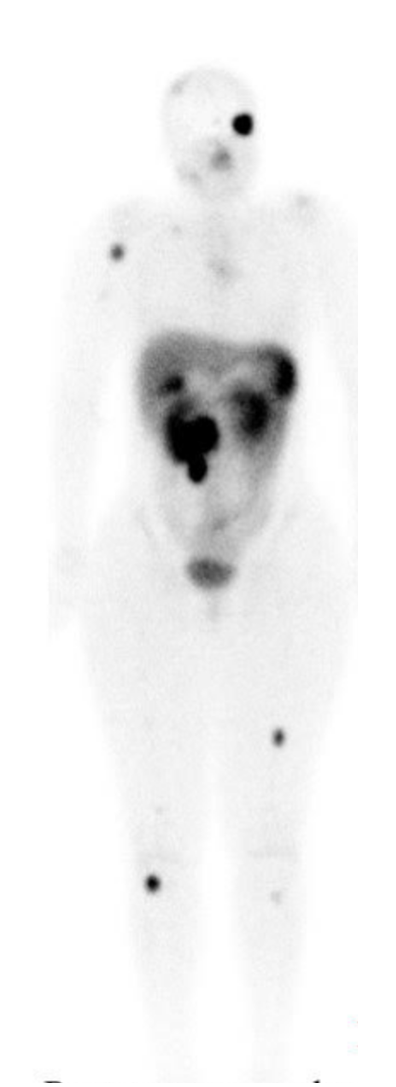
Neuroendocrine Tumor 177Lutetium-Dotatate Therapy
Neuroendocrine Tumor 177Lutetium-Dotatate Therapy
Indications
Neuroendocrine tumors (NETs) are a heterogeneous group of malignancies characterized by various presentations associated with indolent biological behavior. These tumors originate from neuroendocrine cells that are distributed throughout various areas of the body. Lutetium-177 (177Lu)-dotatate therapy or peptide receptor radioligand therapy (PRRT) is indicated for treating gastroenteropancreatic NETs and NET of the prostate gland. Studies have shown that the use of Lu-dotatate in the treatment of advanced, grade 2 to 3, well-differentiated gastroenteropancreatic NETs doubles the median progression-free survival time from approximately 9 months to approximately 23 months.[1][2] Attempts to improve responses with the concurrent administration of Capecitabine were unsuccessful.[3] The surgical literature still advocates their role as primary, with PRRT, eg, the beta-emitting Lu-dotatate, relegated to an adjunctive status in metastatic liver disease.[4]
NETs account for <1% of all malignancies and comprise a diverse family throughout the endocrine system, including the gastrointestinal tract, bronchi, thymus, and adrenal glands. The most common locations are carcinoid gastrointestinal and pancreatic NETs. These are classified based on the degree of differentiation into well-differentiated, moderately differentiated, or poorly differentiated tumors. Well-differentiated NETs arising within the digestive system are referred to as carcinoids. Gastrointestinal neuroendocrine tumors (GNETS) and pancreatic neuroendocrine tumors (PNETs) may have similar histological characteristics, but exhibit variable clinical behavior and biology.[5]
Pancreatic NETs have a relatively worse prognosis than GINETs and respond differently to therapies, with most agents demonstrating somewhat higher responses with PNETs than those with GINETs.[6] The most common site of metastasis is the liver.[6] Management is often dependent on the resectability of the tumor. Patients presenting with resectable or early-stage cancer have excellent clinical outcomes and control of symptoms. However, with unresectable disease, the initial treatment is somatostatin analogs, eg, octreotide or lanreotide, to control symptoms and tumor growth.
Patients with symptomatic disease are often treated initially with somatostatin analogs that are highly effective in controlling the symptoms of carcinoid syndrome, but also control tumor growth. Treatment decisions are based on the patient's clinical presentation, including hormonal symptoms, and the type of treatment received before disease progression. NET patients need staging evaluation with Gallium-68 (68Ga) dotatate or Indium111 pentetreotide imaging to assess the expression of somatostatin receptors. Recently, 68Ga-dotatate imaging has supplanted Indium111 pentetreotide (ie, Octreoscan) imaging based on the availability of PET imaging. Copper64 (Cu64) is a PET isotope similar to 68Ga that can be produced in quantities to meet the clinical needs of imaging centers without the supply shortages of nuclear-generator-based positron emission tomography (PET) isotopes. Cu64-dotatate has advantages over 68Ga-dotatate in detecting significantly more NET lesions than 68Ga-dotatate. The shelf life of Cu64 is over 24 hours, and the scanning window of at least 3 hours makes Cu64-dotatate logistically more favorable to use in the outpatient clinical setting.[7] A subset of patients with clinical or radiological progression after somatostatin analogs are managed with debulking palliative surgery, liver-directed interventions (eg, chemoembolization), or systemic radiolabeled somatostatin analogs, eg, 177Lu-dotatate.[7][8] Most clinical studies reporting efficacy with radiolabeled somatostatin analogs have included PNETs and GINETs.[9]
177Lu-dotatate also has efficacy in treating NET in other nonendocrine organs, eg, the prostate.[10] When prostate cancer has progressed to an androgen-resistant state, one such result is neuroendocrine prostate cancer (NEPC).[11] This is due to the amplification of the NMYC and AURKA genes at the genetic level. NEPC is a highly aggressive variant with more frequent distant metastases, the development of drug resistance, more frequent loss of RB1 and TP53 genes, and patient death within a year. Studies have demonstrated a response to 177Lu-dotatate with this tumor.[12][13][14] Despite dose-related renal toxicity, 177Lu-dotatate is deemed safe and effective in NEPC.
Mechanism of Action
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Mechanism of Action
177Lu-dotatate therapy is a form of endo-radiotherapy that combines a radionuclide with a peptide that specifically binds to peptide receptors. The high affinity of these somatostatin receptors (SSTRs) causes the internalization of the receptor–peptide complex through endocytosis. These peptide receptors (ie, somatostatin receptors) are expressed at high concentrations on tumor cells as assessed by 68Ga positron emission tomography (PET) scans.[15]
The FDA approved 177Lu-dotatate therapy in 2018 to treat somatostatin receptor (SSTR) positive gastroenteropancreatic NETs. Most NETs show relatively high expression levels of SSTRs, which allows imaging and therapy using radiolabeled somatostatin analogs. 177Lu is a commonly used radionucleotide for targeted radiation therapy. The combination of radionucleotide 177Lu with the somatostatin analog dotatate delivers ionizing radiation that targets tumor cells expressing somatostatin receptors, causing radiation-induced single- and double-stranded DNA breaks that lead to apoptosis. This action is more specific for somatostatin receptor-positive lesions.[16] Based on recent clinical studies, this treatment has yielded a significant response rate, accompanied by substantial symptomatic improvement.[17] However, long-term effects include renal dysfunction, pancytopenia, and myelodysplastic syndrome. This treatment is commonly indicated for well-differentiated tumors with relatively high expression of somatostatin receptors. 177Lu-dotatate is the treatment of choice for well-differentiated NETs, especially those that progress despite using long-acting somatostatin analogs. This treatment is favored over monitoring or administering targeted therapies (eg, everolimus or sunitinib).
177Lu-dotatate is administered as an intravenous infusion in an outpatient setting. The recommended dose is 7.4 GBq (200 mCi) every 8 weeks for 4 doses. Dose adjustments may be needed based on patients' renal and hematological parameters. Long-acting somatostatin analogs will be discontinued for at least 4 weeks before initiating this therapy. However, short-acting octreotide is prescribed as needed for symptom control. After treatment, long-acting somatostatin analogs are continued every 4 weeks until the disease progresses.[18]
The global phase 3 NETTER-1 trial is the most compelling evidence of clinical benefit for 177Lu-dotatate in midgut GINETs. In this study, 229 patients with inoperable, somatostatin-receptor-positive midgut NETs who experienced progressive disease on standard doses of long-acting octreotide were randomly assigned to 4 doses of 177Lu-dotatate every 8 weeks in 116 patients and long-acting octreotide 60 mg administered intramuscularly every 28 days in 113 patients.[18] In the 177Lu-dotatate group, patients continued to receive long-acting octreotide at 30 mg after each infusion of 177Lu-dotatate and then monthly after completing all 4 treatments. The study met the primary endpoint of progression-free survival (PFS). The estimated PFS rate at month 20 was significantly higher with 177Lu-dotatate, at 65.2% compared to 10.8%, which reached statistical significance. Among patients evaluated for radiographic response, 177Lu-dotatate showed a significantly higher objective response rate of 18% compared to 3% for octreotide. The treatment-related serious adverse events are more common with 177Lu-dotatate at 9% versus 1%. The most common adverse event in the 177Lu-dotatate was nausea, possibly due to the amino acid infusions administered during therapy for renal protection. 177Lu-dotatate is associated with hematologic toxicities due to irradiation of the bone marrow. The Netter-1 study reported mild degrees of thrombocytopenia in 25% of participants, lymphopenia in 18%, anemia in 14%, and leukopenia in 10% of participants that occurred 4 to 6 weeks after each infusion and resolved within 8 weeks.[19]
This treatment also has an impact on the quality of life. A study concluded that the time to deterioration in the quality of life was significantly longer with 177Lu-dotatate compared with octreotide in several domains, including global health status, physical functioning, role functioning, fatigue, pain, diarrhea, disease-related worries, and body image. The differences were clinically significant in global health status, with median times to deterioration in quality of life of 28.8 months versus 6.1 months and physical functioning of 25.2 months versus 11.5 months.[20] In a later analysis, 5 years after the last patient, long-term outcomes were randomized with a median follow-up of 76 months in both groups.[21][55] Notably, 12% of the 177 patients in the 177Lu-dotatate group received further PRRT, whereas 36% of patients in the control group had a crossover to 177Lu-dotatate. The final overall survival in the intent-to-treat population was not significantly different between the study groups (HR, 0.84; 95% CI, 0.60-1.17), likely due to the crossover design.
Myelodysplastic syndrome (MDS) was reported with this treatment. Based on this data from the NETTER-1 trial, 177Lu-dotatate was approved to treat somatostatin-receptor-positive gastroenteropancreatic NETs in adults. The recommended dosage is 7.4 GBq (200 mCi), administered via intravenous infusion every 8 weeks for a total of 4 doses. No definitive selection criteria for 177Lu-dotatate have been established. The European Society guidelines suggested 177Lu-dotatate therapy in patients with an inoperable metastatic grade 1 and 2 well-differentiated NET with sufficient tumor uptake on diagnostic somatostatin receptor-based imaging and adequate renal and bone marrow reserve.[22]
Administration
The most common adverse effect associated with 177Lu-dotatate therapy is nausea with an incidence of up to 60%, associated with the amino acid infusions administered along with the treatment for renal protective effects. Beyond the administration day, the incidence of nausea is substantially lower. Hematological toxicities, including thrombocytopenia, lymphopenia, anemia, or leukopenia, were also reported with an incidence of approximately 10% to 25%, typically occurring 4 weeks after the infusions begin and lasting an average of 8 weeks.[19]
PRRT has associated radiation risks that require close monitoring and counseling. Following each treatment, low radiation activity levels occur due to the ongoing decay of radionucleotide disintegration. The day after treatments, the amount of radiation released is not harmful to surrounding individuals; however, this limited radiation could trigger alarms at international airports.[20] The most severe long-term toxicity associated with PRRT, with a median incidence of 0.5% to 2% 1 year after completion of PRRT, is therapy-related myeloid neoplasms (eg, myelodysplastic syndrome, acute leukemia, or myeloproliferative neoplasms).[23][24] The definitive patient-related risk factors that could potentially place them at higher risk of developing therapy-related neoplasms are unknown. However, advanced age and bone metastasis are the potential risk factors that need further confirmation.[25]
Therefore, the patients at high risk for developing myeloid neoplasms require close monitoring with complete blood counts for at least the first 2 years after 177Lu-dotatate therapy. Renal dysfunction occurs in up to 20% of patients secondary to glomerular damage by radiation with PRRT.[26] No clear guidelines have been established for continuing somatostatin analogs as maintenance therapy after PRRT. Fewer studies have indicated possible clinical benefits of combining PRRT with somatostatin analogs, preferably in patients with functional NETs.[27][28] The limitations of 177Lu-dotatate at present also include the complexity of administration. The 177Lu-dotatate infusion is currently administered in the nuclear medicine department and needs thorough communication with medical oncology.
Adverse Effects
PRRT is associated with improved clinical outcomes, including overall and progression-free survival. PRRT is also related to improvements in clinical symptoms, quality of life parameters (eg, global health status, body image, and physical functioning), and other symptoms (eg, fatigue and diarrhea). The median time for quality of life deterioration is significantly higher with PRRT, with a difference of over 22 months.[20]
A long-term retrospective study on 468 advanced NET patients who underwent at least 2 cycles of 177Lu-dotatate PRRT showed substantially improved quality of life and biochemical markers in most patients. With a median follow-up of 46 months, most patients achieved excellent disease control. The overall survival rate at 7 years was approximately 80%.[29] 177Lu-dotatate PRRT was reported as a safe therapeutic option in heavily pretreated somatostatin receptor-positive bronchial NET patients with a comparable efficacy and safety profile to other systemic treatment options. A retrospective analysis of 25 bronchial NET patients showed a median PFS of 17 months and an OS of 42 months.[30]
Contraindications
Few limitations to 177Lu-dotatate therapy have been identified. The treatment is quite expensive and complex in terms of administration. Ongoing clinical trials continue to compare 177Lu-dotatate therapy with other targeted systemic treatments. Also, 177Lu-dotatate therapy is currently only available at tertiary centers, limiting its widespread availability. Additionally, the optimal selection of the candidates has been challenging.
Monitoring
An interprofessional team provides an integrated approach to treatment decisions, aiming to achieve the best possible clinical outcomes. Clinicians in interventional radiology, medical oncology, and surgical oncology are critical stakeholders in individualized, patient-centered treatment decisions. Randomized clinical trials with a head-to-head comparison of other treatment options, eg, targeted therapies, are still being conducted; therefore, evidence on the optimal sequence of metastatic neuroendocrine tumor treatment is unavailable. Eligible patients are encouraged to enroll in open clinical trials.
Toxicity
PRRT involves complex clinical and navigational patient needs, as well as thorough interdepartmental communication, to prepare a patient for each therapy. A nurse navigator is vital in providing a cohesive solution to address the multifaceted operational requirements associated with PRRT. The navigator provides critical ambulatory support to each patient throughout the treatment journey by delivering interprofessional coordination and patient education, monitoring treatment-related adverse effects, and adhering to radiation safety protocols.
Enhancing Healthcare Team Outcomes
NETs are a rare and heterogeneous group of malignancies arising from neuroendocrine cells throughout the body, most commonly in the gastrointestinal tract and pancreas. These tumors range from indolent, well-differentiated lesions to aggressive, poorly differentiated variants. Management depends on tumor type, stage, and somatostatin receptor expression. For advanced, somatostatin receptor-positive NETs, PRRT with 177Lu-dotatate has demonstrated significant improvements in progression-free survival, symptom control, and quality of life. Treatment selection requires careful evaluation of imaging, clinical status, and prior therapies, with close monitoring for hematologic and renal toxicities.
Effective care of NET patients requires a coordinated, interprofessional approach. Physicians and advanced practitioners are responsible for diagnosis, treatment planning, and monitoring therapeutic response, while nurses and navigators provide patient education, symptom management, and adherence support. Pharmacists ensure safe handling and dosing of PRRT and supportive medications. Collaboration with radiology, oncology, and surgical teams is essential to optimize sequencing of therapies, monitor adverse effects, and ensure safe delivery of treatment. This integrated strategy enhances patient-centered care, improves clinical outcomes, and maintains safety while fostering efficient team performance.
Media
(Click Image to Enlarge)
References
Singh S, Halperin D, Myrehaug S, Herrmann K, Pavel M, Kunz PL, Chasen B, Tafuto S, Lastoria S, Capdevila J, García-Burillo A, Oh DY, Yoo C, Halfdanarson TR, Falk S, Folitar I, Zhang Y, Aimone P, de Herder WW, Ferone D, all the NETTER-2 Trial Investigators. [(177)Lu]Lu-DOTA-TATE plus long-acting octreotide versus high‑dose long-acting octreotide for the treatment of newly diagnosed, advanced grade 2-3, well-differentiated, gastroenteropancreatic neuroendocrine tumours (NETTER-2): an open-label, randomised, phase 3 study. Lancet (London, England). 2024 Jun 29:403(10446):2807-2817. doi: 10.1016/S0140-6736(24)00701-3. Epub 2024 Jun 5 [PubMed PMID: 38851203]
Level 1 (high-level) evidenceHolzgreve A, Unterrainer LM, Tiling M, Mansour N, Spitzweg C, Brendel M, Ricke J, Unterrainer M, Kunz WG, Mehrens D. Cost-Effectiveness of [(177)Lu]Lu-DOTATATE for the Treatment of Newly Diagnosed Advanced Gastroenteropancreatic Neuroendocrine Tumors: An Analysis Based on Results of the NETTER-2 Trial. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 2025 Jul 1:66(7):1075-1081. doi: 10.2967/jnumed.124.269416. Epub 2025 Jul 1 [PubMed PMID: 40473457]
Satapathy S, Aggarwal P, Sood A, Chandekar KR, Das CK, Gupta R, Khosla D, Das N, Kapoor R, Kumar R, Singh H, Shukla J, Kumar A, Mittal BR. (177)Lu-DOTATATE Plus Capecitabine Versus (177)Lu-DOTATATE Alone in Patients with Advanced Grade 1/2 Gastroenteropancreatic Neuroendocrine Tumors (LuCAP): A Randomized, Phase 2 Trial. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 2025 Feb 3:66(2):238-244. doi: 10.2967/jnumed.124.268617. Epub 2025 Feb 3 [PubMed PMID: 39778968]
Level 1 (high-level) evidenceKenney LM, Hughes M. Surgical Management of Gastroenteropancreatic Neuroendocrine Tumors. Cancers. 2025 Jan 23:17(3):. doi: 10.3390/cancers17030377. Epub 2025 Jan 23 [PubMed PMID: 39941746]
Duerr EM, Chung DC. Molecular genetics of neuroendocrine tumors. Best practice & research. Clinical endocrinology & metabolism. 2007 Mar:21(1):1-14 [PubMed PMID: 17382262]
Level 3 (low-level) evidenceDasari A, Shen C, Halperin D, Zhao B, Zhou S, Xu Y, Shih T, Yao JC. Trends in the Incidence, Prevalence, and Survival Outcomes in Patients With Neuroendocrine Tumors in the United States. JAMA oncology. 2017 Oct 1:3(10):1335-1342. doi: 10.1001/jamaoncol.2017.0589. Epub [PubMed PMID: 28448665]
Johnbeck CB, Knigge U, Loft A, Berthelsen AK, Mortensen J, Oturai P, Langer SW, Elema DR, Kjaer A. Head-to-Head Comparison of (64)Cu-DOTATATE and (68)Ga-DOTATOC PET/CT: A Prospective Study of 59 Patients with Neuroendocrine Tumors. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 2017 Mar:58(3):451-457. doi: 10.2967/jnumed.116.180430. Epub 2016 Sep 22 [PubMed PMID: 27660147]
Kwekkeboom DJ, de Herder WW, Kam BL, van Eijck CH, van Essen M, Kooij PP, Feelders RA, van Aken MO, Krenning EP. Treatment with the radiolabeled somatostatin analog [177 Lu-DOTA 0,Tyr3]octreotate: toxicity, efficacy, and survival. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2008 May 1:26(13):2124-30. doi: 10.1200/JCO.2007.15.2553. Epub [PubMed PMID: 18445841]
Level 1 (high-level) evidenceImhof A, Brunner P, Marincek N, Briel M, Schindler C, Rasch H, Mäcke HR, Rochlitz C, Müller-Brand J, Walter MA. Response, survival, and long-term toxicity after therapy with the radiolabeled somatostatin analogue [90Y-DOTA]-TOC in metastasized neuroendocrine cancers. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2011 Jun 10:29(17):2416-23. doi: 10.1200/JCO.2010.33.7873. Epub 2011 May 9 [PubMed PMID: 21555692]
Ichikawa Y, Kobayashi N, Takano S, Kato I, Endo K, Inoue T. Neuroendocrine tumor theranostics. Cancer science. 2022 Jun:113(6):1930-1938. doi: 10.1111/cas.15327. Epub 2022 Apr 11 [PubMed PMID: 35271754]
Xie Y, Ning S, Hu J. Molecular mechanisms of neuroendocrine differentiation in prostate cancer progression. Journal of cancer research and clinical oncology. 2022 Jul:148(7):1813-1823. doi: 10.1007/s00432-022-04061-7. Epub 2022 May 28 [PubMed PMID: 35633416]
Assadi M, Pirayesh E, Rekabpour SJ, Zohrabi F, Jafari E, Nabipour I, Esmaili A, Amini A, Ahmadzadehfar H. 177Lu-PSMA and 177Lu-DOTATATE Therapy in a Patient With Metastatic Castration-Resistant Prostate Cancer and Neuroendocrine Differentiation. Clinical nuclear medicine. 2019 Dec:44(12):978-980. doi: 10.1097/RLU.0000000000002824. Epub [PubMed PMID: 31689280]
Liu C, Liu T, Zhang J, Baum RP, Yang Z. Excellent Response to 177Lu-DOTATATE Peptide Receptor Radionuclide Therapy in a Patient With Progressive Metastatic Castration-Resistant Prostate Cancer With Neuroendocrine Differentiation After 177Lu-PSMA Therapy. Clinical nuclear medicine. 2019 Nov:44(11):876-878. doi: 10.1097/RLU.0000000000002780. Epub [PubMed PMID: 31584494]
Nesari Javan F, Aryana K, Askari E. Prostate Cancer With Neuroendocrine Differentiation Recurring After Treatment With 177Lu-PSMA: A Chance for 177Lu-DOTATATE Therapy? Clinical nuclear medicine. 2021 Sep 1:46(9):e480-e482. doi: 10.1097/RLU.0000000000003685. Epub [PubMed PMID: 34028407]
Hennrich U, Kopka K. Lutathera(®): The First FDA- and EMA-Approved Radiopharmaceutical for Peptide Receptor Radionuclide Therapy. Pharmaceuticals (Basel, Switzerland). 2019 Jul 29:12(3):. doi: 10.3390/ph12030114. Epub 2019 Jul 29 [PubMed PMID: 31362406]
Feijtel D, Doeswijk GN, Verkaik NS, Haeck JC, Chicco D, Angotti C, Konijnenberg MW, de Jong M, Nonnekens J. Inter and intra-tumor somatostatin receptor 2 heterogeneity influences peptide receptor radionuclide therapy response. Theranostics. 2021:11(2):491-505. doi: 10.7150/thno.51215. Epub 2021 Jan 1 [PubMed PMID: 33391488]
Urso L, Nieri A, Uccelli L, Castello A, Artioli P, Cittanti C, Marzola MC, Florimonte L, Castellani M, Bissoli S, Porto F, Boschi A, Evangelista L, Bartolomei M. Lutathera(®) Orphans: State of the Art and Future Application of Radioligand Therapy with (177)Lu-DOTATATE. Pharmaceutics. 2023 Mar 31:15(4):. doi: 10.3390/pharmaceutics15041110. Epub 2023 Mar 31 [PubMed PMID: 37111596]
Strosberg J, El-Haddad G, Wolin E, Hendifar A, Yao J, Chasen B, Mittra E, Kunz PL, Kulke MH, Jacene H, Bushnell D, O'Dorisio TM, Baum RP, Kulkarni HR, Caplin M, Lebtahi R, Hobday T, Delpassand E, Van Cutsem E, Benson A, Srirajaskanthan R, Pavel M, Mora J, Berlin J, Grande E, Reed N, Seregni E, Öberg K, Lopera Sierra M, Santoro P, Thevenet T, Erion JL, Ruszniewski P, Kwekkeboom D, Krenning E, NETTER-1 Trial Investigators. Phase 3 Trial of (177)Lu-Dotatate for Midgut Neuroendocrine Tumors. The New England journal of medicine. 2017 Jan 12:376(2):125-135. doi: 10.1056/NEJMoa1607427. Epub [PubMed PMID: 28076709]
Brabander T, van der Zwan WA, Teunissen JJM, Kam BLR, Feelders RA, de Herder WW, van Eijck CHJ, Franssen GJH, Krenning EP, Kwekkeboom DJ. Long-Term Efficacy, Survival, and Safety of [(177)Lu-DOTA(0),Tyr(3)]octreotate in Patients with Gastroenteropancreatic and Bronchial Neuroendocrine Tumors. Clinical cancer research : an official journal of the American Association for Cancer Research. 2017 Aug 15:23(16):4617-4624. doi: 10.1158/1078-0432.CCR-16-2743. Epub 2017 Apr 20 [PubMed PMID: 28428192]
Strosberg J, Wolin E, Chasen B, Kulke M, Bushnell D, Caplin M, Baum RP, Kunz P, Hobday T, Hendifar A, Oberg K, Sierra ML, Thevenet T, Margalet I, Ruszniewski P, Krenning E, NETTER-1 Study Group. Health-Related Quality of Life in Patients With Progressive Midgut Neuroendocrine Tumors Treated With (177)Lu-Dotatate in the Phase III NETTER-1 Trial. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2018 Sep 1:36(25):2578-2584. doi: 10.1200/JCO.2018.78.5865. Epub 2018 Jun 7 [PubMed PMID: 29878866]
Level 2 (mid-level) evidenceStrosberg JR, Caplin ME, Kunz PL, Ruszniewski PB, Bodei L, Hendifar A, Mittra E, Wolin EM, Yao JC, Pavel ME, Grande E, Van Cutsem E, Seregni E, Duarte H, Gericke G, Bartalotta A, Mariani MF, Demange A, Mutevelic S, Krenning EP, NETTER-1 investigators. (177)Lu-Dotatate plus long-acting octreotide versus high‑dose long-acting octreotide in patients with midgut neuroendocrine tumours (NETTER-1): final overall survival and long-term safety results from an open-label, randomised, controlled, phase 3 trial. The Lancet. Oncology. 2021 Dec:22(12):1752-1763. doi: 10.1016/S1470-2045(21)00572-6. Epub 2021 Nov 15 [PubMed PMID: 34793718]
Level 1 (high-level) evidenceHicks RJ, Kwekkeboom DJ, Krenning E, Bodei L, Grozinsky-Glasberg S, Arnold R, Borbath I, Cwikla J, Toumpanakis C, Kaltsas G, Davies P, Hörsch D, Tiensuu Janson E, Ramage J, Antibes Consensus Conference participants. ENETS Consensus Guidelines for the Standards of Care in Neuroendocrine Neoplasia: Peptide Receptor Radionuclide Therapy with Radiolabeled Somatostatin Analogues. Neuroendocrinology. 2017:105(3):295-309. doi: 10.1159/000475526. Epub 2017 Apr 13 [PubMed PMID: 28402980]
Level 3 (low-level) evidenceBergsma H, van Lom K, Raaijmakers MHGP, Konijnenberg M, Kam BLBLR, Teunissen JJM, de Herder WW, Krenning EP, Kwekkeboom DJ. Persistent Hematologic Dysfunction after Peptide Receptor Radionuclide Therapy with (177)Lu-DOTATATE: Incidence, Course, and Predicting Factors in Patients with Gastroenteropancreatic Neuroendocrine Tumors. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 2018 Mar:59(3):452-458. doi: 10.2967/jnumed.117.189712. Epub 2017 Aug 3 [PubMed PMID: 28775205]
Sonbol MB, Halfdanarson TR, Hilal T. Assessment of Therapy-Related Myeloid Neoplasms in Patients With Neuroendocrine Tumors After Peptide Receptor Radionuclide Therapy: A Systematic Review. JAMA oncology. 2020 Jul 1:6(7):1086-1092. doi: 10.1001/jamaoncol.2020.0078. Epub [PubMed PMID: 32297906]
Level 1 (high-level) evidenceBrieau B, Hentic O, Lebtahi R, Palazzo M, Ben Reguiga M, Rebours V, Maire F, Hammel P, Ruszniewski P, Fenaux P. High risk of myelodysplastic syndrome and acute myeloid leukemia after 177Lu-octreotate PRRT in NET patients heavily pretreated with alkylating chemotherapy. Endocrine-related cancer. 2016 May:23(5):L17-23. doi: 10.1530/ERC-15-0543. Epub 2016 Mar 1 [PubMed PMID: 26932783]
Bergsma H, Konijnenberg MW, van der Zwan WA, Kam BL, Teunissen JJ, Kooij PP, Mauff KA, Krenning EP, Kwekkeboom DJ. Nephrotoxicity after PRRT with (177)Lu-DOTA-octreotate. European journal of nuclear medicine and molecular imaging. 2016 Sep:43(10):1802-11. doi: 10.1007/s00259-016-3382-9. Epub 2016 May 10 [PubMed PMID: 27160225]
Yordanova A, Wicharz MM, Mayer K, Brossart P, Gonzalez-Carmona MA, Strassburg CP, Fimmers R, Essler M, Ahmadzadehfar H. The Role of Adding Somatostatin Analogues to Peptide Receptor Radionuclide Therapy as a Combination and Maintenance Therapy. Clinical cancer research : an official journal of the American Association for Cancer Research. 2018 Oct 1:24(19):4672-4679. doi: 10.1158/1078-0432.CCR-18-0947. Epub 2018 Jun 27 [PubMed PMID: 29950352]
Pavel M, Öberg K, Falconi M, Krenning EP, Sundin A, Perren A, Berruti A, ESMO Guidelines Committee. Electronic address: clinicalguidelines@esmo.org. Gastroenteropancreatic neuroendocrine neoplasms: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Annals of oncology : official journal of the European Society for Medical Oncology. 2020 Jul:31(7):844-860. doi: 10.1016/j.annonc.2020.03.304. Epub 2020 Apr 6 [PubMed PMID: 32272208]
Level 1 (high-level) evidenceSitani K, Parghane RV, Talole S, Basu S. Long-term outcome of indigenous (177)Lu-DOTATATE PRRT in patients with Metastatic Advanced Neuroendocrine Tumours: a single institutional observation in a large tertiary care setting. The British journal of radiology. 2021 Jan 1:94(1117):20201041. doi: 10.1259/bjr.20201041. Epub 2020 Oct 29 [PubMed PMID: 33095671]
Mirvis E, Toumpanakis C, Mandair D, Gnanasegaran G, Caplin M, Navalkissoor S. Efficacy and tolerability of peptide receptor radionuclide therapy (PRRT) in advanced metastatic bronchial neuroendocrine tumours (NETs). Lung cancer (Amsterdam, Netherlands). 2020 Dec:150():70-75. doi: 10.1016/j.lungcan.2020.10.005. Epub 2020 Oct 14 [PubMed PMID: 33075738]