses reported to 13 poison centers in the U.S. found that most cases with mild exposure did not result in hypoglycemia, except in pediatric patients. The predominant presenting symptoms were nausea, vomiting, and dizziness. However, intentional overdose can lead to hypoglycemia, vomiting, confusion, hypertension, tachycardia, and urinary incontinence.[73] A case report documented euglycemia despite ertugliflozin overdose in a patient who ingested 150 mg, whereas the maximum recommended dose is 15 mg.
According to standard hypoglycemia protocols, treatment requires immediate reversal of low glucose levels. Oral glucose should be administered if the patient can safely swallow. Intravenous dextrose (25 g) is administered to patients with impaired consciousness. Glucagon (0.5 to 1 mg) should be administered subcutaneously or intramuscularly when intravenous access cannot be established. Subcutaneous or intravenous octreotide has been used in cases of refractory hypoglycemia. Consultation with the poison control center is recommended in complicated overdose cases.[74]
Enhancing Healthcare Team Outcomes
SGLT2 inhibitors act on SGLT2 transporters expressed in the proximal convoluted tubules of the kidney. Four agents—canagliflozin, dapagliflozin, empagliflozin, and ertugliflozin—are FDA-approved for treating adults with T2DM to improve glycemic control as an adjunct to diet and exercise. Adverse events associated with decreased intravascular volume occur more frequently in older adults receiving these agents, making routine monitoring of blood glucose and blood pressure in this population essential to reduce risk.
Female individuals of childbearing potential should be counseled on the possible risks of SGLT2 inhibitor use during pregnancy, particularly in the 2nd and 3rd trimesters. Management of glycemic control in patients with T2DM and associated renal and cardiovascular complications requires interprofessional coordination. Optimal care involves collaboration between a primary care physician (PCP), endocrinologist, cardiologist, nephrologist, nurses, and a pharmacist.
A comprehensive clinical evaluation should be conducted to determine the appropriateness of SGLT2 inhibitor therapy. Baseline blood glucose, HbA1c, renal function, and volume status must be assessed before initiation. The PCP and the interprofessional care team should remain updated on current guidelines for the management of T2DM and the appropriate use of SGLT2 inhibitors. Kidney function requires ongoing monitoring, and collaboration between the PCP and nephrologist is essential to evaluate therapeutic benefits in patients with CKD and select the most suitable agent with proven efficacy.[75] In patients with T2DM and concomitant heart failure (New York Heart Association classes II-IV) with reduced ejection fraction, evaluation by both the PCP and cardiologist is necessary to guide the use of SGLT2 inhibitors.[76]
Endocrinologists play a critical role in achieving glycemic targets, managing complications such as euglycemic DKA, and preventing both macrovascular and microvascular complications of diabetes. Pharmacists are responsible for counseling patients on the potential adverse effects of SGLT2 inhibitors. The interprofessional care team should advise patients to avoid concurrent use of insulin and insulin secretagogues with SGLT2 inhibitors.
Nurses should monitor adherence and educate patients on the signs and symptoms of hypoglycemia, particularly when combination therapy is prescribed, so that prescribers can adjust management when necessary. Dietitians can counsel patients on adherence to dietary recommendations from the ADA or AHA, tailored to individual comorbidities. Infectious disease physicians may provide essential care for patients who develop UTIs or recurrent fungal infections.
Early surgical consultation is essential in cases of Fournier gangrene. In the setting of overdose, emergency medicine physicians must stabilize the patient promptly, and consultation with a medical toxicologist is recommended for up-to-date management strategies. In cases of intentional overdose, psychiatric and psychological consultation is necessary to address underlying mental health concerns. An interprofessional team of prescribing clinicians, including medical doctors, doctors of osteopathic medicine, nurse practitioners, and physician assistants, working alongside specialists such as endocrinologists, cardiologists, and nephrologists, as well as pharmacists, specialty-trained nurses, and dietitians, can optimize outcomes in patients receiving SGLT2 inhibitor therapy.
Media
(Click Image to Enlarge)
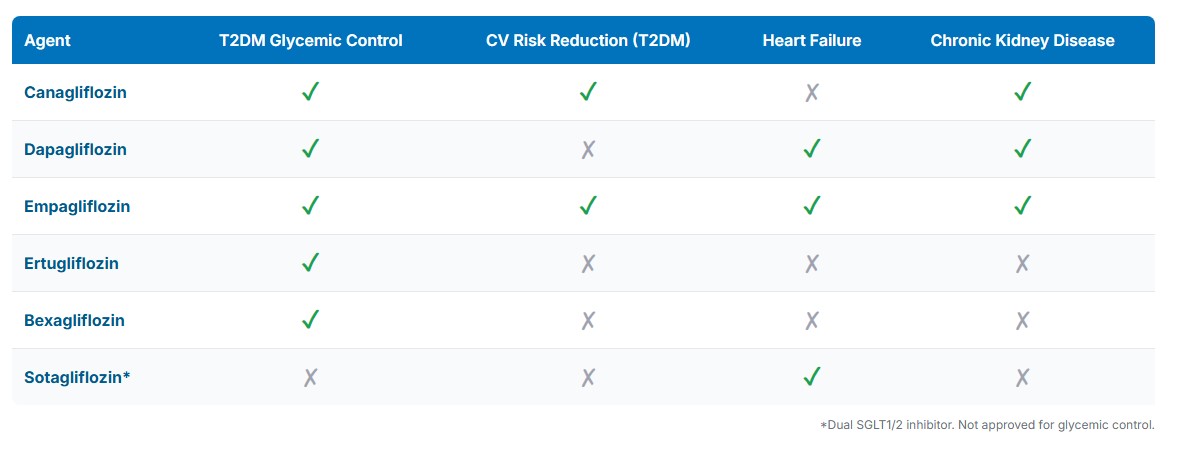
U.S. Food and Drug Administration-Approved Indications of Sodium-Glucose Cotransporter 2 and Sodium-Glucose Cotransporter 1/2 Inhibitors. This table compares major therapeutic uses for approved agents as of mid-2025. Most SGLT2 inhibitors are approved for type 2 diabetes mellitus glycemic control, with select drugs also indicated for cardiovascular risk reduction, heart failure, and chronic kidney disease. Sotagliflozin, a dual sodium-glucose cotransporter 1/2 inhibitor, is approved only for heart failure and not for glycemic control.
Contributed by Mayur S. Parmar, Ph.D.
(Click Image to Enlarge)
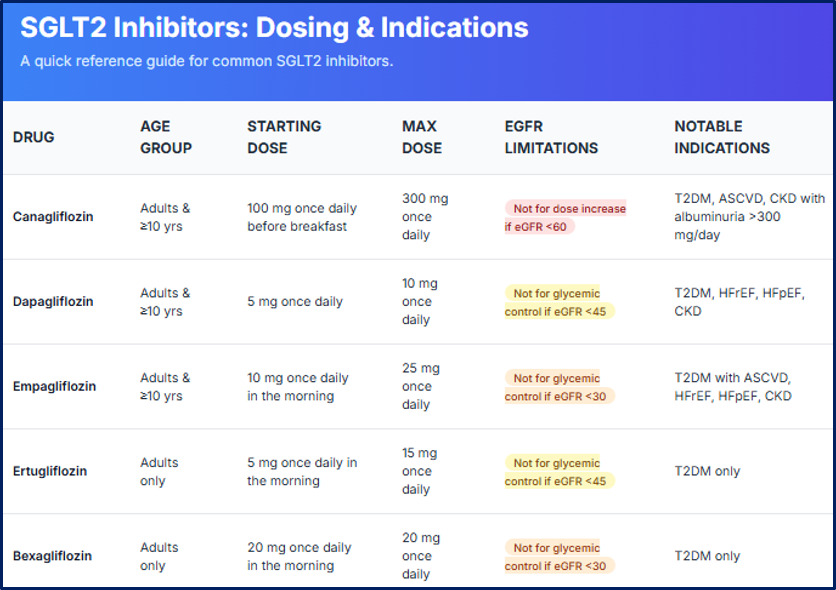
Dosing and Indications of Sodium-Glucose Cotransporter 2 Inhibitors. This image summarizes key dosing regimens, starting and maximum doses, age group eligibility, estimated glomerular filtration rate limitations, and notable U.S. Food and Drug Administration–approved indications for canagliflozin, dapagliflozin, empagliflozin, ertugliflozin, and bexagliflozin (updated in June 2025). Renal function thresholds guide glycemic use, and clinical indications include type 2 diabetes mellitus, atherosclerotic cardiovascular disease, heart failure, and chronic kidney disease.
Contributed by Mayur S. Parmar
References
Nespoux J, Vallon V. Renal effects of SGLT2 inhibitors: an update. Current opinion in nephrology and hypertension. 2020 Mar:29(2):190-198. doi: 10.1097/MNH.0000000000000584. Epub [PubMed PMID: 31815757]
Level 3 (low-level) evidenceNeal B, Perkovic V, Mahaffey KW, de Zeeuw D, Fulcher G, Erondu N, Shaw W, Law G, Desai M, Matthews DR, CANVAS Program Collaborative Group. Canagliflozin and Cardiovascular and Renal Events in Type 2 Diabetes. The New England journal of medicine. 2017 Aug 17:377(7):644-657. doi: 10.1056/NEJMoa1611925. Epub 2017 Jun 12 [PubMed PMID: 28605608]
Level 1 (high-level) evidenceMahaffey KW, Jardine MJ, Bompoint S, Cannon CP, Neal B, Heerspink HJL, Charytan DM, Edwards R, Agarwal R, Bakris G, Bull S, Capuano G, de Zeeuw D, Greene T, Levin A, Pollock C, Sun T, Wheeler DC, Yavin Y, Zhang H, Zinman B, Rosenthal N, Brenner BM, Perkovic V. Canagliflozin and Cardiovascular and Renal Outcomes in Type 2 Diabetes Mellitus and Chronic Kidney Disease in Primary and Secondary Cardiovascular Prevention Groups. Circulation. 2019 Aug 27:140(9):739-750. doi: 10.1161/CIRCULATIONAHA.119.042007. Epub 2019 Jul 11 [PubMed PMID: 31291786]
Wiviott SD, Raz I, Bonaca MP, Mosenzon O, Kato ET, Cahn A, Silverman MG, Zelniker TA, Kuder JF, Murphy SA, Bhatt DL, Leiter LA, McGuire DK, Wilding JPH, Ruff CT, Gause-Nilsson IAM, Fredriksson M, Johansson PA, Langkilde AM, Sabatine MS, DECLARE–TIMI 58 Investigators. Dapagliflozin and Cardiovascular Outcomes in Type 2 Diabetes. The New England journal of medicine. 2019 Jan 24:380(4):347-357. doi: 10.1056/NEJMoa1812389. Epub 2018 Nov 10 [PubMed PMID: 30415602]
Wheeler DC, Stefánsson BV, Jongs N, Chertow GM, Greene T, Hou FF, McMurray JJV, Correa-Rotter R, Rossing P, Toto RD, Sjöström CD, Langkilde AM, Heerspink HJL, DAPA-CKD Trial Committees and Investigators. Effects of dapagliflozin on major adverse kidney and cardiovascular events in patients with diabetic and non-diabetic chronic kidney disease: a prespecified analysis from the DAPA-CKD trial. The lancet. Diabetes & endocrinology. 2021 Jan:9(1):22-31. doi: 10.1016/S2213-8587(20)30369-7. Epub [PubMed PMID: 33338413]
Zannad F, Ferreira JP, Pocock SJ, Anker SD, Butler J, Filippatos G, Brueckmann M, Ofstad AP, Pfarr E, Jamal W, Packer M. SGLT2 inhibitors in patients with heart failure with reduced ejection fraction: a meta-analysis of the EMPEROR-Reduced and DAPA-HF trials. Lancet (London, England). 2020 Sep 19:396(10254):819-829. doi: 10.1016/S0140-6736(20)31824-9. Epub 2020 Aug 30 [PubMed PMID: 32877652]
Level 1 (high-level) evidenceCorbin KD, Dagogo-Jack S, Cannon CP, Cherney DZI, Cosentino F, Frederich R, Liu J, Pong A, Lin J, Cater NB, Pratley RE. Cardiorenal outcomes by indices of liver steatosis and fibrosis in individuals with type 2 diabetes and atherosclerotic cardiovascular disease: Analyses from VERTIS CV, a randomized trial of the sodium-glucose cotransporter-2 inhibitor ertugliflozin. Diabetes, obesity & metabolism. 2023 Mar:25(3):758-766. doi: 10.1111/dom.14923. Epub 2022 Dec 26 [PubMed PMID: 36394384]
Level 1 (high-level) evidenceHadd MJ, Bienhoff SE, Little SE, Geller S, Ogne-Stevenson J, Dupree TJ, Scott-Moncrieff JC. Safety and effectiveness of the sodium-glucose cotransporter inhibitor bexagliflozin in cats newly diagnosed with diabetes mellitus. Journal of veterinary internal medicine. 2023 May-Jun:37(3):915-924. doi: 10.1111/jvim.16730. Epub 2023 May 6 [PubMed PMID: 37148170]
Heidenreich PA, Bozkurt B, Aguilar D, Allen LA, Byun JJ, Colvin MM, Deswal A, Drazner MH, Dunlay SM, Evers LR, Fang JC, Fedson SE, Fonarow GC, Hayek SS, Hernandez AF, Khazanie P, Kittleson MM, Lee CS, Link MS, Milano CA, Nnacheta LC, Sandhu AT, Stevenson LW, Vardeny O, Vest AR, Yancy CW, ACC/AHA Joint Committee Members. 2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2022 May 3:145(18):e895-e1032. doi: 10.1161/CIR.0000000000001063. Epub 2022 Apr 1 [PubMed PMID: 35363499]
Level 1 (high-level) evidenceMaddox TM, Januzzi JL Jr, Allen LA, Breathett K, Brouse S, Butler J, Davis LL, Fonarow GC, Ibrahim NE, Lindenfeld J, Masoudi FA, Motiwala SR, Oliveros E, Walsh MN, Wasserman A, Yancy CW, Youmans QR. 2024 ACC Expert Consensus Decision Pathway for Treatment of Heart Failure With Reduced Ejection Fraction: A Report of the American College of Cardiology Solution Set Oversight Committee. Journal of the American College of Cardiology. 2024 Apr 16:83(15):1444-1488. doi: 10.1016/j.jacc.2023.12.024. Epub 2024 Mar 8 [PubMed PMID: 38466244]
Level 3 (low-level) evidenceLevin A, Ahmed SB, Carrero JJ, Foster B, Francis A, Hall RK, Herrington WG, Hill G, Inker LA, Kazancıoğlu R, Lamb E, Lin P, Madero M, McIntyre N, Morrow K, Roberts G, Sabanayagam D, Schaeffner E, Shlipak M, Shroff R, Tangri N, Thanachayanont T, Ulasi I, Wong G, Yang CW, Zhang L, Robinson KA, Wilson L, Wilson RF, Kasiske BL, Cheung M, Earley A, Stevens PE. Executive summary of the KDIGO 2024 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease: known knowns and known unknowns. Kidney international. 2024 Apr:105(4):684-701. doi: 10.1016/j.kint.2023.10.016. Epub [PubMed PMID: 38519239]
Level 1 (high-level) evidencePlosker GL. Canagliflozin: a review of its use in patients with type 2 diabetes mellitus. Drugs. 2014 May:74(7):807-24. doi: 10.1007/s40265-014-0225-5. Epub [PubMed PMID: 24831734]
Lytvyn Y, Bjornstad P, Udell JA, Lovshin JA, Cherney DZI. Sodium Glucose Cotransporter-2 Inhibition in Heart Failure: Potential Mechanisms, Clinical Applications, and Summary of Clinical Trials. Circulation. 2017 Oct 24:136(17):1643-1658. doi: 10.1161/CIRCULATIONAHA.117.030012. Epub [PubMed PMID: 29061576]
Chilton R, Tikkanen I, Cannon CP, Crowe S, Woerle HJ, Broedl UC, Johansen OE. Effects of empagliflozin on blood pressure and markers of arterial stiffness and vascular resistance in patients with type 2 diabetes. Diabetes, obesity & metabolism. 2015 Dec:17(12):1180-93. doi: 10.1111/dom.12572. Epub 2015 Oct 9 [PubMed PMID: 26343814]
Li H, Shin SE, Seo MS, An JR, Choi IW, Jung WK, Firth AL, Lee DS, Yim MJ, Choi G, Lee JM, Na SH, Park WS. The anti-diabetic drug dapagliflozin induces vasodilation via activation of PKG and Kv channels. Life sciences. 2018 Mar 15:197():46-55. doi: 10.1016/j.lfs.2018.01.032. Epub 2018 Feb 1 [PubMed PMID: 29409796]
Solini A, Giannini L, Seghieri M, Vitolo E, Taddei S, Ghiadoni L, Bruno RM. Dapagliflozin acutely improves endothelial dysfunction, reduces aortic stiffness and renal resistive index in type 2 diabetic patients: a pilot study. Cardiovascular diabetology. 2017 Oct 23:16(1):138. doi: 10.1186/s12933-017-0621-8. Epub 2017 Oct 23 [PubMed PMID: 29061124]
Level 3 (low-level) evidenceJanuzzi JL Jr, Butler J, Jarolim P, Sattar N, Vijapurkar U, Desai M, Davies MJ. Effects of Canagliflozin on Cardiovascular Biomarkers in Older Adults With Type 2 Diabetes. Journal of the American College of Cardiology. 2017 Aug 8:70(6):704-712. doi: 10.1016/j.jacc.2017.06.016. Epub 2017 Jun 12 [PubMed PMID: 28619659]
de Boer IH, Khunti K, Sadusky T, Tuttle KR, Neumiller JJ, Rhee CM, Rosas SE, Rossing P, Bakris G. Diabetes Management in Chronic Kidney Disease: A Consensus Report by the American Diabetes Association (ADA) and Kidney Disease: Improving Global Outcomes (KDIGO). Diabetes care. 2022 Dec 1:45(12):3075-3090. doi: 10.2337/dci22-0027. Epub [PubMed PMID: 36189689]
Level 3 (low-level) evidenceMcMurray JJV, Solomon SD, Inzucchi SE, Køber L, Kosiborod MN, Martinez FA, Ponikowski P, Sabatine MS, Anand IS, Bělohlávek J, Böhm M, Chiang CE, Chopra VK, de Boer RA, Desai AS, Diez M, Drozdz J, Dukát A, Ge J, Howlett JG, Katova T, Kitakaze M, Ljungman CEA, Merkely B, Nicolau JC, O'Meara E, Petrie MC, Vinh PN, Schou M, Tereshchenko S, Verma S, Held C, DeMets DL, Docherty KF, Jhund PS, Bengtsson O, Sjöstrand M, Langkilde AM, DAPA-HF Trial Committees and Investigators. Dapagliflozin in Patients with Heart Failure and Reduced Ejection Fraction. The New England journal of medicine. 2019 Nov 21:381(21):1995-2008. doi: 10.1056/NEJMoa1911303. Epub 2019 Sep 19 [PubMed PMID: 31535829]
Yang F, Meng R, Zhu DL. Cardiovascular effects and mechanisms of sodium-glucose cotransporter-2 inhibitors. Chronic diseases and translational medicine. 2020 Dec:6(4):239-245. doi: 10.1016/j.cdtm.2020.07.002. Epub 2020 Aug 15 [PubMed PMID: 33336169]
Ferrannini E, Mark M, Mayoux E. CV Protection in the EMPA-REG OUTCOME Trial: A "Thrifty Substrate" Hypothesis. Diabetes care. 2016 Jul:39(7):1108-14. doi: 10.2337/dc16-0330. Epub [PubMed PMID: 27289126]
Yau K, Dharia A, Alrowiyti I, Cherney DZI. Prescribing SGLT2 Inhibitors in Patients With CKD: Expanding Indications and Practical Considerations. Kidney international reports. 2022 Jul:7(7):1463-1476. doi: 10.1016/j.ekir.2022.04.094. Epub 2022 May 5 [PubMed PMID: 35812300]
Larmour K, Levin A. Slowing Progression in CKD: DAPA CKD and Beyond. Clinical journal of the American Society of Nephrology : CJASN. 2021 Jul:16(7):1117-1119. doi: 10.2215/CJN.20211220. Epub 2021 Mar 10 [PubMed PMID: 33692116]
Scheen AJ. Pharmacokinetics, Pharmacodynamics and Clinical Use of SGLT2 Inhibitors in Patients with Type 2 Diabetes Mellitus and Chronic Kidney Disease. Clinical pharmacokinetics. 2015 Jul:54(7):691-708. doi: 10.1007/s40262-015-0264-4. Epub [PubMed PMID: 25805666]
Wright EM. SGLT2 Inhibitors: Physiology and Pharmacology. Kidney360. 2021 Dec 30:2(12):2027-2037. doi: 10.34067/KID.0002772021. Epub 2021 Sep 17 [PubMed PMID: 35419546]
Kidney Disease: Improving Global Outcomes (KDIGO) Diabetes Work Group. KDIGO 2022 Clinical Practice Guideline for Diabetes Management in Chronic Kidney Disease. Kidney international. 2022 Nov:102(5S):S1-S127. doi: 10.1016/j.kint.2022.06.008. Epub [PubMed PMID: 36272764]
Level 1 (high-level) evidenceAthyros VG, Boutari C, Karagiannis A. Ertugliflozin + metformin as a treatment option for type 2 diabetes. Expert opinion on pharmacotherapy. 2021 Nov:22(16):2105-2111. doi: 10.1080/14656566.2021.1939676. Epub 2021 Jul 15 [PubMed PMID: 34130582]
Level 3 (low-level) evidenceMarrs JC, Anderson SL. Ertugliflozin in the treatment of type 2 diabetes mellitus. Drugs in context. 2020:9():. doi: 10.7573/dic.2020-7-4. Epub 2020 Nov 30 [PubMed PMID: 33293984]
Jakher H, Chang TI, Tan M, Mahaffey KW. Canagliflozin review - safety and efficacy profile in patients with T2DM. Diabetes, metabolic syndrome and obesity : targets and therapy. 2019:12():209-215. doi: 10.2147/DMSO.S184437. Epub 2019 Feb 1 [PubMed PMID: 30787627]
American Diabetes Association Professional Practice Committee. 9. Pharmacologic Approaches to Glycemic Treatment: Standards of Medical Care in Diabetes-2022. Diabetes care. 2022 Jan 1:45(Suppl 1):S125-S143. doi: 10.2337/dc22-S009. Epub [PubMed PMID: 34964831]
Heidenreich PA, Bozkurt B, Aguilar D, Allen LA, Byun JJ, Colvin MM, Deswal A, Drazner MH, Dunlay SM, Evers LR, Fang JC, Fedson SE, Fonarow GC, Hayek SS, Hernandez AF, Khazanie P, Kittleson MM, Lee CS, Link MS, Milano CA, Nnacheta LC, Sandhu AT, Stevenson LW, Vardeny O, Vest AR, Yancy CW. 2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Journal of the American College of Cardiology. 2022 May 3:79(17):e263-e421. doi: 10.1016/j.jacc.2021.12.012. Epub 2022 Apr 1 [PubMed PMID: 35379503]
Level 1 (high-level) evidenceKittleson MM, Panjrath GS, Amancherla K, Davis LL, Deswal A, Dixon DL, Januzzi JL Jr, Yancy CW. 2023 ACC Expert Consensus Decision Pathway on Management of Heart Failure With Preserved Ejection Fraction: A Report of the American College of Cardiology Solution Set Oversight Committee. Journal of the American College of Cardiology. 2023 May 9:81(18):1835-1878. doi: 10.1016/j.jacc.2023.03.393. Epub 2023 Apr 19 [PubMed PMID: 37137593]
Level 3 (low-level) evidenceMenne J, Dumann E, Haller H, Schmidt BMW. Acute kidney injury and adverse renal events in patients receiving SGLT2-inhibitors: A systematic review and meta-analysis. PLoS medicine. 2019 Dec:16(12):e1002983. doi: 10.1371/journal.pmed.1002983. Epub 2019 Dec 9 [PubMed PMID: 31815931]
Level 1 (high-level) evidenceMacha S, Rose P, Mattheus M, Cinca R, Pinnetti S, Broedl UC, Woerle HJ. Pharmacokinetics, safety and tolerability of empagliflozin, a sodium glucose cotransporter 2 inhibitor, in patients with hepatic impairment. Diabetes, obesity & metabolism. 2014 Feb:16(2):118-23. doi: 10.1111/dom.12183. Epub 2013 Aug 19 [PubMed PMID: 23859534]
Hsiang JC, Wong VW. SGLT2 Inhibitors in Liver Patients. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2020 Sep:18(10):2168-2172.e2. doi: 10.1016/j.cgh.2020.05.021. Epub 2020 May 16 [PubMed PMID: 32428710]
Sacks DA, Feig DS. Caring for pregnant women whose diabetes antedates pregnancy: is there room for improvement? Diabetologia. 2018 May:61(5):1022-1026. doi: 10.1007/s00125-018-4565-7. Epub 2018 Feb 7 [PubMed PMID: 29411042]
. ACOG Practice Bulletin No. 190: Gestational Diabetes Mellitus. Obstetrics and gynecology. 2018 Feb:131(2):e49-e64. doi: 10.1097/AOG.0000000000002501. Epub [PubMed PMID: 29370047]
. Empagliflozin. Drugs and Lactation Database (LactMed®). 2006:(): [PubMed PMID: 30000031]
. Ertugliflozin. Drugs and Lactation Database (LactMed®). 2006:(): [PubMed PMID: 30000032]
. Dapagliflozin. Drugs and Lactation Database (LactMed®). 2006:(): [PubMed PMID: 30000030]
. Canagliflozin. Drugs and Lactation Database (LactMed®). 2006:(): [PubMed PMID: 29999683]
Lunati ME, Cimino V, Gandolfi A, Trevisan M, Montefusco L, Pastore I, Pace C, Betella N, Favacchio G, Bulgheroni M, Bucciarelli L, Massari G, Mascardi C, Girelli A, Morpurgo PS, Folli F, Luzi L, Mirani M, Pintaudi B, Bertuzzi F, Berra C, Fiorina P. SGLT2-inhibitors are effective and safe in the elderly: The SOLD study. Pharmacological research. 2022 Sep:183():106396. doi: 10.1016/j.phrs.2022.106396. Epub 2022 Aug 12 [PubMed PMID: 35970329]
Halimi S, Vergès B. Adverse effects and safety of SGLT-2 inhibitors. Diabetes & metabolism. 2014 Dec:40(6 Suppl 1):S28-34. doi: 10.1016/S1262-3636(14)72693-X. Epub [PubMed PMID: 25554069]
Engelhardt K, Ferguson M, Rosselli JL. Prevention and Management of Genital Mycotic Infections in the Setting of Sodium-Glucose Cotransporter 2 Inhibitors. The Annals of pharmacotherapy. 2021 Apr:55(4):543-548. doi: 10.1177/1060028020951928. Epub 2020 Aug 18 [PubMed PMID: 32808541]
Li D, Wang T, Shen S, Fang Z, Dong Y, Tang H. Urinary tract and genital infections in patients with type 2 diabetes treated with sodium-glucose co-transporter 2 inhibitors: A meta-analysis of randomized controlled trials. Diabetes, obesity & metabolism. 2017 Mar:19(3):348-355. doi: 10.1111/dom.12825. Epub 2016 Dec 19 [PubMed PMID: 27862830]
Level 1 (high-level) evidenceFisher A, Fralick M, Filion KB, Dell'Aniello S, Douros A, Tremblay É, Shah BR, Ronksley PE, Alessi-Severini S, Hu N, Bugden SC, Ernst P, Lix LM, Canadian Network for Observational Drug Effect Studies (CNODES) Investigators. Sodium-glucose co-transporter-2 inhibitors and the risk of urosepsis: A multi-site, prevalent new-user cohort study. Diabetes, obesity & metabolism. 2020 Sep:22(9):1648-1658. doi: 10.1111/dom.14082. Epub 2020 Jun 4 [PubMed PMID: 32383792]
Hall V, Kwong J, Johnson D, Ekinci EI. Caution advised with dapagliflozin in the setting of male urinary tract outlet obstruction. BMJ case reports. 2017 May 22:2017():. pii: bcr-2017-219335. doi: 10.1136/bcr-2017-219335. Epub 2017 May 22 [PubMed PMID: 28536217]
Level 3 (low-level) evidenceKhouri C, Cracowski JL, Roustit M. SGLT-2 inhibitors and the risk of lower-limb amputation: Is this a class effect? Diabetes, obesity & metabolism. 2018 Jun:20(6):1531-1534. doi: 10.1111/dom.13255. Epub 2018 Mar 12 [PubMed PMID: 29430814]
Patel S, Hickman A, Frederich R, Johnson S, Huyck S, Mancuso JP, Gantz I, Terra SG. Safety of Ertugliflozin in Patients with Type 2 Diabetes Mellitus: Pooled Analysis of Seven Phase 3 Randomized Controlled Trials. Diabetes therapy : research, treatment and education of diabetes and related disorders. 2020 Jun:11(6):1347-1367. doi: 10.1007/s13300-020-00803-3. Epub 2020 May 5 [PubMed PMID: 32372382]
Level 1 (high-level) evidenceDouros A, Lix LM, Fralick M, Dell'Aniello S, Shah BR, Ronksley PE, Tremblay É, Hu N, Alessi-Severini S, Fisher A, Bugden SC, Ernst P, Filion KB, Canadian Network for Observational Drug Effect Studies (CNODES) Investigators. Sodium-Glucose Cotransporter-2 Inhibitors and the Risk for Diabetic Ketoacidosis : A Multicenter Cohort Study. Annals of internal medicine. 2020 Sep 15:173(6):417-425. doi: 10.7326/M20-0289. Epub 2020 Jul 28 [PubMed PMID: 32716707]
Chandrakumar HP, Chillumuntala S, Singh G, McFarlane SI. Postoperative Euglycemic Ketoacidosis in Type 2 Diabetes Associated with Sodium-Glucose Cotransporter 2 Inhibitor: Insights Into Pathogenesis and Management Strategy. Cureus. 2021 Jun 8:13(6):e15533. doi: 10.7759/cureus.15533. Epub 2021 Jun 8 [PubMed PMID: 34123681]
Peters AL, Buschur EO, Buse JB, Cohan P, Diner JC, Hirsch IB. Euglycemic Diabetic Ketoacidosis: A Potential Complication of Treatment With Sodium-Glucose Cotransporter 2 Inhibition. Diabetes care. 2015 Sep:38(9):1687-93. doi: 10.2337/dc15-0843. Epub 2015 Jun 15 [PubMed PMID: 26078479]
Chen G, Li X, Cui Q, Zhou Y, Zhao B, Mei D, Xuemei. Acute kidney injury following SGLT2 inhibitors among diabetic patients: a pharmacovigilance study. International urology and nephrology. 2022 Nov:54(11):2949-2957. doi: 10.1007/s11255-022-03211-7. Epub 2022 May 17 [PubMed PMID: 35579781]
Horii T, Oikawa Y, Kunisada N, Shimada A, Atsuda K. Real-world risk of hypoglycemia-related hospitalization in Japanese patients with type 2 diabetes using SGLT2 inhibitors: a nationwide cohort study. BMJ open diabetes research & care. 2020 Nov:8(2):. doi: 10.1136/bmjdrc-2020-001856. Epub [PubMed PMID: 33246930]
Chowdhury T, Gousy N, Bellamkonda A, Dutta J, Zaman CF, Zakia UB, Tasha T, Dutta P, Deb Roy P, Gomez AM, Mainali A. Fournier's Gangrene: A Coexistence or Consanguinity of SGLT-2 Inhibitor Therapy. Cureus. 2022 Aug:14(8):e27773. doi: 10.7759/cureus.27773. Epub 2022 Aug 8 [PubMed PMID: 36106208]
McGill JB, Subramanian S. Safety of Sodium-Glucose Co-Transporter 2 Inhibitors. The American journal of cardiology. 2019 Dec 15:124 Suppl 1():S45-S52. doi: 10.1016/j.amjcard.2019.10.029. Epub [PubMed PMID: 31741440]
Watts NB, Bilezikian JP, Usiskin K, Edwards R, Desai M, Law G, Meininger G. Effects of Canagliflozin on Fracture Risk in Patients With Type 2 Diabetes Mellitus. The Journal of clinical endocrinology and metabolism. 2016 Jan:101(1):157-66. doi: 10.1210/jc.2015-3167. Epub 2015 Nov 18 [PubMed PMID: 26580237]
Fralick M, Kim SC, Schneeweiss S, Kim D, Redelmeier DA, Patorno E. Fracture Risk After Initiation of Use of Canagliflozin: A Cohort Study. Annals of internal medicine. 2019 Feb 5:170(3):155-163. doi: 10.7326/M18-0567. Epub 2019 Jan 1 [PubMed PMID: 30597484]
Tang H, Dai Q, Shi W, Zhai S, Song Y, Han J. SGLT2 inhibitors and risk of cancer in type 2 diabetes: a systematic review and meta-analysis of randomised controlled trials. Diabetologia. 2017 Oct:60(10):1862-1872. doi: 10.1007/s00125-017-4370-8. Epub 2017 Jul 19 [PubMed PMID: 28725912]
Level 1 (high-level) evidenceGarcía M, Arteche-Martinez U, Lertxundi U, Aguirre C. SGLT2 Inhibitors and Bladder Cancer: Analysis of Cases Reported in the European Pharmacovigilance Database. Journal of clinical pharmacology. 2021 Feb:61(2):187-192. doi: 10.1002/jcph.1722. Epub 2020 Aug 21 [PubMed PMID: 32827151]
Level 3 (low-level) evidenceValdivielso JM, Balafa O, Ekart R, Ferro CJ, Mallamaci F, Mark PB, Rossignol P, Sarafidis P, Del Vecchio L, Ortiz A. Hyperkalemia in Chronic Kidney Disease in the New Era of Kidney Protection Therapies. Drugs. 2021 Sep:81(13):1467-1489. doi: 10.1007/s40265-021-01555-5. Epub 2021 Jul 27 [PubMed PMID: 34313978]
Weir MR, Kline I, Xie J, Edwards R, Usiskin K. Effect of canagliflozin on serum electrolytes in patients with type 2 diabetes in relation to estimated glomerular filtration rate (eGFR). Current medical research and opinion. 2014 Sep:30(9):1759-68. doi: 10.1185/03007995.2014.919907. Epub 2014 May 22 [PubMed PMID: 24786834]
Level 1 (high-level) evidenceSzekeres Z, Toth K, Szabados E. The Effects of SGLT2 Inhibitors on Lipid Metabolism. Metabolites. 2021 Feb 1:11(2):. doi: 10.3390/metabo11020087. Epub 2021 Feb 1 [PubMed PMID: 33535652]
Armstrong GP. Empagliflozin-Mediated Lithium Excretion: A Case Study and Clinical Applications. The American journal of case reports. 2020 Jun 10:21():e923311. doi: 10.12659/AJCR.923311. Epub 2020 Jun 10 [PubMed PMID: 32518219]
Level 3 (low-level) evidenceDevineni D, Manitpisitkul P, Vaccaro N, Bernard A, Skee D, Mamidi RN, Tian H, Weiner S, Stieltjes H, Sha S, Rothenberg P. Effect of canagliflozin, a sodium glucose co-transporter 2 inhibitor, on the pharmacokinetics of oral contraceptives, warfarin, and digoxin in healthy participants. International journal of clinical pharmacology and therapeutics. 2015 Jan:53(1):41-53. doi: 10.5414/CP202157. Epub [PubMed PMID: 25345427]
Level 1 (high-level) evidenceScheen AJ. Drug-drug interactions with sodium-glucose cotransporters type 2 (SGLT2) inhibitors, new oral glucose-lowering agents for the management of type 2 diabetes mellitus. Clinical pharmacokinetics. 2014 Apr:53(4):295-304. doi: 10.1007/s40262-013-0128-8. Epub [PubMed PMID: 24420910]
Vallon V. The mechanisms and therapeutic potential of SGLT2 inhibitors in diabetes mellitus. Annual review of medicine. 2015:66():255-70. doi: 10.1146/annurev-med-051013-110046. Epub 2014 Oct 17 [PubMed PMID: 25341005]
Kim WJ, Park CY. 1,5-Anhydroglucitol in diabetes mellitus. Endocrine. 2013 Feb:43(1):33-40. doi: 10.1007/s12020-012-9760-6. Epub 2012 Jul 31 [PubMed PMID: 22847316]
Level 3 (low-level) evidenceDeedwania P. Hypertension, dyslipidemia, and insulin resistance in patients with diabetes mellitus or the cardiometabolic syndrome: benefits of vasodilating β-blockers. Journal of clinical hypertension (Greenwich, Conn.). 2011 Jan:13(1):52-9. doi: 10.1111/j.1751-7176.2010.00386.x. Epub 2010 Nov 8 [PubMed PMID: 21214722]
Scheen AJ. An update on the safety of SGLT2 inhibitors. Expert opinion on drug safety. 2019 Apr:18(4):295-311. doi: 10.1080/14740338.2019.1602116. Epub 2019 Apr 16 [PubMed PMID: 30933547]
Level 3 (low-level) evidenceAmerican Diabetes Association Professional Practice Committee. 6. Glycemic Targets: Standards of Medical Care in Diabetes-2022. Diabetes care. 2022 Jan 1:45(Suppl 1):S83-S96. doi: 10.2337/dc22-S006. Epub [PubMed PMID: 34964868]
Rossing P, Caramori ML, Chan JCN, Heerspink HJL, Hurst C, Khunti K, Liew A, Michos ED, Navaneethan SD, Olowu WA, Sadusky T, Tandon N, Tuttle KR, Wanner C, Wilkens KG, Zoungas S, Craig JC, Tunnicliffe DJ, Tonelli MA, Cheung M, Earley A, de Boer IH. Executive summary of the KDIGO 2022 Clinical Practice Guideline for Diabetes Management in Chronic Kidney Disease: an update based on rapidly emerging new evidence. Kidney international. 2022 Nov:102(5):990-999. doi: 10.1016/j.kint.2022.06.013. Epub [PubMed PMID: 36272755]
Level 1 (high-level) evidenceSchaeffer SE, DesLauriers C, Spiller HA, Aleguas A, Baeza S, Ryan ML. Retrospective review of SGLT2 inhibitor exposures reported to 13 poison centers. Clinical toxicology (Philadelphia, Pa.). 2018 Mar:56(3):204-208. doi: 10.1080/15563650.2017.1357824. Epub 2017 Aug 16 [PubMed PMID: 28812381]
Level 2 (mid-level) evidenceBaig MA, Nogar J. Euglycemia despite a sodium glucose co-transporter 2 inhibitor overdose. World journal of emergency medicine. 2022:13(2):147-148. doi: 10.5847/wjem.j.1920-8642.2022.050. Epub [PubMed PMID: 35237371]
Scheen AJ. Pharmacodynamics, efficacy and safety of sodium-glucose co-transporter type 2 (SGLT2) inhibitors for the treatment of type 2 diabetes mellitus. Drugs. 2015 Jan:75(1):33-59. doi: 10.1007/s40265-014-0337-y. Epub [PubMed PMID: 25488697]
Dhillon S. Dapagliflozin: A Review in Type 2 Diabetes. Drugs. 2019 Jul:79(10):1135-1146. doi: 10.1007/s40265-019-01148-3. Epub [PubMed PMID: 31236801]