Osteopathic Manipulative Treatment: 5 Diaphragm Procedure
Osteopathic Manipulative Treatment: 5 Diaphragm Procedure
Introduction
Assessment and treatment of the 5 diaphragms fall within the respiratory-circulatory model of osteopathic medicine. Body diaphragms are traditionally defined as anatomical structures occupying a horizontal plane. Considering the 3-dimensional organization of the human body, the diaphragms are more accurately described as regions in close anatomical continuity capable of reciprocal influence: the tentorium cerebelli, the tongue, the thoracic outlet, the diaphragm, and the pelvic floor. This activity reviews the anatomical interconnections of these regions, methods of assessment, and manual treatment strategies, emphasizing potential clinical applications.
The objective of the osteopathic manual approach is to create functional space between anatomical structures. Manual manipulation does not reshape the tissues themselves. Expansion of these spaces facilitates movement and physiologic function. To paraphrase Leonardo da Vinci, “space is life.”
Anatomy and Physiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Anatomy and Physiology
Osteopathic medicine was established in the U.S. by Dr. A. T. Still in the late 1800s. Evaluation and manual treatment strategies may be developed from the 5 osteopathic models: biomechanical-structural, respiratory-circulatory, neurological, metabolic-nutritional, and behavioral-biopsychosocial. These models serve as foundational frameworks rather than constraints, and integration across models is essential. The 5 diaphragms are components of the respiratory-circulatory model, which emphasizes optimization of body fluid circulation to support overall patient health.[1] The first study delineating the anatomical and physiological relationships of the 5 diaphragms, providing a clinical rationale for osteopathic manual treatment, was published in 2013 and identified the tentorium cerebelli, tongue, thoracic outlet, diaphragm, and pelvic floor as key regions.[2]
Tentorium Cerebelli
The tentorium cerebelli is a meningeal structure situated in the posterior cranial fossa. This structure forms a semicircular transverse septum with a concave anterior margin and a convex posterior margin, separating the cerebral hemispheres from the cerebellum. The falx cerebri converges with the anterior aspect of the tentorium, while the falx cerebelli lies inferiorly. The attachments of the tentorium cerebelli extend to the internal occipital protuberance, occipital bone, parietal bone, and temporal bone. The superior petrosal sinuses and the straight sinus course along its margins, facilitating venous drainage, while the glymphatic system contributes to lymphatic clearance.
The supratentorial portion of the tentorium cerebelli is innervated by the nervus tentorii, a branch of the ophthalmic nerve, including the tentorial notch and regions adjacent to the straight and transverse sinuses. Parasympathetic fibers supplying this area likely originate from the sphenopalatine ganglion and vascular fibers associated with the meningeal blood supply. Sympathetic fibers arise from the middle meningeal artery, itself receiving input from the stellate ganglion, and terminate in the supratentorial region. Afferents from the nervus tentorii project to the dorsal horns at the C2 spinal level, whereas sympathetic and parasympathetic autonomic afferents from the dural vascular plexus terminate in the medullary region of C1 to C3.
The subtentorial region receives innervation from the spinal roots of C1 to C4, sympathetic fibers of the sympathetic trunk, and cranial nerves IX (glossopharyngeal), X (vagus), and XII (hypoglossal). Additional innervation described in the literature includes branches of cranial nerve VII (facial), parasympathetic fibers, and neuronal elements of cranial nerve IV (trochlear nerve) associated with meningeal vessels.
The subtentorial region is associated with 3 of the 4 suboccipital muscles—rectus capitis posterior minor, oblique capitis inferior, and rectus capitis posterior major—which contribute to the myodural bridge. The suboccipital muscles form an integral component of the thoracolumbar myofascial system. Through a fibrous structure referred to as the to-be-named ligament (TBNL), the nuchal ligament maintains anatomical continuity with the tentorium cerebelli.[3][4]
Tongue
The suprahyoid and infrahyoid muscles interact with and are influenced by tongue movements. The intrinsic and extrinsic muscles of the tongue function cooperatively, forming the lingual complex.
The lingual complex impacts the occipitocervical region, particularly the anterior cervical tract, and contributes to functions such as mastication, respiration, and other physiologic processes. Intrinsic muscles include the transversus linguae, verticalis linguae, inferior longitudinalis, and superior longitudinalis. Extrinsic muscles comprise the genioglossus, styloglossus, hyoglossus, palatoglossus, glossopharyngeus, and chondroglossus.
At the central level, the tongue musculature is represented in specific regions, including the limbic area, the somatosensory cortex, the medulla oblongata, and the midbrain. Innervation of the tongue involves the hypoglossal nerve, the lingual nerve (a branch of the mandibular division of the trigeminal nerve or cranial nerve V), and parasympathetic fibers originating from the mandibular ganglion. The hypoglossal and lingual nerves anastomose within the lingual musculature and in adjacent regions. The glossopharyngeal nerve, along with parasympathetic branches, contributes additional innervation, forming anastomoses with the hypoglossal and lingual nerves within the tongue. The facial nerve provides afferent input, while sympathetic fibers arising from the superior cervical ganglion supply the tongue, reflecting integrated myofascial relationships.
The lingual complex interacts directly with multiple muscles, including the suprahyoid and infrahyoid muscles, masseter, temporalis, buccinator, mylohyoid, pterygoid, digastric, and superior pharyngeal constrictor. Connective tissue spanning the craniocervical tract to the thoracic outlet remains functionally continuous with the lingual complex.[5][6]
Thoracic Outlet
The thoracic outlet comprises musculoskeletal structures including the sternum, 1st and 2nd ribs, clavicle, scapula, 1st and 2nd thoracic vertebrae, trapezius, subclavian, pectoralis major and minor, intercostal and deep dorsocervical musculature, and scalene muscles. The brachial neurovascular bundle (C1 to T1) traverses 3 confined passages: between the anterior and middle scalene muscles, beneath the clavicle and 1st rib, and beneath the pectoralis minor muscle. This region also contains components of the autonomic nervous system, notably the stellate ganglion and the vagus nerve with its parasympathetic fibers. The thoracic outlet includes the fusion of cervical and thoracic fascial bands and provides passage for visceral structures, including the pleural dome.[7]
Diaphragm
The diaphragm muscle attaches to the xiphoid process of the sternum, the last 6 ribs, the anterior aspects of the bodies of vertebrae T11 to L4, and the transverse processes of L1. Major structures traversing the diaphragm include the inferior vena cava, esophagus, aorta, azygos and hemiazygos veins, as well as the lymphatic system via the cisterna chyli. Innervation arises from the phrenic and vagus nerves, with sympathetic input from subdiaphragmatic ganglia. The diaphragm maintains functional continuity with the abdominal muscles, psoas, quadratus lumborum, and the fascial networks of the thorax, abdomen, and lumbosacral region.[8]
Pelvic Floor
The pelvic floor is a muscle complex composed of the levator ani muscle—including the puborectalis, pubococcygeus, and iliococcygeus muscles—and the ischiococcygeus muscle. The gluteus maximus contributes to the levator ani via a fascial septum at the posterior ischioanal fossa. The pelvic floor encompasses the abdominopelvic cavity, pubic symphysis, and coccyx.
Innervation is provided by spinal segments S2 to S4 through the pudendal nerve and the nucleus of Onuf in the sacral spinal cord. Parasympathetic fibers also arise from S2 to S4, whereas sympathetic fibers derive from the lumbar chain. The pelvic floor integrates with multiple fascial structures, including the transversalis fascia, prevertebral fascia, iliac fascia, pectineal fascia, and thoracolumbar fascia, and maintains functional continuity with contractile regions such as the adductor muscles, tensor fascia lata, and pyramidalis. Inferior to the pelvic floor lies the triangular fascial formation known as the urogenital diaphragm.[9]
Central Control and Coordinated Function of the Five Diaphragms During Respiration
During quiet inhalation, a central pattern generator (CPG) located in the brainstem and spinal cord becomes active. The hypoglossal nerve is recruited during the preinhalation and inspiratory phases to dilate the upper airway, producing a dual movement in which the hyoid portion displaces anteriorly and the posterior and superior portions move caudally and posteriorly. The tongue is perceived as retracting posteriorly during inspiration.
During the continuation of inhalation, the phrenic nerve activates the diaphragm, the vagus nerve regulates the esophageal hiatus depending on the presence of a food bolus, and the external intercostal muscles contract. The abdominal muscles, internal intercostal muscles, and pelvic floor muscles are generally inhibited by the central pattern generator, although they maintain baseline tone.
Pelvic floor musculature descends synchronously with diaphragmatic movement. The movements are reversed during exhalation. Diaphragmatic activity places all myofascial systems of the 5 diaphragms under tension, simultaneously stimulating fluid dynamics throughout the body.[10][11][12]
Indications
Chronic diseases frequently demonstrate recurrence of related disorders and symptoms. Patients with diagnosed obstructive sleep apnea (OSA) present with a range of associated manifestations, including headache, neurophysiological alterations of the lingual complex, morphological and functional changes of the diaphragm, and impaired control of the pelvic floor muscles.[13][14][15][16][17]
Common complications of chronic obstructive pulmonary disease include persistent headache, OSA, diaphragmatic dysfunction, and pelvic floor muscle impairment.[18][19] Patients with longstanding cervical pain may exhibit headache, functional alterations of the lingual complex, respiratory dysfunction, and postural changes affecting the pelvic region.[20][21][22][23] Chronic kidney disease is associated with comorbidities such as recurrent headache, OSA, impaired diaphragmatic function, and pelvic floor weakness.[24][25][26] These manifestations are specific to the underlying pathology. Clinical evidence supports the anatomical and neurological interconnections of the 5 diaphragms, emphasizing system integration as a fundamental principle of osteopathic philosophy. The human body functions as a continuous network of interrelated systems.[27]
Understanding the application and clinical relevance of the osteopathic 5 diaphragm approach requires knowledge of the anatomy and physiology underlying its interconnections. Osteopathic assessment considers not only the presenting symptom but also the underlying causative factors. Shoulder pain may arise secondary to diaphragmatic dysfunction. The phrenic nerve transmits electrical and biochemical signals bidirectionally, communicating with the brachial plexus along its course, including the axillary nerve, which supplies the shoulder musculature.
Persistent diaphragmatic dysfunction, resulting from conditions such as trauma or emotional factors, induces local production of paracrine signaling molecules, including cytokines, which propagate centrally toward the medulla, affecting all structures connected to the phrenic nerve. Functional alterations of the diaphragm have been associated with impaired shoulder arthrokinematics and pain through somatosomatic reflexes. Dysfunctional diaphragmatic reflexes may also arise via the fascial continuum. Diaphragmatic descent during inhalation exerts caudal tension on the connected fascial system, facilitating arm flexion during inhalation relative to exhalation. Evaluation and treatment of the 5 diaphragms may therefore contribute to the management of shoulder disorders, as discussed in subsequent sections.
Abnormal traction of the suboccipital muscles, such as from trauma to the sacral region, can transmit forces through the posterior fascial sheet, generating mechanical and metabolic tension in the subtentorial dural region. Dural tissue can release irritant substances that influence the trigeminal system, producing cranial pain. Mechanical dural receptors are concentrated near vascular pathways, and nonphysiological stimulation may induce chronic inflammation affecting surrounding neural structures. Given that the subtentorial area shares innervation with the lingual complex, motor and sensory dysfunctions in this region are plausible. Additionally, fascial continuity between the tentorium cerebelli, the lingual complex, the cervical tract, and the occipital region contributes to the integration of these structures and their functional interdependence.
Dysfunction of a fascial tract can alter biomechanical and metabolic dynamics both at proximal and distant sites, such as following sacral trauma. The thoracolumbar fascia encompasses the deep spinal musculature, including the suboccipital muscles, and abnormal traction of the sacrum generates excessive tension within these cervical muscles.[28][29][30]
Application of the 5 diaphragm approach is recommended in the presence of chronic, local, or systemic dysfunction. During the acute phase, techniques may be applied while respecting tissue tolerance and the patient’s comfort. In cases of unhealed wounds, these interventions may be ineffective, and manual contact may provoke discomfort or exacerbate symptoms.
Contraindications
The literature does not report specific contraindications for the use of the 5 diaphragms in osteopathic medicine. The primary contraindications pertain to high-velocity, low-amplitude techniques. The techniques described in this context are gentle and do not present a risk of harm.
Equipment
The necessary equipment includes a treatment table. All relevant previous clinical data should also be available.
Personnel
The osteopathic approach should be performed by a clinician trained in osteopathic medicine. Patients must be informed of potential risks associated with the procedure and provide informed consent. Clinicians should perform only those interventions indicated by the patient’s history and physical examination.
Preparation
The clinician must assess the anatomical regions comprising the 5 diaphragms before proceeding with osteopathic manual treatment. The evaluation proceeds in 2 stages: an initial general assessment followed by a detailed, comprehensive examination.
General Listening
The patient is positioned supine while the clinician is seated at the head of the table to palpate the tentorium cerebelli. The little fingers contact the inion, or external occipital protuberance, while the remaining fingers form a semicircle approximating the tentorium’s internal curvature, ending with the index finger on the asterion. This bony landmark represents the junction of the occipital, parietal, and temporal bones. Assessment includes evaluation of tissue texture, presence of pain, temperature variations, and any structural abnormalities or restrictions. The clinician may detect subtle movements of the tentorium, characterized by expansion and subsequent recoil, independent of cardiac or respiratory rhythms.
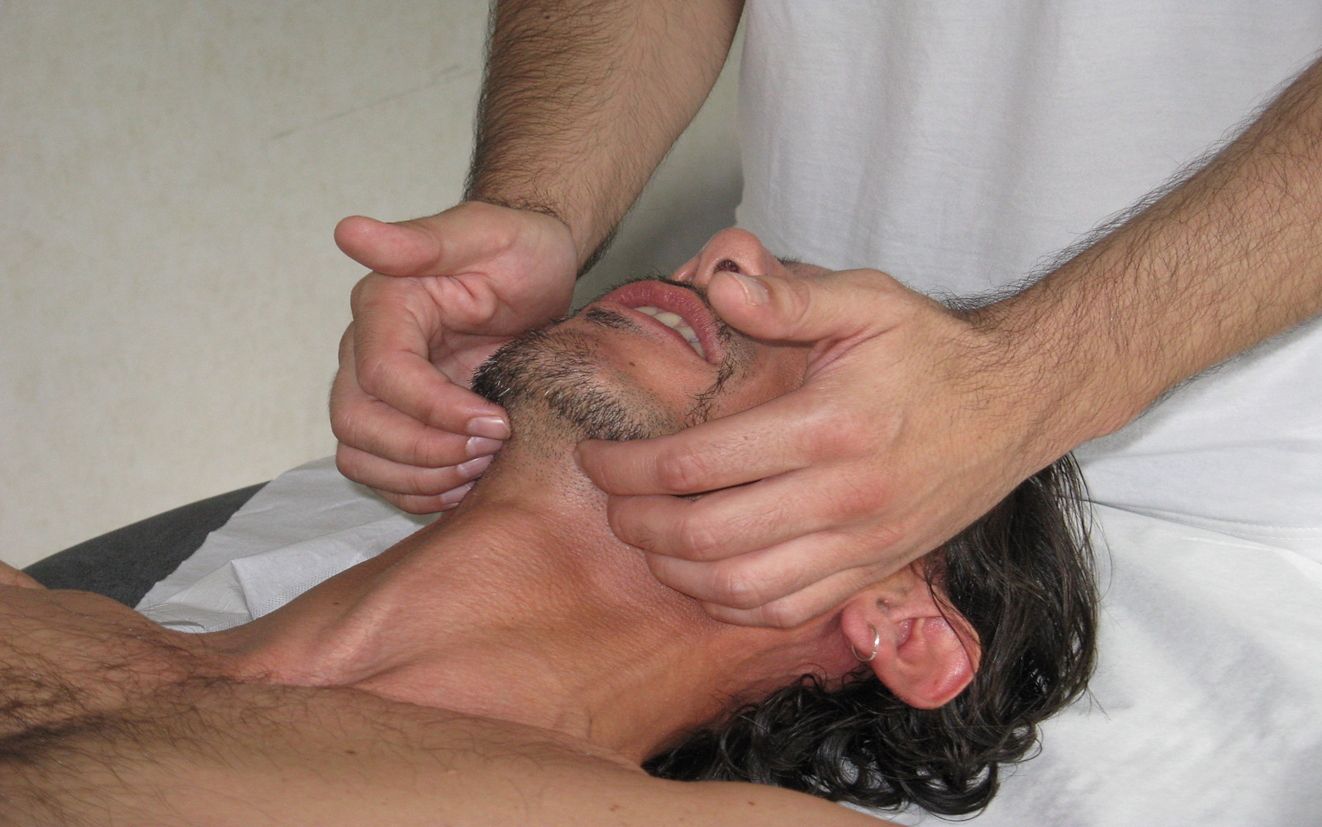
For evaluation of the lingual complex, one hand is placed beneath the floor of the mouth to detect abnormal tensions and small movements. Alternatively, the tongue may be assessed with one hand on the greater horns of the hyoid bone while the other remains beneath the buccal floor, allowing simultaneous palpation of structural and functional dynamics (see Image. Buccal Floor Assessment).
The thoracic outlet is assessed with the clinician seated at the head of the table. The index finger is placed above the clavicle, the middle finger beneath the same bone, and the thumb near the transverse process of C7, while the remaining 2 fingers rest on the chest. Evaluation includes tissue texture, temperature, pain, and any structural restrictions or entrainments. Palpation aims to detect subtle opening and closing movements of the thoracic outlet, which occur independently of cardiac and respiratory rhythms.
For general evaluation of the diaphragm, the clinician stands beside the patient. Hands are positioned posterolaterally, corresponding to the greatest diaphragmatic displacement, while the anterior portion moves minimally during calm respiration. Assessment includes tissue texture, temperature, pain, and any restrictions. Observed alterations in diaphragmatic movement should be noted, with recognition that the right hemidiaphragm exhibits reduced displacement due to the presence of the liver.
The final step of the general assessment targets the pelvic floor, with the clinician standing. Palms are placed on the iliac wings, and small oscillatory movements are applied toward the bed to facilitate a subtle opening of the pelvis. This technique allows identification of restrictions in movement, pain, or dysfunction in the muscles, joints, and visceral components of the pelvic floor.
The initial assessment highlights areas exhibiting limited mobility or symptomatic responses such as pain or discomfort. Identification of regions requiring focused osteopathic treatment necessitates simple inhibitory tests. For example, dysfunctions may be detected in the respiratory diaphragm and the lingual complex. One hand is positioned on the diaphragmatic rib area while the other contacts the floor of the mouth. Gentle pressure applied with the cranial hand masks afferent input from the lingual region, revealing diaphragmatic dysfunction. The procedure is reversed to assess lingual function relative to the diaphragm. Anatomical regions where dysfunction persists are likely the primary targets for treatment.[31][32][33]
Targeted Manual Evaluation
The 5 diaphragms play a central role in maintaining integrated physiological and postural function. Techniques for the manual assessment of these regions are described below.
Tentorium cerebelli
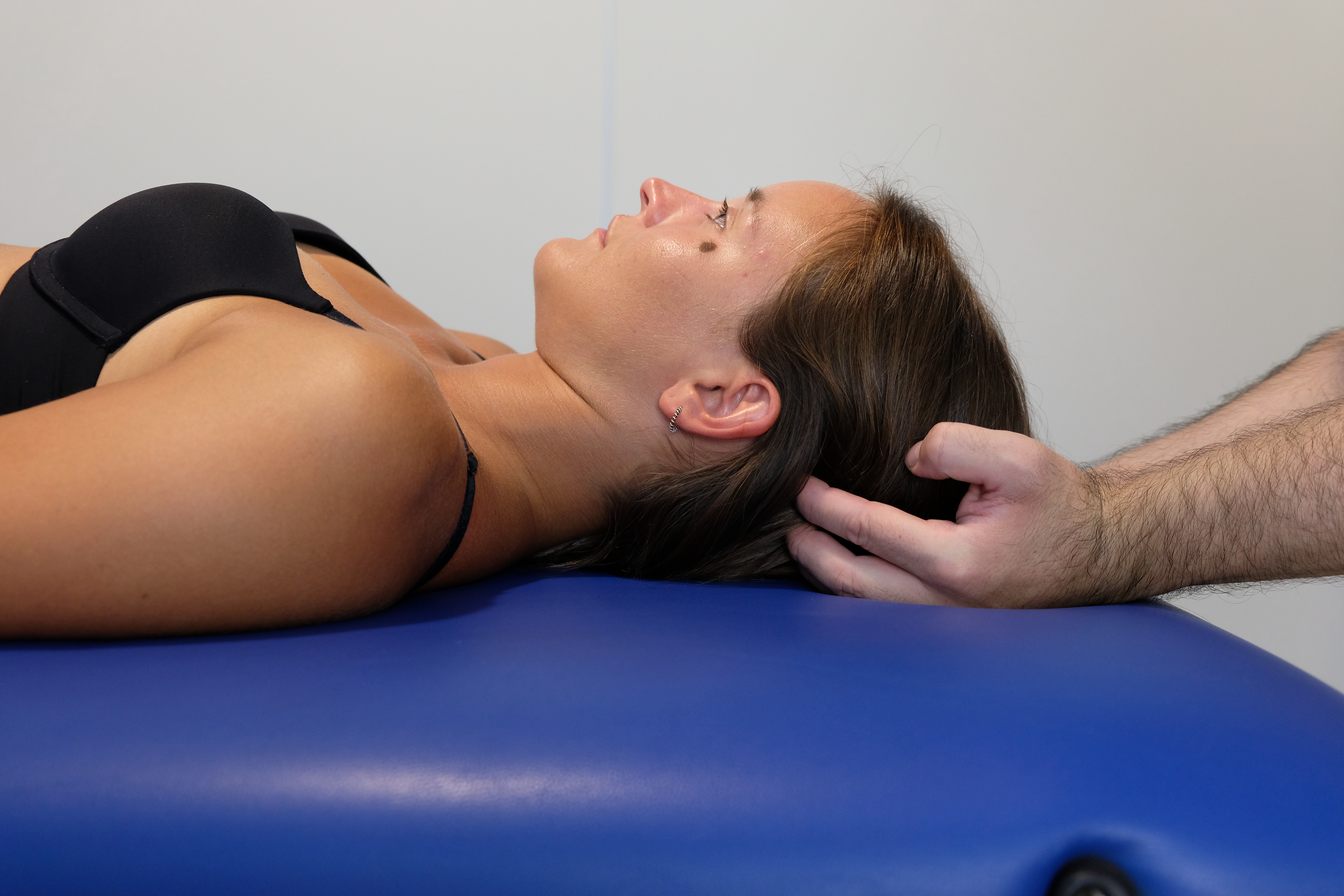
Evaluation of the tentorium cerebelli involves assessment of multiple interrelated structures (see Image. Tentorium Cerebelli Assessment Technique). The tone of the suboccipital muscles and the range of motion of the cervical vertebrae, from C1 to C4, as well as the dorsal nerve exits of the cervical plexus up to C3, should be examined, with attention to subtle palpable nodules. Movements of the temporal bones and their associated sutures—occipitomastoid, sphenosquamosal, petrojugular, petroccipital, sphenopetrosal, temporozygomatic, parietomastoid, and temporomandibular—should also be evaluated.
Tension within the tentorium cerebelli may be assessed by applying anterior, oblique, and slightly caudal traction to the ear lobe. The area exhibiting the greatest resistance corresponds to the site of potential tentorial dysfunction. Palpation should include other meningeal membranes to determine whether observed tentorial tension represents a primary dysfunction or a secondary effect of adjacent membranous structures. In this context, the quality of palpatory perception is more relevant than the specific technique employed.
Lingual complex
Evaluation of the lingual complex begins with visual observation of the tongue and the patient’s mouth opening, noting any mandibular deviations or temporomandibular joint noises. Cervical spine behavior should be assessed during mouth opening and with tongue protrusion or retrusion. Dental occlusion and classification should also be considered to understand how the tongue interacts with the oral cavity. Active tongue movements are examined with the mouth open, including anterior protrusion, dorsiflexion, ventroflexion, cranial elevation, inferior depression, lateral movements, and posterior retrusion. Tongue strength is assessed using a gloved finger to provide minimal resistance against the same movements, allowing evaluation of neuromotor function and muscular coordination.
Tongue movement is further evaluated by instructing the patient to rotate the head to the right and left while maintaining tongue protrusion. The same movements are observed with the cervical spine in flexion and extension, providing insight into fascial and embryological correlations between the tongue and neck. Postural integration of the tongue is assessed using the Fukuda balance test, performed with the patient’s eyes closed and the tongue tip positioned against the upper incisors. The test is repeated with the tongue in its resting position. Improvement in postural stability with the tongue at the designated spot indicates proper integration of lingual afferents, whereas unchanged or worsened balance suggests potential dysfunction of the tongue.
With the tongue in dorsiflexion, thigh strength is assessed by asking the patient to extend the knee while applying manual resistance to the leg. The procedure is repeated on the opposite limb. An increase in strength indicates preserved lingual integration, whereas unchanged or decreased strength with the tongue at the palatine spot suggests a nonphysiological lingual response. Limb strength parallels lingual contractility, with reductions in one corresponding to declines in the other.
Muscular function of the lips is evaluated by asking the patient to smile or purse the lips, reflecting the neuromotor collaboration between tongue and lip muscles during speech. Weakness in these muscles may indicate impaired lingual coordination. Chewing muscle tone is palpated with a gloved hand, and the hyoid bone is examined during swallowing, breathing, mouth opening, and tongue protrusion or retrusion. A preliminary noninstrumental assessment of tongue function may be performed using the Performance Tongue Test (PTT).[34]
Thoracic outlet
Evaluation of the thoracic outlet includes assessment of bony, muscular, and fascial components (see Image. Manual Palpation of the Thoracic Outlet). Examination begins with the sternum, sternoclavicular and 1st rib joints, the clavicle-scapula articulation, C7 to T2 vertebrae, and the 1st and 2nd ribs. Scapular motion is observed both actively and passively, along with the movement of the 1st rib. Muscular assessment encompasses the scalene muscles, pectoralis major and minor, subclavian, and trapezius muscles, while shoulder mobility is also evaluated. The pleural dome and its associated fascial system are palpated to identify restrictions or abnormal tension. Neurological evaluation may include the Elevated Arm Stress Test (EAST) and the Upper Limb Tension Test (ULTT) of Elvey to detect possible nerve dysfunctions. No manual test reliably assesses vascular function within the thoracic outlet.
Diaphragm muscle
Diaphragm muscle evaluation is performed with the patient in a supine position. The clinician places the hands laterally along the ribs in the diaphragmatic region to palpate active and passive rib movements, applying small oscillations. The xiphoid process is palpated to assess tenderness, mobility, and coordination with the ribs. Assessment of the right and left diaphragmatic domes involves placing the hands on the anterolateral ribs with the thumbs beneath the inferior ribs, applying gentle cranial pressure to evaluate tissue elasticity, with the forearm positioned parallel to the abdomen. The forearm may be adjusted to a 45° angle to apply pressure toward the bed, facilitating assessment of the posterolateral muscle mass.
The medial and intermediate pillars are evaluated by stimulating movement from T11 to L4 vertebrae and the last rib. The phrenic nerve is palpated along its passage through the thoracic outlet, adjacent to the anterior scalene muscle and lateral to the sternocleidomastoid clavicular head, to identify any tender or restricted areas.
Manual evaluation of the diaphragm (MED) may be performed using the MED Scale, alongside a test to assess neurological integration with the body. The patient performs a Fukuda test with deep inhalation, followed by repetition during normal breathing. Improvement in balance indicates proper diaphragmatic function, whereas a latent imbalance suggests dysfunction. Additional confirmation involves assessing thigh strength during flexion with the patient standing, applying manual resistance over the thigh during active movement, combined with deep inhalation. Improvement in strength indicates proper integration of the diaphragm, while unchanged or decreased strength suggests functional dysfunction. This assessment is referred to as the Bordoni Diaphragmatic Test (BDT).[35]
Pelvic floor
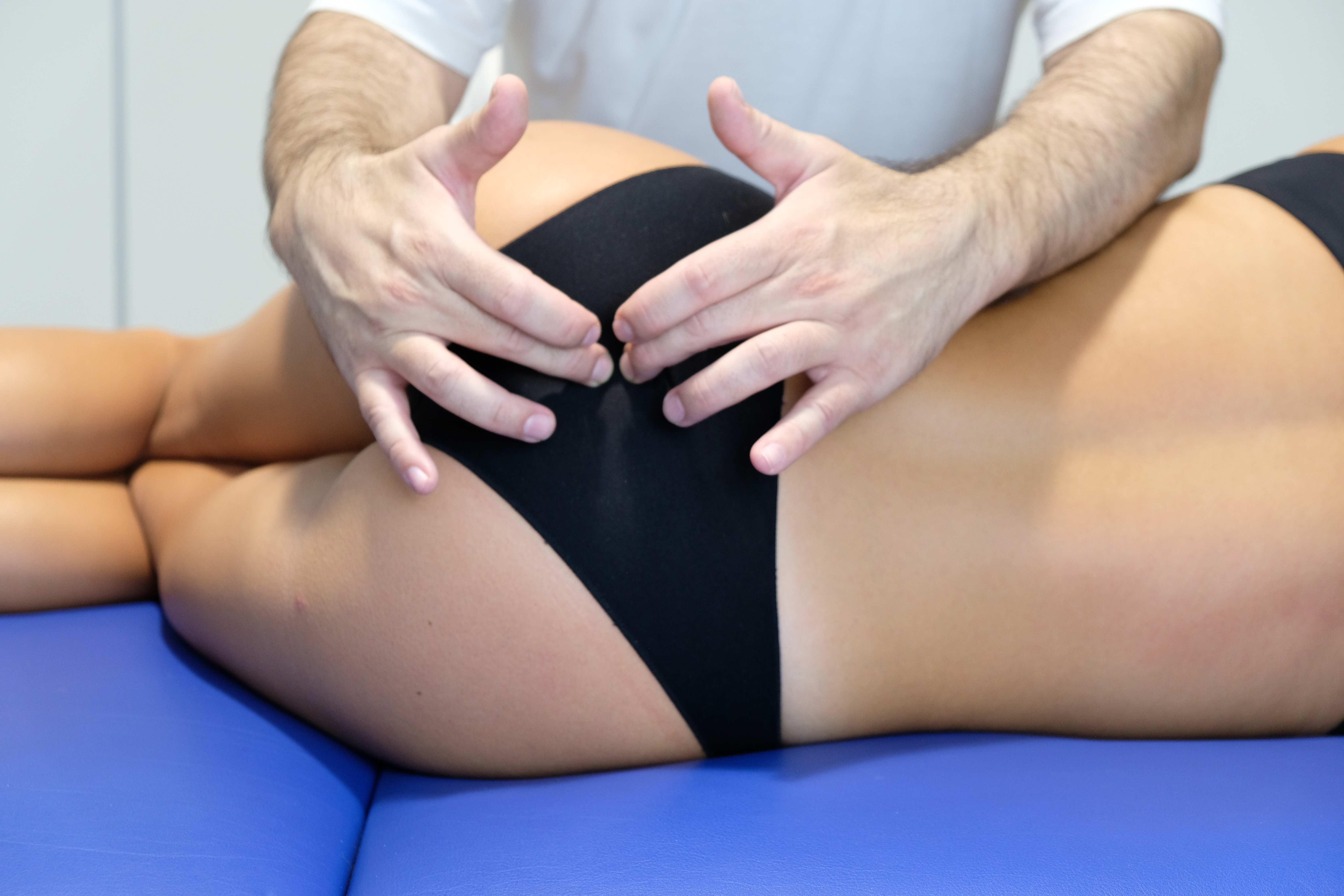
The patient is initially positioned supine for assessment of the pelvic floor region. Evaluation includes palpation of the symphysis pubis, suprapubic area for trigger points, ilioinguinal ligament, obturator nerve, adductor and hip rotator muscle strength, pelvic floor muscles, pudendal nerve, sacral base at L5, and sacroiliac joints (see Image. Evaluation of the Sacrococcygeal Joint). External examination of the bladder and uterus is also performed. The patient is then placed in lateral decubitus to allow further assessment of the pelvic floor muscles, the iliolumbar ligament, the iliac bone, and the coccyx.
Afterward, the patient assumes a prone position for evaluation of the sacrum using the spring test, thoracolumbar fascia trigger points, gluteal and piriformis muscles, long dorsal sacroiliac ligament, and pelvic floor muscles, with external assessment of the rectum. The clinician must adapt evaluation methods to the patient’s capabilities when standard positions are not feasible.[36][37]
Technique or Treatment
Unwinding represents one of the most effective techniques in osteopathic manipulation, originally developed by the American osteopath Dr. Viola Frymann. This approach may be applied to all patients, including pediatric populations, by utilizing the patient’s intrinsic forces without requiring additional manual force from the clinician.
The clinician may target individual components of a specific dysfunctional diaphragm or address multiple diaphragms simultaneously. Selection of the technique for each component, or for a single component within a diaphragm, depends on clinical judgment and the intended therapeutic outcome.
When addressing multiple diaphragms, the unwinding technique may be applied by placing one hand on the most cranially located diaphragm and the other on the most caudal. The diaphragms treated may be anatomically close, such as the tentorium and tongue, or distant, such as the tentorium and pelvic floor. The duration of treatment and manual grip are determined by patient comfort and clinician expertise. If all diaphragms require intervention, each may be engaged sequentially with a manual grip before proceeding to the next.
The clinician must avoid introducing external force or initiating movement during application. The hands follow the intrinsic motion of the tissues. Initial movements may appear chaotic or asymmetrical. The technique concludes when intrinsic tissue movements achieve a symmetrical pattern in amplitude, magnitude, and duration. The hands may appear to move but remain passive, responding to tissue dynamics rather than directing them.[38]
Complications
Current scientific literature indicates that unwinding techniques are not associated with complications in patients. The technique may be applied even in areas with incompletely healed wounds. Patient dignity and privacy must be prioritized, and informed consent must be obtained prior to treatment.
Clinical Significance
Dural pain may result from dysfunction of the lingual complex. Tooth pulp inflammation, for example, can induce tongue pain through retrograde electrical and biochemical signaling from the gums to the structures innervating the lingual complex. From an electrophysiological and mechano-metabolic perspective, the precise location of symptoms cannot always be predicted due to the integrated networks of bodily innervations that communicate extensively.
Mechanical problems of the tongue following cervical trauma, such as whiplash, may trigger antidromic signaling from the trigeminal system to the Gasser ganglion. This ganglion contains neural cells, macrophages, lymphocytes, and glial cells, which increase the trigeminal network’s receptivity to mechano-metabolic signals, ultimately contributing to trigeminal pain.
Mechanical receptors are located near arterial pathways at the dural level of the tentorium cerebelli. Activation of these receptors can cause vasodilation and trigeminal pain. Dysfunction of the tongue can thus transmit nonphysiological signals to the trigeminal ganglion in the Meckel cave, stimulating abnormal activity in the dural area and producing pain localized in the dura or referred to other cranial locations, such as the eye, due to connections with the nervus tentorii.[39][40]
Manual treatment of the 5 diaphragms has multiple objectives, including the improvement of body fluid circulation. The meningeal system encloses venous vessels and the glymphatic system, which is primarily drained via the cribriform plate, continuing to the oral mucosa, tongue, cervical tract, and ultimately to the thoracic outlet.
Cranial dural tissue may modify its tension state, which, in turn, alters fluid passage for various reasons. Mechanical tension originating from the suboccipital muscles, for example, can affect the tension of the tentorium, creating an inflammatory environment. Arterial tone contributes to the extent of this inflammatory response. Lighter fluids, such as lymph and interstitial fluids, are displaced predominantly by the heartbeat, which is reflected in arterial vascular tone. Altered tension of the tentorium and arterial vessels may slow glymphatic flow and adversely affect immunoregulation.
Animal models demonstrate that lymphatic vessels are located near cranial nerves, including those supplying branches involved in tongue function. Nonphysiological mechanical tension combined with an altered metabolic environment can thus promote inflammation or infection of the lingual complex, both by disrupting neural conductivity and modifying transport of biological substances to the tongue, as validated by the literature. Clinical reasoning supports evaluating and treating the tentorium to address or assist in resolving dysfunctions of the lingual system.[41][42][43]
Paresthesia of the 5th finger resulting from nonphysiological pressure on the ulnar nerve may originate from abnormal tension in the diaphragm muscle. Palpation can direct the osteopath’s attention to the respiratory diaphragm, even when the suspected cause is linked to thoracic outlet syndrome.
The right or left phrenic nerve can carry inflammatory substances produced by contractile fibers or connective tissue retrogradely to the medulla through an antidromic mechanism, thereby affecting the motor neurons of the subclavian nerve. This nerve can generate nonphysiological muscle tension (hypertonus) in the subclavian muscle and keep the 1st rib in an inspiratory position, compressing the ulnar nerve and producing a neuropathic picture.
Literature demonstrates that diaphragmatic herniation may also contribute to shoulder joint pain by involving the axillary nerve. Chronic pathologies affecting the diaphragm frequently coexist with thoracic outlet syndromes, although the precise nature of this relationship is not always clearly defined or illustrated.
Myocardial infarction can alter the genetic transcription of certain diaphragm proteins, particularly in aging, inducing subclinical or overt respiratory dysfunction. This dysfunction may lead to the production of inflammatory substances by the diaphragm, which are transported via the phrenic nerve and negatively impact brachial nerve function. Chronic inspiratory positioning of the diaphragm can subject the phrenic nerve to persistent nonphysiological traction, prompting the nerve tissue to release biological substances that affect the brachial medullary nerves, as occurs in traction neuropathies.[44][45][46]
In some patients, the vagus nerve has a close anatomical relationship with the omohyoid muscle, which lies medial to the jugular vein. In certain cases, the omohyoid may span both clavicles or attach to the mastoid process. The muscle can be positioned above the superior transverse scapular ligament or laterally beneath it, where the suprascapular nerve (C5-C6) passes.
Movements of the tongue activate the omohyoid muscle and likely provide proprioceptive input for scapular motion. Anatomical studies using dye injection have demonstrated a close relationship between the suprascapular nerve and the phrenic nerve. When the tongue tends to remain elevated toward the hard palate, the omohyoid shows increased electromyographic activity. This heightened activation may alter the mechanical environment around the suprascapular nerve and potentially impact phrenic nerve function.
The innervation of the omohyoid muscle (C1-C3) could further modify the neurological input reaching the suboccipital muscles. Additionally, the vagus nerve may experience abnormal tension from the subhyoid musculature, generating localized disturbances, such as at the thoracic outlet, that can propagate to other diaphragms. Altered scapular positioning, as observed in hyperkyphotic postures, can produce similar mechanical and neurological effects.[47][48][49][50]
Hypertonicity of the muscles comprising the pelvic floor can generate pain in this anatomical region, particularly during pelvic movements, inhalation, or physical exertion, such as lifting or sneezing. Chronic pain or associated visceral dysfunction may impair diaphragmatic excursion, reducing functional capacity during inhalation and potentially contributing to chronic lower back pain. Osteopathic evaluation requires awareness of the anatomy of the 5 diaphragms and avoidance of focusing solely on the presenting symptom. In this example, treatment should initially target the pelvic floor muscles rather than the diaphragm.[51][52][53]
Previous cardiac surgery, such as median sternotomy, can induce iatrogenic effects on the phrenic nerve, including permanent partial injury. In cases of complete and extensive lesions, patients may require a second diaphragm plication to restore some function of the primary respiratory muscle.
One observed consequence following cardiovascular surgery, particularly in the presence of partial or total phrenic nerve plegia, is altered movement of the ipsilateral shoulder, most frequently on the left side and less commonly on the right. This phenomenon may be explained by the antidromic activity of the phrenic nerve, which transmits biochemical mediators toward the medulla and involves the brachial plexus, including the axillary nerve.
Mechanical tension distribution is altered if the myofascial continuum experiences functional discontinuity due to a compromised anatomical region, such as a hemidiaphragm, resulting in reduced shoulder range of motion. Clinically, this scenario highlights that osteopathic intervention can provide meaningful relief despite the presence of permanent phrenic nerve injury or postplication changes, including diaphragmatic scarring. Such intervention does not fully resolve structural damage but serves as a moment for maximal clinical adaptability, enhancing patient functional capacity while recognizing the inherent limits of treatment.
In patients with obesity, the diaphragm frequently exhibits dysfunction, as evidenced by decreased spirometry values. These parameters may suggest a restrictive pattern, although they do not necessarily indicate intrinsic lung disease. A substantial proportion of individuals with obesity experience dyspnea, attributable both to altered respiratory rhythms regulated by central respiratory centers and to reduced diaphragmatic excursion with phenotypic changes, including an increased proportion of oxidative yet functionally impaired fibers.[54] Dysfunction of the respiratory centers can impair all muscles involved in breathing, including the diaphragms, producing symptoms that are often difficult to classify.[55]
Diaphragm dysfunction can manifest in a wide range of clinical symptoms, including neck pain, profuse night sweats, daytime fatigue, sleep apnea, unexplained chest pain, gastroesophageal reflux, the Hoover sign, dysphagia, low back and pelvic pain, trigeminal pain, chronic cough, hoarseness, chronic atelectasis, and recurrent lung infections.[56][57][58][59][60][61] Application of the osteopathic 5 diaphragm approach allows clinicians to manually identify the most dysfunctional region, focus treatment accordingly, or refer patients for further instrumental evaluation by medical specialists.
Enhancing Healthcare Team Outcomes
Ailments, such as disease and pain, may affect the entirety of a patient's personal sphere. Both chronic and acute dysfunctions can involve psychic and emotional domains. Osteopathic manipulative treatment may not always produce the desired outcomes due to multiple factors, such as patient nonadherence or underlying emotional disturbances that extend beyond observable physical dysfunction. Engaging the patient as the central participant in the treatment process remains essential in all cases. Comprehensive care requires attention to the mind, body, and spirit, including referral to other healthcare professionals, such as psychologists or psychiatrists, when indicated. Clinicians must recognize the limits of their expertise and determine when other skill sets are more appropriate to continue care.
Addressing mental health constitutes a critical component of optimizing patient outcomes. Enhancing clinical proficiency involves acknowledging professional limits and engaging in interprofessional collaboration. Evidence demonstrates that osteopathic outcomes improve when clinicians operate within an interprofessional team.[62][63][64]
Nursing, Allied Health, and Interprofessional Team Interventions
The 5 diaphragms encompass distinct anatomical regions while maintaining a continuous functional and structural relationship. Involvement of different healthcare professionals is essential, as their assessment and management of related systems, including pulmonary and circulatory functions, provide critical information for the treatment of patients presenting with diaphragmatic restrictions.
Nursing, Allied Health, and Interprofessional Team Monitoring
Each team member should apply their specialized skills to support and inform the treatment objectives of others. Referral to healthcare professionals with expertise in the relevant anatomical region or specific pathology, or ongoing monitoring of the therapeutic course, is critical to validating the outcomes of osteopathic manual interventions.
Media
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)

Manual Palpation of the Thoracic Outlet. The image demonstrates proper hand placement on the thoracic outlet region as part of a global evaluation using osteopathic palpation techniques. This approach allows for assessment of tissue quality, structure, and mobility.
Contributed by Bruno Bordoni, PhD.
References
Bordoni B. The Five Diaphragms in Osteopathic Manipulative Medicine: Myofascial Relationships, Part 1. Cureus. 2020 Apr 23:12(4):e7794. doi: 10.7759/cureus.7794. Epub 2020 Apr 23 [PubMed PMID: 32461863]
Bordoni B, Zanier E. Anatomic connections of the diaphragm: influence of respiration on the body system. Journal of multidisciplinary healthcare. 2013:6():281-91. doi: 10.2147/JMDH.S45443. Epub 2013 Jul 25 [PubMed PMID: 23940419]
Bordoni B, Simonelli M, Lagana MM. Tentorium Cerebelli: Muscles, Ligaments, and Dura Mater, Part 1. Cureus. 2019 Sep 9:11(9):e5601. doi: 10.7759/cureus.5601. Epub 2019 Sep 9 [PubMed PMID: 31700714]
Ita MI, Bordoni B. Neuroanatomy, Tentorium Cerebelli. StatPearls. 2025 Jan:(): [PubMed PMID: 32491772]
McCausland T, Bordoni B. Anatomy, Head and Neck: Genioglossus Muscle. StatPearls. 2025 Jan:(): [PubMed PMID: 31424725]
Bordoni B, Morabito B, Mitrano R, Simonelli M, Toccafondi A. The Anatomical Relationships of the Tongue with the Body System. Cureus. 2018 Dec 5:10(12):e3695. doi: 10.7759/cureus.3695. Epub 2018 Dec 5 [PubMed PMID: 30838167]
Bordoni B. The Five Diaphragms in Osteopathic Manipulative Medicine: Neurological Relationships, Part 2. Cureus. 2020 Jun 20:12(6):e8713. doi: 10.7759/cureus.8713. Epub 2020 Jun 20 [PubMed PMID: 32699708]
Bordoni B. The Five Diaphragms in Osteopathic Manipulative Medicine: Neurological Relationships, Part 1. Cureus. 2020 Jun 19:12(6):e8697. doi: 10.7759/cureus.8697. Epub 2020 Jun 19 [PubMed PMID: 32699694]
Bordoni B. The Five Diaphragms in Osteopathic Manipulative Medicine: Myofascial Relationships, Part 2. Cureus. 2020 Apr 23:12(4):e7795. doi: 10.7759/cureus.7795. Epub 2020 Apr 23 [PubMed PMID: 32467780]
Bordoni B. Comment on "Comparison of Diaphragmatic Stretch Technique and Manual Diaphragm Release Technique on Diaphragmatic Excursion in Chronic Obstructive Pulmonary Disease: A Randomized Crossover Trial". Pulmonary medicine. 2020:2020():7437019. doi: 10.1155/2020/7437019. Epub 2020 May 18 [PubMed PMID: 32518696]
Level 3 (low-level) evidenceBordoni B, Morabito B, Simonelli M. Ageing of the Diaphragm Muscle. Cureus. 2020 Jan 13:12(1):e6645. doi: 10.7759/cureus.6645. Epub 2020 Jan 13 [PubMed PMID: 32064216]
Bordoni B, Sugumar K, Leslie SW. Anatomy, Abdomen and Pelvis, Pelvic Floor. StatPearls. 2025 Jan:(): [PubMed PMID: 29489277]
Kirsch DB. Obstructive Sleep Apnea. Continuum (Minneapolis, Minn.). 2020 Aug:26(4):908-928. doi: 10.1212/CON.0000000000000885. Epub [PubMed PMID: 32756228]
Ruehland WR, Rochford PD, Pierce RJ, Trinder J, Jordan AS, Cori JM, O'Donoghue FJ. Genioglossus muscle responses to resistive loads in severe OSA patients and healthy control subjects. Journal of applied physiology (Bethesda, Md. : 1985). 2019 Dec 1:127(6):1586-1598. doi: 10.1152/japplphysiol.00186.2019. Epub 2019 Oct 24 [PubMed PMID: 31647723]
Pazarlı AC, Özmen Z, İnönü Köseoğlu H, Ekiz T. Ultrasonographic measurement of the diaphragm thickness in patients with obstructive sleep apnea syndrome. Sleep & breathing = Schlaf & Atmung. 2020 Mar:24(1):89-94. doi: 10.1007/s11325-019-01931-2. Epub 2019 Aug 28 [PubMed PMID: 31463778]
Chuang SY, Teng A, Butler J, Gandevia S, Narang I, Briggs N, Selvadurai H, Jaffe A. Quantitative assessment of nocturnal neural respiratory drive in children with and without obstructive sleep apnoea using surface EMG. Experimental physiology. 2019 May:104(5):755-764. doi: 10.1113/EP087441. Epub 2019 Mar 20 [PubMed PMID: 30821402]
Misraï V, Charbonneau H, Attias D, Pathak A. Obstructive sleep apnea syndrome should always be screened in patients complaining of nocturia. World journal of urology. 2019 Dec:37(12):2801-2802. doi: 10.1007/s00345-018-2534-x. Epub 2018 Oct 22 [PubMed PMID: 30350015]
Fuentes-Alonso M, López-de-Andrés A, Palacios-Ceña D, Jimenez-Garcia R, Lopez-Herranz M, Hernandez-Barrera V, Perez-Farinos N, Ji Z, de-Miguel-Diez J. COPD is Associated with Higher Prevalence of Back Pain: Results of a Population-Based Case-Control Study, 2017. Journal of pain research. 2020:13():2763-2773. doi: 10.2147/JPR.S271713. Epub 2020 Nov 2 [PubMed PMID: 33173326]
Level 2 (mid-level) evidencede Miguel-Díez J, López-de-Andrés A, Hernandez-Barrera V, Jimenez-Trujillo I, Del Barrio JL, Puente-Maestu L, Martinez-Huedo MA, Jimenez-García R. Prevalence of Pain in COPD Patients and Associated Factors: Report From a Population-based Study. The Clinical journal of pain. 2018 Sep:34(9):787-794. doi: 10.1097/AJP.0000000000000598. Epub [PubMed PMID: 29485534]
Elizagaray-Garcia I, Beltran-Alacreu H, Angulo-Díaz S, Garrigós-Pedrón M, Gil-Martínez A. Chronic Primary Headache Subjects Have Greater Forward Head Posture than Asymptomatic and Episodic Primary Headache Sufferers: Systematic Review and Meta-analysis. Pain medicine (Malden, Mass.). 2020 Oct 1:21(10):2465-2480. doi: 10.1093/pm/pnaa235. Epub [PubMed PMID: 33118601]
Level 1 (high-level) evidenceBordoni B, Marelli F, Morabito B. The tongue after whiplash: case report and osteopathic treatment. International medical case reports journal. 2016:9():179-82. doi: 10.2147/IMCRJ.S111147. Epub 2016 Jul 7 [PubMed PMID: 27462180]
Level 3 (low-level) evidenceYeampattanaporn O, Mekhora K, Jalayondeja W, Wongsathikun J. Immediate effects of breathing re-education on respiratory function and range of motion in chronic neck pain. Journal of the Medical Association of Thailand = Chotmaihet thangphaet. 2014 Jul:97 Suppl 7():S55-9 [PubMed PMID: 25141528]
Yim J, Park J, Lohman E, Do K. Comparison of cervical muscle activity and spinal curvatures in the sitting position with 3 different sloping seats. Medicine. 2020 Jul 10:99(28):e21178. doi: 10.1097/MD.0000000000021178. Epub [PubMed PMID: 32664159]
Somkearti P, Chattakul P, Khamsai S, Limpawattana P, Chindaprasirt J, Chotmongkol V, Sawanyawisuth K. Predictors of chronic kidney disease in obstructive sleep apnea patients. Multidisciplinary respiratory medicine. 2020 Jan 28:15(1):470. doi: 10.4081/mrm.2020.470. Epub 2020 Jan 28 [PubMed PMID: 32153778]
Wang B, Yin Q, Wang YY, Tu Y, Han Y, Gao M, Pan M, Yang Y, Xue Y, Zhang L, Zhang L, Liu H, Tang R, Zhang X, Xiao J, Wang XH, Liu BC. Diaphragmatic dysfunction associates with dyspnoea, fatigue, and hiccup in haemodialysis patients: a cross-sectional study. Scientific reports. 2019 Dec 18:9(1):19382. doi: 10.1038/s41598-019-56035-4. Epub 2019 Dec 18 [PubMed PMID: 31853002]
Level 2 (mid-level) evidenceBharucha AE, Dunivan G, Goode PS, Lukacz ES, Markland AD, Matthews CA, Mott L, Rogers RG, Zinsmeister AR, Whitehead WE, Rao SS, Hamilton FA. Epidemiology, pathophysiology, and classification of fecal incontinence: state of the science summary for the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) workshop. The American journal of gastroenterology. 2015 Jan:110(1):127-36. doi: 10.1038/ajg.2014.396. Epub 2014 Dec 23 [PubMed PMID: 25533002]
Bordoni B, Escher AR Jr. Osteopathic Principles: The Inspiration of Every Science Is Its Change. Cureus. 2021 Jan 4:13(1):e12478. doi: 10.7759/cureus.12478. Epub 2021 Jan 4 [PubMed PMID: 33425556]
King BW, Skedros JG, Glasgow RE, Morrell DG. Resolution of Chronic Shoulder Pain after Repair of a Posttraumatic Diaphragmatic Hernia: A 22-Year Delay in Diagnosis and Treatment. Case reports in orthopedics. 2020:2020():7984936. doi: 10.1155/2020/7984936. Epub 2020 Jan 6 [PubMed PMID: 31976108]
Level 3 (low-level) evidenceBuzzi MG, Dimitriadou V, Theoharides TC, Moskowitz MA. 5-Hydroxytryptamine receptor agonists for the abortive treatment of vascular headaches block mast cell, endothelial and platelet activation within the rat dura mater after trigeminal stimulation. Brain research. 1992 Jun 26:583(1-2):137-49 [PubMed PMID: 1324091]
Level 3 (low-level) evidenceBordoni B, Zanier E. Skin, fascias, and scars: symptoms and systemic connections. Journal of multidisciplinary healthcare. 2013:7():11-24. doi: 10.2147/JMDH.S52870. Epub 2013 Dec 28 [PubMed PMID: 24403836]
Bordoni B, Morabito B, Simonelli M, Nicoletti L, Rinaldi R, Tobbi F, Caiazzo P. Osteopathic approach with a patient undergoing cardiac transplantation: the five diaphragms. International medical case reports journal. 2019:12():303-308. doi: 10.2147/IMCRJ.S204829. Epub 2019 Sep 5 [PubMed PMID: 31564994]
Level 3 (low-level) evidenceBordoni B, Zanier E. The continuity of the body: hypothesis of treatment of the five diaphragms. Journal of alternative and complementary medicine (New York, N.Y.). 2015 Apr:21(4):237-42. doi: 10.1089/acm.2013.0211. Epub 2015 Mar 16 [PubMed PMID: 25775273]
Bicalho E, Vieira L, Makita DK, Rivas L. Inhibitory Tests as Assessment Tools for Somatic Dysfunctions: Mechanisms and Practical Applications. Cureus. 2020 Apr 16:12(4):e7700. doi: 10.7759/cureus.7700. Epub 2020 Apr 16 [PubMed PMID: 32431979]
Bordoni B, Escher AR. Non-Instrumental Test for the Evaluation of Tongue Function. Cureus. 2021 Sep:13(9):e18333. doi: 10.7759/cureus.18333. Epub 2021 Sep 27 [PubMed PMID: 34603903]
Bordoni B, Escher AR. Functional evaluation of the diaphragm with a noninvasive test. Journal of osteopathic medicine. 2021 Sep 15:121(11):835-842. doi: 10.1515/jom-2021-0101. Epub 2021 Sep 15 [PubMed PMID: 34523291]
Bordoni B, Simonelli M. Chronic Obstructive Pulmonary Disease: Proprioception Exercises as an Addition to the Rehabilitation Process. Cureus. 2020 May 13:12(5):e8084. doi: 10.7759/cureus.8084. Epub 2020 May 13 [PubMed PMID: 32542139]
Bordoni B, Morabito B. The Diaphragm Muscle Manual Evaluation Scale. Cureus. 2019 Apr 30:11(4):e4569. doi: 10.7759/cureus.4569. Epub 2019 Apr 30 [PubMed PMID: 31281752]
Racca V, Bordoni B, Castiglioni P, Modica M, Ferratini M. Osteopathic Manipulative Treatment Improves Heart Surgery Outcomes: A Randomized Controlled Trial. The Annals of thoracic surgery. 2017 Jul:104(1):145-152. doi: 10.1016/j.athoracsur.2016.09.110. Epub 2017 Jan 18 [PubMed PMID: 28109570]
Level 1 (high-level) evidenceKanno K, Shimizu K, Shinoda M, Hayashi M, Takeichi O, Iwata K. Role of macrophage-mediated Toll-like receptor 4-interleukin-1R signaling in ectopic tongue pain associated with tooth pulp inflammation. Journal of neuroinflammation. 2020 Oct 21:17(1):312. doi: 10.1186/s12974-020-01995-y. Epub 2020 Oct 21 [PubMed PMID: 33081813]
Stone D, Bogaardt H, Linnstaedt SD, Martin-Harris B, Smith AC, Walton DM, Ward E, Elliott JM. Whiplash-Associated Dysphagia: Considerations of Potential Incidence and Mechanisms. Dysphagia. 2020 Jun:35(3):403-413. doi: 10.1007/s00455-019-10039-4. Epub 2019 Aug 3 [PubMed PMID: 31377863]
Hershenhouse KS, Shauly O, Gould DJ, Patel KM. Meningeal Lymphatics: A Review and Future Directions From a Clinical Perspective. Neuroscience insights. 2019:14():1179069519889027. doi: 10.1177/1179069519889027. Epub 2019 Dec 31 [PubMed PMID: 32363346]
Level 3 (low-level) evidenceLohrberg M, Wilting J. The lymphatic vascular system of the mouse head. Cell and tissue research. 2016 Dec:366(3):667-677 [PubMed PMID: 27599481]
Ananian SG, Gvetadze SR, Ilkaev KD, Mochalnikova VV, Zayratiants GO, Mkhitarov VA, Yang X, Ciciashvili AM. Anatomic-histologic study of the floor of the mouth: the lingual lymph nodes. Japanese journal of clinical oncology. 2015 Jun:45(6):547-54. doi: 10.1093/jjco/hyv029. Epub 2015 Mar 13 [PubMed PMID: 25770836]
Bosco F, Musolino V, Gliozzi M, Nucera S, Carresi C, Zito MC, Scarano F, Scicchitano M, Reale F, Ruga S, Maiuolo J, Macrì R, Guarnieri L, Coppoletta AR, Mollace R, Muscoli C, Palma E, Mollace V. The muscle to bone axis (and viceversa): An encrypted language affecting tissues and organs and yet to be codified? Pharmacological research. 2021 Mar:165():105427. doi: 10.1016/j.phrs.2021.105427. Epub 2021 Jan 13 [PubMed PMID: 33453372]
Yegorova S, Yegorov O, Ferreira LF. RNA-sequencing reveals transcriptional signature of pathological remodeling in the diaphragm of rats after myocardial infarction. Gene. 2021 Feb 20:770():145356. doi: 10.1016/j.gene.2020.145356. Epub 2020 Dec 15 [PubMed PMID: 33333219]
Holtman JR Jr. Localization of substance P immunoreactivity in phrenic primary afferent neurons. Peptides. 1989 Jan-Feb:10(1):53-6 [PubMed PMID: 2473451]
Level 3 (low-level) evidenceSonne JWH. Report of a non-looped variant of ansa cervicalis with omohyoid innervation from accessory nerve branch and omohyoid attachment to mastoid process. European archives of oto-rhino-laryngology : official journal of the European Federation of Oto-Rhino-Laryngological Societies (EUFOS) : affiliated with the German Society for Oto-Rhino-Laryngology - Head and Neck Surgery. 2019 Jul:276(7):2105-2108. doi: 10.1007/s00405-019-05436-2. Epub 2019 Apr 26 [PubMed PMID: 31028533]
Castro HA, Resende LA, Bérzin F, König B. Electromyographic analysis of the superior belly of the omohyoid muscle and anterior belly of the digastric muscle in tongue and head movements. Journal of electromyography and kinesiology : official journal of the International Society of Electrophysiological Kinesiology. 1999 Jun:9(3):229-32 [PubMed PMID: 10328418]
Yang HJ, Gil YC, Jin JD, Ahn SV, Lee HY. Topographical anatomy of the suprascapular nerve and vessels at the suprascapular notch. Clinical anatomy (New York, N.Y.). 2012 Apr:25(3):359-65. doi: 10.1002/ca.21248. Epub 2011 Aug 18 [PubMed PMID: 21853468]
Sehmbi H, Johnson M, Dhir S. Ultrasound-guided subomohyoid suprascapular nerve block and phrenic nerve involvement: a cadaveric dye study. Regional anesthesia and pain medicine. 2019 May:44(5):561-564. doi: 10.1136/rapm-2018-100075. Epub 2019 Mar 13 [PubMed PMID: 30867276]
Grimes WR, Stratton M. Pelvic Floor Dysfunction. StatPearls. 2025 Jan:(): [PubMed PMID: 32644672]
Hwang UJ, Lee MS, Jung SH, Ahn SH, Kwon OY. Effect of pelvic floor electrical stimulation on diaphragm excursion and rib cage movement during tidal and forceful breathing and coughing in women with stress urinary incontinence: A randomized controlled trial. Medicine. 2021 Jan 8:100(1):e24158. doi: 10.1097/MD.0000000000024158. Epub [PubMed PMID: 33429797]
Level 1 (high-level) evidenceFinta R, Boda K, Nagy E, Bender T. Does inspiration efficiency influence the stability limits of the trunk in patients with chronic low back pain? Journal of rehabilitation medicine. 2020 Mar 31:52(3):jrm00038. doi: 10.2340/16501977-2645. Epub 2020 Mar 31 [PubMed PMID: 31974590]
Bordoni B, Escher AR. Obesity and the Importance of Breathing. Cureus. 2025 Jan:17(1):e77431. doi: 10.7759/cureus.77431. Epub 2025 Jan 14 [PubMed PMID: 39811724]
Bordoni B, Escher AR. Muscles and Central Neural Networks Involved in Breathing: State of the Art. Cureus. 2025 Mar:17(3):e80599. doi: 10.7759/cureus.80599. Epub 2025 Mar 15 [PubMed PMID: 40091907]
Bordoni B, Mapelli L, Toccafondi A, Di Salvo F, Cannadoro G, Gonella M, Escher AR, Morici N. Post-Myocardial Infarction Rehabilitation: The Absence in the Rehabilitation Process of the Diaphragm Muscle. International journal of general medicine. 2024:17():3201-3210. doi: 10.2147/IJGM.S470878. Epub 2024 Jul 22 [PubMed PMID: 39070222]
Bordoni B. Diaphragm and chronic neck pain: Letter to editor. Musculoskeletal science & practice. 2024 Jun:71():102932. doi: 10.1016/j.msksp.2024.102932. Epub 2024 Mar 12 [PubMed PMID: 38520878]
Level 3 (low-level) evidenceBordoni B, Escher AR. Motor Dysfunctions in Fibromyalgia Patients: The Importance of Breathing. Open access rheumatology : research and reviews. 2024:16():55-66. doi: 10.2147/OARRR.S442327. Epub 2024 Mar 7 [PubMed PMID: 38476512]
Bordoni B, Kotha R, Escher AR. Symptoms Arising From the Diaphragm Muscle: Function and Dysfunction. Cureus. 2024 Jan:16(1):e53143. doi: 10.7759/cureus.53143. Epub 2024 Jan 29 [PubMed PMID: 38288323]
Bordoni B, Escher A, Compalati E, Mapelli L, Toccafondi A. The Importance of the Diaphragm in Neuromotor Function in the Patient with Chronic Obstructive Pulmonary Disease. International journal of chronic obstructive pulmonary disease. 2023:18():837-848. doi: 10.2147/COPD.S404190. Epub 2023 May 11 [PubMed PMID: 37197600]
Bordoni B, Escher AR, Toccafondi A, Mapelli L, Banfi P. Obstructive Sleep Apnea and Role of the Diaphragm. Cureus. 2022 Sep:14(9):e29004. doi: 10.7759/cureus.29004. Epub 2022 Sep 10 [PubMed PMID: 36159353]
Marin T, Maxel X, Robin A, Stubbe L. Evidence-based assessment of potential therapeutic effects of adjunct osteopathic medicine for multidisciplinary care of acute and convalescent COVID-19 patients. Explore (New York, N.Y.). 2021 Mar-Apr:17(2):141-147. doi: 10.1016/j.explore.2020.09.006. Epub 2020 Sep 25 [PubMed PMID: 33158784]
Zarucchi A, Vismara L, Frazzitta G, Mauro A, Priano L, Maestri R, Bergna A, Tarantino AG. Efficacy of Osteopathic Manipulative Treatment on postural control in Parkinsonian patients with Pisa syndrome: A pilot randomized placebo-controlled trial. NeuroRehabilitation. 2020:46(4):529-537. doi: 10.3233/NRE-203068. Epub [PubMed PMID: 32538880]
Level 3 (low-level) evidenceGelinne A, Thakrar R, Tranmer BI, Durham SR, Jewell RP, Penar PL, Lollis SS. Differential Patterns of Referral to Neurosurgery: A Comparison of Allopathic Physicians, Osteopathic Physicians, Nurse Practitioners, Physician Assistants, and Chiropractors. World neurosurgery. 2019 Jun:126():e564-e569. doi: 10.1016/j.wneu.2019.02.095. Epub 2019 Mar 1 [PubMed PMID: 30831280]