Introduction
Acute pediatric emergencies arise from diverse etiologies, including respiratory failure, cardiac arrest, shock, and severe infections. Newly delivered infants experiencing critical events often present with apnea, bradycardia, hypotonia, and central cyanosis due to incomplete cardiopulmonary transition. In contrast, clinical decompensation in older children typically manifests as altered consciousness, tachypnea, hypotension, and evidence of respiratory or cardiac failure, reflecting distinct pathophysiological mechanisms. Prompt recognition and implementation of age-targeted resuscitative measures are essential to optimize survival and long-term outcomes.
Neonatal and pediatric resuscitation employs structured, algorithmic methods to restore spontaneous circulation and stabilize critical physiologic derangements in infants and children. Although the framework parallels adult resuscitation, emergency interventions in the pediatric population must account for unique considerations, including age-appropriate differential diagnoses, weight- and age-specific medication dosing, specialized procedural techniques, and tailored postresuscitation care.
The American Heart Association (AHA) and American Academy of Pediatrics (AAP) provide evidence-based guidelines that outline stepwise evaluation, airway management, ventilation, chest compressions, vascular access, and pharmacologic support. Mastery of these procedures enables clinicians to respond efficiently, reduce morbidity and mortality, and deliver safe, high-quality care across diverse clinical settings.
Clinical Considerations During Neonatal Resuscitation
The initiation of pulmonary ventilation upon birth transforms circulatory dynamics. Pulmonary vascular resistance falls abruptly as lung expansion and oxygen uptake enhance pulmonary blood flow. Rising left-atrial pressure leads to functional closure of the foramen ovale.[1] Increased oxygen tension and reduced prostaglandin levels trigger constriction of the ductus arteriosus, redirecting flow through the pulmonary circuit. The ductus venosus and umbilical vein, along with the paired umbilical arteries, lose patency as placental circulation ceases.[2] These coordinated adaptations establish distinct pulmonary and systemic circuits essential for neonatal survival.
Most newborns achieve this transition effectively shortly after birth without intervention. For these infants, standard resuscitation steps include providing warmth, drying, clearing secretions if needed, and positioning the airway to support spontaneous respirations. Gentle stimulation is performed to encourage effective breathing, while heart rate, tone, and color are monitored. Ongoing observation ensures maintenance of normal respiration, heart rate above 100 bpm, and appropriate oxygen saturation within target ranges.[3]
Various maternal, fetal, and intrapartum conditions can complicate the transition at birth and increase the likelihood of requiring neonatal resuscitation. Such conditions include the following:
- Gestational age concerns: Preterm birth younger than 36 weeks, postterm birth older than 40 weeks, and multiple pregnancy before 35 weeks
- Maternal conditions: Hypertension, preeclampsia or eclampsia, infection, and exposure to magnesium or general anesthesia
- Fetal growth and anomalies: Intrauterine growth restriction, macrosomia, anemia, hydrops, and major congenital malformations
- Amniotic fluid abnormalities: Polyhydramnios, oligohydramnios, or meconium-stained amniotic fluid
- Placental and cord complications: Placental abruption, intrapartum bleeding, prolapsed umbilical cord, and abnormal fetal heart rate patterns
- Labor and delivery complications: Emergency cesarean section, breech or other malpresentation, and shoulder dystocia
- Medication exposures: Maternal narcotics administered within 4 hours of delivery
- Social factors: Absence of prenatal care [4]
Anticipating these risk factors allows the resuscitation team to prepare appropriate personnel, equipment, and interventions before delivery.
Contexts of Pediatric Resuscitation Beyond the Perinatal Period
Unlike in adults, cardiac arrest after the perinatal period in children is usually due to respiratory failure or shock rather than primary cardiac disease. The mnemonic "Hs and Ts" summarizes the reversible causes:
- Hs: Hypovolemia, hypoxia, hydrogen ion imbalance, hypokalemia/hyperkalemia, hypothermia
- Ts: Tension pneumothorax, tamponade, toxins, thrombosis, trauma
Among these conditions, hypoxia and hypovolemia account for the majority of pediatric arrests. Toxins, severe acidosis, and electrolyte disturbances usually arise in specific contexts such as ingestion, sepsis, renal disease, or metabolic disorders.
Out-of-hospital cardiac arrest (OHCA), defined as the cessation of cardiac activity occurring outside a healthcare facility, typically follows progressive respiratory compromise and presents with hypoxemia, bradycardia, and eventual pulselessness, often with delayed recognition and intervention. In-hospital cardiac arrest (IHCA), defined as cardiac arrest occurring in patients under active medical care within a healthcare facility, arises in children with comorbidities such as sepsis, congenital heart disease, or postoperative instability and is often preceded by abnormal vital signs or metabolic deterioration detected on monitoring. Identifying underlying causes and prearrest physiology before and during resuscitation enables targeted interventions that enhance the potential for return of spontaneous circulation (ROSC) and favorable neurological outcomes.[5]
Anatomy and Physiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Anatomy and Physiology
Anatomical differences between pediatric and adult patients critically influence positioning and resuscitative procedures during emergency care. These distinctions demand tailored strategies to optimize stabilization and recovery.
In children, a proportionally larger tongue, prominent occiput, shorter neck, and anteriorly positioned vocal cords complicate alignment of the oral, laryngeal, and tracheal axes, while a short hypopharynx and cephalad larynx increase the risk of obstruction during manipulation.[6][7] The subglottic region represents the narrowest airway segment, and even minor edema or inflammation can dramatically increase airflow impedance, given that airway resistance correlates inversely with the 4th power of the radius.[8]
Pediatric respiratory physiology increases vulnerability during resuscitation. A higher metabolic rate and oxygen consumption, along with lower functional residual capacity and limited oxygen reserve, cause rapid oxygen desaturation when ventilation ceases.[9] Diaphragmatic-dominant breathing and a highly compliant thorax reduce ventilatory efficiency and predispose to rapid fatigue during assisted ventilation. These factors markedly shorten the duration of safely tolerated apnea, making timely airway management and effective ventilation critical.[10]
Neonatal circulation is uniquely influenced by transitional physiology, including closure of fetal shunts, which alters preload, afterload, and pulmonary blood flow. A smaller circulating blood volume (about 80-100 mL/kg) and limited stroke volume reserve make cardiac output highly heart rate-dependent.[11][12] Immature autonomic regulation in neonates further reduces compensatory responses to hypovolemia or hypotension, increasing vulnerability to rapid hemodynamic deterioration. In contrast, stroke volume in older children is more stable, and autonomic responses are more mature. However, the smaller blood volume relative to adults still predisposes to rapid shock progression.
Exploiting key pediatric anatomic and physiologic characteristics can improve the success of resuscitative interventions. The extensive alveolar-capillary surface area allows certain drugs to be absorbed via the endotracheal tube (ETT) when intravenous access is delayed, providing a critical pharmacologic route. Intraosseous access offers rapid, noncollapsible entry into the central circulation, with preferred sites including the proximal tibia, distal tibia, and proximal humerus, facilitating timely administration of fluids and medications during emergency resuscitation.
Pediatric critical care emphasizes the sequence of airway, breathing, and circulation (ABC) due to hypoxia being the primary cause of arrest. This order contrasts with that used in adult resuscitation, where lifesaving interventions generally follow a circulation-first order (CAB), as cardiac arrest in this population more commonly results from arrhythmias.
Indications
Resuscitation should be initiated immediately in the presence of cyanosis, asystole, respiratory arrest, or a heart rate below 60 bpm in a critically ill child. Assisted ventilation is indicated when respiratory failure is impending or the patient has a pulse but respiratory effort is insufficient. The recommended ventilation rate is 1 breath every 2 to 3 seconds (20 to 30 breaths/min).
Other pediatric emergencies, such as pulseless tachycardia and septic shock, have specific protocols outlined in the AHA 2020 Pediatric Advanced Life Support (PALS) guidelines (see Technique or Treatment).[13][14] Intensive care and advanced support are necessary in approximately 1% of pediatric cases.
Contraindications
The 2020 AHA and AAP guidelines list no conditions that absolutely preclude initiating resuscitation in neonates or children. Resuscitation should begin promptly for any child who is not breathing normally or lacks signs of life. Exceptions include severe congenital anomalies incompatible with life or valid advanced directives indicating no resuscitation. Decisions should involve the healthcare team, parents, and ethics consultation when appropriate, ensuring interventions align with the child’s best interests and parental wishes while prioritizing early, lifesaving measures.
Equipment
Effective neonatal and pediatric stabilization requires a range of specialized equipment, outlined in the table, Equipment Needed for Neonatal and Pediatric Resuscitation (see Image. Equipment Needed for Neonatal and Pediatric Resuscitation).[15][16] Cuffed ETTs are preferred over uncuffed types in the latest PALS update, as they reduce the need for tube replacement. The appropriate tube size may be estimated using the formula:
ETT size (mm) = 3.5 + (age in years / 4)
A 3.0-mm tube may be required for patients younger than 3 months. Correct placement should be confirmed by auscultation and end-tidal carbon dioxide measurement.
Routine application of cricoid pressure is discouraged, as it does not reliably prevent regurgitation and may impede intubation.[17] Miller laryngoscope blades are commonly used to lift the U-shaped epiglottis and visualize the vocal cords, while Mackintosh blades remain a viable alternative depending on practitioner preference and availability. Video laryngoscopy or bronchoscopy may assist in managing difficult airways. Following intubation, tidal volumes slightly below the physiologic norm for age or ideal body weight (approximately 5-6 mL/kg) are recommended, with standard positive end-expiratory pressure settings maintained without adjustment.[18]
Personnel
Effective neonatal and pediatric resuscitation requires a coordinated team with clearly defined roles. Ideally, the following personnel should be present:
- Team leader: Directs resuscitation and communicates decisions
- Airway manager: Secures and maintains the airway
- Chest compression provider: Performs high-quality compressions
- Medication and vascular access provider: Establishes intravenous or intraosseous access and administers drugs
- Recorder/monitoring: Documents interventions, timing, and vital signs
- Additional support: Nurses, respiratory therapists, or specialists as needed
Roles may be combined when personnel are limited, with team members multitasking while ensuring that interventions are performed according to established guidelines. In neonatal resuscitation, the focus on airway and ventilation often requires immediate airway expertise, whereas in older children, circulation support may demand earlier allocation of chest compressions and vascular access. Regardless of the setting, a clear leader and organized task assignment are essential to optimize outcomes and ensure efficient use of available resources.
Preparation
All personnel participating in neonatal or pediatric resuscitation should maintain current Neonatal Resuscitation Program (NRP) and PALS certification to ensure familiarity with age-specific algorithms, equipment, and medication dosing. A fully stocked and regularly inspected resuscitation cart should be immediately available, containing airway equipment, medications, fluids, and defibrillation devices appropriate for neonatal and pediatric patients. Facilities providing care for pediatric patients are encouraged to establish a pediatric early warning system (PEWS) and form a rapid response team (RRT) to facilitate timely intervention.
Providers may carry a single-use pocket mask with a 1-way valve to safely perform ventilation during out-of-hospital or unexpected resuscitation. Personal preparedness tools, such as pocket reference cards and portable digital resources, are also recommended for rapid recognition and initiation of resuscitation in such settings until full support is available.
Technique or Treatment
Initial Evaluation
Initial assessment of pediatric patients prior to resuscitation focuses on airway patency, breathing, and circulation, along with evaluation of heart rate, oxygen saturation, and level of consciousness. Rapid identification of respiratory distress, hypoxemia, bradycardia, or hemodynamic compromise guides immediate intervention. Assessment should also consider congenital anomalies, underlying medical conditions, and, for neonates, gestational age, enabling selection of age-appropriate airway management, ventilation strategies, and pharmacologic support to optimize resuscitation outcomes.
Airway and Breathing
The initial step for airway support involves the administration of supplemental oxygen via a nasal cannula, starting at 30% and titrated to maintain oxygen saturation above 94%. Persistent hypoxia should prompt consideration of alternative diagnoses, including congenital airway malformations, cardiac anomalies, or metabolic disorders. In such cases, bag-mask ventilation at a rate of 20 to 30 breaths/min is recommended for both infants and children.
Advanced airway management, including laryngeal mask airway insertion or endotracheal intubation, should be considered if patients fail to respond. During intubation, the head-tilt-chin-lift maneuver may be used to optimize airway access. A jaw thrust is preferred for suspected spinal injury, with head-tilt-chin-lift reserved as a secondary option while maintaining cervical spine immobilization.[19] Administration of 100% oxygen via a high-concentration mask or a bag-valve-mask is recommended before intubation to optimize oxygen saturation.
Household objects and ingested foreign bodies are common causes of pediatric airway compromise. Coughing or wheezing may be observed in patients with partial obstruction, whereas cyanosis or silence indicates complete obstruction. Objects that are visible and easily accessible should be removed immediately. If removal is not possible, back blows, abdominal thrusts, and chest compressions should be performed to dislodge the obstruction.
Cardiopulmonary Resuscitation
High-quality cardiopulmonary resuscitation (CPR) forms the foundation of effective resuscitation and is defined by optimal chest compression rate and depth, minimal interruptions, full chest recoil, and avoidance of excessive ventilation. The recommended compression rate is approximately 100 to 120/min.[20][21]
In neonates, compressions may be performed using either the 2-finger or 2-thumb technique, both considered acceptable for infant CPR. In other pediatric patients, compressions are applied to the lower 1/2 of the sternum using the heel of one or both hands. Compression depth varies with age, as follows:
- Infants: Approximately 4 cm (1.5 inches)
- Children: 5 cm (2 inches)
- Adolescents 5 to 6 cm, not exceeding 6 cm (2.4 inches) per adult recommendations
The chest should be compressed at least 1/3 of its anteroposterior diameter with each compression. For infants and children requiring an advanced airway or receiving rescue breaths while maintaining a pulse, a ventilation rate of 20 to 30 breaths/min is recommended.[22][23][24]
Circulation
Assessment of fluid status is essential in resuscitation management. Rapid fluid administration is the primary intervention for shock, as hypotension indicates a late and severe stage.[25] Diastolic blood pressure is a critical parameter during resuscitation. Maintaining a diastolic blood pressure greater than 25 mm Hg in infants and greater than 30 mm Hg in children is associated with a significant survival benefit, with approximately 70% survival, and a 60% improvement in favorable neurologic outcomes.[26]
Isotonic crystalloids are the preferred fluids for resuscitating children in shock. An initial bolus of 20 mL/kg is recommended for signs of shock, with repeated boluses if systemic perfusion does not improve. Resuscitation for hypovolemia or sepsis may require 40 to 60 mL/kg. Children who remain in shock after 60 mL/kg of intravenous fluids generally require initiation of vasopressor therapy (see Image. Medications Used in Neonatal Resuscitation and Pediatric Advanced Life Support.[27]
Automated External Defibrillator
Biphasic attenuated defibrillators are preferred over standard adult automated external defibrillators, which do not provide energy attenuation. A pediatric dose attenuator should be employed in patients younger than 8 years. Adult defibrillators may be used if pediatric-specific devices are unavailable. Defibrillation is indicated for pulseless ventricular tachycardia and ventricular fibrillation, with an energy dose of 2 to 4 J/kg. Synchronized cardioversion is recommended for unstable tachyarrhythmias, using 0.5 to 2 J/kg.[28]
Medications
Pediatric medication doses are calculated based on the patient’s weight. When a child’s weight is unknown, length-based resuscitation tapes (eg, Broselow tape) can provide a rapid estimation. Preprinted medication cards should be readily available at all pediatric care sites. Intravenous access is preferred, though intraosseous access is a suitable alternative during emergencies. Lidocaine, epinephrine, atropine, and naloxone (mnemonic: LEAN) may be administered endotracheally when vascular access is unavailable.[29] For neonates younger than 14 days, a 5F catheter in the umbilical vein is a reliable 1st-line option.
Fluid-refractory shock necessitates initiation of vasopressor therapy. These agents may be administered via peripheral access if no other option is available. However, transition to central access should occur as soon as feasible. Norepinephrine (for warm shock) and epinephrine (for cold shock) are 1st-line agents, both starting at 0.05 mcg/kg/min. Dopamine serves as a 2nd-line option in both scenarios, typically initiated at 10 mcg/kg/min.
The table, Vasopressors Used in Neonatal Resuscitation and Pediatric Advanced Life Support, summarizes medications commonly employed in neonatal and pediatric resuscitation (see Image. Vasopressors Used in Neonatal Resuscitation and Pediatric Advanced Life Support). This table does not represent an exhaustive list of all agents used in pediatric emergencies. Although most of these medications are generally available in emergency departments and hospitals, outpatient clinics may have limited access. Continuous patient monitoring and frequent reassessment are essential following each intervention.
Neonatal Resuscitation Guidelines and Key Updates
Neonatal resuscitation should be performed according to the 2020 AHA guidelines (see Image. Neonatal Resuscitation Algorithm). Updates from the AHA and AAP in 2023, outlined below, were prompted by new research demonstrating the impact of cord clamping timing, positive pressure ventilation (PPV) methods, and airway device selection on neonatal outcomes.
- In term and late preterm newborns at least 34 weeks’ gestation who do not require resuscitation, delaying cord clamping for at least 30 seconds significantly improves outcomes compared to clamping earlier than 30 seconds.
- In term and late preterm newborns at least 34 weeks’ gestation who do not require resuscitation, intact cord milking has not demonstrated additional benefit compared with delayed cord clamping.
- In late preterm newborns 35 to 42 weeks’ gestation requiring resuscitation, intact cord milking may be considered if early clamping would otherwise be performed.
- In preterm newborns younger than 34 weeks’ gestation who do not require resuscitation, delayed cord clamping is preferred over early clamping.
- In preterm newborns 28 to 34 weeks’ gestation who do not require resuscitation and cannot undergo delayed clamping, intact cord milking may be reasonable.
- In extreme preterm newborns younger than 28 weeks’ gestation, intact cord milking is not recommended.
- PPV remains the highest priority for newborns requiring respiratory support immediately after birth.
- A T-piece resuscitator is preferred over a self-inflating bag for effective delivery of PPV.
- Since both T-piece resuscitators and flow-inflating bags rely on a compressed gas source, a self-inflating bag should always be available in case of gas source failure.
- Supraglottic airway devices may be considered as the primary interface for PPV in newborns delivered at least 34 0/7 weeks’ gestation.[30]
Delaying cord clamping for at least 30 seconds, and up to 60 seconds when clinically appropriate, is a straightforward intervention that can decrease the need for cardiovascular support. This approach mitigates the risk of early hemodynamic decompensation in newborns. In cases where a neonate presents in significant distress at birth, immediate resuscitation takes priority over delayed cord clamping.[31][32]
Pediatric Resuscitation Recommendations and Recent Amendments
The primary objective of PALS is to prevent cardiopulmonary failure and arrest through early recognition and management of respiratory distress, respiratory failure, and shock. Several algorithms exist to assist healthcare providers in delivering timely and systematic interventions across different pediatric emergencies (see Images. Pediatric Cardiac Arrest Algorithm; Pediatric Bradycardia with a Pulse Algorithm; Pediatric Tachycardia with a Pulse Algorithm; Pediatric Septic Shock Algorithm). The key updates below include modifications to chest compression technique, ventilation rates, airway management, pharmacologic interventions, and post-ROSC care:
- High-quality CPR is essential for effective resuscitation and is defined by an optimal chest compression rate and depth, minimal interruptions, full chest recoil, and avoidance of excessive ventilation.
- For infants and children with an advanced airway or receiving rescue breaths while maintaining a pulse, a respiratory rate of 20 to 30 breaths/min is recommended.
- Early administration of epinephrine following initiation of CPR in nonshockable rhythms is associated with improved survival outcomes.
- Cuffed ETTs are preferred in pediatric patients, as their use reduces the need for tube replacement.
- Routine application of cricoid pressure provides no benefit in preventing regurgitation and may impede successful intubation.
- In OHCA, bag-mask ventilation produces outcomes comparable to advanced airway techniques, including endotracheal intubation.
- Post-ROSC care is critical and should include targeted temperature management, continuous electroencephalographic monitoring, and correction of hypotension or hypertension, hypoxia or hyperoxia, and hypocapnia or hypercapnia.
- Survivors of pediatric cardiac arrest may experience ongoing physical, neurocognitive, and emotional impairments, necessitating structured follow-up and rehabilitation.
- Naloxone effectively reverses opioid-induced respiratory arrest but does not demonstrate benefit in patients already in cardiac arrest.
- Fluid resuscitation for septic shock should be guided by patient response and frequent reassessment. Balanced crystalloids, unbalanced crystalloids, and colloids are all acceptable, and vasopressors such as epinephrine or norepinephrine are indicated for fluid-refractory cases.[33]
As mentioned, ROSC postresuscitation requires prompt stabilization, including optimized ventilation, hemodynamic support, temperature management, and neurologic monitoring. If circulation is not restored, continuous high-quality CPR, reassessment of reversible causes, and consideration of advanced therapies such as extracorporeal support may be warranted (see Image. Management of Pediatric Shock After Return of Spontaneous Circulation).
Complications
Initial stabilization may be performed in most healthcare facilities. However, definitive management and long-term care are generally restricted to tertiary or quaternary centers with specialized pediatric services.
Extracorporeal membrane oxygenation should be considered early in IHCA cases. Evidence for OHCAs remains limited, although this modality may be appropriate based on clinician judgment.
While older age often correlates with improved outcomes, postarrest hypoxic-ischemic brain injury continues to contribute substantially to morbidity and mortality in pediatric patients. Continuous EEG monitoring is recommended following ROSC in high-risk or comatose patients to detect subclinical seizures. Anticonvulsants are indicated only when seizures are observed. Autopsy with potential genetic testing should be strongly considered in cases of unexplained death.
Clinical Significance
Effective neonatal resuscitation requires meticulous preparation and anticipation of potential complications. In the U.S., approximately 10% of newborns require some assistance at birth, and 1% warrant intensive resuscitative intervention.[34]
An estimated 20,000 children in the U.S. experience cardiac arrest annually, including roughly 7,000 out-of-hospital events in 2015. OHCAs predominantly result from respiratory causes, whereas IHCAs are more frequently cardiogenic, reflecting the concentration of complex cases in medical facilities. Survival to hospital discharge approaches 40% for IHCAs, compared with approximately 11% for OHCAs. Survival also varies by age, with younger children demonstrating a lower likelihood of favorable outcomes.[35] These data underscore the necessity for all healthcare personnel to maintain familiarity with evidence-based, population-specific resuscitation practices.
Enhancing Healthcare Team Outcomes
Effective team training improves coordination and outcomes during pediatric resuscitation, enhancing the likelihood of ROSC. Key components include accurate pediatric weight-based medication dosing, assignment of specific roles during resuscitation, and delivery of high-quality CPR. Closed-loop communication and clearly designated responsibilities facilitate efficient code management.
At the institutional level, structured educational protocols are necessary to attain and maintain NRP and PALS proficiency and remain current with the latest AHA guidelines for neonatal and pediatric resuscitation. Anticipatory preparation, coordinated teamwork, and precise communication are strongly associated with improved resuscitation outcomes.[36]
Media
(Click Image to Enlarge)
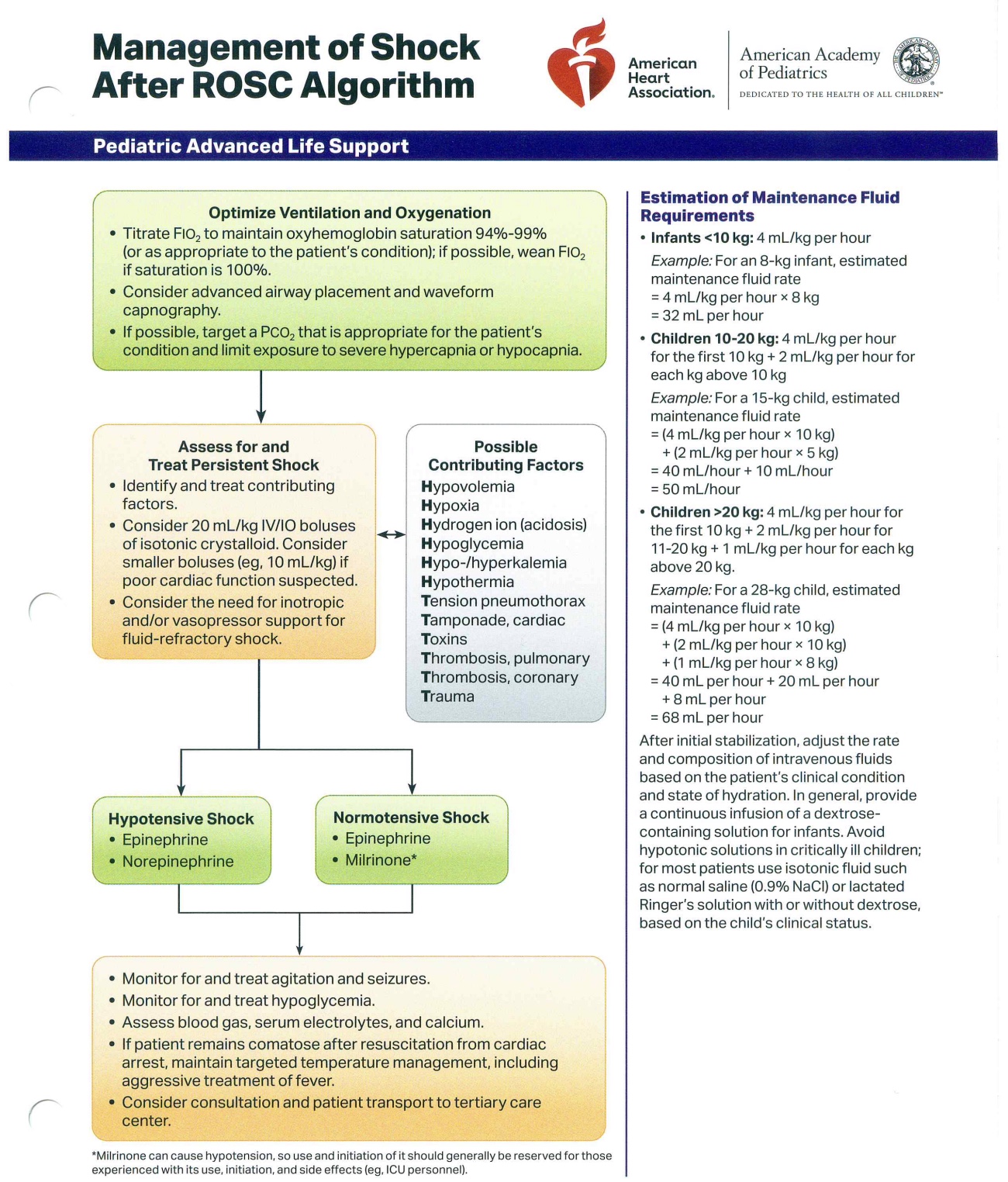
Management of Pediatric Shock After Return of Spontaneous Circulation. This flowchart, based on an American Heart Association and American Academy of Pediatrics algorithm, provides a guide for managing pediatric shock after the return of spontaneous circulation. The algorithm outlines the initial steps, including optimizing ventilation and oxygenation. Details on the assessment and treatment of persistent shock are also included, with a focus on identifying and correcting potential contributing factors. The chart also provides specific guidance for the estimation of maintenance fluid requirements based on a patient's weight.
I have not obtained copyright/ permission yet
(Click Image to Enlarge)
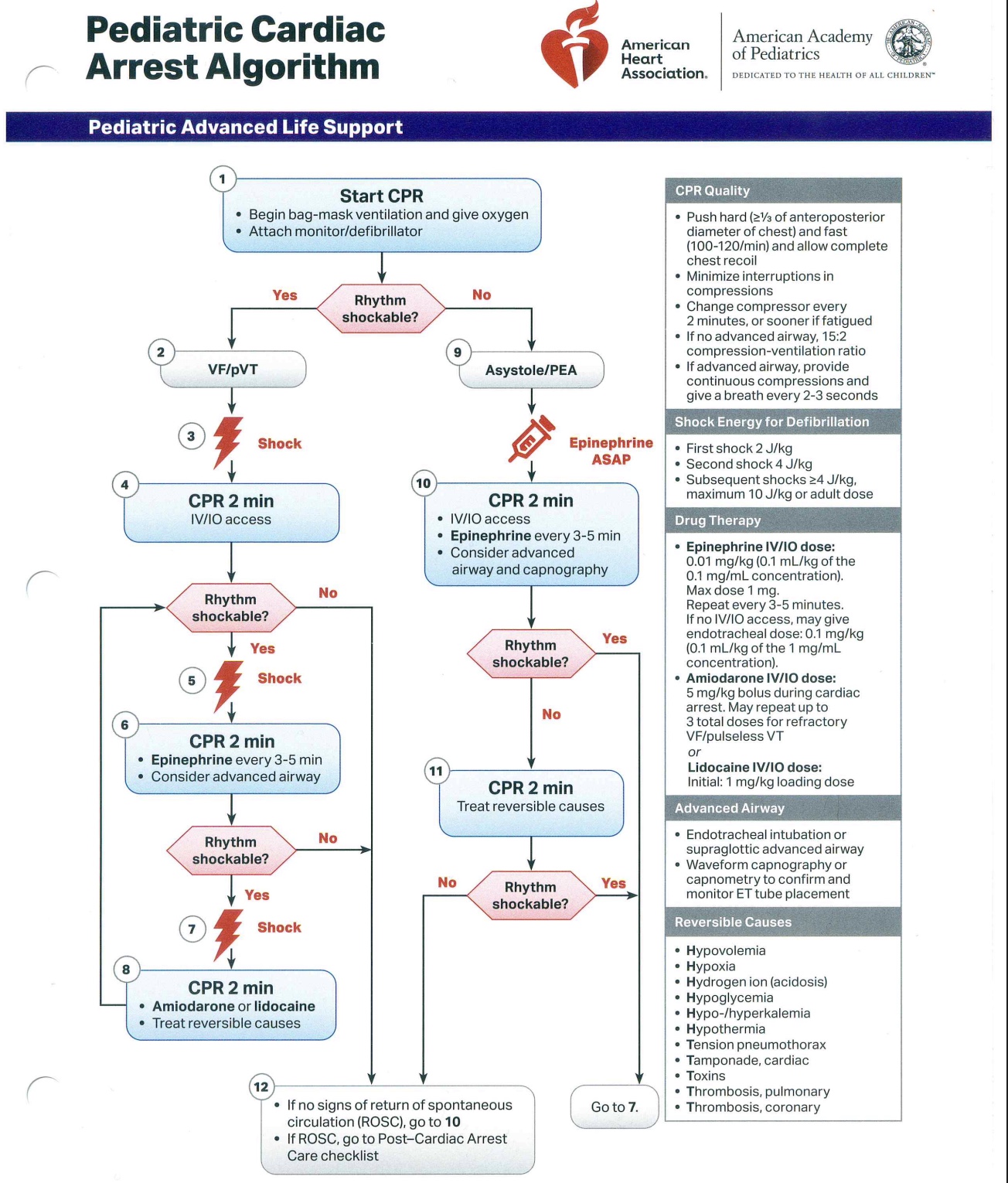
Pediatric Cardiac Arrest Algorithm. Flowchart depicting the management of pediatric cardiac arrest, distinguishing shockable from nonshockable rhythms to guide treatment. The chart includes the recommended sequence for cardiopulmonary resuscitation, pharmacologic interventions, and defibrillator use. Reversible causes of cardiac arrest for targeted correction are listed.
I have not obtained copyright/ permission yet
(Click Image to Enlarge)
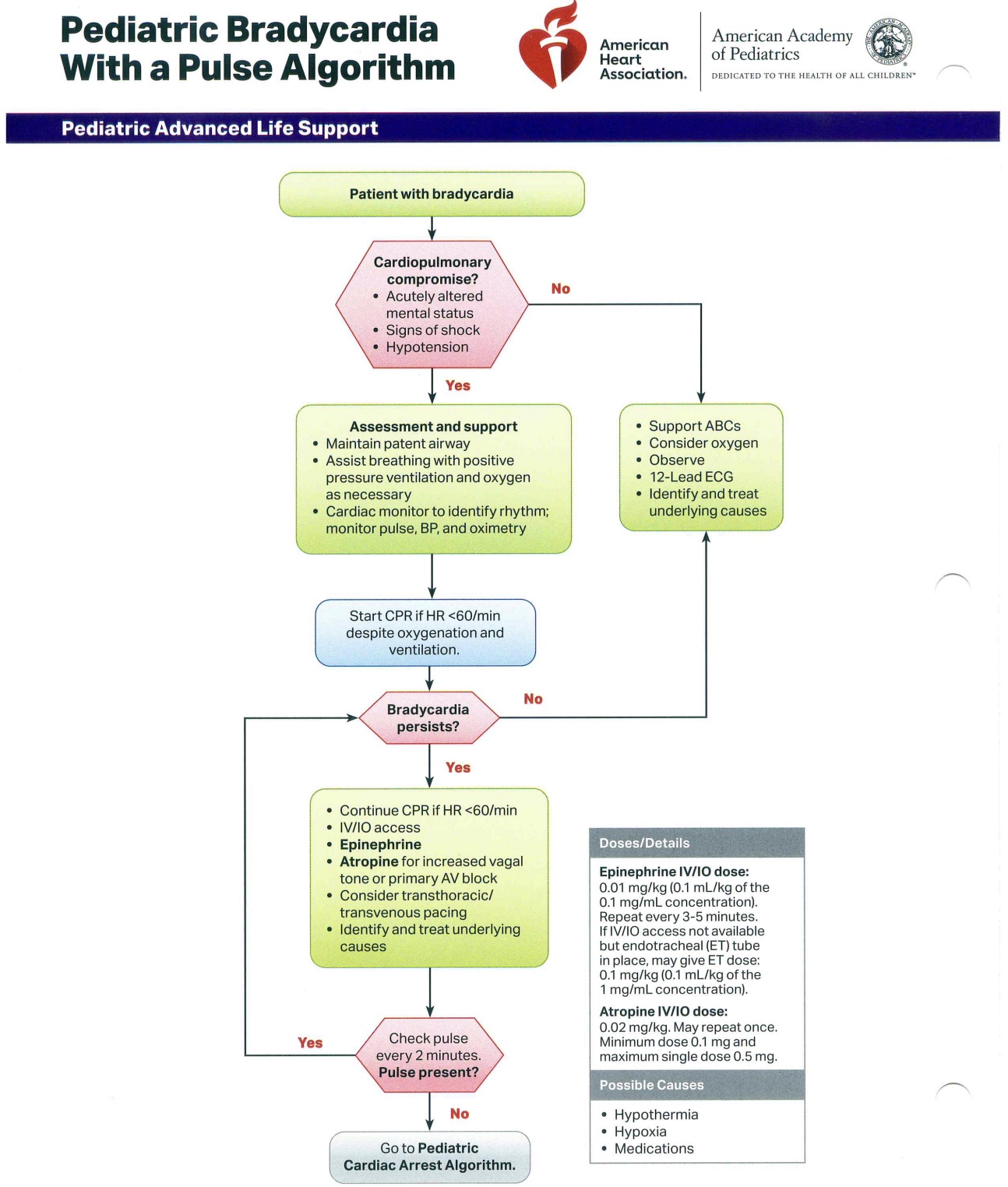
Pediatric Bradycardia with a Pulse Algorithm. This flowchart guides the management of bradycardia with a palpable pulse in pediatric patients. Initial assessment focuses on identifying cardiopulmonary compromise, followed by evaluation of causes and appropriate supportive measures. The algorithm includes specific medication dosages for targeted treatment and outlines steps for escalation when necessary.
I have not obtained copyright/ permission yet
(Click Image to Enlarge)
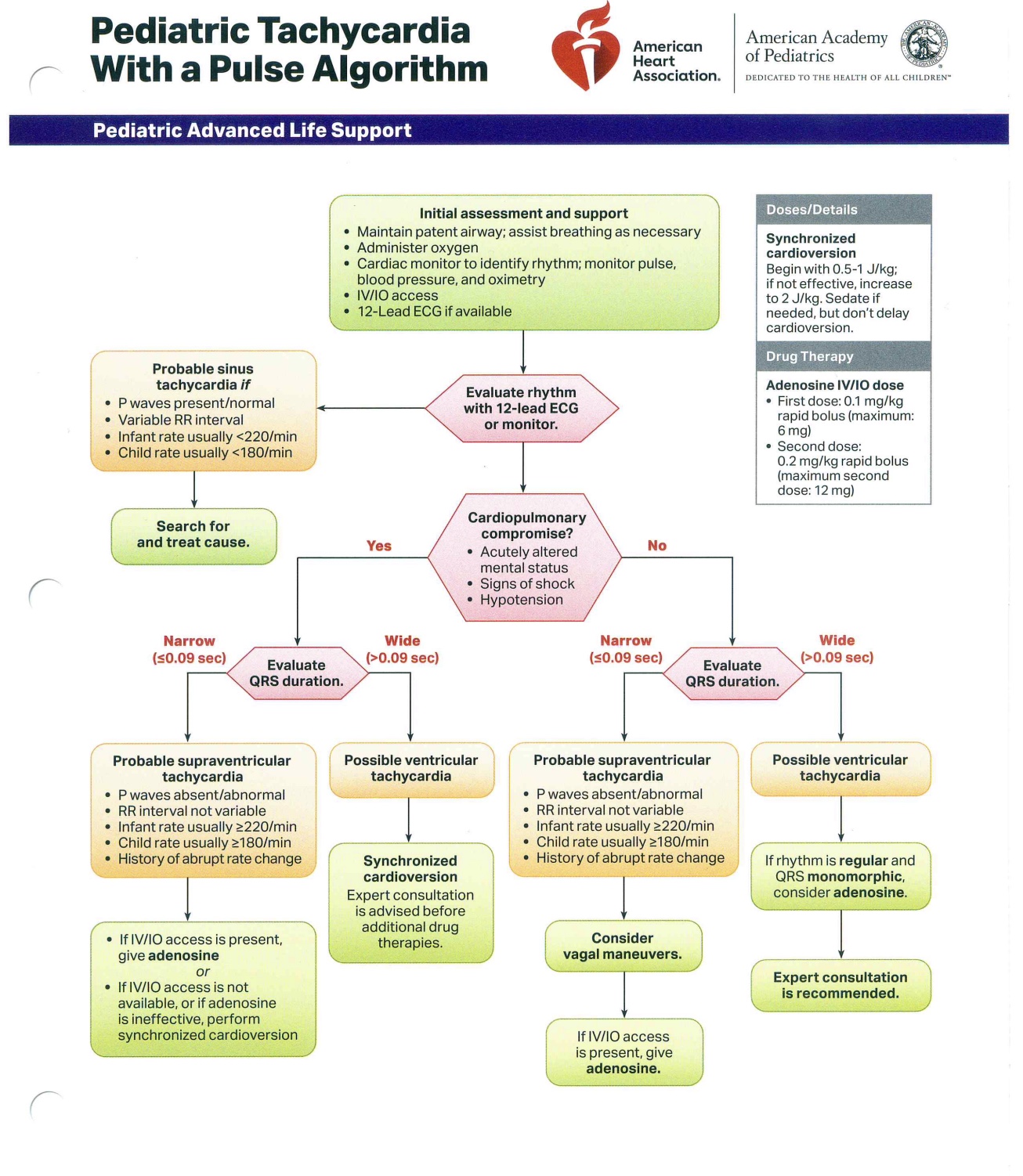
Pediatric Tachycardia with a Pulse Algorithm. This flowchart guides the management of tachycardia and a palpable pulse in pediatric patients. Initial assessment determines the presence of cardiopulmonary compromise. The algorithm directs the evaluation of QRS duration and presents probable rhythm diagnoses. Specific medication dosages and intervention recommendations are included to facilitate targeted treatment.
I have not obtained copyright/ permission yet
(Click Image to Enlarge)

Pediatric Septic Shock Algorithm. This flowchart provides a structured guide for the management of pediatric septic shock. The diagram begins by outlining the clinical signs used to identify the condition and details steps for initial stabilization, including fluid resuscitation and laboratory evaluation. The algorithm further guides clinicians through obtaining critical care consultation and initiating vasoactive therapy with epinephrine or norepinephrine as indicated.
I have not obtained copyright/ permission yet
(Click Image to Enlarge)
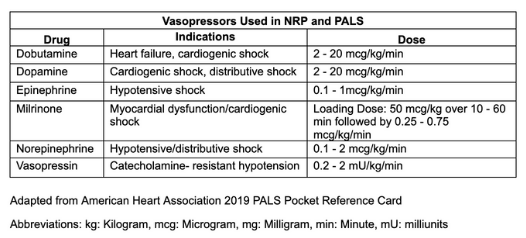
Vasopressors Used in Neonatal Resuscitation and Pediatric Advanced Life Support. This table summarizes the vasopressors employed in neonatal resuscitation and pediatric advanced life support. Listed are the individual drugs, their specific indications, and recommended dosages. The information is adapted from the 2019 American Heart Association Pediatric Advanced Life Support pocket reference card.
Adapted from American Heart Association 2019 PALS Pocket Reference Card
(Click Image to Enlarge)
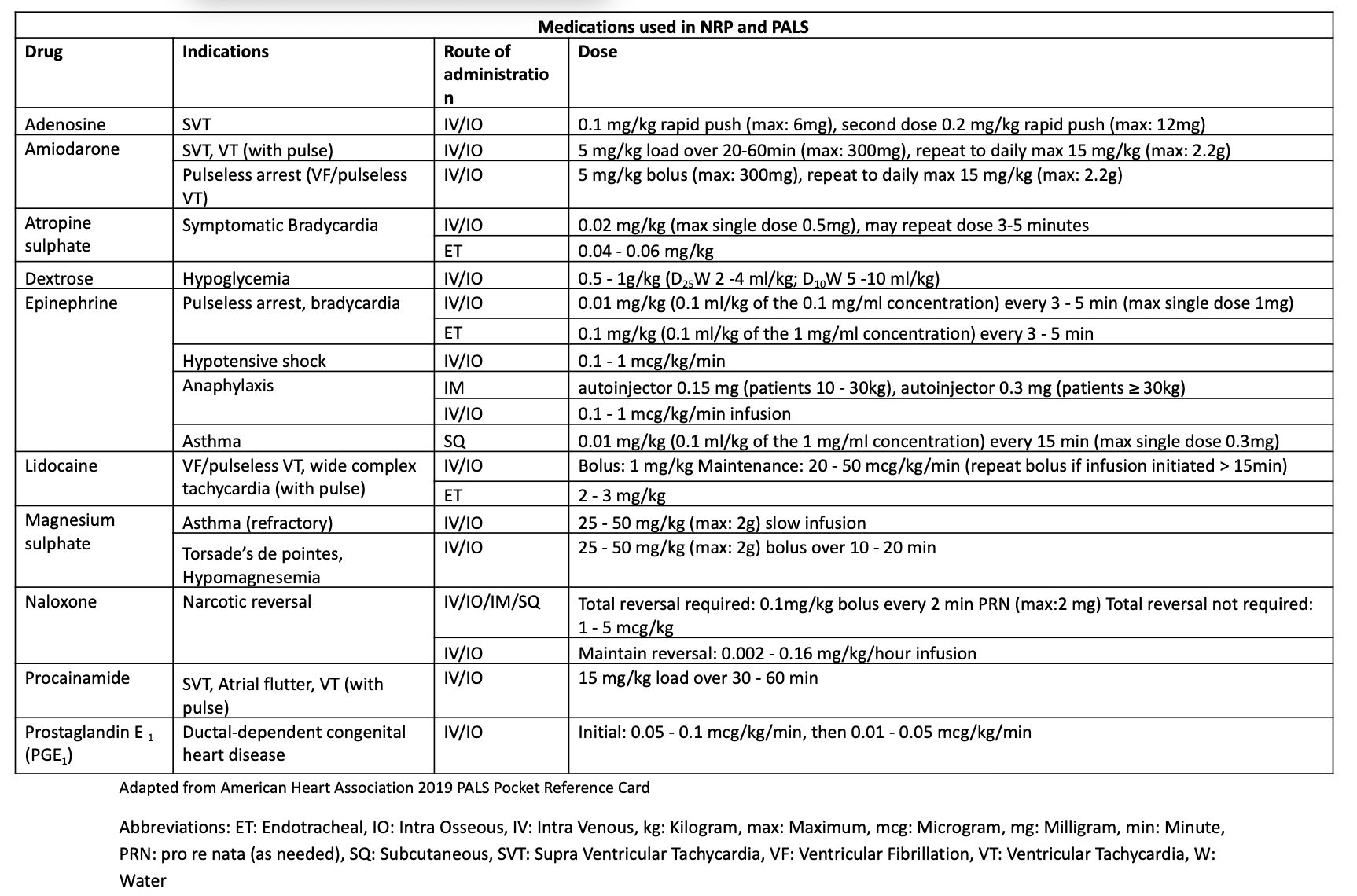
Medications Used in Neonatal Resuscitation and Pediatric Advanced Life Support. This table provides a summary of medications, their indications, and dosages used in neonatal resuscitation and pediatric advanced life support. The table includes details on the route of administration for each drug. The information is adapted from a 2019 American Heart Association Pediatric Advanced Life Support pocket reference card.
Adapted from American Heart Association 2019 PALS Pocket Reference Card
(Click Image to Enlarge)
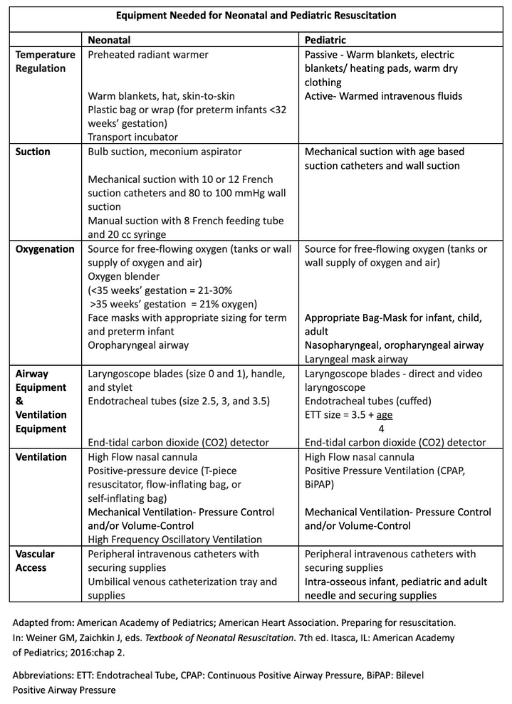
Equipment Needed for Neonatal and Pediatric Resuscitation. This table provides a comprehensive list of equipment for neonatal and pediatric resuscitation. The list is organized by function, including sections for temperature regulation, suction, and oxygenation. It also details the necessary airway equipment, ventilation devices, and vascular access tools. The equipment types and sizes are specified for both neonatal and pediatric patients.
Adapted from: American Academy of Pediatrics; American Heart Association. Preparing for resuscitation. In: Weiner GM, Zaichkin J, eds. Textbook of Neonatal Resuscitation. 7th ed. Itasca, IL: American Academy of Pediatrics; 2016:chap 2.
(Click Image to Enlarge)
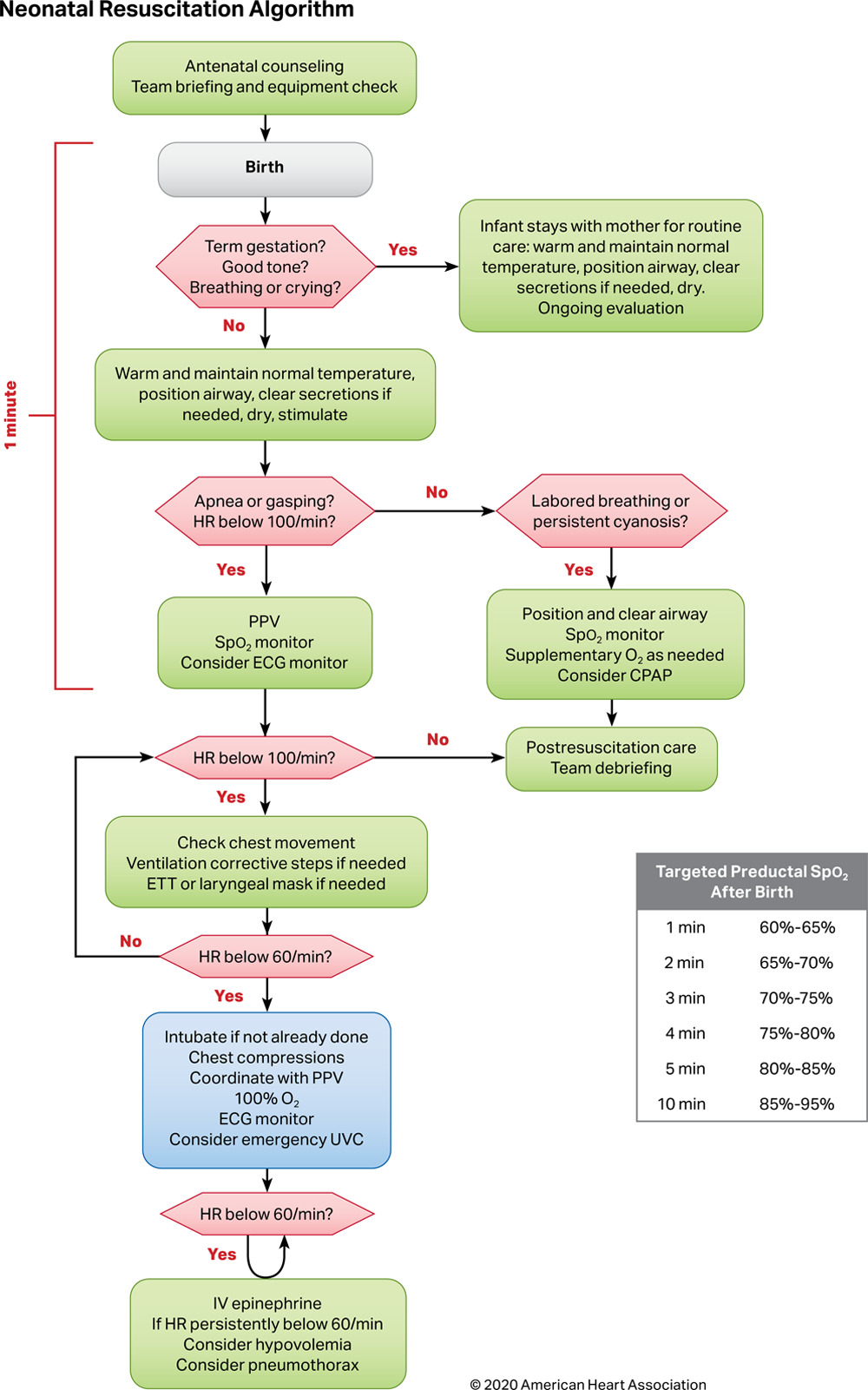
Neonatal Resuscitation Algorithm. This flowchart provides a guide for the resuscitation of a neonate based on the 2020 American Heart Association recommendations. The chart begins with the initial steps of assessment and care during the 1st minute of life. The algorithm then directs clinicians to specific interventions based on the neonate's heart rate and breathing. The chart includes a reference table for targeted oxygen saturation levels after birth.
AHA, I have not obtained copy right or receive permission yet.
References
Munneke AG, Lumens J, Delhaas T. Cardiovascular fetal-to-neonatal transition: an in silico model. Pediatric research. 2022 Jan:91(1):116-128. doi: 10.1038/s41390-021-01401-0. Epub 2021 Mar 17 [PubMed PMID: 33731808]
Tan CMJ, Lewandowski AJ. The Transitional Heart: From Early Embryonic and Fetal Development to Neonatal Life. Fetal diagnosis and therapy. 2020:47(5):373-386. doi: 10.1159/000501906. Epub 2019 Sep 18 [PubMed PMID: 31533099]
Kariuki E, Sutton C, Leone TA. Neonatal resuscitation: current evidence and guidelines. BJA education. 2021 Dec:21(12):479-485. doi: 10.1016/j.bjae.2021.07.008. Epub 2021 Sep 15 [PubMed PMID: 34840820]
Aziz K, Chadwick M, Baker M, Andrews W. Ante- and intra-partum factors that predict increased need for neonatal resuscitation. Resuscitation. 2008 Dec:79(3):444-52. doi: 10.1016/j.resuscitation.2008.08.004. Epub 2008 Oct 25 [PubMed PMID: 18952348]
Lovik K, Sasaki J, Edemekong PF. Cardiopulmonary Arrest in Children. StatPearls. 2025 Jan:(): [PubMed PMID: 28613789]
Harless J, Ramaiah R, Bhananker SM. Pediatric airway management. International journal of critical illness and injury science. 2014 Jan:4(1):65-70. doi: 10.4103/2229-5151.128015. Epub [PubMed PMID: 24741500]
Miller KA, Goldman MP, Nagler J. Management of the Difficult Airway. Pediatric emergency care. 2023 Mar 1:39(3):192-200. doi: 10.1097/PEC.0000000000002916. Epub 2023 Feb 16 [PubMed PMID: 36790950]
Hsu G, von Ungern-Sternberg BS, Engelhardt T. Pediatric airway management. Current opinion in anaesthesiology. 2021 Jun 1:34(3):276-283. doi: 10.1097/ACO.0000000000000993. Epub [PubMed PMID: 33935175]
Level 3 (low-level) evidenceVijayasekaran S. Pediatric Airway Pathology. Frontiers in pediatrics. 2020:8():246. doi: 10.3389/fped.2020.00246. Epub 2020 Jun 4 [PubMed PMID: 32582586]
Fiadjoe J, Stricker P. Pediatric difficult airway management: current devices and techniques. Anesthesiology clinics. 2009 Jun:27(2):185-95. doi: 10.1016/j.anclin.2009.06.002. Epub [PubMed PMID: 19703672]
Arumainathan R, Stendall C, Visram A. Management of fluids in neonatal surgery. BJA education. 2018 Jul:18(7):199-203. doi: 10.1016/j.bjae.2018.03.006. Epub 2018 May 21 [PubMed PMID: 33456833]
Saikia D, Mahanta B. Cardiovascular and respiratory physiology in children. Indian journal of anaesthesia. 2019 Sep:63(9):690-697. doi: 10.4103/ija.IJA_490_19. Epub [PubMed PMID: 31571681]
Duff JP, Topjian AA, Berg MD, Chan M, Haskell SE, Joyner BL Jr, Lasa JJ, Ley SJ, Raymond TT, Sutton RM, Hazinski MF, Atkins DL. 2019 American Heart Association Focused Update on Pediatric Advanced Life Support: An Update to the American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Pediatrics. 2020 Jan:145(1):. pii: e20191361. doi: 10.1542/peds.2019-1361. Epub 2019 Nov 14 [PubMed PMID: 31727859]
Craig-Brangan KJ, Day MP. AHA update: BLS, ACLS, and PALS. Nursing. 2021 Jun 1:51(6):24-30. doi: 10.1097/01.NURSE.0000751340.92329.ae. Epub [PubMed PMID: 34014872]
Sawyer T, Lee HC, Aziz K. Anticipation and preparation for every delivery room resuscitation. Seminars in fetal & neonatal medicine. 2018 Oct:23(5):312-320. doi: 10.1016/j.siny.2018.06.004. Epub 2018 Jul 6 [PubMed PMID: 30369405]
Zaichkin J, Mccarney L, Weiner G. NRP 7th Edition: Are You Prepared? Neonatal network : NN. 2016:35(4):184-91. doi: 10.1891/0730-0832.35.4.184. Epub [PubMed PMID: 27461196]
King BR, Baker MD, Braitman LE, Seidl-Friedman J, Schreiner MS. Endotracheal tube selection in children: a comparison of four methods. Annals of emergency medicine. 1993 Mar:22(3):530-4 [PubMed PMID: 8442540]
Cheifetz IM. Pediatric ARDS. Respiratory care. 2017 Jun:62(6):718-731. doi: 10.4187/respcare.05591. Epub [PubMed PMID: 28546374]
Krishnaprasadh D, Joyce T, Huecker MR. Pediatric Abusive Head Trauma. StatPearls. 2025 Jan:(): [PubMed PMID: 29763011]
Sutton RM, French B, Nishisaki A, Niles DE, Maltese MR, Boyle L, Stavland M, Eilevstjønn J, Arbogast KB, Berg RA, Nadkarni VM. American Heart Association cardiopulmonary resuscitation quality targets are associated with improved arterial blood pressure during pediatric cardiac arrest. Resuscitation. 2013 Feb:84(2):168-72. doi: 10.1016/j.resuscitation.2012.08.335. Epub 2012 Sep 6 [PubMed PMID: 22960227]
Level 2 (mid-level) evidenceSutton RM, Reeder RW, Landis W, Meert KL, Yates AR, Berger JT, Newth CJ, Carcillo JA, McQuillen PS, Harrison RE, Moler FW, Pollack MM, Carpenter TC, Notterman DA, Holubkov R, Dean JM, Nadkarni VM, Berg RA, Eunice Kennedy Shriver National Institute of Child Health and Human Development Collaborative Pediatric Critical Care Research Network (CPCCRN) Investigators. Chest compression rates and pediatric in-hospital cardiac arrest survival outcomes. Resuscitation. 2018 Sep:130():159-166. doi: 10.1016/j.resuscitation.2018.07.015. Epub 2018 Jul 18 [PubMed PMID: 30031055]
Sutton RM, Niles D, Nysaether J, Arbogast KB, Nishisaki A, Maltese MR, Bishnoi R, Helfaer MA, Nadkarni V, Donoghue A. Pediatric CPR quality monitoring: analysis of thoracic anthropometric data. Resuscitation. 2009 Oct:80(10):1137-41. doi: 10.1016/j.resuscitation.2009.06.031. Epub 2009 Jul 31 [PubMed PMID: 19647359]
Level 2 (mid-level) evidenceKao PC, Chiang WC, Yang CW, Chen SJ, Liu YP, Lee CC, Hsidh MJ, Ko PC, Chen SC, Ma MH. What is the correct depth of chest compression for infants and children? A radiological study. Pediatrics. 2009 Jul:124(1):49-55. doi: 10.1542/peds.2008-2536. Epub [PubMed PMID: 19564282]
Sutton RM, French B, Niles DE, Donoghue A, Topjian AA, Nishisaki A, Leffelman J, Wolfe H, Berg RA, Nadkarni VM, Meaney PA. 2010 American Heart Association recommended compression depths during pediatric in-hospital resuscitations are associated with survival. Resuscitation. 2014 Sep:85(9):1179-84. doi: 10.1016/j.resuscitation.2014.05.007. Epub 2014 May 16 [PubMed PMID: 24842846]
Mendelson J. Emergency Department Management of Pediatric Shock. Emergency medicine clinics of North America. 2018 May:36(2):427-440. doi: 10.1016/j.emc.2017.12.010. Epub 2018 Feb 10 [PubMed PMID: 29622332]
Berg RA, Sutton RM, Reeder RW, Berger JT, Newth CJ, Carcillo JA, McQuillen PS, Meert KL, Yates AR, Harrison RE, Moler FW, Pollack MM, Carpenter TC, Wessel DL, Jenkins TL, Notterman DA, Holubkov R, Tamburro RF, Dean JM, Nadkarni VM, Eunice Kennedy Shriver National Institute of Child Health and Human Development Collaborative Pediatric Critical Care Research Network (CPCCRN) PICqCPR (Pediatric Intensive Care Quality of Cardio-Pulmonary Resuscitation) Investigators. Association Between Diastolic Blood Pressure During Pediatric In-Hospital Cardiopulmonary Resuscitation and Survival. Circulation. 2018 Apr 24:137(17):1784-1795. doi: 10.1161/CIRCULATIONAHA.117.032270. Epub 2017 Dec 26 [PubMed PMID: 29279413]
Level 2 (mid-level) evidenceWeiss SL, Keele L, Balamuth F, Vendetti N, Ross R, Fitzgerald JC, Gerber JS. Crystalloid Fluid Choice and Clinical Outcomes in Pediatric Sepsis: A Matched Retrospective Cohort Study. The Journal of pediatrics. 2017 Mar:182():304-310.e10. doi: 10.1016/j.jpeds.2016.11.075. Epub 2017 Jan 4 [PubMed PMID: 28063688]
Level 2 (mid-level) evidenceNeumar RW, Otto CW, Link MS, Kronick SL, Shuster M, Callaway CW, Kudenchuk PJ, Ornato JP, McNally B, Silvers SM, Passman RS, White RD, Hess EP, Tang W, Davis D, Sinz E, Morrison LJ. Part 8: adult advanced cardiovascular life support: 2010 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2010 Nov 2:122(18 Suppl 3):S729-67. doi: 10.1161/CIRCULATIONAHA.110.970988. Epub [PubMed PMID: 20956224]
Hegenbarth MA, American Academy of Pediatrics Committee on Drugs. Preparing for pediatric emergencies: drugs to consider. Pediatrics. 2008 Feb:121(2):433-43. doi: 10.1542/peds.2007-3284. Epub [PubMed PMID: 18245435]
Yamada NK, Szyld E, Strand ML, Finan E, Illuzzi JL, Kamath-Rayne BD, Kapadia VS, Niermeyer S, Schmölzer GM, Williams A, Weiner GM, Wyckoff MH, Lee HC, American Heart Association and American Academy of Pediatrics. 2023 American Heart Association and American Academy of Pediatrics Focused Update on Neonatal Resuscitation: An Update to the American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2024 Jan 2:149(1):e157-e166. doi: 10.1161/CIR.0000000000001181. Epub 2023 Nov 16 [PubMed PMID: 37970724]
Chandrasekharan P, Vento M, Trevisanuto D, Partridge E, Underwood MA, Wiedeman J, Katheria A, Lakshminrusimha S. Neonatal Resuscitation and Postresuscitation Care of Infants Born to Mothers with Suspected or Confirmed SARS-CoV-2 Infection. American journal of perinatology. 2020 Jun:37(8):813-824. doi: 10.1055/s-0040-1709688. Epub 2020 Apr 8 [PubMed PMID: 32268381]
Kim SY, Shim GH, O'Reilly M, Cheung PY, Lee TF, Schmölzer GM. Asphyxiated Female and Male Newborn Piglets Have Similar Outcomes With Different Cardiopulmonary Resuscitation Interventions. Frontiers in pediatrics. 2020:8():602228. doi: 10.3389/fped.2020.602228. Epub 2020 Dec 3 [PubMed PMID: 33425814]
Topjian AA, Raymond TT, Atkins D, Chan M, Duff JP, Joyner BL Jr, Lasa JJ, Lavonas EJ, Levy A, Mahgoub M, Meckler GD, Roberts KE, Sutton RM, Schexnayder SM, Pediatric Basic and Advanced Life Support Collaborators. Part 4: Pediatric Basic and Advanced Life Support: 2020 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2020 Oct 20:142(16_suppl_2):S469-S523. doi: 10.1161/CIR.0000000000000901. Epub 2020 Oct 21 [PubMed PMID: 33081526]
Aziz K, Lee CHC, Escobedo MB, Hoover AV, Kamath-Rayne BD, Kapadia VS, Magid DJ, Niermeyer S, Schmölzer GM, Szyld E, Weiner GM, Wyckoff MH, Yamada NK, Zaichkin J. Part 5: Neonatal Resuscitation 2020 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Pediatrics. 2021 Jan:147(Suppl 1):. pii: e2020038505E. doi: 10.1542/peds.2020-038505E. Epub 2020 Oct 21 [PubMed PMID: 33087555]
Topjian AA, Berg RA, Nadkarni VM. Pediatric cardiopulmonary resuscitation: advances in science, techniques, and outcomes. Pediatrics. 2008 Nov:122(5):1086-98. doi: 10.1542/peds.2007-3313. Epub [PubMed PMID: 18977991]
Level 3 (low-level) evidenceFernandez Castelao E, Russo SG, Riethmüller M, Boos M. Effects of team coordination during cardiopulmonary resuscitation: a systematic review of the literature. Journal of critical care. 2013 Aug:28(4):504-21. doi: 10.1016/j.jcrc.2013.01.005. Epub 2013 Apr 16 [PubMed PMID: 23602030]
Level 1 (high-level) evidence