Introduction
Corneal endotheliitis, first described by Khodadoust and Attarzadeh in 1982, is characterized by corneal edema, keratic precipitates, mild anterior chamber inflammation, and endothelial dysfunction. Classification is based on the distribution of keratic precipitates and associated stromal and epithelial edema, with 4 recognized patterns: linear, sectoral, disciform, and diffuse.[1]
Several viruses, including herpes simplex virus (HSV), varicella-zoster virus (VZV), and human herpesvirus 7 (HHV-7), have been implicated in corneal endotheliitis. In 2006, Koizumi reported the first case attributed to cytomegalovirus (CMV).[2][3][4] CMV corneal endotheliitis may manifest with linear or coin-shaped keratic precipitates, with or without corneal edema, in immunocompetent individuals.[5][6] Misdiagnosis is common due to clinical overlap with other viral endotheliitides. Timely and accurate recognition is essential, as delayed antiviral therapy can result in irreversible endothelial cell loss and permanent visual impairment.
CMV corneal endotheliitis is an emerging cause of endothelial dysfunction, recurrent anterior uveitis, and secondary glaucoma, with major implications for vision preservation. The corneal pathogenicity of CMV in immunocompetent individuals was first recognized in the early 2000s. Subsequent reports from East Asia, particularly Japan, Taiwan, and Singapore, demonstrated that CMV accounts for a substantial proportion of presumed viral endotheliitis previously attributed to HSV or VZV.
In nonendemic regions, the condition is likely underdiagnosed because of limited aqueous humor polymerase chain reaction (PCR) testing, low clinical suspicion, and overlapping clinical features. Accurate recognition is critical, as management strategies differ from other viral etiologies, and inappropriate therapy can result in irreversible endothelial cell loss, corneal decompensation, and permanent visual impairment.[7]
The pathogenesis of CMV corneal endotheliitis involves direct viral replication within corneal endothelial cells, inducing cytopathic changes and local immune-mediated inflammation. CMV establishes latency in ocular tissues, with reactivation precipitated by immunosenescence, ocular surgery, corticosteroid exposure, or systemic immune dysregulation.
Reactivation results in endothelial barrier dysfunction, stromal hydration, and progressive endothelial loss, amplified by cytotoxic T-cell activity and pro-inflammatory cytokines such as interleukin 6 (IL-6) and tumor necrosis factor α (TNF-α). Trabecular meshwork inflammation may impair aqueous outflow, accounting for the frequent association with ocular hypertension and secondary glaucoma. Surgical procedures, including penetrating keratoplasty, endothelial keratoplasty, and trabeculectomy, as well as long-term corticosteroid use, contribute to a microenvironment that favors viral persistence and reactivation.[8]
Clinically, CMV corneal endotheliitis presents as recurrent, unilateral blurred vision, often with halos, glare, or photophobia. The hallmark slit-lamp finding is localized corneal edema with sharply demarcated “coin-shaped” keratic precipitates, although diffuse or granulomatous forms may occur. Anterior chamber inflammation is generally mild to moderate, and iris atrophy can develop in chronic or recurrent cases. Intraocular pressure (IOP) spikes are common during active episodes, and repeated uncontrolled elevations may cause glaucomatous optic neuropathy. The disease follows a chronic, relapsing course with asymptomatic intervals. Differential diagnosis includes HSV and VZV endotheliitis, Fuchs endothelial corneal dystrophy, idiopathic corneal edema, and postsurgical immune reactions such as graft rejection.[9]
The gold standard for diagnosis is aqueous humor PCR for CMV DNA, with quantitative PCR providing additional value for viral load assessment and treatment monitoring. Adjunctive tools include specular microscopy to evaluate endothelial cell density (ECD) and morphology, anterior segment OCT (AS-OCT) to monitor corneal thickness and edema resolution, and in vivo confocal microscopy (IVCM) to detect endothelial abnormalities and inflammatory infiltration. Limited access to PCR in many settings underscores the importance of clinical suspicion for early recognition. Delayed diagnosis and treatment accelerate endothelial loss and markedly increase the risk of corneal decompensation and keratoplasty.[10]
Management of CMV corneal endotheliitis centers on viral suppression and inflammation control. Topical ganciclovir 0.15% gel is the 1st-line agent, applied 5 times daily for induction and tapered to maintenance dosing to reduce recurrence. Systemic valganciclovir is reserved for severe, bilateral, or recurrent disease, typically initiated at 900 mg twice daily and tapered to 450 to 900 mg once daily. Corticosteroids must be used cautiously, always with antiviral coverage, to control inflammation without promoting viral replication.
Secondary glaucoma is managed with topical hypotensive agents and, in refractory cases, surgical interventions such as trabeculectomy or glaucoma drainage devices. Endothelial keratoplasty is indicated for irreversible decompensation, although recurrence in grafts is common and requires perioperative antiviral prophylaxis. Long-term follow-up is critical, as relapse is frequent without maintenance therapy, and early detection of reactivation is essential.
The prognosis of CMV corneal endotheliitis depends on timely diagnosis and initiation of targeted antiviral therapy. Early recognition and treatment allow many patients to preserve corneal clarity and functional vision, whereas delayed or missed diagnosis often leads to visual loss from corneal decompensation and secondary glaucoma. The chronic, relapsing course underscores the need for patient education, adherence to therapy, and regular monitoring.
Optimal outcomes require an interprofessional approach involving ophthalmologists, optometrists, infectious disease specialists, and primary care providers to ensure accurate diagnosis, appropriate antiviral use, monitoring for drug toxicity, and management of comorbidities that predispose to reactivation. Educational initiatives to improve recognition and standardize management can enhance provider competence, improve outcomes, and reduce the burden of this vision-threatening disease.
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
Human CMV, a ubiquitous lymphotropic herpesvirus, is increasingly recognized as a major cause of corneal endotheliitis.[11][12] Since Koizumi first reported CMV DNA in the aqueous humor of an affected individual, subsequent PCR-based studies have provided further evidence for CMV as an etiological agent.[13][14]
Higher aqueous CMV viral load has been correlated with accelerated corneal endothelial cell loss and elevated IOP.[15][16] An association has also been observed between CMV endotheliitis and prior corneal transplantation.[17][18]
CMV corneal endotheliitis primarily results from reactivation of latent infection within the anterior segment, leading to endothelial inflammation. CMV, a member of the Herpesviridae family (betaherpesvirus subfamily), establishes lifelong latency after primary infection and may reactivate under conditions of reduced systemic immunity, such as postorgan transplantation, HIV infection, and iatrogenic systemic immunosuppression, where impaired immune surveillance facilitates viral reactivation.
Local ocular immune modulation also contributes to the condition's development, particularly after glaucoma surgery, keratoplasty, or prolonged corticosteroid use, which can reduce local antiviral defense. Although CMV infection is widespread in the general population, corneal endotheliitis typically manifests in individuals with disrupted ocular immune privilege, such as those who have undergone keratoplasty, glaucoma surgery, or corticosteroid therapy.
In immunocompetent individuals, CMV endotheliitis is generally localized and reflects anterior chamber viral replication. In immunosuppressed patients, including transplant recipients and people with HIV, this ocular condition may present as part of systemic CMV disease.
Surgical triggers, such as penetrating keratoplasty and endothelial keratoplasty procedures (eg, Descemet stripping automated endothelial keratoplasty or DSAEK; Descemet membrane endothelial keratoplasty or DMEK), may alter endothelial barrier function and increase susceptibility, while coinfections with HSV or VZV can further compromise endothelial integrity and facilitate CMV invasion. Endothelial infection is promoted by CMV immune evasion mechanisms, including downregulation of major histocompatibility complex (MHC) molecules and inhibition of antigen presentation, resulting in chronic low-grade infection with episodic viral replication and inflammation.[19]
Epidemiology
CMV seroprevalence varies by ethnicity, age, and socioeconomic status, ranging from about 50% in the U.S. to nearly 100% in parts of Africa, Asia, and South America.[20] Despite this high global prevalence, CMV corneal endotheliitis has been reported predominantly in Asian populations.[21] CMV accounts for approximately 25% of all corneal endotheliitis cases. Recurrence occurs in about 50% to 60% of patients even with antiviral therapy, and 20% to 60% progress to bullous keratopathy.[22] In East Asia, particularly Japan, Taiwan, China, and South Korea, CMV PCR positivity has been documented in 20% to 25% of presumed viral endotheliitis cases, reflecting both higher disease recognition and widespread use of anterior chamber paracentesis.
The Japan Corneal Endotheliitis Study, the largest series with 106 cases, found CMV endotheliitis most often in middle-aged and older men (mean age 66.9 years, 80.2% men).[23] CMV has also been implicated in endothelial rejection following penetrating keratoplasty in Southeast Asia.[24][25][26] In a retrospective analysis of 1,194 corneal transplants for bullous keratopathy, CMV endotheliitis developed in 1.26% of postoperative cases, typically within the first year.[27] Male sex, prior anterior uveitis, and glaucoma were significant risk factors for post-keratoplasty CMV endotheliitis.
Reports from Southeast Asia, including Singapore, Thailand, Malaysia, and the Philippines, show PCR positivity rates of 15% to 20%, with increasing recognition due to improved molecular diagnostics. In South Asia, particularly India and Sri Lanka, published cases remain limited but are likely underdiagnosed because of frequent misclassification as HSV or VZV endotheliitis.
Most studies on CMV corneal endotheliitis originate from Southeast Asia and the U.S., while data from other regions are largely limited to case reports, making global prevalence difficult to determine. In Europe, CMV accounts for fewer than 10% of viral endotheliitis cases, and diagnosis is usually confined to tertiary centers. In North America, cases are rarely reported and most often occur in immunocompromised patients, suggesting underrecognition in routine clinical practice. Published reports are scarce in Oceania, and little to no epidemiological data exist in Africa, reflecting limited access to molecular testing.
CMV corneal endotheliitis is particularly recognized in Asia as a subset of anterior segment viral infections that primarily involve the corneal endothelium. CMV seroprevalence ranges from 40% to 60% in developed nations to more than 90% in parts of Asia, Africa, and Latin America. Primary CMV infection is usually asymptomatic in immunocompetent hosts, but the virus establishes lifelong latency with potential for reactivation under immune modulation.
In ophthalmology, CMV reactivation within the anterior segment, especially the corneal endothelium, is now identified as a cause of chronic or recurrent keratouveitis. This recognition stems from the increased use of molecular diagnostics such as PCR of aqueous humor samples, which have clarified the viral etiology in cases once labeled idiopathic or presumed herpetic.[28]
Epidemiologically, CMV corneal endotheliitis appears to have a higher prevalence among middle-aged to older men, with studies from Japan, Singapore, Taiwan, and India reporting a male predominance of up to 70%. The typical patient demographic falls within the 40- to 70-year age range, although younger adults may also be affected, particularly those with immunosuppression. The male predilection remains incompletely understood, with hormonal, genetic, and occupational factors postulated. Geographic trends reveal that CMV-related anterior segment disease is reported more frequently in East and Southeast Asia than in Western countries, likely reflecting both higher CMV seroprevalence and greater use of anterior chamber paracentesis in the diagnostic workup for uveitis in these regions.
The risk factors for CMV corneal endotheliitis are heterogeneous. Immunosuppression, whether systemic (eg, postorgan transplantation, HIV/AIDS, long-term corticosteroid therapy) or local (eg, prolonged topical corticosteroid use after keratoplasty or cataract surgery), can precipitate viral reactivation. Individuals with a history of Posner–Schlossman syndrome (PSS) or Fuchs uveitis syndrome may also have unrecognized CMV involvement, as PCR-based studies have demonstrated a substantial proportion of such patients to have documented CMV infection. The overlap between CMV endotheliitis and these anterior uveitic entities suggests that CMV is a more common cause of hypertensive anterior uveitis than previously recognized.[29]
From a disease burden perspective, CMV corneal endotheliitis has significant implications for visual morbidity. The condition is characterized by recurrent or chronic endothelial inflammation, leading to progressive endothelial cell loss. Epidemiological studies indicate that without prompt diagnosis and antiviral therapy, 40% to 50% of affected eyes may develop irreversible corneal decompensation requiring keratoplasty. Recurrence after corneal transplantation is not uncommon if the viral etiology is unrecognized and untreated, contributing to graft failure and increasing the need for repeat surgical interventions.[30]
The condition also poses unique diagnostic challenges. Anterior chamber paracentesis and PCR testing are not routinely performed in many parts of the world, particularly low-resource settings, leading to underdiagnosis and misclassification of CMV cases as HSV or VZV endotheliitis. Epidemiological data from such regions likely underestimate the true burden of disease. Wider adoption of multiplex PCR assays in tertiary eye care centers over the last decade has revealed that CMV is an underrecognized but important cause of viral anterior segment disease.[31]
In terms of public health relevance, CMV corneal endotheliitis is a potentially preventable cause of corneal blindness if identified and managed early. Epidemiological evidence supports targeted screening in high-risk groups, including patients with recurrent hypertensive anterior uveitis, unexplained corneal edema with keratic precipitates, or graft failure following keratoplasty. Longitudinal cohort studies from Asia indicate that early initiation of systemic or topical ganciclovir therapy reduces recurrence and slows endothelial cell loss, thereby altering the disease course.
Pathophysiology
The pathophysiology of CMV endotheliitis is not fully elucidated. Suzuki and Ohashi hypothesized that the disease may be related to anterior chamber-associated immune deviation (ACAID). Latent CMV infection in the trabecular meshwork and ciliary body can become intermittently reactivated, releasing small amounts of virus into the anterior chamber. Repeated viral release may promote ACAID against viral antigens, a mechanism supported by studies inducing ACAID against HSV in a rabbit model of herpetic corneal endotheliitis.[32]
CMV reactivates within the anterior segment, primarily targeting corneal endothelial cells. Following primary infection, typically acquired in early life through mucosal contact, the virus establishes lifelong latency in vascular endothelial cells, monocytes, and ocular tissues. The cornea’s immune-privileged status permits CMV to remain dormant for years without eliciting a significant immune response.
Reactivation occurs when immune surveillance is compromised, as mentioned. Afterward, the virus infects endothelial cells via direct cell-to-cell spread or aqueous humor seeding, binding host receptors, including integrins and epidermal growth factor receptor, through viral glycoproteins gB and gH/gL. Viral replication hijacks host DNA polymerase, producing viral particles and inducing cytopathic changes, including endothelial cell enlargement, dense intranuclear “owl’s-eye” inclusions, and perinuclear halos. These alterations impair endothelial pump and barrier functions, leading to stromal and epithelial edema.[33]
The host immune response amplifies the pathophysiologic cascade. Infected endothelial cells release proinflammatory cytokines (IL-1β, IL-6, TNF-α) and chemokines that recruit natural killer cells and CMV-specific CD8+ T lymphocytes. These immune cells contribute to bystander endothelial destruction, compounding direct viral cytotoxicity. Inflammation may extend to the trabecular meshwork, causing trabeculitis, reduced aqueous outflow, and secondary ocular hypertension or glaucomatous optic nerve damage. Disruption of the blood–aqueous barrier can allow leukocytes and fibrin into the anterior chamber. The combination of viral replication, cytopathic effects, and immune-mediated injury results in an often recurrent disease course.
Clinically, CMV corneal endotheliitis is distinguished from HSV and VZV endotheliitis by chronic, low-grade inflammation and characteristic “coin-shaped” or linear keratic precipitates. Lesions may be localized or diffuse, with minimal stromal infiltrate. IOP elevation is common due to trabecular involvement and may persist between episodes. Each reactivation causes cumulative endothelial cell loss. Persistent edema and, in advanced cases, bullous keratopathy ensue when the residual population falls below the critical threshold for corneal deturgescence.[34]
Thus, the long-term impact of CMV corneal endotheliitis arises from persistent viral replication, immune-mediated injury, and secondary glaucomatous damage. Early recognition and accurate diagnosis, including aqueous humor PCR, are essential to guide sustained antiviral therapy and preserve endothelial function. Prompt intervention may reduce the risk of recurrence, structural damage, and vision-threatening sequelae.[35]
Histopathology
Histopathological studies of cadaveric eyes demonstrate that CMV inclusion bodies localize predominantly to the iris, ciliary body, and the endothelial cells lining the Schlemm canal.[36] However, detailed histopathology of corneal endothelium affected by CMV remains limited.
Histopathological examination of corneal tissue reveals cytomegalic endothelial cells with large nuclei containing dense intranuclear “owl’s-eye” inclusions surrounded by a perinuclear halo. These inclusions, representing viral nucleoprotein aggregates, are considered pathognomonic but require confirmation via immunohistochemistry (IHC) or PCR. The cytoplasm may appear swollen and granular due to viral replication complexes.
During the early active phase, focal attenuation or denudation of the endothelial layer exposes the posterior stroma to aqueous humor. The underlying stroma may show mild-to-moderate edema with widened collagen lamellae. Inflammatory infiltration, primarily lymphocytes and macrophages with occasional neutrophils, is seen along the Descemet membrane and in the posterior stroma. Chronic or recurrent episodes may cause stromal thickening, hyaline degeneration, and deposition of extracellular matrix material.
Endothelial loss and viral infection can induce focal irregular thickening, duplication, or nodular excrescences in the Descemet membrane, corresponding clinically to keratic precipitates. These keratic precipitates often appear unusually large, coin-shaped, and arranged in linear or circular patterns, reflecting clusters of virally infected endothelial cells. Trabecular meshwork involvement manifests as trabeculitis, with endothelial swelling, inflammatory infiltration of trabecular beams, and extracellular debris in the Schlemm canal, underlying secondary ocular hypertension or glaucoma. The iris may exhibit low-grade stromal inflammation with occasional pigment dispersion.[37]
IHC using monoclonal antibodies against CMV immediate-early or late antigens highlights infected endothelial cells, particularly when inclusions are sparse. Electron microscopy confirms enlarged endothelial cells containing intranuclear inclusions with central dense cores and perinuclear halos, smaller cytoplasmic inclusions, and viral particles (~150-200 nm) budding from the nuclear membrane. Mitochondrial swelling, endoplasmic reticulum dilatation, and loss of cytoplasmic microvilli may also be observed.[38]
Long-standing disease reflects cumulative viral and immune-mediated damage. The endothelium may be markedly depleted, with surviving cells exhibiting pleomorphism and abnormal orientation. Posterior stroma may show chronic inflammatory aggregates, fibroblastic proliferation, and neovascularization. The Descemet membrane may be thickened and irregular, with prior breaks or scarring. These changes correspond clinically to corneal decompensation and, in advanced stages, bullous keratopathy.[39]
Corneal grafts from patients undergoing endothelial keratoplasty for CMV-induced decompensation frequently reveal recurrent CMV infection in the donor endothelium, with inclusion bodies and inflammatory features, especially in patients on postoperative corticosteroids. This observation emphasizes CMV’s persistence in the anterior segment and potential to reinfect transplanted tissue.[40]
Overall, histopathology demonstrates a triad of direct viral cytopathic effects, immune-mediated posterior corneal injury, and secondary stromal or Descemet membrane changes. Correlation with clinical findings and molecular diagnostics provides critical insight into disease mechanisms and informs medical and surgical management.
Toxicokinetics
Toxicokinetics in CMV corneal endotheliitis pertains to the pharmacokinetic behavior and ocular disposition of antiviral agents, particularly ganciclovir, valganciclovir, and foscarnet. These drugs’ absorption, distribution, metabolism, and elimination determine both therapeutic efficacy and potential toxicity to corneal and intraocular structures.
Topical ganciclovir gel (0.15%) attains high concentrations in the tear film and corneal epithelium, with measurable penetration into the stroma and aqueous humor. The drug is eliminated via aqueous outflow and systemic metabolism, and frequent dosing is required due to minimal systemic absorption. Chronic use may lead to local irritation and punctate keratopathy. Intracameral or intravitreal injections bypass epithelial and stromal barriers, rapidly achieving therapeutic levels in the corneal endothelium, the primary site of CMV replication. These routes achieve direct high levels at the endothelial surface and provide rapid therapeutic concentrations. High osmolarity formulations should be avoided. Repeated high-dose intracameral injections may induce endothelial stress or cell loss due to localized toxicity or osmolarity effects.[41]
Oral valganciclovir, a prodrug converted to ganciclovir in the liver and gut, distributes systemically and to ocular tissues via aqueous humor turnover. The drug achieves systemic distribution with aqueous humor penetration, reaching the corneal endothelium via the circulation. Valganciclovir undergoes renal excretion as ganciclovir, has good bioavailability, and requires dose adjustment in renal impairment. Systemic exposure carries risks of myelosuppression, renal impairment, and gastrointestinal adverse effects, with cumulative toxicity increasing during prolonged therapy.
Foscarnet is administered via the intraocular (intracameral or intravitreal) route. The drug inhibits viral DNA polymerase but can directly damage endothelial cells at high concentrations, necessitating careful dosing and monitoring. Drug elimination from ocular tissues occurs primarily via aqueous outflow and systemic clearance, with renal excretion contributing to systemic elimination. Impaired renal function prolongs systemic half-life and amplifies toxicity risk. Foscarnet exhibits excellent local penetration, has a narrow therapeutic index, and requires dose adjustment based on renal status. Potential toxicity includes endothelial cell injury, corneal edema, and local inflammation.[42]
Cidofovir is rarely used in CMV corneal endotheliitis but may be administered intravitreally. This agent exhibits a prolonged intraocular half-life and binds extensively to ocular tissues. Clearance from the eye is slow, with systemic elimination via renal excretion. Although long-acting, cidofovir carries a high risk of ocular adverse effects, including dose-dependent toxicity, anterior uveitis, and hypotony.
Toxicokinetic management in CMV corneal endotheliitis requires balancing sustained therapeutic intraocular drug levels with the risk of local endothelial injury and systemic adverse effects, necessitating individualized dosing and close monitoring of ocular and systemic parameters.[43]
Ocular Distribution and Safety Profiles of Antiviral Agents in Cytomegalovirus Corneal Endotheliitis
Antiviral administration routes in CMV corneal endotheliitis exhibit distinct toxicokinetic profiles that influence onset, peak ocular concentrations, duration of effect, and safety. Topical ganciclovir 0.15% gel achieves therapeutic concentrations in the tear film and corneal epithelium within hours, with moderate stromal and aqueous penetration. The drug's effect is short-lived, necessitating frequent dosing. The route is minimally invasive and has low systemic absorption, but penetration to deep stroma and endothelium is limited.
Intracameral administration of ganciclovir or foscarnet provides immediate high concentrations in the corneal endothelium and aqueous humor, with short-to-moderate duration (days). Advantages include direct delivery to the target site and bypass of corneal barriers, whereas risks include endothelial toxicity if overdosed, procedural complications, and limited duration of effect.
Intravitreal delivery of ganciclovir, foscarnet, or cidofovir achieves peak concentrations in the vitreous and aqueous within hours, with indirect endothelial exposure and moderate-to-long duration (days to weeks). This route maintains sustained intraocular drug levels and is particularly useful for posterior segment CMV, but carries risks of intraocular inflammation, retinal toxicity, hypotony, and procedural complications.
Systemic administration, such as in oral valganciclovir or intravenous ganciclovir, reaches moderate concentrations in the anterior segment via aqueous humor turnover within 1 to 2 days and maintains continuous drug levels with maintenance dosing. This route effectively treats bilateral or extraocular CMV and systemic infection, but exhibits slower corneal penetration and carries systemic toxicity risks, including myelosuppression and renal impairment, requiring renal dose adjustment.
History and Physical
A history of glaucoma or ocular hypertension is particularly relevant, as CMV endotheliitis frequently coexists with trabeculitis, causing episodic IOP elevations that may be resistant to conventional therapy. Patients often experience IOP spikes exceeding 30 mm Hg, which may contribute to cumulative optic nerve damage if unrecognized. Prior episodes of anterior uveitis, especially those labeled as “viral” or “hypertensive,” should prompt evaluation for possible missed CMV infection. Travel to endemic regions or exposure to stagnant water may be relevant in rare cases of primary ocular CMV acquisition.[44]
Unlike CMV retinitis, which primarily affects immunocompromised patients, CMV endotheliitis typically occurs in immunocompetent individuals. Patients commonly report unilateral photophobia and visual disturbances. According to the Japan Endotheliitis Study, nearly half had a prior history of anterior uveitis, Posner-Schlossman syndrome, or secondary glaucoma or ocular hypertension, and over half had undergone corneal transplantation, glaucoma surgery, or cataract surgery.[45] Recurrent or chronic unilateral blurred vision, photophobia, and mild redness are particularly suggestive of relapsing viral endotheliitis rather than bacterial keratitis.
A history of systemic or local immunosuppression is highly relevant, as CMV endotheliitis is more common in patients with compromised immune function. Risk factors include organ transplantation, systemic corticosteroid use, immunomodulatory therapy, hematologic malignancies, and HIV infection. Nevertheless, CMV endotheliitis can also occur in immunocompetent individuals, particularly in East Asian populations. Prior ocular surgeries—such as penetrating keratoplasty, DSAEK, or DMEK—are significant, particularly in patients with long-term topical steroid use, as CMV reactivation may occur from infection of the donor endothelium or reactivation triggered by immune suppression.[46]
A meticulous history and physical examination are essential, given CMV endotheliitis’s variable presentation and frequent misdiagnosis as herpetic or idiopathic anterior uveitis. History-taking should document symptom onset, duration, and progression. Patients typically present with recurrent or chronic unilateral blurred vision, ocular discomfort, photophobia, and mild redness. Pain is generally less severe than in acute bacterial keratitis but may be more pronounced than in early Fuchs endothelial corneal dystrophy. Episodes often show intermittent symptom resolution followed by recurrence, sometimes with increasing severity—a pattern that should raise suspicion for viral etiology, particularly in patients with prior negative fungal or bacterial cultures.[47]
On physical examination, slit-lamp biomicroscopy reveals characteristic but variable findings. The hallmark sign is localized or diffuse corneal stromal and epithelial edema overlying endothelial lesions, often visible as keratic precipitates on the endothelium. These lesions are often large, coin-shaped, or nummular, and may appear in clusters, rings, or occasionally linear arrangements along the inferior endothelium. The lesions are more sharply demarcated and elevated than the diffuse areas of involvement seen in herpetic uveitis or granulomatous inflammation (see Image. Cytomegalovirus Endotheliitis on Slit-Lamp Examination).
Disc-shaped or coin-shaped endothelial lesions are observed in approximately 70.6% of eyes, whereas linear patterns are seen in 8.3%, according to the Japan Endotheliitis Study.[48] IVCM demonstrates highly reflective bodies within endothelial cells, corresponding to the classic “owl’s eye” appearance, which may also be visualized with specular microscopy.
Anterior chamber inflammation is usually mild to moderate, typically without hypopyon, with fine to medium-sized inflammatory cells. Iris atrophy is uncommon, helping differentiate CMV endotheliitis from HSV or VZV uveitis, where sectoral atrophy is frequent. IOP measurement is essential, as trabeculitis may cause episodic spikes exceeding 30 mm Hg, potentially leading to optic nerve damage. Gonioscopy, when feasible, can reveal fine inflammatory precipitates on the trabecular meshwork.
Specular or confocal microscopy often demonstrates localized or generalized endothelial cell loss, pleomorphism, polymegathism, and hyperreflective deposits corresponding to endothelial lesions. Corneal edema is typically sectoral or central in early stages, becoming diffuse and persistent as the disease progresses, potentially leading to irreversible endothelial decompensation. In pseudophakic eyes, edema may be misattributed to pseudophakic bullous keratopathy unless endothelial lesion distribution is carefully assessed.[49]
Fundus examination is generally unremarkable unless concurrent CMV retinitis is present in immunocompromised patients. Optical coherence tomography (OCT) of the macula may be indicated to exclude cystoid macular edema in cases of unexplained visual loss. Systemic examination for signs of immunosuppression, including lymphadenopathy, skin lesions, and hepatosplenomegaly, may be warranted, though systemic involvement is rare in isolated ocular CMV endotheliitis.
Diagnosis is supported by targeted investigations, most notably aqueous humor PCR for CMV DNA, which offers high specificity. Characteristic historical and physical findings generally guide confirmatory testing. Early clinical suspicion, based on recurrent, often unilateral hypertensive anterior uveitis, coin-shaped keratic precipitates, and sectoral corneal edema, is critical to prevent irreversible endothelial damage. Careful history-taking, slit-lamp evaluation, and IOP assessment remain the cornerstone for identifying patients who require timely molecular testing and initiation of targeted antiviral therapy.
Evaluation
Corneal endotheliitis is primarily a clinical diagnosis. The presence of coin-shaped keratic precipitates with overlying corneal edema has a high positive predictive value (90.9%) for CMV endotheliitis.[50] Definitive diagnosis is established via qualitative or quantitative PCR for CMV DNA in aqueous humor. Multiplex PCR for CMV, HSV, and VZV may aid in guiding therapy, and repeat sampling may be considered if clinical suspicion remains high despite an initial negative result.[51] Viral load has been shown to correlate with IOP elevation, recurrent inflammation, presence of coin-shaped lesions, and endothelial cell loss.
According to the Japan Endotheliitis Study, typical CMV endotheliitis is characterized by corneal endotheliitis presenting with a coin-shaped or linear lesion, detection of CMV DNA by PCR in the aqueous humor, and the absence of HSV and VZV DNA. Atypical CMV endotheliitis involves localized corneal edema with keratic precipitates accompanied by at least 2 of the following:
- Recurrent or chronic anterior uveitis
- Ocular hypertension or secondary glaucoma
- Corneal endothelial cell loss in the presence of PCR-confirmed CMV DNA and absence of HSV and VZV DNA
CMV-specific antibodies may be measured using enzyme-linked immunoassay (ELISA). The Goldmann-Witmer coefficient (GWc), calculated as the aqueous-serum antibody ratio adjusted for total globulins, suggests intraocular antibody production when exceeding 3.[52] Combining PCR and antibody testing may improve diagnostic yield, as PCR positivity is typically limited to active disease, whereas antibody detection may persist throughout the disease course.[53]
In addition, noninvasive imaging, including AS-OCT and IVCM, can assist in the diagnosis and monitoring of disease progression. Using AS-OCT, Kobayashi et al demonstrated a sawtooth appearance of protruding structures and high reflectivity of the posterior cornea in CMV endotheliitis, which decreased over the course of antiviral therapy. Hashida et al reported distinct AS-OCT patterns depending on the causative virus.[54][55]
A coin-shaped lesion in CMV endotheliitis presents with quadrilateral or elliptical morphology, compared to HSV endotheliitis, which shows small protrusions with low reflectivity, and VZV endotheliitis, which exhibits larger pigmented keratic precipitates. Owl’s eye morphological features detected with IVCM, considered pathognomonic for CMV endotheliitis, may serve as an adjunct diagnostic clue. Rarely, owl’s eye morphology has been reported in HSV keratitis and in longstanding corneal grafts.[56][57]
Evaluation of CMV corneal endotheliitis requires a systematic approach integrating clinical examination, targeted laboratory testing, advanced ocular imaging, and adherence to national and international guidelines for viral anterior segment infections.[58]
Initial assessment begins with comprehensive slit-lamp biomicroscopy to document keratic precipitate morphology, corneal edema distribution, and anterior chamber inflammation. Detailed IOP measurement is essential to identify hypertensive uveitis, a hallmark of CMV-associated trabeculitis. Goldmann or rebound tonometry may be used, and gonioscopy performed when corneal clarity allows to evaluate angle structures and detect inflammatory precipitates on the trabecular meshwork.[59]
At triage, documentation should include onset, recurrence, prior steroid use, keratoplasty history, and any IOP spikes, as recurrent unilateral hypertensive uveitis raises suspicion for CMV.
Laboratory diagnosis relies on PCR-based detection of CMV DNA in aqueous humor, which remains the gold standard with high sensitivity and specificity. Quantitative real-time PCR can assess viral load and monitor treatment response. Early sampling is recommended before prolonged corticosteroid therapy to prevent diagnostic masking, particularly in atypical or recurrent cases.
In immunocompromised patients, systemic evaluation may include CMV serology (immunoglobulins M and G), complete blood count (CBC), liver function tests, and plasma CMV PCR to detect systemic reactivation. Guidelines from the Japanese Ocular Inflammation Society and the American Academy of Ophthalmology (AAO) recommend early aqueous sampling for PCR in suspected viral hypertensive anterior uveitis.[60]
Imaging studies support diagnosis and follow-up. Specular microscopy quantifies ECD and reveals pleomorphism or polymegathism, while IVCM can visualize hyperreflective endothelial deposits corresponding to keratic precipitates. AS-OCT documents corneal thickness changes and localizes edema, facilitating longitudinal monitoring.[61] These adjunctive tests establish a baseline for follow-up and help track progression or response to therapy.
International practice guidelines emphasize integrating laboratory confirmation with imaging findings before initiating long-term antiviral therapy. The Asia-Pacific Academy of Ophthalmology (APAO) and the Japanese Ocular Inflammation Society recommend that all atypical anterior uveitis cases with elevated IOP, sectoral corneal edema, and coin-shaped keratic precipitates undergo aqueous humor PCR testing for CMV, HSV, and VZV. Repeat testing is advised in recurrent or treatment-resistant cases to ensure diagnostic accuracy.[62] Interim management while awaiting PCR may include initiation of topical ganciclovir (0.15%) and aggressive IOP control, while avoiding high-dose steroids until CMV is confirmed. Escalation to intracameral or intravitreal antivirals should be considered for persistent edema, high viral load, or steroid-dependency.
Evaluation requires correlating characteristic slit-lamp findings and IOP measurements with confirmatory aqueous humor PCR, supported by endothelial imaging and systemic assessment when indicated. Adherence to guideline-based protocols facilitates timely diagnosis, prevents irreversible endothelial decompensation, and optimizes visual outcomes.[63] Monitoring should include visual acuity, IOP, slit-lamp documentation of keratic precipitates and edema, and periodic ECD measurements, with repeat PCR testing if relapse occurs.
Treatment / Management
Optimal management of CMV corneal endotheliitis necessitates targeted antiviral therapy alongside meticulous IOP control, with individualized, long-term follow-up due to the high risk of recurrent episodes. The critical aspects of treatment are explained below.
Antiviral Therapy
First-line therapy consists of topical ganciclovir 0.15% gel, administered 5 times daily and tapered to 2 to 3 times daily for maintenance. Topical therapy effectively reduces viral replication, achieves good corneal penetration, and is relatively safe for long-term use. Compounded topical valganciclovir may be considered when ganciclovir is unavailable. Management addresses both the infectious and inflammatory components, with antivirals, including intravenous or oral ganciclovir and valganciclovir, acting through inhibition of CMV DNA polymerase to halt viral replication. Acyclovir and valacyclovir are ineffective, as CMV lacks viral thymidine kinase.
Systemic therapy with oral valganciclovir is indicated for recurrent, bilateral, or refractory disease. Induction dosing is 900 mg twice daily for 2 to 3 weeks, followed by maintenance at 450 to 900 mg once daily for 3 to 6 months. Blood counts and renal function should be monitored regularly due to risks of neutropenia, anemia, and renal impairment.[64]
Intracameral or intravitreal ganciclovir (2 mg/0.1 mL) may be employed in resistant or recurrent cases, achieving high local drug concentrations while minimizing systemic toxicity. Repeated injections may be administered if relapses occur.
Corticosteroids
Anti-inflammatory therapy is primarily achieved with low-potency topical corticosteroids, such as loteprednol and fluorometholone, administered in combination with antivirals to mitigate inflammation. Corticosteroids should never be used as monotherapy due to the risk of viral reactivation and must be tapered gradually to prevent rebound inflammation. In the Japan Endotheliitis Study, 104 of 109 eyes received either systemic anti-CMV therapy (ganciclovir or valganciclovir), topical ganciclovir, or a combination of both.[65][66] Combined systemic and topical treatment was more effective in reducing corneal edema and keratic precipitates, although no significant difference was observed compared to systemic or topical therapy alone.(B3)
A review by Wong et al summarized 12 case series and reports published between January 2000 and January 2020, highlighting that suppression of inflammation in CMV endotheliitis requires caution.[67][68] In a case report and 3 case series encompassing 32 eyes, the majority of patients achieved favorable outcomes with topical ganciclovir combined with topical prednisolone. Successful treatment with intravitreal ganciclovir injections has also been documented.[69][70](B3)
A multicenter phase 3 trial in Japan evaluating topical 0.15% ganciclovir gel (ROH-101) demonstrated that 63.6% of patients achieved a substantial reduction in aqueous CMV DNA after 12 weeks of therapy. Clinical improvement was observed in all treated eyes, with stable ECD and no progression to corneal decompensation. The regimen was well tolerated, with only mild adverse events reported in a few cases, further supporting the efficacy and safety of topical antiviral therapy in CMV endotheliitis.[71]
Intraocular Pressure Control
CMV endotheliitis frequently presents with secondary glaucoma, necessitating careful IOP management. First-line topical therapy includes β-blockers, carbonic anhydrase inhibitors, and α2 agonists. Prostaglandin analogs should be avoided due to the potential exacerbation of inflammation. Refractory cases may warrant surgical interventions, such as trabeculectomy or glaucoma drainage device implantation, which must be combined with antiviral therapy to prevent recurrence.[72]
Consensus on an optimal anti-CMV regimen for CMV endotheliitis is lacking. However, commonly reported protocols include systemic and topical antivirals. Oral valganciclovir is typically administered as induction therapy at 900 mg twice daily for 21 days, followed by maintenance at 900 mg once daily. Intravenous ganciclovir at 5 mg/kg twice daily for 21 days, then 5 mg/kg daily, may be used in cases refractory to oral therapy. Topical ganciclovir 0.15% is applied every 2 hours to 6 times daily initially, then tapered to 3 to 4 times daily, and may be used alone in mild disease. Systemic maintenance is generally discontinued 3 months after clinical resolution, while long-term topical ganciclovir is continued for prophylaxis.
Surgical Considerations
DSAEK, DMEK, or penetrating keratoplasty may be required in cases with irreversible endothelial damage. The risk of CMV recurrence post-keratoplasty is high, making long-term prophylaxis with topical ganciclovir essential to prevent graft failure. Management of CMV endotheliitis in the setting of corneal transplantation involves prompt diagnosis via aqueous PCR and initiation of anti-CMV therapy, typically oral valganciclovir with or without topical ganciclovir. Systemic or topical corticosteroids should be tapered as safely permissible. Therapeutic oral valganciclovir is usually administered for 6 weeks to 3 months, followed by long-term maintenance with topical ganciclovir 0.15%.[73] Further studies are needed to determine optimal treatment regimens and maintenance duration.(B3)
Prophylactic strategies after keratoplasty in patients with a history of CMV infection have included topical ganciclovir alone or combined with short-term oral valganciclovir. Some cases of treatment failure responded better to the addition of systemic therapy.[74][75][76][77] Koizumi et al demonstrated that topical ganciclovir 0.15% achieves therapeutic aqueous concentrations, supporting its use as a prophylactic option, particularly to avoid systemic adverse effects such as myelosuppression and pancytopenia.[78]
Aqueous sampling before or during corneal transplantation may be advantageous in patients with prior CMV infection or unexplained endothelial failure, especially in regions with high CMV seroprevalence.
Monitoring and Follow-Up
Monitoring and follow-up of CMV endotheliitis involve regular slit-lamp examinations to assess keratic precipitates and corneal edema. Serial anterior chamber PCR, when available, can help confirm viral suppression. Long-term prophylactic topical ganciclovir is frequently required to prevent recurrence, and patients receiving systemic valganciclovir should be monitored for potential drug-related toxicities.
Summary of Treatment Protocol
Management of CMV endotheliitis is tailored to disease severity and clinical context. In mild-to-moderate cases, first-line therapy consists of topical ganciclovir 0.15% gel administered 5 times daily with a gradual taper, combined with low-potency topical corticosteroids and topical anti-glaucoma medications as needed. Maintenance topical ganciclovir 2 to 3 times daily is often continued to prevent recurrence.
Severe or recurrent disease may require systemic therapy with oral valganciclovir (induction 900 mg twice daily, followed by daily maintenance), along with low-potency steroids, IOP-lowering drops, and, in selected cases, intracameral ganciclovir. Post-keratoplasty management emphasizes long-term topical ganciclovir with minimal steroid cover, vigilant IOP control, and lifelong prophylaxis to mitigate the high risk of recurrence. Across all stages, therapy must balance viral suppression, inflammation control, and glaucoma management.
Differential Diagnosis
CMV corneal endotheliitis should be suspected in patients presenting with unilateral coin-shaped keratic precipitates, corneal edema, elevated IOP, and mild anterior chamber inflammation. Similar endothelial dysfunction may also result from other herpesviruses, including HSV, VZV, mumps, and Epstein-Barr virus (EBV). HSV endotheliitis typically manifests with linear or diffuse small to medium-sized pigmented keratic precipitates, VZV endotheliitis presents with diffuse medium-to-large mutton-fat keratic precipitates, and EBV may cause diffuse corneal edema with multiple fine keratic precipitates. PCR analysis for HSV, VZV, and CMV remains essential for accurate diagnosis.
Inflammatory glaucomatous conditions previously considered idiopathic, such as hypertensive anterior uveitis, Fuchs uveitis syndrome, and PSS, have been increasingly associated with viral etiologies, including CMV infection.[79][80]
Differentiating CMV endotheliitis from corneal graft rejection after transplantation can be challenging, particularly when PCR results are negative. Interventions for graft rejection may trigger latent CMV reactivation, exacerbate CMV endotheliitis, and increase the risk of graft failure. Clinical indicators favoring CMV endotheliitis over allograft rejection include elevated IOP, characteristic keratic precipitates, steroid-refractory graft dysfunction, and sudden unexplained endothelial cell loss.
Diagnostic Clues Distinguishing Cytomegalovirus Endotheliitis From Mimics
Clinicians face challenges in identifying CMV endotheliitis due to overlapping symptoms with other ocular conditions. The key distinguishing features and relevant diagnostic tests that can help providers differentiate CMV endotheliitis from alternative etiologies are explained below.
HSV endotheliitis typically presents with corneal hypoesthesia, dendritic or geographic epithelial ulcers, and stromal scarring, confirmed by PCR and corneal sensation testing. VZV endotheliitis often manifests with a dermatomal rash and sectoral iris atrophy, with PCR confirming viral identity, whereas Fuchs heterochromic iridocyclitis shows chronic low-grade inflammation, stellate keratic precipitates, and heterochromia without IOP spikes or detectable viral DNA.
PSS features recurrent mild anterior chamber inflammation with elevated IOP, usually lacking persistent keratic precipitates and often yielding no viral DNA on PCR analysis.. Chronic nongranulomatous anterior uveitis presents as persistent mild inflammation without viral etiology, while granulomatous anterior uveitis from sarcoid, tuberculosis, or syphilis is characterized by large mutton-fat keratic precipitates and systemic markers. Endothelial graft rejection after keratoplasty is distinguished by a Khodadoust line and immune-mediated signs, whereas pseudophakic bullous keratopathy produces corneal edema without inflammatory deposits.
Acute angle-closure glaucoma shows a mid-dilated pupil, corneal edema, and a shallow anterior chamber with acute pain but no keratic precipitates. Toxic anterior segment syndrome manifests as acute sterile postoperative inflammation, whereas bacterial and fungal keratitis with endothelial involvement present with stromal infiltrates and hypopyon or feathery plaques, respectively, confirmed by culture. Acanthamoeba keratitis is associated with severe pain and ring-shaped infiltrates, detected by confocal microscopy or PCR, and autoimmune endotheliitis arises in systemic autoimmune disease with positive serology but no viral DNA.
Intraocular lymphoma masquerades as chronic, treatment-refractory inflammation, confirmed via cytology and interleukin profiling. Recurrent graft infection occurs post-keratoplasty with localized edema and positive microbiology, while idiopathic hypertensive anterior uveitis shows IOP spikes and mild anterior chamber inflammation with negative PCR results. Rubella-associated uveitis resembles Fuchs heterochromic iridocyclitis and is diagnosed by serology. Immune-mediated trabeculitis produces localized trabecular inflammation without viral DNA, and phacolytic glaucoma is characterized by hypermature cataract, anterior chamber flare, and milky lens material.
Pertinent Studies and Ongoing Trials
Evidence-Based Management of Cytomegalovirus Corneal Endotheliitis
The current evidence base for CMV corneal endotheliitis is primarily derived from observational cohorts, case series, and prospective nonrandomized studies, with large randomized controlled trials (RCTs) still absent. Nevertheless, the literature consistently highlights 3 key points:
- Aqueous-humor PCR-guided diagnosis enhances diagnostic accuracy and reduces inappropriate corticosteroid exposure.
- Ganciclovir-based therapy, administered topically or intraocularly with the addition of oral valganciclovir when indicated, effectively reduces corneal edema, keratic precipitates, and IOP spikes.
- Relapse prevention requires adequate duration of therapy, maintenance treatment, and close virologic monitoring.
Topical 0.15% ganciclovir gel has been reported in multiple prospective and retrospective series to achieve a rapid reduction in endothelial inflammation and corneal edema in PCR-confirmed cases, with concomitant IOP control. Clinical response typically occurs within days to weeks, and incomplete responders frequently benefit from escalation to intracameral or intravitreal ganciclovir or foscarnet. Intracameral administration has been employed as rescue or induction therapy in severe, steroid-dependent, or recurrent cases, providing high anterior chamber drug levels and measurable clinical improvement. Safety depends critically on careful dosing and osmolarity to preserve endothelial integrity.
Systemic antiviral therapy with oral valganciclovir, administered as induction followed by maintenance, is supported by prospective cohorts for recalcitrant, bilateral, or frequently relapsing disease, as well as in cases with concomitant posterior segment CMV involvement. Studies consistently demonstrate reduced relapse frequency while therapy is ongoing, with recurrences clustering after discontinuation, underscoring the importance of time-limited maintenance guided by clinical response and PCR monitoring. Hematologic and renal function monitoring is essential to ensure patient safety.[81]
Evidence from multiple cohorts indicates that effective antiviral therapy not only reduces endothelial inflammation but also correlates with improved IOP control, resulting in fewer hypertensive flares and a reduced need for multidrug glaucoma regimens. Despite this overall benefit, a subset of patients progresses to secondary glaucoma, underscoring the importance of early initiation of antiviral therapy combined with judicious corticosteroid use to optimize optic nerve outcomes.[82]
In eyes requiring endothelial keratoplasty due to corneal decompensation, studies demonstrate superior graft survival when antiviral coverage is administered both preoperatively and postoperatively. Aqueous or tissue PCR testing to confirm CMV presence guides the duration of therapy and reduces the risk of recurrence, which is well-documented in the absence of antiviral prophylaxis.[83]
Diagnostic studies further reinforce the value of PCR confirmation, showing that patients managed with PCR-guided therapy experience fewer treatment failures and achieve faster disease control compared with clinically presumed cases. Repeating PCR testing during a flare enhances diagnostic yield and permits monitoring of viral load (cycle threshold values) alongside clinical response, allowing for more precise adjustments to therapy.[84]
Steroid stewardship remains critical, as observational series consistently highlight that early, high-dose corticosteroid use without concurrent antiviral coverage increases the risk of disease relapse. When corticosteroids are indicated, they should be initiated only after antiviral therapy has been established and subsequently tapered to the lowest effective dose to mitigate viral reactivation while controlling immune-mediated inflammation.[85]
Evidence from multiple studies indicates that the safety profile of CMV antiviral therapy is generally predictable and consistent with known pharmacokinetics. Topical ganciclovir is well-tolerated, whereas intraocular administration of ganciclovir or foscarnet requires careful attention to endothelium-safe dosing and osmolarity. Oral valganciclovir necessitates CBC and renal function monitoring, with dose adjustments recommended for patients with impaired renal function to avoid hematologic or nephrotoxic complications.[86]
Recent and ongoing investigations, predominantly single-center studies, are exploring optimized dosing schedules for intracameral ganciclovir and foscarnet, comparative treatment algorithms of topical-only versus combined topical and systemic induction, PCR-guided cessation rules based on viral load thresholds, and post-keratoplasty antiviral prophylaxis to reduce recurrence. Imaging biomarkers, including specular and confocal microscopy and AS-OCT, are being evaluated as surrogate outcomes for endothelial recovery and early detection of subclinical disease. Despite these advances, large multicenter RCTs remain an unmet need due to the relative rarity of CMV corneal endotheliitis and variability in diagnosis.[87]
Although high-level randomized evidence is limited, convergent findings across prospective cohorts and consistent case series support a management strategy incorporating PCR-confirmed diagnosis, ganciclovir-based therapy (topical with or without intraocular administration, adding oral valganciclovir for severe, recurrent, or bilateral disease), judicious corticosteroid use, and structured monitoring of IOP, ECD, and, where available, PCR cycle threshold values. This approach has been associated with reduced relapse rates, preservation of endothelial function, and improved graft survival when surgical intervention is required.[88]
Pertinent Studies Informing the Recommended Therapy for Cytomegalovirus Corneal Endotheliitis
Evidence from prospective and retrospective studies guides the recommended therapy for CMV corneal endotheliitis. Interventions, outcomes, and practical insights that underpin treatment strategies in both routine and complex cases are highlighted below.
- Topical ganciclovir 0.15% with IOP control and judicious steroid after antiviral start: In a single-center prospective cohort of PCR-confirmed CMV endotheliitis (unilateral or bilateral, mixed immune status), this regimen led to consistent clinical improvement within days to weeks. Time to reduction in corneal edema and keratic precipitates was rapid, IOP spikes were reduced, and relapses decreased during follow-up. No comparator was used, and topical therapy was safe.
- Topical ganciclovir versus topical ganciclovir with oral valganciclovir: In a 2-arm prospective cohort of PCR-confirmed hypertensive uveitis phenotype, adding oral valganciclovir lowered relapse frequency in severe, recurrent, or bilateral disease. The study compared topical-only therapy with the combination, reporting outcomes including relapse rate, need for treatment escalation, and safety laboratory evaluations. Hematologic and renal monitoring was required.
- Intracameral ganciclovir (with or without repeat) or intravitreal ganciclovir or foscarnet: In a retrospective series at a tertiary center of refractory or steroid-dependent cases, rescue local therapy rapidly improved short-term corneal clarity and endothelial status while minimizing complications. The intervention was compared with historical topical-only therapy, emphasizing the importance of careful dosing for endothelial safety.
- Aqueous humor PCR panel (CMV with or without HSV or VZV) guiding therapy: A prospective cohort of patients with suspected viral hypertensive anterior uveitis demonstrated that PCR-guided management improved diagnostic accuracy, reduced inappropriate steroid exposure, and shortened the time to initiation of correct therapy. Outcomes included flare control and time to correct therapy, compared with empiric steroid-first management.
- Preoperative and postoperative antiviral coverage with tissue PCR or IHC: Surgical series of eyes requiring endothelial keratoplasty or penetrating keratoplasty for corneal decompensation showed improved graft survival and reduced recurrence on the graft when preoperative and postoperative antiviral coverage was applied. The comparison was with no standardized prophylaxis, highlighting the benefit of PCR or IHC-guided therapy.
- Antiviral regimen with glaucoma medications: In patients with CMV endotheliitis and IOP spikes, cohorts demonstrated that antiviral therapy combined with glaucoma medications reduced hypertensive flares, improved IOP control, and decreased the need for filtering surgery or multiple glaucoma medications. Historical steroid-heavy care served as the comparator.
- Serial specular or confocal microscopy and AS-OCT: Imaging and biomarker cohorts of PCR-confirmed cases tracked ECD trajectories, corneal edema maps, and correlations with viral load (PCR cycle threshold values). Baseline imaging enabled early detection of decompensation, with rising cycle threshold values aligning with clinical improvement.
- Induction followed by maintenance valganciclovir: Safety cohorts evaluating systemic therapy in valganciclovir recipients reported predictable hematologic and renal adverse events, including neutropenia and gastrointestinal effects. Monitoring and dose adjustment were emphasized to mitigate toxicity. No comparator was used.
Data from these cohorts and series reinforce the value of individualized, PCR-confirmed management strategies for CMV endotheliitis. Physicians can integrate these findings into clinical practice to achieve timely viral control, preserve endothelial function, and reduce the risk of postoperative complications.
Ongoing and Recent Trial Themes
Given the relative rarity of CMV corneal endotheliitis and the lack of large multicenter RCTs, ongoing studies are essential to refine treatment strategies and optimize patient outcomes. The following findings summarize current research efforts, including dosing, monitoring, and prophylactic approaches.
- Intracameral ganciclovir versus foscarnet: Single-arm or randomized dose-finding studies compare different intracameral dosing regimens of ganciclovir and foscarnet. Key endpoints include corneal clarity time, ECD changes, and adverse events, aiming to optimize local antiviral therapy.
- Topical-only versus topical with oral valganciclovir: Pragmatic RCTs or prospective cohort studies evaluate topical ganciclovir alone compared with combination therapy including oral valganciclovir. Outcomes measured are relapse rate, time to steroid-free control, and safety, informing strategies for systemic supplementation in severe or recurrent disease.
- Fixed duration versus PCR- or cycle threshold-guided stop rules: Prospective protocols investigate whether stopping therapy based on a fixed duration or guided by viral load (PCR cycle threshold values) affects sustained remission. Primary endpoints focus on relapse-free survival and maintenance of quiescence off therapy.
- Antiviral prophylaxis versus none: Prospective endothelial keratoplasty cohort studies examine the effect of preoperative and postoperative antiviral prophylaxis compared with no prophylaxis on graft outcomes. Endpoints include graft survival and recurrence of CMV on the graft, providing evidence for preventive strategies post-keratoplasty.
- Imaging metrics versus viral load: Observational biomarker studies correlate serial imaging outcomes, such as specular or confocal microscopy and AS-OCT, with viral load. Key endpoints involve validation of surrogate measures, including ECD trajectory and edema mapping, to monitor disease activity and guide therapy.
Collectively, these investigations strengthen the evidence base for current management, highlighting optimal dosing, monitoring, and prophylactic interventions. The results of these studies enable clinicians to implement more precise, patient-centered approaches to prevent relapse and preserve visual function.
Registered Trials Relevant to Cytomegalovirus Corneal Endotheliitis
Contemporary registered trials explore novel approaches to antiviral therapy, dosing, and delivery in CMV anterior segment disease. The following findings provide a framework for understanding how these investigations may inform clinical practice and future management strategies.
- Systemic and Topical Antivirals for Control of Cytomegalovirus Anterior Uveitis: Treatment Outcomes (STACCATO, NCT03586284): This randomized, double-masked pilot study compared oral valganciclovir, topical 2% ganciclovir, and placebo in patients with PCR-proven CMV anterior uveitis, including cases with corneal endothelial involvement across multiple centers. Primary endpoints included the change in aqueous CMV viral load from days 0 to 28, achievement of disease quiescence, and the effect of corticosteroid therapy. Listing and protocol details are summarized by Retina Physician & CenterWatch, with the record updated in December 2024 by UCSF, although the trial status is not clearly displayed in static mirrors.
- Intra-cameral Penetration and Efficacy of Topical 2% Ganciclovir Eye Drops for CMV Anterior Uveitis/Endotheliitis (NCT02943057): This prospective, single-arm study enrolled approximately 25 patients, administering 2% ganciclovir 5 times daily for 6 weeks in PCR-positive CMV anterior segment infection, including uveitis or endotheliitis. Primary outcomes comprised aqueous ganciclovir concentrations and clinical response. Registry summaries describe the study design, and the trial was conducted at the Singapore National Eye Centre.
- Topical 0.15% Ganciclovir Gel (Virgan) for CMV Anterior Uveitis/Endotheliitis (NCT01647529): This prospective, interventional, nonrandomized study included patients with CMV anterior uveitis or endotheliitis. Outcomes focused on pharmacokinetics and clinical response, with site-specific summary data available. The study is registered on ClinicalTrials.gov, and related pharmacokinetic results were published in PLoS ONE.
- Ganciclovir Eye Drop for the Treatment of Cytomegalovirus Anterior Uveitis (UMIN000007127, Japan): This single-arm, open-label study evaluated 0.5% ganciclovir administered 4 times daily in patients with CMV anterior uveitis seen in outpatient or inpatient settings. Primary endpoints included visual acuity, IOP, and anterior segment inflammation. The study was completed, with the record last modified on July 24, 2015, targeting enrollment of 10 participants, and was led by principal investigator Kusuhara at Kobe University.
The ongoing and completed trials illustrate evolving strategies for CMV anterior segment disease management, including topical, systemic, and combination antiviral therapy. Clinicians can use these data to tailor individualized treatment plans, balancing efficacy, safety, and long-term visual outcomes.
Related Clinical Evidence
Related clinical evidence includes a double-masked, randomized, placebo-controlled 4-week study summarizing contemporary therapy choices for CMV anterior uveitis, highlighting the use of topical 2% ganciclovir and systemic valganciclovir.[89] Pharmacokinetic data after topical 0.15% ganciclovir gel demonstrate measurable aqueous levels, supporting the efficacy of topical regimens.[90] Long-term outcomes with 2% ganciclovir solution, including cases of endotheliitis, further inform clinical decision-making.
Treatment Planning
The primary goal in managing CMV corneal endotheliitis is to suppress viral replication, control inflammation, prevent irreversible endothelial cell loss, and mitigate secondary complications such as ocular hypertension and corneal decompensation. Treatment must be individualized according to disease severity, laterality, patient immune status, prior therapy, and comorbid ocular conditions.
A PCR-confirmed diagnosis from aqueous humor sampling should guide initial therapy. In cases of high clinical suspicion, empiric antiviral therapy may be initiated while awaiting results, particularly in sight-threatening presentations. Topical antiviral therapy constitutes the 1st-line management, most commonly using compounded 2% ganciclovir solution or 0.15% ganciclovir gel, administered 5 to 8 times daily during induction. This approach targets viral replication within the anterior segment while minimizing systemic exposure. For patients with severe, bilateral, recurrent, or hypertensive uveitis phenotypes, systemic valganciclovir—900 mg twice daily for a 2- to 3-week induction phase, followed by 450 to 900 mg once daily for maintenance—is indicated to achieve deeper and sustained viral suppression, with careful monitoring of renal function and hematologic parameters.
Adjunctive corticosteroid therapy is necessary to limit immune-mediated endothelial damage but should be initiated only after antiviral coverage is established to prevent exacerbation of viral replication. Low- to moderate-potency topical steroids, such as fluorometholone or loteprednol, are preferred initially, with escalation considered if inflammation persists. Steroid-sparing immunomodulators are generally unnecessary, as the underlying pathology is viral rather than autoimmune.
Ocular hypertension is managed concurrently with aqueous suppressants, including β-blockers, α-agonists, and carbonic anhydrase inhibitors. Prostaglandin analogs are avoided due to their potential to worsen inflammation. In cases where IOP remains uncontrolled despite maximal medical therapy, filtering surgery or minimally invasive glaucoma surgery may be performed once inflammation is quiescent.
Refractory or relapsing cases may require intracameral ganciclovir (2 mg/0.05 mL) or intravitreal ganciclovir or foscarnet, particularly when topical therapy fails to achieve adequate corneal penetration. These interventions demand careful patient selection and informed consent due to procedural risks.
In patients who have received keratoplasty, perioperative antiviral prophylaxis is strongly recommended to prevent graft recurrence, which can compromise graft survival. Maintenance antiviral therapy may be continued for several months postoperatively, adjusted based on PCR results, ECD, and clinical stability.
Long-term follow-up using specular microscopy, AS-OCT, and periodic PCR testing for aqueous CMV DNA is essential to detect subclinical recurrence promptly. Educating patients on treatment adherence, early symptom recognition, and the importance of follow-up is critical to optimizing clinical outcomes.
Toxicity and Adverse Effect Management
Management of CMV corneal endotheliitis necessitates prolonged antiviral therapy, which carries both local and systemic toxicity risks. Effective management of these adverse effects relies on early recognition, routine monitoring, and timely interventions to prevent treatment interruption or irreversible complications.
Topical ganciclovir, administered as 0.15% gel or 2% solution, is generally well-tolerated. Common local adverse effects include superficial punctate keratopathy, ocular surface irritation, conjunctival hyperemia, and mild epithelial toxicity, occurring in approximately 10% to 20% of patients. These effects are usually mild and reversible, and typically manifest within days to weeks of therapy initiation. Adverse events may be minimized by optimizing dosing frequency after induction, using preservative-free artificial tears, and, when necessary, switching to a gel formulation to enhance corneal contact time while reducing instillation frequency.
Systemic valganciclovir, used for severe, bilateral, or recurrent disease, carries a significant risk of hematologic suppression, particularly neutropenia, leukopenia, anemia, and thrombocytopenia, occurring in approximately 10% to 30% of patients, as well as gastrointestinal upset, elevated liver enzymes, and nephrotoxicity, with gastrointestinal symptoms reported in 15% to 20% of cases. Hematologic changes typically develop within 1 to 4 weeks, whereas gastrointestinal effects can appear within days of therapy initiation.
To minimize systemic toxicity, renal function (as indicated by, for example, serum creatinine or estimated glomerular filtration rate) should be assessed before initiation and monitored regularly during therapy, with dose adjustments based on renal clearance. CBC should be performed weekly during induction and monthly thereafter. Therapy should be paused or reduced if significant cytopenia occurs, following baseline and periodic monitoring of CBC, renal, and liver function tests, with dose adjustments for renal impairment. Growth factor support (eg, granulocyte-colony stimulating factor) may be considered in select cases.[91]
Intracameral or intravitreal antivirals (ganciclovir or foscarnet) may rarely induce anterior segment inflammation, endothelial trauma, or a transient IOP rise, occurring in less than 5% of cases with appropriate technique and typically within the 1st week postinjection. These risks may be minimized by using atraumatic injection techniques, appropriate dosing, postprocedural anti-inflammatory coverage, and careful monitoring of IOP.[92]
Corticosteroid-related complications, including steroid-induced ocular hypertension, cataract progression, and delayed epithelial healing, must be monitored closely. IOP rises in up to 30% of patients, and cataract progression may occur over the long term, typically within weeks to months. Management strategies include tapering steroids when possible, switching to lower-potency or soft agents such as loteprednol, and introducing IOP-lowering medications as needed.[93]
Prostaglandin analogs are generally avoided due to their pro-inflammatory potential. However, these agents may be cautiously introduced in quiescent disease under close supervision if other pressure-lowering medications fail.[94] In all cases, patient counseling is essential to encourage prompt reporting of side effects, adherence to follow-up schedules, and avoidance of self-discontinuation of therapy, which can lead to recurrence and irreversible endothelial loss.
Staging
A standardized clinical staging system for CMV corneal endotheliitis has not yet been validated and remains a proposed framework. The classification, outlined below, aims to stratify disease severity based on clinical, virologic, endothelial, and IOP parameters.
- Stage 1 (Early/Mild): Localized coin-shaped or linear keratic precipitates, mild anterior chamber cells, and minimal corneal edema without keratic precipitate confluence characterize early disease. Aqueous PCR shows low-positive viral load, ECD exceeds 2,000 cells/mm², and IOP is normal. Management focuses on topical ganciclovir gel or solution with a short course of low-potency topical steroids, alongside careful monitoring.
- Stage 2 (Moderate/Active): Diffuse or multiple confluent keratic precipitates, moderate corneal edema, anterior chamber reaction, and mild elevation of IOP (21-30 mm Hg) define this stage. PCR demonstrates moderate viral load, and ECD ranges from 1,500 to 2,000 cells/mm². Recommended therapy includes combined topical and systemic valganciclovir, anti-glaucoma medications, and moderate-potency topical steroids under antiviral coverage.
- Stage 3 (Severe/Advanced): Dense keratic precipitate clusters, marked corneal edema, stromal involvement, and recurrent inflammation despite therapy indicate severe disease. Aqueous viral load is high, ECD declines to between 1,000 and 1,500 cells/mm², and IOP is often above 30 mm Hg and difficult to control. Aggressive systemic antiviral therapy, intracameral ganciclovir, and IOP management, including surgical intervention if needed, are recommended.
- Stage 4 (End-stage/Decompensated): Persistent corneal edema, Descemet folds, endothelial failure, corneal decompensation, and possible graft failure in post-keratoplasty patients define the end-stage. Viral activity is often low or undetectable, ECD drops below 1,000 cells/mm², and IOP is variable. Management focuses on penetrating or endothelial keratoplasty with perioperative antiviral prophylaxis, accompanied by long-term maintenance therapy to prevent recurrence.
Although preliminary, this staging framework provides clinicians with a structured approach to assess disease severity and tailor antiviral and adjunctive therapy. Integrating clinical, virologic, and endothelial parameters may enhance monitoring, enable earlier intervention, and help prevent irreversible corneal damage.
Prognosis
The prognosis of CMV corneal endotheliitis is generally favorable when the disease is detected and treated early with effective antiviral therapy. Recurrent episodes, however, remain a concern and may lead to progressively worse outcomes if inadequately managed. Topical ganciclovir appears to reduce the risk of recurrence, although additional studies are required to determine the optimal prophylactic regimen.
Key determinants of prognosis include the timeliness of diagnosis, adequacy and duration of antiviral therapy, the extent of endothelial cell loss at presentation, and control of secondary complications such as ocular hypertension and glaucoma. Early recognition and prompt initiation of targeted antiviral therapy typically result in the resolution of corneal edema, preservation of corneal transparency, and minimization of endothelial cell loss. Patients receiving appropriate maintenance therapy demonstrate lower recurrence rates and a more favorable long-term visual outcome.
Moderate-to-advanced CMV corneal endotheliitis at presentation often carries a guarded prognosis. Persistent or recurrent viral activity results in progressive endothelial cell loss, ultimately leading to irreversible corneal decompensation. Long-term follow-up studies indicate that even with adequate viral suppression, annual endothelial cell attrition may be accelerated relative to age-matched healthy eyes. Secondary glaucoma is a critical prognostic factor, as eyes with uncontrolled IOP are at increased risk of optic nerve damage and vision loss, even after resolution of corneal disease.[95]
Recurrence rates remain significant, with reports of relapse in 30% to 50% of cases when antiviral therapy is tapered or discontinued prematurely. Recurrences are more frequent in immunocompromised individuals, post-keratoplasty eyes, and patients with suboptimal treatment adherence. In grafted eyes, recurrence affecting the donor endothelium substantially compromises graft survival and is a common indication for repeat keratoplasty.
Prognosis is most favorable when diagnosis is confirmed via PCR early in the disease course, antiviral therapy is promptly initiated, IOP is tightly controlled, and patients undergo regular monitoring with specular microscopy and anterior segment imaging to detect subclinical recurrence. With proactive long-term management, many patients maintain functional vision for years, although lifelong surveillance is generally warranted.[96]
Complications
CMV endotheliitis causes irreversible endothelial dysfunction, vision loss, and secondary glaucoma. In the Japan Endotheliitis study, 60.6% of eyes retained corneal clarity without additional corneal transplantation. Systemic treatment carries a risk of complications, including pancytopenia and myelosuppression, which require periodic monitoring. Therapy should be discontinued if pancytopenia or myelosuppression develops.
If not diagnosed and managed promptly, CMV endotheliitis can produce a spectrum of ocular complications, many of which are irreversible. These complications result from persistent viral activity, immune-mediated endothelial damage, secondary structural alterations in the cornea, and sequelae of elevated IOP.
The most significant complication is progressive endothelial cell loss. CMV directly infects endothelial cells, causing cytopathic changes and apoptosis, while chronic inflammation accelerates attrition. This complication is common and progressive in untreated or late-stage cases. Once endothelial reserve drops below the critical threshold (~500-800 cells/mm²), the cornea loses its ability to maintain deturgescence, resulting in stromal edema, Descemet membrane folds, epithelial bullae, and chronic visual blur. Advanced cases may develop painful bullous keratopathy, necessitating keratoplasty.[97][98] Early diagnosis, adequate antiviral therapy, and regular specular microscopy are essential to preserve endothelial function and prevent irreversible decompensation.
Recurrent inflammatory episodes and trabeculitis caused by CMV may lead to ocular hypertension. This complication occurs in 30% to 50% of patients over the disease course. Chronic IOP elevation results in glaucomatous optic neuropathy, which may be the primary cause of irreversible vision loss in some patients. Steroid-induced ocular hypertension, a complication of long-term topical corticosteroid use, further exacerbates the problem. Management requires regular IOP monitoring, appropriate anti-glaucoma therapy, and early surgical intervention in refractory cases.[99]
In patients who develop CMV endotheliitis after penetrating or endothelial keratoplasty, infection of the donor endothelium leads to graft edema, rejection-like episodes, and eventual graft failure. This complication occurs in up to 40% of infected grafts. Recurrence of CMV in the graft significantly reduces graft survival and increases the risk of multiple repeat surgeries. Perioperative antiviral prophylaxis and close graft surveillance are essential to optimize graft outcomes.
Even with adequate antiviral therapy, recurrences occur in 30% to 50% of patients, particularly if treatment is tapered too quickly or discontinued. Recurrence results from incomplete viral suppression or premature cessation of therapy. Chronic low-grade inflammation contributes to ongoing endothelial damage, IOP instability, and gradual visual decline. Long-term maintenance therapy, with a slow taper and strict patient adherence, is essential to minimize recurrence and preserve corneal function.[100]
Visual outcomes vary widely, with advanced cases exhibiting severe and permanent vision loss resulting from corneal decompensation, glaucomatous optic neuropathy, or both. In bilateral or sequential involvement, functional blindness may develop if timely intervention is not implemented.
Long-term systemic valganciclovir can cause neutropenia, anemia, thrombocytopenia, and renal or hepatic toxicity. Topical antivirals may induce superficial punctate keratopathy, epithelial defects, or allergic reactions. Systemic hematologic changes occur in 10% to 30% of patients, while topical ocular effects occur in 10% to 20%. These complications result from bone marrow suppression, renal or hepatic toxicity, and epithelial injury. Monitoring of CBC and renal and liver function, along with the use of artificial tears and dose adjustment, is essential to minimize adverse effects. Corticosteroid use increases the risk of cataract formation and ocular hypertension.[101]
Long-term corticosteroid use in CMV endotheliitis increases the risk of ocular hypertension and cataract formation. Steroid-induced ocular hypertension occurs in up to 30% of patients, while cataract develops with prolonged therapy. These complications result from altered aqueous outflow and lens opacity due to chronic steroid exposure. Mitigation strategies include using the lowest effective steroid dose, switching to soft steroids when possible, and performing regular IOP monitoring.
Chronic epithelial compromise, bullous keratopathy, or prior surgical interventions can predispose to secondary bacterial or fungal keratitis, which may be vision-threatening and complicate the management of CMV endotheliitis. This complication is rare (<5%) but occurs more frequently in eyes with epithelial compromise. Pathogenesis involves a breach in the epithelial barrier that allows microbial entry. Prevention and management include ocular surface protection, prophylactic antibiotics following surgery, and prompt treatment of any epithelial defects.[102][103]
In some cases, endothelial failure progresses even after the virus has been eradicated, due to residual structural and functional damage. This complication occurs variably, often in end-stage disease. These eyes may require keratoplasty despite achieving virologic cure.[104] Endothelial keratoplasty, when indicated, helps maintain corneal quiescence prior to surgery.
Postoperative and Rehabilitation Care
Postoperative and rehabilitation care following management of CMV corneal endotheliitis, whether via medical therapy, keratoplasty, or a combination, is essential to preserve graft integrity, prevent viral recurrence, maintain visual function, and optimize patient quality of life. Effective care requires an interprofessional approach involving ophthalmologists, optometrists, rehabilitation specialists, and, when indicated, infectious disease experts.
Immediate Postoperative Phase
During the immediate postoperative phase, the focus is on graft protection, inflammation control, and prevention of CMV reactivation. Patients undergoing penetrating keratoplasty, DSAEK, or DMEK require close slit-lamp evaluation within the first 24 to 48 hours.
Key measures include the cautious use of topical corticosteroids, such as prednisolone acetate 1% or loteprednol etabonate in steroid responders, to maintain graft clarity while minimizing the risk of viral reactivation. Antiviral prophylaxis with topical ganciclovir 0.15% gel or systemic valganciclovir, adjusted for renal function, should be continued for at least 3 to 6 months postoperatively in high-risk patients. IOP should be closely monitored to detect steroid- or inflammation-induced elevations, which are managed with topical hypotensive agents as needed. Broad-spectrum topical antibiotics are recommended for 1 to 2 weeks to prevent secondary infection.[105]
Intermediate Recovery Phase
During the intermediate recovery phase, spanning 2 weeks to 3 months postsurgery, care emphasizes minimizing recurrence and ensuring graft integration. Corticosteroids are gradually tapered under antiviral cover, guided by clinical response and, if available, aqueous humor PCR results. Serial specular microscopy is performed at 1, 3, and 6 months to monitor ECD, while AS-OCT evaluates the graft-host interface, resolution of edema, and early signs of detachment in lamellar keratoplasty. Patients are educated on recognizing symptoms of recurrence, such as sudden vision blur, glare, or halos, and the importance of prompt reporting to facilitate timely intervention.
Long-Term Maintenance and Rehabilitation
Long-term management of CMV corneal endotheliitis extends beyond infection control to include visual rehabilitation and prevention of irreversible ocular damage. Maintenance antiviral therapy is recommended for individuals with recurrent episodes or an increased risk to prevent reactivation, while regular glaucoma surveillance, including IOP measurement, optic nerve evaluation, and visual field testing every 3 to 6 months, helps preserve long-term visual function.
Optical rehabilitation may involve prescription spectacles, rigid gas-permeable lenses, scleral lenses, or customized contact lenses to correct residual irregular astigmatism. For patients with irreversible optic nerve or retinal damage, low-vision aids can facilitate functional independence. Psychosocial support and counseling are also important to address quality-of-life concerns associated with visual disability.[106]
Special Considerations for Bilateral Disease
Bilateral CMV corneal endotheliitis, or sequential involvement of both eyes, necessitates more aggressive prophylactic strategies and careful coordination of care. Early initiation of low-vision rehabilitation and minimization of downtime between surgical interventions are critical to optimizing functional outcomes.
Interprofessional Collaboration
Optimal long-term rehabilitation requires a coordinated interprofessional approach. Cornea specialists guide surgical management and follow-up, infectious disease physicians oversee systemic antiviral dosing and monitoring, optometrists and orthoptists provide visual rehabilitation, and glaucoma specialists manage IOP. Collaborative care ensures comprehensive management of both ocular and systemic aspects of the disease, maximizing the likelihood of preserved vision and functional independence.
Consultations
Management of CMV corneal endotheliitis often requires an interprofessional approach to optimize patient outcomes, prevent complications, and address systemic comorbidities. Early engagement of relevant subspecialists ensures comprehensive care.
The cornea and external disease specialist is central to diagnosis, treatment planning, and long-term follow-up. This specialist coordinates antiviral therapy, surgical interventions such as endothelial keratoplasty, and monitoring for recurrence through serial specular microscopy and AS-OCT.[107] A glaucoma specialist becomes essential when IOP remains high due to CMV-induced trabeculitis, steroid response, or angle damage from chronic inflammation. The roles of these providers encompass pharmacologic management, laser procedures such as selective laser trabeculoplasty when indicated, and surgical interventions, including trabeculectomy and glaucoma drainage device placement.[108]
An infectious disease physician provides critical support, particularly for immunocompromised patients or those with systemic CMV involvement. These providers guide systemic antiviral dosing, monitor renal and hepatic function, and manage potential drug interactions or toxicities.[109] Consultation with a retina specialist is warranted if posterior segment CMV involvement is suspected, such as CMV retinitis, or if secondary retinal complications arise from ocular hypertension, including optic nerve head damage or retinal vascular occlusions.[110] An optometrist or contact lens specialist contributes to optical rehabilitation following treatment or corneal transplantation, particularly in patients with irregular astigmatism, high corneal toricity, or anisometropia. The prescription of rigid gas-permeable, scleral, or hybrid lenses can substantially improve visual function.[111]
A low-vision rehabilitation specialist is essential for patients who have sustained irreversible vision loss due to advanced corneal decompensation, optic nerve damage, or graft failure. These professionals provide guidance on low-vision aids, assistive technologies, and vocational rehabilitation.[112] The primary care physician coordinates systemic health monitoring for patients on long-term systemic antivirals or corticosteroids, ensuring regular assessment of renal, hepatic, and hematologic parameters. In patients with autoimmune conditions or immune dysregulation, consultation with immunology or rheumatology specialists is important to optimize systemic immunosuppression while minimizing the risk of infection.
Deterrence and Patient Education
Patient education is critical in the management of CMV corneal endotheliitis, as the condition is prone to relapse. Patients should be instructed on the importance of adherence to antiviral therapy and self-monitoring for symptoms, such as blurred vision, ocular redness, and photophobia. Delayed treatment or recurrent episodes may result in progressive endothelial damage, corneal decompensation, and worsening visual outcomes. Patients should also be counseled regarding potential adverse effects associated with systemic or topical anti-CMV therapy.
Preventive strategies focus on reducing disease onset in at-risk individuals, minimizing recurrence after successful treatment, and mitigating long-term vision-threatening complications. Patients should understand that CMV infection can lead to chronic endothelial inflammation, endothelial cell loss, and secondary glaucoma if untreated or inadequately controlled. In post-corneal transplantation patients, education regarding the risk of CMV reactivation and its potential to precipitate graft failure is essential, with strict postoperative surveillance emphasized to optimize graft survival and visual outcomes.
Key preventive strategies in CMV corneal endotheliitis should focus on strict adherence to antiviral therapy, whether topical or systemic, for the full prescribed duration, even after symptomatic improvement. Regular follow-up visits are essential for slit-lamp examination, IOP measurement, and, when indicated, anterior chamber fluid PCR testing to detect subclinical viral activity.
Corticosteroids should be used judiciously, as overuse without concurrent antiviral therapy can trigger viral replication and exacerbate endothelial damage. Patients must promptly report new ocular symptoms, such as blurred vision, halos, photophobia, and redness, which may signify disease recurrence or treatment complications.
In immunocompromised individuals, including transplant recipients, patients with HIV, or those receiving systemic immunosuppressants, education should emphasize systemic infection control and routine laboratory monitoring to balance antiviral efficacy with potential toxicity. Counseling on lifestyle modifications, including minimizing ocular trauma, maintaining proper contact lens hygiene, and optimizing general health through adequate hydration and nutrition, can further support ocular outcomes.[113]
The use of educational materials, including illustrated brochures, digital resources, and interactive counseling, enhances patient understanding and adherence. In recurrent or bilateral cases, involving family members in the education process provides additional support for medication compliance and follow-up scheduling. An informed and engaged patient is more likely to maintain therapy, attend regular monitoring, and achieve optimal visual outcomes.[114]
Pearls and Other Issues
Clinical Pearls
CMV corneal endotheliitis should be considered in any patient presenting with unilateral, recurrent, coin-shaped keratic precipitates associated with localized corneal edema and minimal anterior chamber inflammation, particularly in immunocompetent middle-aged men. Aqueous humor PCR for CMV DNA is the diagnostic gold standard and should be performed prior to initiating long-term antiviral therapy to prevent unnecessary drug exposure. Early combination of corticosteroids with antiviral therapy mitigates endothelial damage caused by immune-mediated inflammation while effectively suppressing viral replication. In post-keratoplasty patients, CMV endotheliitis often manifests subtly. Routine endothelial cell counts and specular microscopy facilitate early detection before graft failure occurs.
Disposition
Most patients achieve favorable outcomes with prompt initiation of topical ganciclovir, systemic valganciclovir, or both. However, relapses are common if antiviral therapy is tapered prematurely. Chronic or recurrent cases may necessitate prolonged low-dose antiviral prophylaxis. Long-term follow-up with regular IOP monitoring is essential, given the high risk of secondary glaucoma associated with CMV corneal endotheliitis.[115]
Pitfalls
Misdiagnosis of CMV corneal endotheliitis as herpetic endotheliitis may delay appropriate antiviral therapy, resulting in irreversible endothelial cell loss. Excessive reliance on corticosteroids without concurrent antiviral coverage can exacerbate disease and precipitate rapid endothelial decompensation. Additionally, failure to adjust systemic valganciclovir dosing in patients with renal impairment can lead to severe hematologic toxicity, including bone marrow suppression.
Prevention
Preventive strategies include the judicious use of postoperative corticosteroids in high-risk corneal graft recipients, ensuring concurrent antiviral coverage. Patient education on early recognition of symptoms and prompt medical evaluation in suspected recurrences is critical. In immunocompromised individuals, monitoring for systemic CMV reactivation and timely management may reduce the risk of ocular involvement.
Enhancing Healthcare Team Outcomes
Management of CMV corneal endotheliitis requires systemic antiviral therapy coordinated by an interprofessional healthcare team to optimize outcomes, minimize recurrence, and prevent vision loss. Ophthalmologists and infectious disease specialists lead diagnosis and treatment planning, ensuring timely initiation of antiviral therapy and monitoring for systemic CMV involvement. Advanced practitioners, including optometrists, nurse practitioners, and physician assistants, provide continuity of care through regular monitoring of IOP, corneal clarity, and treatment adherence, identifying early signs of recurrence or complications.
Nurses support patient education, reinforce adherence to topical and systemic antivirals, provide postoperative instructions, and monitor for adverse drug effects such as bone marrow suppression and renal dysfunction, escalating concerns promptly. Pharmacists ensure correct dosing, particularly in patients with renal impairment, review potential drug interactions, and counsel patients regarding proper medication use and side effects.
Ethical considerations involve balancing aggressive antiviral therapy to preserve vision against systemic toxicity risks, such as those associated with valganciclovir, with shared decision-making to ensure patient understanding of risks, benefits, and follow-up requirements. Interprofessional communication through electronic health records, secure messaging, and case conferences enables timely information sharing, reducing duplicated testing and treatment delays.
For immunocompromised patients, including transplant recipients, coordination with systemic healthcare providers aligns ocular management with systemic CMV prophylaxis protocols. Leveraging the expertise of each team member and maintaining open communication enhances patient-centered care, improves adherence, reduces recurrence, and preserves long-term visual function in CMV corneal endotheliitis.
Media
(Click Image to Enlarge)
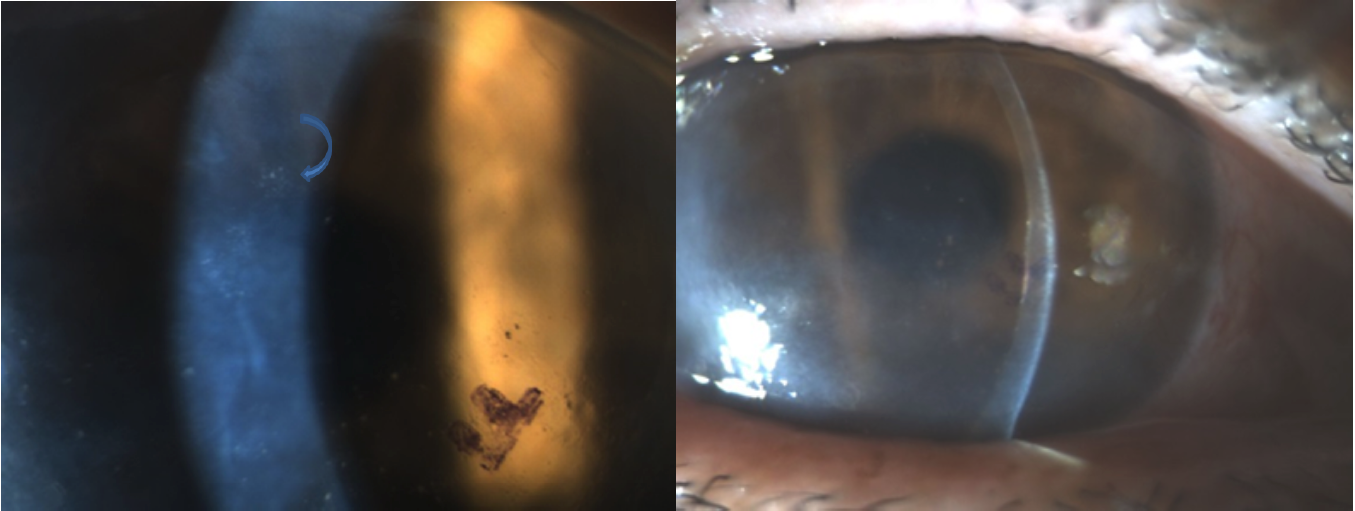
Cytomegalovirus Endotheliitis on Slit-Lamp Examination. The left image shows discrete, coin-shaped keratic precipitates on the corneal endothelium, a hallmark of cytomegalovirus-associated endotheliitis. The right image demonstrates localized corneal edema and corresponding endothelial deposits, illustrating sectoral involvement typical of this condition.
Contributed by Nathaniel Nataneli, MD
References
Alfawaz A. Cytomegalovirus-related corneal endotheliitis: A review article. Saudi journal of ophthalmology : official journal of the Saudi Ophthalmological Society. 2013 Jan:27(1):47-9. doi: 10.1016/j.sjopt.2011.10.001. Epub 2011 Oct 10 [PubMed PMID: 23964187]
Koizumi N, Yamasaki K, Kawasaki S, Sotozono C, Inatomi T, Mochida C, Kinoshita S. Cytomegalovirus in aqueous humor from an eye with corneal endotheliitis. American journal of ophthalmology. 2006 Mar:141(3):564-5 [PubMed PMID: 16490509]
Level 3 (low-level) evidenceBaltatzis S, Romero-Rangel T, Foster CS. Sectorial keratitis and uveitis: differential diagnosis. Graefe's archive for clinical and experimental ophthalmology = Albrecht von Graefes Archiv fur klinische und experimentelle Ophthalmologie. 2003 Jan:241(1):2-7 [PubMed PMID: 12545285]
Level 3 (low-level) evidenceOhashi Y, Yamamoto S, Nishida K, Okamoto S, Kinoshita S, Hayashi K, Manabe R. Demonstration of herpes simplex virus DNA in idiopathic corneal endotheliopathy. American journal of ophthalmology. 1991 Oct 15:112(4):419-23 [PubMed PMID: 1656756]
Level 3 (low-level) evidenceKhodabande A. Varicella endotheliitis: a case report. European journal of ophthalmology. 2009 Nov-Dec:19(6):1076-8 [PubMed PMID: 19882562]
Level 3 (low-level) evidenceInoue T, Kandori M, Takamatsu F, Hori Y, Maeda N. Corneal endotheliitis with quantitative polymerase chain reaction positive for human herpesvirus 7. Archives of ophthalmology (Chicago, Ill. : 1960). 2010 Apr:128(4):502-3. doi: 10.1001/archophthalmol.2010.35. Epub [PubMed PMID: 20385952]
Level 3 (low-level) evidenceEhara F, Nagase D, Kai M, Adachi K, Miyake H, Shimizu Y, Inoue Y, Miyazaki D. Roles played by IL-8 in altering dynamics of trabecular meshwork cells after human cytomegalovirus infection. Frontiers in cellular and infection microbiology. 2025:15():1550509. doi: 10.3389/fcimb.2025.1550509. Epub 2025 Apr 25 [PubMed PMID: 40353220]
Hwang YS, Wu PY, Kang EY, Wu WC, Chen LY, Lai CC, Choi KS. Comprehensive insights into cytomegalovirus anterior segment infections: A narrative review. Taiwan journal of ophthalmology. 2025 Apr-Jun:15(2):212-217. doi: 10.4103/tjo.TJO-D-25-00032. Epub 2025 Jun 10 [PubMed PMID: 40584195]
Level 3 (low-level) evidenceWang J, Rabiee B, Patel C, Jafri M, Hussain H, Chaudhry A, Chaudhry I, Kamoun L, Chaudhry I, Oh L, Bobat FI, Shukla D, Farooq AV. Herpesvirus Infections of the Corneal Endothelium. Microorganisms. 2025 Mar 28:13(4):. doi: 10.3390/microorganisms13040778. Epub 2025 Mar 28 [PubMed PMID: 40284615]
Ma L, Li Y, Wang X, Hong J. Cytomegalovirus Corneal Endotheliitis After Acanthamoeba Keratitis: A Case Report. Ocular immunology and inflammation. 2025 Apr:33(3):474-477. doi: 10.1080/09273948.2024.2400617. Epub 2025 Feb 24 [PubMed PMID: 39992328]
Level 3 (low-level) evidenceWong AHY, Kua WN, Young AL, Wan KH. Management of cytomegalovirus corneal endotheliitis. Eye and vision (London, England). 2021 Jan 14:8(1):3. doi: 10.1186/s40662-020-00226-y. Epub 2021 Jan 14 [PubMed PMID: 33441165]
Moshirfar M, Murri MS, Shah TJ, Skanchy DF, Tuckfield JQ, Ronquillo YC, Birdsong OC, Hofstedt D, Hoopes PC. A Review of Corneal Endotheliitis and Endotheliopathy: Differential Diagnosis, Evaluation, and Treatment. Ophthalmology and therapy. 2019 Jun:8(2):195-213. doi: 10.1007/s40123-019-0169-7. Epub 2019 Mar 11 [PubMed PMID: 30859513]
Chee SP, Bacsal K, Jap A, Se-Thoe SY, Cheng CL, Tan BH. Corneal endotheliitis associated with evidence of cytomegalovirus infection. Ophthalmology. 2007 Apr:114(4):798-803 [PubMed PMID: 17207531]
Level 2 (mid-level) evidenceKandori M, Inoue T, Takamatsu F, Kojima Y, Hori Y, Maeda N, Tano Y. Prevalence and features of keratitis with quantitative polymerase chain reaction positive for cytomegalovirus. Ophthalmology. 2010 Feb:117(2):216-22. doi: 10.1016/j.ophtha.2009.06.059. Epub 2009 Dec 6 [PubMed PMID: 19969369]
Level 2 (mid-level) evidenceMiyanaga M, Sugita S, Shimizu N, Morio T, Miyata K, Maruyama K, Kinoshita S, Mochizuki M. A significant association of viral loads with corneal endothelial cell damage in cytomegalovirus anterior uveitis. The British journal of ophthalmology. 2010 Mar:94(3):336-40. doi: 10.1136/bjo.2008.156422. Epub 2009 Sep 3 [PubMed PMID: 19734135]
Kandori M, Miyazaki D, Yakura K, Komatsu N, Touge C, Ishikura R, Inoue Y. Relationship between the number of cytomegalovirus in anterior chamber and severity of anterior segment inflammation. Japanese journal of ophthalmology. 2013 Nov:57(6):497-502. doi: 10.1007/s10384-013-0268-2. Epub 2013 Aug 9 [PubMed PMID: 23928983]
Level 2 (mid-level) evidenceSuzuki T, Hara Y, Uno T, Ohashi Y. DNA of cytomegalovirus detected by PCR in aqueous of patient with corneal endotheliitis after penetrating keratoplasty. Cornea. 2007 Apr:26(3):370-2 [PubMed PMID: 17413969]
Level 3 (low-level) evidenceAnshu A, Chee SP, Mehta JS, Tan DT. Cytomegalovirus endotheliitis in Descemet's stripping endothelial keratoplasty. Ophthalmology. 2009 Apr:116(4):624-30. doi: 10.1016/j.ophtha.2008.10.031. Epub 2009 Feb 4 [PubMed PMID: 19195708]
Level 3 (low-level) evidenceWaduthantri S, Zhou L, Chee SP. Aqueous humour concentration of topically applied 2.0% ganciclovir eye drops in eyes with cytomegalovirus anterior uveitis and endotheliitis. Clinical & experimental ophthalmology. 2025 May-Jun:53(4):384-390. doi: 10.1111/ceo.14469. Epub 2024 Nov 25 [PubMed PMID: 39587428]
Adland E, Klenerman P, Goulder P, Matthews PC. Ongoing burden of disease and mortality from HIV/CMV coinfection in Africa in the antiretroviral therapy era. Frontiers in microbiology. 2015:6():1016. doi: 10.3389/fmicb.2015.01016. Epub 2015 Sep 24 [PubMed PMID: 26441939]
Joye A, Gonzales JA. Ocular manifestations of cytomegalovirus in immunocompetent hosts. Current opinion in ophthalmology. 2018 Nov:29(6):535-542. doi: 10.1097/ICU.0000000000000521. Epub [PubMed PMID: 30281031]
Level 3 (low-level) evidenceYoo WS, Kwon LH, Eom Y, Thng ZX, Or C, Nguyen QD, Kim SJ. Cytomegalovirus Corneal Endotheliitis: A Comprehensive Review. Ocular immunology and inflammation. 2024 Nov:32(9):2228-2237. doi: 10.1080/09273948.2024.2320704. Epub 2024 Feb 28 [PubMed PMID: 38417101]
Koizumi N, Inatomi T, Suzuki T, Shiraishi A, Ohashi Y, Kandori M, Miyazaki D, Inoue Y, Soma T, Nishida K, Takase H, Sugita S, Mochizuki M, Kinoshita S, Japan Corneal Endotheliitis Study Group. Clinical features and management of cytomegalovirus corneal endotheliitis: analysis of 106 cases from the Japan corneal endotheliitis study. The British journal of ophthalmology. 2015 Jan:99(1):54-8. doi: 10.1136/bjophthalmol-2013-304625. Epub 2014 Jul 29 [PubMed PMID: 25075122]
Level 3 (low-level) evidenceTan TE, Tan DTH. Cytomegalovirus Corneal Endotheliitis After Descemet Membrane Endothelial Keratoplasty. Cornea. 2019 Apr:38(4):413-418. doi: 10.1097/ICO.0000000000001847. Epub [PubMed PMID: 30614903]
Nakagawa S, Ishii H, Takamoto M, Kaburaki T, Ishii K, Miyai T. Diagnosis of cytomegalovirus corneal endotheliitis using surgically removed Descemet's membrane and endothelium despite negative results with aqueous humor PCR: a case report. BMC ophthalmology. 2021 May 1:21(1):194. doi: 10.1186/s12886-021-01962-y. Epub 2021 May 1 [PubMed PMID: 33933006]
Level 3 (low-level) evidenceGandhi N, Lalitha P, Radhakrishnan N, Kumar A, Siva Ganesa Karthikeyan R, Rameshkumar G, Prajna NV. Cytomegalovirus Corneal Endotheliitis After Penetrating Keratoplasty. Cornea. 2022 May 1:41(5):e13-e14. doi: 10.1097/ICO.0000000000002944. Epub [PubMed PMID: 35383623]
Watanabe M, Kitazawa K, Fukuoka H, Wakimasu K, Inatomi T, Yoshii K, Koizumi N, Kinoshita S, Sotozono C. Predictors of cytomegalovirus corneal endotheliitis postcorneal transplantation. The British journal of ophthalmology. 2025 May 30:109(6):638-644. doi: 10.1136/bjo-2024-326055. Epub 2025 May 30 [PubMed PMID: 39797632]
Okumura N, Tanaka T, Fukui Y, Koizumi N. Pharmacokinetics of ganciclovir eye drops: a comparative study of solutions prepared from ganciclovir for intravenous infusion and ganciclovir gel. Japanese journal of ophthalmology. 2024 Nov:68(6):764-770. doi: 10.1007/s10384-024-01106-x. Epub 2024 Aug 31 [PubMed PMID: 39215881]
Level 2 (mid-level) evidenceBüyüktepe TÇ, Yalçındağ N. Cytomegalovirus Endotheliitis After Penetrating Keratoplasty. Turkish journal of ophthalmology. 2020 Oct 30:50(5):304-307. doi: 10.4274/tjo.galenos.2020.47568. Epub [PubMed PMID: 33342198]
Newman A, McCluskey P. Epidemiological spectrum of infectious uveitis in the Asia-Pacific. Taiwan journal of ophthalmology. 2025 Apr-Jun:15(2):157-181. doi: 10.4103/tjo.TJO-D-25-00052. Epub 2025 Jun 10 [PubMed PMID: 40584206]
Level 2 (mid-level) evidenceBenador-Shen C, Shantha J, Lee J, Qian Y, Doan T, Gonzales JA. Multiple Anterior Chamber Paracenteses May Be Needed to Identify Cytomegalovirus Anterior Uveitis. American journal of ophthalmology. 2025 Jan:269():189-194. doi: 10.1016/j.ajo.2024.08.025. Epub 2024 Aug 31 [PubMed PMID: 39218387]
Level 2 (mid-level) evidenceZheng X, Yamaguchi M, Goto T, Okamoto S, Ohashi Y. Experimental corneal endotheliitis in rabbit. Investigative ophthalmology & visual science. 2000 Feb:41(2):377-85 [PubMed PMID: 10670465]
Level 3 (low-level) evidenceKobayashi R, Hashida N. Overview of Cytomegalovirus Ocular Diseases: Retinitis, Corneal Endotheliitis, and Iridocyclitis. Viruses. 2024 Jul 11:16(7):. doi: 10.3390/v16071110. Epub 2024 Jul 11 [PubMed PMID: 39066272]
Level 3 (low-level) evidencePisitpayat P, Mentreddy A, Pekmezci M, Hwang D, Shantha J, Benador-Shen C, Terry M, Pothikamjorn T, Gonzales J. Stromal Keratitis Associated With Cytomegalovirus Anterior Uveitis. Cornea. 2024 Jul 1:43(7):903-908. doi: 10.1097/ICO.0000000000003487. Epub 2024 Jan 31 [PubMed PMID: 38294900]
Ng JM, Hsiao CH. Bilateral herpes zoster ophthalmicus in an immunocompetent patient. European journal of ophthalmology. 2024 Jan:34(1):NP32-NP34. doi: 10.1177/11206721231177895. Epub 2023 May 21 [PubMed PMID: 37211648]
Daicker B. Cytomegalovirus panuveitis with infection of corneo-trabecular endothelium in AIDS. Ophthalmologica. Journal international d'ophtalmologie. International journal of ophthalmology. Zeitschrift fur Augenheilkunde. 1988:197(4):169-75 [PubMed PMID: 2852787]
Sakamoto K, Fukuoka H, Otsuki Y, Sotozono C. Cytomegalovirus Corneal Endotheliitis Presenting As Bullous Keratopathy: Resolution With Antiviral Therapy Alone. Cureus. 2025 May:17(5):e84175. doi: 10.7759/cureus.84175. Epub 2025 May 15 [PubMed PMID: 40519499]
Ocular Infection Group of Chinese Ophthalmologist Association. [Chinese expert consensus on diagnosis and treatment of viral corneal endotheliitis (2023)]. [Zhonghua yan ke za zhi] Chinese journal of ophthalmology. 2023 Jan 11:59(1):13-19. doi: 10.3760/cma.j.cn112142-20220407-00163. Epub [PubMed PMID: 36631052]
Level 3 (low-level) evidenceMori K, Ye Y, Yokogawa H, Nishino T, Kobayashi A, Mori N, Takemoto Y, Sugiyama K. Clinical Features of Glaucoma Associated with Cytomegalovirus Corneal Endotheliitis. Clinical ophthalmology (Auckland, N.Z.). 2022:16():2705-2711. doi: 10.2147/OPTH.S376039. Epub 2022 Aug 19 [PubMed PMID: 36017508]
Kobayashi R, Hashida N, Maruyama K, Nishida K. Clinical Findings of Specular Microscopy Images in Cytomegalovirus Corneal Endotheliitis. Asia-Pacific journal of ophthalmology (Philadelphia, Pa.). 2022 May 1:11(3):273-278. doi: 10.1097/APO.0000000000000522. Epub 2022 May 1 [PubMed PMID: 35772085]
Chen PJ, Lin IH, Chi YC, Lai CC, Hung JH, Tseng SH, Huang YH. Long-Term Outcome of Treatment with 2% Topical Ganciclovir Solution in Cytomegalovirus Anterior Uveitis and Corneal Endotheliitis. Infection and drug resistance. 2022:15():3395-3403. doi: 10.2147/IDR.S370905. Epub 2022 Jun 29 [PubMed PMID: 35791348]
Lee SH, Chun YS, Kim KW. Coin-shaped corneal endothelial scar in herpes zoster ophthalmicus: a case report. Journal of medical case reports. 2022 Mar 17:16(1):107. doi: 10.1186/s13256-022-03319-5. Epub 2022 Mar 17 [PubMed PMID: 35296348]
Level 3 (low-level) evidenceYoshida M, Yokokura S, Hariya T, Kobayashi W, Hashimoto K, Nakazawa T. Ripasudil Eyedrops Ameliorated Bullous Keratopathy Complicated with Cytomegalovirus Corneal Endotheliitis: A Case Report. Ocular immunology and inflammation. 2023 Jan:31(1):207-210. doi: 10.1080/09273948.2021.1988114. Epub 2021 Nov 2 [PubMed PMID: 34726564]
Level 3 (low-level) evidenceMersch J, Mouthuy M, Otjacques L, Forez S, Öztürk N. Unilateral mumps-associated corneal endotheliitis: A case report of a rare ocular complication of mumps. Journal francais d'ophtalmologie. 2021 Dec:44(10):e609-e611. doi: 10.1016/j.jfo.2021.01.016. Epub 2021 Jul 7 [PubMed PMID: 34244001]
Level 3 (low-level) evidenceDos Reis C, Miranda BA, da Cunha Afonso AF, Malta E Cunha LH, Trindade BC, Vasconcelos-Santos DV. Cytomegalovirus as a possibly overlooked agent of hypertensive anterior uveitis and endotheliitis in immunocompetent patients in Brazil. Revista do Instituto de Medicina Tropical de Sao Paulo. 2021:63():e84. doi: 10.1590/S1678-9946202163084. Epub 2021 Dec 6 [PubMed PMID: 34878042]
Moriyama H, Takada T, Aoki A, Shima K, Kikuchi T. Is giant cell interstitial pneumonia pathognomonic for hard metal lung diseases?-pathological and elemental analyses of 84 cases. Histopathology. 2025 Aug 15:():. doi: 10.1111/his.15537. Epub 2025 Aug 15 [PubMed PMID: 40815182]
Level 3 (low-level) evidenceUotani R, Miyazaki D, Shimizu Y, Ohtani F, Haruki T, Sasaki SI, Koyama A, Inoue Y, Suzutani T. Antiviral cytotoxic T lymphocyte responses for long term prognosis of corneal infection by cytomegalovirus in immunocompetent subjects. Scientific reports. 2022 Mar 30:12(1):5419. doi: 10.1038/s41598-022-09312-8. Epub 2022 Mar 30 [PubMed PMID: 35354878]
Yokogawa H, Kobayashi A, Sugiyama K. Mapping owl's eye cells of patients with cytomegalovirus corneal endotheliitis using in vivo laser confocal microscopy. Japanese journal of ophthalmology. 2013 Jan:57(1):80-4. doi: 10.1007/s10384-012-0189-5. Epub 2012 Nov 3 [PubMed PMID: 23124832]
Level 3 (low-level) evidenceMutwaly RF Sr. Corneal Endothelial Polymegathism and Pleomorphism Induced by Daily-Wear Soft Contact Lenses. Cureus. 2024 Nov:16(11):e74187. doi: 10.7759/cureus.74187. Epub 2024 Nov 21 [PubMed PMID: 39583607]
Hwang YS, Shen CR, Chang SH, Lai CC, Liu CL, Chen KJ, Lin KK, Chen TL, Hsiao CH. The validity of clinical feature profiles for cytomegaloviral anterior segment infection. Graefe's archive for clinical and experimental ophthalmology = Albrecht von Graefes Archiv fur klinische und experimentelle Ophthalmologie. 2011 Jan:249(1):103-10. doi: 10.1007/s00417-010-1510-y. Epub 2010 Sep 21 [PubMed PMID: 20857136]
Level 2 (mid-level) evidenceMartín Ramírez A, Cardeñoso Domingo L, González Guijarro JJ. PCR Multiplex for CMV Detection in Patients with Anterior Uveitis. Ocular immunology and inflammation. 2019:27(2):197-202. doi: 10.1080/09273948.2018.1438633. Epub 2018 Feb 23 [PubMed PMID: 29474137]
De Groot-Mijnes JD, Rothova A, Van Loon AM, Schuller M, Ten Dam-Van Loon NH, De Boer JH, Schuurman R, Weersink AJ. Polymerase chain reaction and Goldmann-Witmer coefficient analysis are complimentary for the diagnosis of infectious uveitis. American journal of ophthalmology. 2006 Feb:141(2):313-8 [PubMed PMID: 16458686]
Level 3 (low-level) evidenceRelvas LJM, Antoun J, de Groot-Mijnes JDF, Motulsky E, Ten Dam-Van Loon NH, Makhoul D, Willermain F, Caspers L. Diagnosis of Cytomegalovirus Anterior Uveitis in Two European Referral Centers. Ocular immunology and inflammation. 2018:26(1):116-121. doi: 10.1080/09273948.2017.1411952. Epub [PubMed PMID: 29377783]
Kobayashi R, Hashida N, Soma T, Koh S, Miki A, Usui S, Maeda N, Nishida K. Clinical Findings of Anterior Segment Spectral Domain Optical Coherence Tomography Images in Cytomegalovirus Corneal Endotheliitis. Cornea. 2017 Apr:36(4):411-414. doi: 10.1097/ICO.0000000000001103. Epub [PubMed PMID: 27941387]
Hashida N, Asao K, Maruyama K, Nishida K. Cornea Findings of Spectral Domain Anterior Segment Optical Coherence Tomography in Uveitic Eyes of Various Etiologies. Cornea. 2019 Oct:38(10):1299-1304. doi: 10.1097/ICO.0000000000002065. Epub [PubMed PMID: 31335529]
Hillenaar T, Weenen C, Wubbels RJ, Remeijer L. Endothelial involvement in herpes simplex virus keratitis: an in vivo confocal microscopy study. Ophthalmology. 2009 Nov:116(11):2077-86.e1-2. doi: 10.1016/j.ophtha.2009.04.022. Epub 2009 Sep 10 [PubMed PMID: 19744733]
Kobayashi A, Yokogawa H, Higashide T, Nitta K, Sugiyama K. Clinical significance of owl eye morphologic features by in vivo laser confocal microscopy in patients with cytomegalovirus corneal endotheliitis. American journal of ophthalmology. 2012 Mar:153(3):445-53. doi: 10.1016/j.ajo.2011.07.026. Epub 2011 Oct 25 [PubMed PMID: 22030352]
Level 3 (low-level) evidenceZhang J, Kamoi K, Zong Y, Yang M, Zou Y, Miyagaki M, Ohno-Matsui K. Cytomegalovirus Retinitis: Clinical Manifestations, Diagnosis and Treatment. Viruses. 2024 Sep 7:16(9):. doi: 10.3390/v16091427. Epub 2024 Sep 7 [PubMed PMID: 39339903]
Ye Z, Yang Y, Ke W, Li Y, Wang K, Chen M. Overview and update on cytomegalovirus-associated anterior uveitis and glaucoma. Frontiers in public health. 2023:11():1117412. doi: 10.3389/fpubh.2023.1117412. Epub 2023 Mar 1 [PubMed PMID: 36935679]
Level 3 (low-level) evidenceLawrence SM, Goshia T, Sinha M, Fraley SI, Williams M. Decoding human cytomegalovirus for the development of innovative diagnostics to detect congenital infection. Pediatric research. 2024 Jan:95(2):532-542. doi: 10.1038/s41390-023-02957-9. Epub 2023 Dec 25 [PubMed PMID: 38146009]
Kaur K, Gurnani B. Specular Microscopy. StatPearls. 2025 Jan:(): [PubMed PMID: 36256774]
Miller JR, Hanumunthadu D. Inflammatory eye disease: An overview of clinical presentation and management. Clinical medicine (London, England). 2022 Mar:22(2):100-103. doi: 10.7861/clinmed.2022-0046. Epub [PubMed PMID: 35304367]
Level 3 (low-level) evidenceGurnani B, Kaur K. Pythium Keratitis. StatPearls. 2025 Jan:(): [PubMed PMID: 34424645]
Zhang XJ, Zhang JX, Qu Y, Peng RM, Zhang P, Hong J. Cytokine analysis of aqueous humor in patients with cytomegalovirus corneal endotheliitis. Graefe's archive for clinical and experimental ophthalmology = Albrecht von Graefes Archiv fur klinische und experimentelle Ophthalmologie. 2024 Aug:262(8):2593-2600. doi: 10.1007/s00417-024-06417-w. Epub 2024 Mar 6 [PubMed PMID: 38446197]
Pavan-Langston D, Welch CL, Zegans ME. Ganciclovir gel for cytomegalovirus keratouveitis. Ophthalmology. 2012 Nov:119(11):2411. doi: 10.1016/j.ophtha.2012.07.024. Epub [PubMed PMID: 23122464]
Level 3 (low-level) evidenceFan NW, Chung YC, Liu YC, Liu CJ, Kuo YS, Lin PY. Long-Term Topical Ganciclovir and Corticosteroids Preserve Corneal Endothelial Function in Cytomegalovirus Corneal Endotheliitis. Cornea. 2016 May:35(5):596-601. doi: 10.1097/ICO.0000000000000791. Epub [PubMed PMID: 26967107]
Hwang JH, Ha M, Park Y, Chung SH. The Effect of Topical Ganciclovir and Corticosteroid on Cytomegalovirus Corneal Endotheliitis in Korean Patients. Ocular immunology and inflammation. 2019:27(2):338-344. doi: 10.1080/09273948.2018.1486436. Epub 2018 Jun 28 [PubMed PMID: 29953306]
Koizumi N, Miyazaki D, Inoue T, Ohtani F, Kandori-Inoue M, Inatomi T, Sotozono C, Nakagawa H, Horikiri T, Ueta M, Nakamura T, Inoue Y, Ohashi Y, Kinoshita S. The effect of topical application of 0.15% ganciclovir gel on cytomegalovirus corneal endotheliitis. The British journal of ophthalmology. 2017 Feb:101(2):114-119. doi: 10.1136/bjophthalmol-2015-308238. Epub 2016 May 3 [PubMed PMID: 27142389]
Choi WS, Cho JH, Kim HK, Kim HS, Shin YJ. A case of CMV endotheliitis treated with intravitreal ganciclovir injection. Korean journal of ophthalmology : KJO. 2013 Apr:27(2):130-2. doi: 10.3341/kjo.2013.27.2.130. Epub 2013 Feb 27 [PubMed PMID: 23543604]
Level 3 (low-level) evidenceYu T, Peng RM, Xiao GG, Feng LN, Hong J. Clinical Evaluation of Intravitreal Injection of Ganciclovir in Refractory Corneal Endotheliitis. Ocular immunology and inflammation. 2020:28(2):270-280. doi: 10.1080/09273948.2019.1573261. Epub 2019 Feb 26 [PubMed PMID: 30806530]
Koizumi N, Miyazaki D, Sugita S, Sotozono C, Inatomi T, Goto H, Shiraishi A, Eguchi S, Ito SI, Hori Y, Uchio E, Soma T, Fukuchi T, Hayashi K, Takeuchi Y, Inoue Y. Efficacy and safety of ROH-101 (0.15% ganciclovir gel) for cytomegalovirus corneal endotheliitis: an open-label, uncontrolled, phase 3 study in Japan. Japanese journal of ophthalmology. 2025 Mar:69(2):296-307. doi: 10.1007/s10384-025-01168-5. Epub 2025 Apr 9 [PubMed PMID: 40202692]
Ding K, Nataneli N. Cytomegalovirus Corneal Endotheliitis. StatPearls. 2025 Jan:(): [PubMed PMID: 35201702]
Dockery PW, Joubert KP, Parker JS, Parker JS. Comment on: "Cytomegalovirus Corneal Endotheliitis After Descemet Membrane Endothelial Keratoplasty". Cornea. 2019 Oct:38(10):e48. doi: 10.1097/ICO.0000000000002079. Epub [PubMed PMID: 31498245]
Level 3 (low-level) evidenceHasan A, Quintero-Estades JA, Nataneli N. A Woman With Intraocular Inflammation After Descemet Membrane Endothelial Keratoplasty. JAMA ophthalmology. 2020 Sep 1:138(9):1000-1001. doi: 10.1001/jamaophthalmol.2020.1337. Epub [PubMed PMID: 32729891]
Kitazawa K, Jongkhajornpong P, Inatomi T, Koizumi N, Kayukawa K, Wakimasu K, Sotozono C, Kinoshita S. Topical ganciclovir treatment post-Descemet's stripping automated endothelial keratoplasty for patients with bullous keratopathy induced by cytomegalovirus. The British journal of ophthalmology. 2018 Sep:102(9):1293-1297. doi: 10.1136/bjophthalmol-2017-311145. Epub 2018 Jan 23 [PubMed PMID: 29363530]
Basilious A, Chew HF. Topical Ganciclovir for Prophylaxis of Cytomegalovirus Endotheliitis in Endothelial Keratoplasty. Cornea. 2019 Jan:38(1):120-122. doi: 10.1097/ICO.0000000000001797. Epub [PubMed PMID: 30379718]
Chew MC, Tan DT, Chee SP, Li L. Optimising graft survival in endothelial keratoplasty for endothelial failure secondary to cytomegalovirus endotheliitis. Journal of ophthalmic inflammation and infection. 2019 Aug 2:9(1):15. doi: 10.1186/s12348-019-0180-0. Epub 2019 Aug 2 [PubMed PMID: 31375951]
Fellay J, Venetz JP, Aubert JD, Seydoux C, Pascual M, Meylan PR. Treatment of cytomegalovirus infection or disease in solid organ transplant recipients with valganciclovir. Transplantation proceedings. 2005 Mar:37(2):949-51 [PubMed PMID: 15848585]
Yoo WS, Kim GN, Chung I, Cho MC, Han YS, Kang SS, Yun SP, Seo SW, Kim SJ. Clinical characteristics and prognostic factors in hypertensive anterior uveitis diagnosed with polymerase chain reaction. Scientific reports. 2021 Apr 23:11(1):8814. doi: 10.1038/s41598-021-87931-3. Epub 2021 Apr 23 [PubMed PMID: 33893358]
Chee SP, Bacsal K, Jap A, Se-Thoe SY, Cheng CL, Tan BH. Clinical features of cytomegalovirus anterior uveitis in immunocompetent patients. American journal of ophthalmology. 2008 May:145(5):834-40. doi: 10.1016/j.ajo.2007.12.015. Epub 2008 Feb 6 [PubMed PMID: 18255045]
Level 2 (mid-level) evidencePatil AJ, Sharma A, Kenney MC, Kuppermann BD. Valganciclovir in the treatment of cytomegalovirus retinitis in HIV-infected patients. Clinical ophthalmology (Auckland, N.Z.). 2010 Mar 4:4():111-9 [PubMed PMID: 20234777]
Noecker RJ. The management of glaucoma and intraocular hypertension: current approaches and recent advances. Therapeutics and clinical risk management. 2006 Jun:2(2):193-206 [PubMed PMID: 18360593]
Level 3 (low-level) evidenceYang K, Zhao Y, Lu H, Zang Y, Mao Y, Hong J, Jie Y. Graft survival and endothelial outcomes after penetrating keratoplasty and Descemet stripping automated endothelial keratoplasty: A systematic review and meta-analysis. Experimental and therapeutic medicine. 2020 Sep:20(3):2794-2804. doi: 10.3892/etm.2020.9010. Epub 2020 Jul 13 [PubMed PMID: 32765774]
Level 1 (high-level) evidenceTeymouri M, Mollazadeh S, Mortazavi H, Naderi Ghale-Noie Z, Keyvani V, Aghababaei F, Hamblin MR, Abbaszadeh-Goudarzi G, Pourghadamyari H, Hashemian SMR, Mirzaei H. Recent advances and challenges of RT-PCR tests for the diagnosis of COVID-19. Pathology, research and practice. 2021 May:221():153443. doi: 10.1016/j.prp.2021.153443. Epub 2021 Apr 14 [PubMed PMID: 33930607]
Level 3 (low-level) evidenceDong Y, Zhu Y, Ma C, Zhao H. Steroid-antivirals treatment versus steroids alone for the treatment of Bell's palsy: a meta-analysis. International journal of clinical and experimental medicine. 2015:8(1):413-21 [PubMed PMID: 25785012]
Level 1 (high-level) evidence. Morbidity and toxic effects associated with ganciclovir or foscarnet therapy in a randomized cytomegalovirus retinitis trial. Studies of ocular complications of AIDS Research Group, in collaboration with the AIDS Clinical Trials Group. Archives of internal medicine. 1995 Jan 9:155(1):65-74 [PubMed PMID: 7802522]
Level 1 (high-level) evidenceFan JJ, Tao Y, Hwang DK. Comparison of intravitreal ganciclovir monotherapy and combination with foscarnet as initial therapy for cytomegalovirus retinitis. International journal of ophthalmology. 2018:11(10):1638-1642. doi: 10.18240/ijo.2018.10.10. Epub 2018 Oct 18 [PubMed PMID: 30364196]
Hoh HB, Hurley C, Claoue C, Viswalingham M, Easty DL, Goldschmidt P, Collum LM. Randomised trial of ganciclovir and acyclovir in the treatment of herpes simplex dendritic keratitis: a multicentre study. The British journal of ophthalmology. 1996 Feb:80(2):140-3 [PubMed PMID: 8814744]
Level 1 (high-level) evidenceTakhar JS, Joye AS, Somkijrungroj T, Laovirojjanakul W, Lin CP, Lietman TM, Porco TC, Keenan JD, Gebreegziabher EA, Seitzman GD, Rose-Nussbaumer J, Doan TA, Acharya NR, Gonzales JA. A double masked randomised 4-week, placebo-controlled study in the USA, Thailand and Taiwan to compare the efficacy of oral valganciclovir and topical 2% ganciclovir in the treatment of cytomegalovirus anterior uveitis: study protocol. BMJ open. 2019 Dec 19:9(12):e033175. doi: 10.1136/bmjopen-2019-033175. Epub 2019 Dec 19 [PubMed PMID: 31862739]
Level 1 (high-level) evidenceWaduthantri S, Zhou L, Chee SP. Intra-cameral level of ganciclovir gel, 0.15% following topical application for cytomegalovirus anterior segment infection: A pilot study. PloS one. 2018:13(1):e0191850. doi: 10.1371/journal.pone.0191850. Epub 2018 Jan 29 [PubMed PMID: 29377953]
Level 3 (low-level) evidenceGalar A, Valerio M, Catalán P, García-González X, Burillo A, Fernández-Cruz A, Zataráin E, Sousa-Casasnovas I, Anaya F, Rodríguez-Ferrero ML, Muñoz P, Bouza E. Valganciclovir-Ganciclovir Use and Systematic Therapeutic Drug Monitoring. An Invitation to Antiviral Stewardship. Antibiotics (Basel, Switzerland). 2021 Jan 15:10(1):. doi: 10.3390/antibiotics10010077. Epub 2021 Jan 15 [PubMed PMID: 33467490]
Level 1 (high-level) evidenceHuang CY, Kang EY, Cheng YC, Hwang YS, Hsiao CH. Efficacy of Intravitreal Ganciclovir Injection with or without Oral Valganciclovir versus Topical 2% Ganciclovir for Cytomegalovirus Anterior Segment Infection. Ocular immunology and inflammation. 2025 Jun 2:():1-7. doi: 10.1080/09273948.2025.2508402. Epub 2025 Jun 2 [PubMed PMID: 40456106]
Paulbuddhe V, Addya S, Gurnani B, Singh D, Tripathy K, Chawla R. Sympathetic Ophthalmia: Where Do We Currently Stand on Treatment Strategies? Clinical ophthalmology (Auckland, N.Z.). 2021:15():4201-4218. doi: 10.2147/OPTH.S289688. Epub 2021 Oct 20 [PubMed PMID: 34707340]
Ricciotti E, FitzGerald GA. Prostaglandins and inflammation. Arteriosclerosis, thrombosis, and vascular biology. 2011 May:31(5):986-1000. doi: 10.1161/ATVBAHA.110.207449. Epub [PubMed PMID: 21508345]
Level 3 (low-level) evidenceNishant P, Gurnani B, Singh P, Sinha S, Kaur K, Kumar A, Sinha RK. Current concepts and recent trends in endothelial keratoplasty. World journal of transplantation. 2025 Jun 18:15(2):102507. doi: 10.5500/wjt.v15.i2.102507. Epub [PubMed PMID: 40535500]
Goodfellow JF, Nabili S, Jones MN, Nguyen DQ, Armitage WJ, Cook SD, Tole DM. Antiviral treatment following penetrating keratoplasty for herpetic keratitis. Eye (London, England). 2011 Apr:25(4):470-4. doi: 10.1038/eye.2010.237. Epub 2011 Jan 28 [PubMed PMID: 21274012]
Gurnani B, Czyz CN, Mahabadi N, Havens SJ. Corneal Graft Rejection. StatPearls. 2025 Jan:(): [PubMed PMID: 30085585]
Gurnani B, Kaur K, Lalgudi VG, Tripathy K. Risk Factors for Descemet Membrane Endothelial Keratoplasty Rejection: Current Perspectives- Systematic Review. Clinical ophthalmology (Auckland, N.Z.). 2023:17():421-440. doi: 10.2147/OPTH.S398418. Epub 2023 Feb 1 [PubMed PMID: 36755886]
Level 1 (high-level) evidenceOkoye GS, Bonabe D, Obasi CU, Munikrishna D, Osho F, Mutali M, Ogwumu K, Oke-Ifidon EO, Nathan IG, Enaholo ES, Suleman AI, Chukwuyem C, Enang AE, Oji RC, Ogechukwu VN, Chidera SP, Ogechukwu HC, Kaur K, Gurnani B. Visual outcomes and complications after phacoemulsification and small incision manual cataract surgery in two eye hospitals. Journal francais d'ophtalmologie. 2025 Jan:48(1):104353. doi: 10.1016/j.jfo.2024.104353. Epub 2024 Nov 18 [PubMed PMID: 39561679]
Pahwa R, Goyal A, Jialal I. Chronic Inflammation. StatPearls. 2025 Jan:(): [PubMed PMID: 29630225]
Lee SY, Gurnani B, Mesfin FB. Blindness. StatPearls. 2025 Jan:(): [PubMed PMID: 28846303]
Gurnani B, Christy J, Narayana S, Rajkumar P, Kaur K, Gubert J. Retrospective multifactorial analysis of Pythium keratitis and review of literature. Indian journal of ophthalmology. 2021 May:69(5):1095-1101. doi: 10.4103/ijo.IJO_1808_20. Epub [PubMed PMID: 33913840]
Level 2 (mid-level) evidenceGurnani B, Kaur K, Venugopal A, Srinivasan B, Bagga B, Iyer G, Christy J, Prajna L, Vanathi M, Garg P, Narayana S, Agarwal S, Sahu S. Pythium insidiosum keratitis - A review. Indian journal of ophthalmology. 2022 Apr:70(4):1107-1120. doi: 10.4103/ijo.IJO_1534_21. Epub [PubMed PMID: 35325996]
Feizi S. Corneal endothelial cell dysfunction: etiologies and management. Therapeutic advances in ophthalmology. 2018 Jan-Dec:10():2515841418815802. doi: 10.1177/2515841418815802. Epub 2018 Dec 7 [PubMed PMID: 30560230]
Level 3 (low-level) evidencePrice MO, Feng MT, Scanameo A, Price FW Jr. Loteprednol Etabonate 0.5% Gel Vs. Prednisolone Acetate 1% Solution After Descemet Membrane Endothelial Keratoplasty: Prospective Randomized Trial. Cornea. 2015 Aug:34(8):853-8. doi: 10.1097/ICO.0000000000000475. Epub [PubMed PMID: 26020827]
Level 1 (high-level) evidenceDeemer AD, Goldstein JE, Ramulu PY. Approaching rehabilitation in patients with advanced glaucoma. Eye (London, England). 2023 Jul:37(10):1993-2006. doi: 10.1038/s41433-022-02303-z. Epub 2022 Dec 16 [PubMed PMID: 36526861]
Gurnani B, Kaur K. Changing drug regimen during COVID-19 pandemic lockdown: An experience from a Tertiary Eye Care Hospital as a cornea specialist. Indian journal of pharmacology. 2020 Sep-Oct:52(5):435-436. doi: 10.4103/ijp.IJP_688_20. Epub [PubMed PMID: 33283776]
Gurnani B, Feroze KB, Patel BC. Neurotrophic Keratitis. StatPearls. 2025 Jan:(): [PubMed PMID: 28613758]
Castro Ochoa KJ, Gurnani B. Ophthalmia Neonatorum. StatPearls. 2025 Jan:(): [PubMed PMID: 31855399]
Gurnani B, Kaur K, Chaudhary S, Gandhi AS, Balakrishnan H, Mishra C, Gosalia H, Dhiman S, Joshi S, Nagtode AH, Jain S, Aguiar M, Rustagi IM. Nystagmus in Clinical Practice: From Diagnosis to Treatment-A Comprehensive Review. Clinical ophthalmology (Auckland, N.Z.). 2025:19():1617-1657. doi: 10.2147/OPTH.S523224. Epub 2025 May 17 [PubMed PMID: 40401036]
Gurnani B, Kaur K. Contact Lenses. StatPearls. 2025 Jan:(): [PubMed PMID: 35593861]
Gurnani B, Kaur K, Sivakumar P, Bhandari S. Retrospective analysis of low vision assistive products - A 6-year review. Saudi journal of ophthalmology : official journal of the Saudi Ophthalmological Society. 2023 Jan-Mar:37(1):32-37. doi: 10.4103/sjopt.sjopt_253_21. Epub 2023 Mar 9 [PubMed PMID: 36968774]
Level 2 (mid-level) evidenceRay A, Gurnani B. Pediatric Cataract. StatPearls. 2025 Jan:(): [PubMed PMID: 34283446]
Aremu TO, Oluwole OE, Adeyinka KO, Schommer JC. Medication Adherence and Compliance: Recipe for Improving Patient Outcomes. Pharmacy (Basel, Switzerland). 2022 Aug 28:10(5):. doi: 10.3390/pharmacy10050106. Epub 2022 Aug 28 [PubMed PMID: 36136839]
Hänsli C, Meier F, Barthelmes D, Böni C. [Prophylaxis of Recurrent Cytomegalovirus Uveitis with Topical Ganciclovir]. Klinische Monatsblatter fur Augenheilkunde. 2019 Apr:236(4):511-515. doi: 10.1055/a-0816-5598. Epub 2019 Feb 14 [PubMed PMID: 30763957]