Sonography Vascular Peripheral Arterial Assessment, Protocols, and Interpretation
Sonography Vascular Peripheral Arterial Assessment, Protocols, and Interpretation
Introduction
The most common disease process affecting the peripheral arterial system is atherosclerosis. Peripheral arterial disease (PAD) is a steno-occlusive process with occasional aneurysmal dilation of the involved vessels that involves over 8.5 million people in the United States.[1] Common symptomatology from compromised peripheral arterial perfusion ranges from intermittent claudication to rest pain. As the disease process progresses, it can lead to ulceration, gangrene, and limb loss. PAD more commonly affects the lower extremities. This topic evaluates lower extremity arterial disease; however, a similar approach can be applied to the upper extremities.
Noninvasive physiological examinations are often the first line of testing, consisting of a physical exam, ankle-brachial index, or segmental limb pressures. Duplex ultrasonography is a complementary modality that can help confirm the diagnosis, evaluate the extent of disease, monitor progression, and identify complications. Performing and interpreting a peripheral arterial ultrasound requires a thorough understanding of the normal anatomy and caliber of arterial vessels, the pathophysiology of arterial disease, and the hemodynamics of both normal and abnormal flow. Moreover, the exam requires a baseline knowledge of various ultrasonographic modalities, as well as appropriate settings and techniques. Both the sonographer and the interpreting physician need to recognize what constitutes an adequate study.
Ultrasound is widely available, exposes the patient to no ionizing radiation, is typically less expensive, and has a smaller footprint than other cross-sectional imaging modalities. Drawbacks include the poor conductivity of sound waves through air and bone, as well as the attenuation of sound waves as they propagate through soft tissues, particularly in patients with larger body habitus. These constraints are most pronounced for the mediastinal and visceral vasculature. Peripheral assessment, however, has its limitations, notably in patients with extensive edema, wounds, or scarring.
Anatomy and Physiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Anatomy and Physiology
The abdominal aorta bifurcates into the common iliac arteries at L4-L5. The common iliac arteries (average diameter 8-10 mm) run anterior to the iliac veins. After the internal iliac artery origin, the common iliac continues as the external iliac artery. The external iliac artery ends at the inferior epigastric artery origin, medially, and the deep iliac circumflex artery origin laterally, becoming the common femoral artery (average diameter 5 to 9 mm). The common femoral artery and vein are contained within the femoral sheath, with the femoral nerve immediately lateral. After a short course, the common femoral artery bifurcates into the superficial femoral and profunda femoris arteries. The profunda femoris artery is posterolateral and takes a deep course. The superficial femoral artery is the predominant arterial supply to the thigh, and it courses through the adductor hiatus, becoming the popliteal artery as it emerges from Hunters canal.
At the proximal calf, the popliteal artery bifurcates into the anterior tibial artery (supplying the lower leg's anterior compartment and terminating as the dorsalis pedis artery) and the tibioperoneal trunk. The short tibioperoneal trunk then bifurcates, giving off the posterior tibial artery (supplying the posterior calf and coursing posterior to the medial malleolus, where it forms the plantar arch of the foot) and the peroneal artery (supplying the lateral calf musculature and coursing posteromedially to the fibula). For a single vessel in continuity, blood flow should be equal between any 2 points. If the cross-sectional area decreases, velocity must increase proportionally to maintain flow. While the vascular system can accommodate a degree of stenosis through variable vascular wall compliance and collateral formation, this principle remains largely intact. It is the basis for ultrasonographic grading of stenosis.[2] When blood is flowing normally through a non-diseased artery, the flow is laminar, and the spectral waveform appears sharp and distinct. Just distal to a stenosis, blood flow becomes nonlaminar and turbulent with erythrocytes moving at different velocities and in multiple directions. The spectral waveform is broadened to encompass the various velocities at 1 point in time.
It is also necessary to understand the basic principles of and the different modes of ultrasound (B-mode, Doppler, color, and power Doppler) used during arterial evaluation. Ultrasonography interrogates tissues via high-frequency sound waves. The speed of sound through tissues is assumed to be 1540 m/s. The ultrasound probe estimates depth based on the time elapsed between the outgoing pulse and its reflection from the target. Brightness depicted on the grayscale ultrasound depends on the intensity of the reflected wave. B-mode (also known as brightness mode) is also referred to as grayscale ultrasound. It is used for anatomical localization, measuring vessel size, and characterizing atherosclerotic plaque. Both intraluminal abnormalities, such as intimal dissection flaps or thrombi, and extraluminal abnormalities, such as hematomas, can usually be identified. The highest-quality images are obtained by positioning the transducer at a near-perpendicular angle to the vessel of interest.
Doppler ultrasound (also referred to as spectral or pulsed-wave ultrasound) utilizes the Doppler effect to estimate the velocity of flowing blood. A sound wave frequency is shifted when it encounters a moving reflector (erythrocyte), increasing when the reflector moves toward the source and decreasing when it moves away. The change in frequency is maximum when the angle of the ultrasound beam is directed against the axis of blood flow (parallel to it). There is no Doppler effect when the sound wave is precisely perpendicular. A Doppler angle of 30 to 60 degrees is ideal. Errors get amplified the closer the transducer is to 90 degrees. Additionally, at an angle of fewer than 30 degrees, the reflection of sound waves from the vessel is less likely to be recorded. Spectral analysis refers to the graphical display of recorded velocity plotted against the elapsed time.
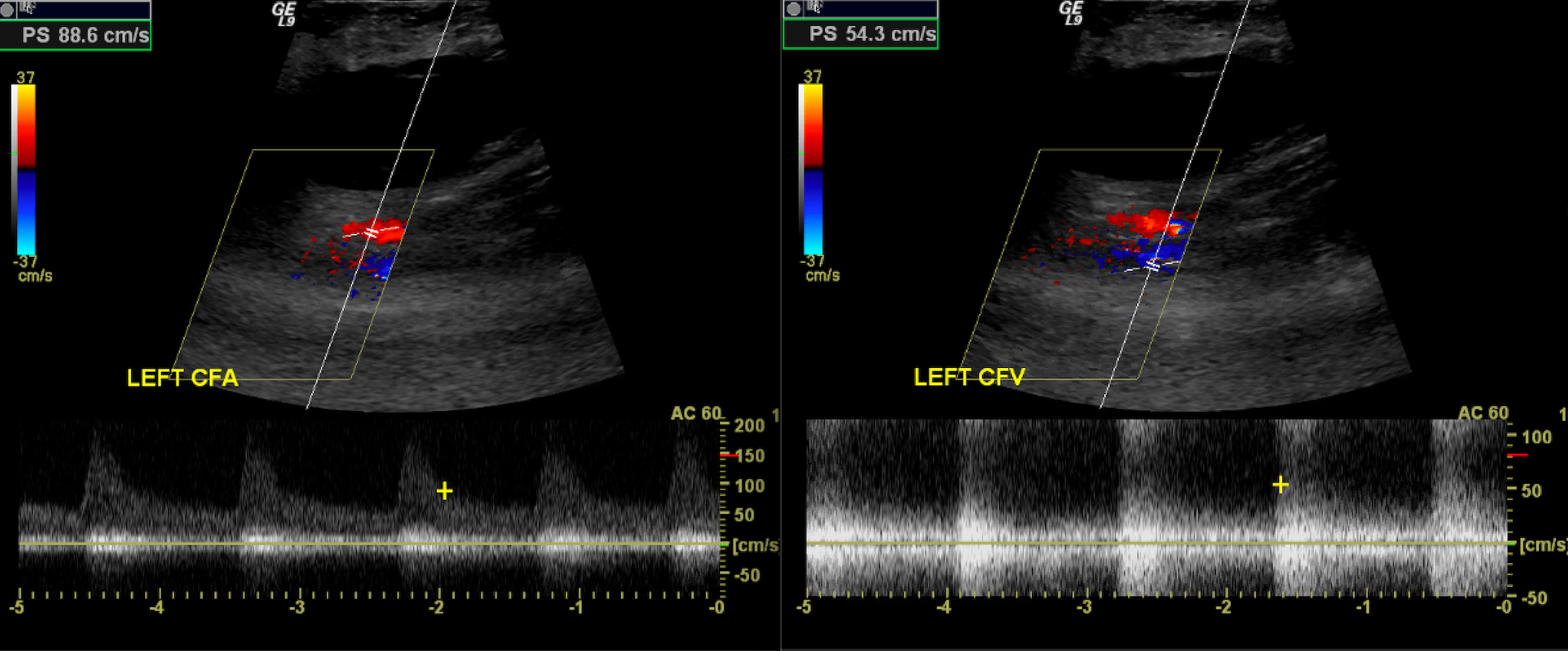
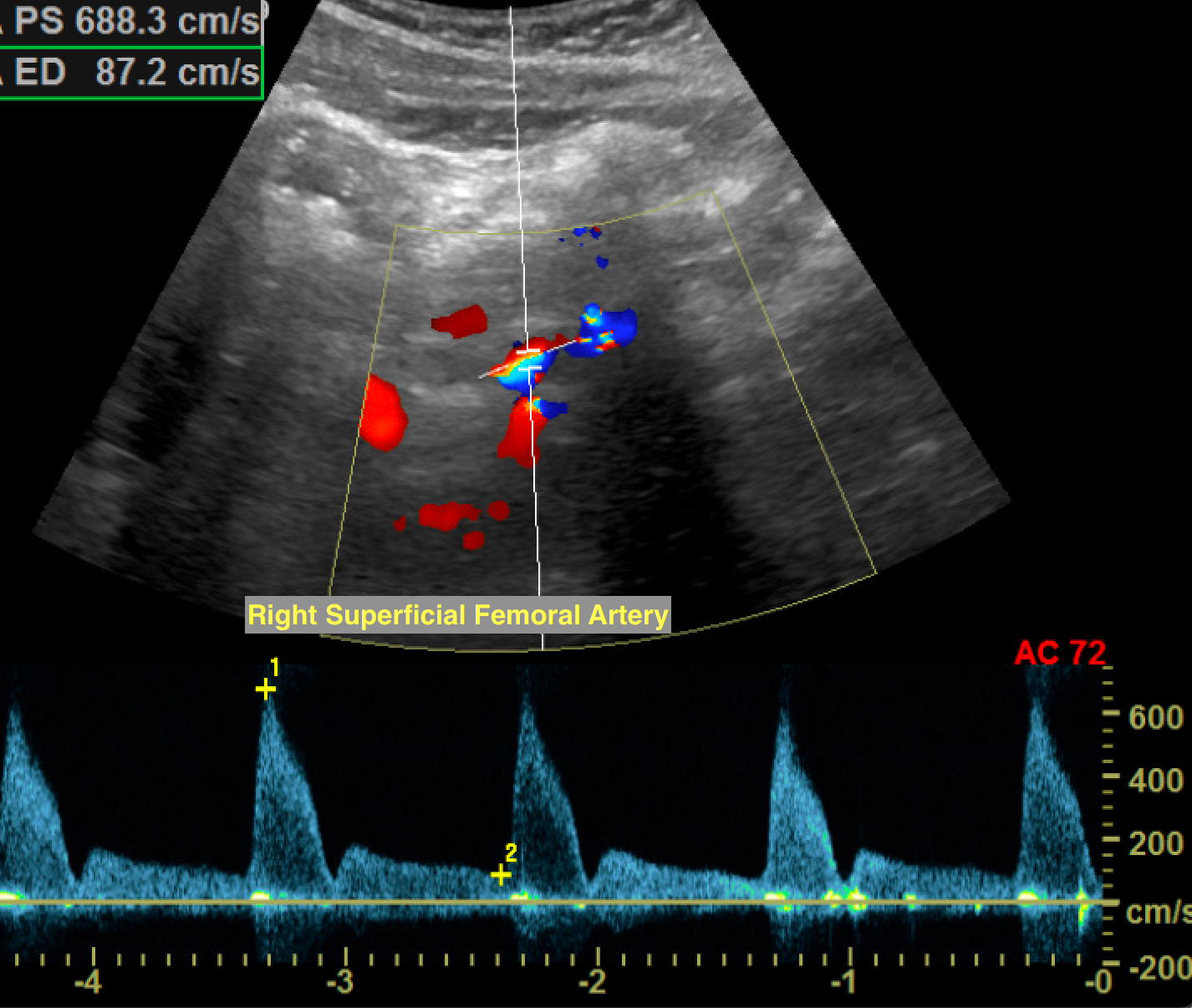
The term duplex refers to the use of 2 ultrasound modes concurrently, most often B-mode and Doppler. Duplex ultrasound is used to image the direction and characteristics of blood vessels. Central, continuous blood flow is commonly observed in veins, whereas peripheral, pulsatile flow is typically seen in normal arteries. Arterial vascular beds are further classified as low-resistance or high-resistance systems. Low-resistance waveforms exhibit antegrade blood flow during diastole, as the organs they perfuse (such as the kidneys, brain, and testes) require continuous blood flow. These waveforms are classically monophasic, characterized by a slower and gentler systolic upstroke. High-resistance waveforms often have a brief reversal of flow during diastole as the organs they perfuse do not require continuous blood flow (musculature). This results in triphasic waveforms with a brisk systolic upstroke and a brief period of flow reversal (see Image. Normal Triphasic Arterial Waveform).
Color Doppler maps demonstrate flow information over a selected region of interest. Flow towards and away from the transducer is mapped using colors, most commonly red and blue, with differing hues related to the magnitude of the frequency shift (velocity). Color Doppler helps quickly identify and localize vascular structures. Furthermore, it provides an overview of the vessel's parameters and highlights turbulent areas through dramatic color changes. The absence of expected color flow can also signify thromboembolic disease or an intimal dissection flap.
Power duplex uses Doppler shift information to map the amount of moving red blood cells rather than the direction or velocity. Since both high and low velocities, regardless of whether they are moving toward or away from the transducer, are equally registered, power Doppler is much more sensitive to slower flow. In turn, the increased sensitivity causes flash artifacts from tissue vibration/motion to become more apparent.[3] A normal spectral arterial waveform is triphasic, consisting of a sharp systolic upstroke, early diastolic flow reversal, and late diastolic return to antegrade flow. The waveform should be sharply outlined. Biphasic waveforms may be considered normal as long as they maintain a sharp systolic upstroke unless there is an abrupt change from triphasic waveforms.[4][5]
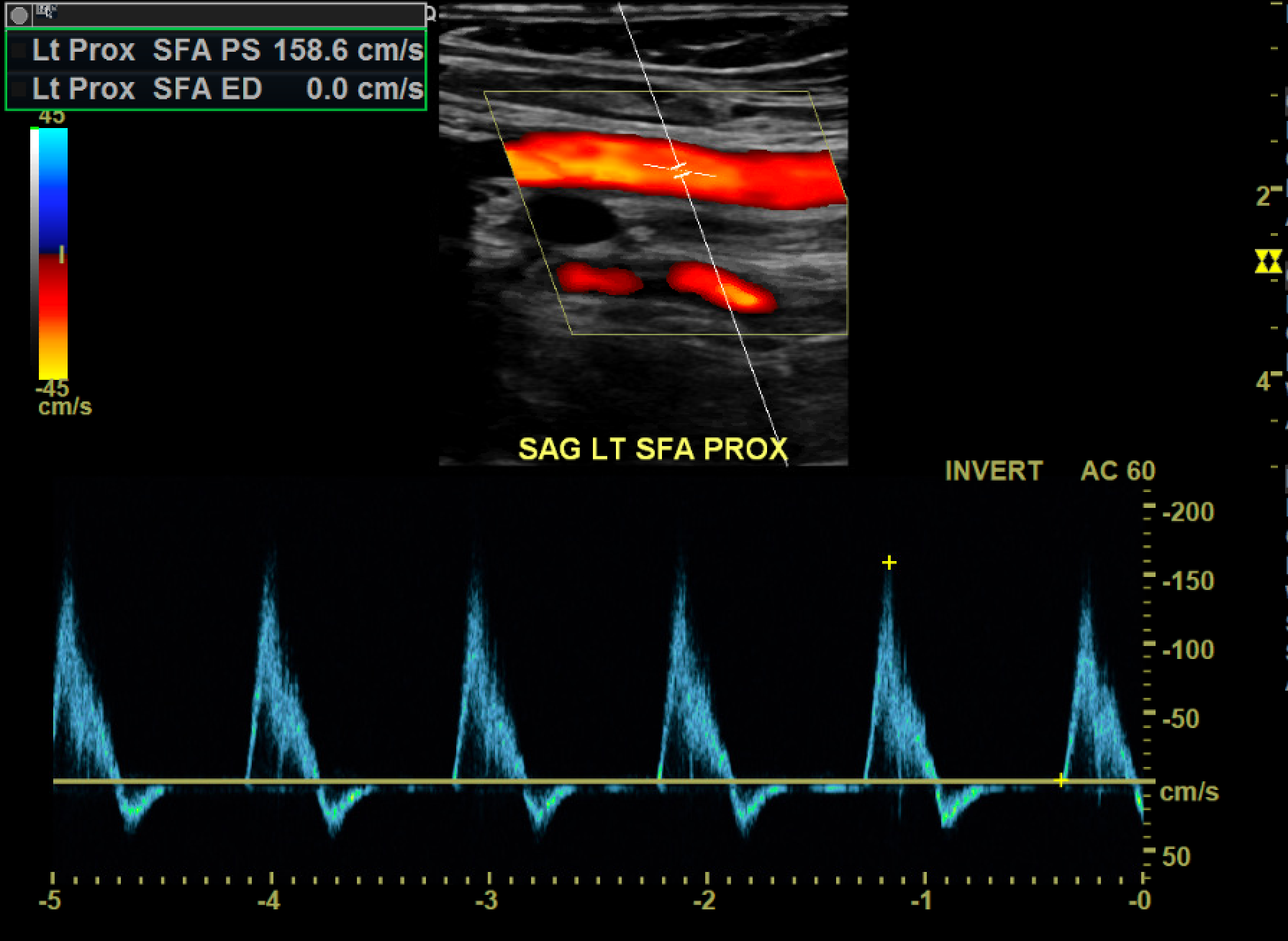
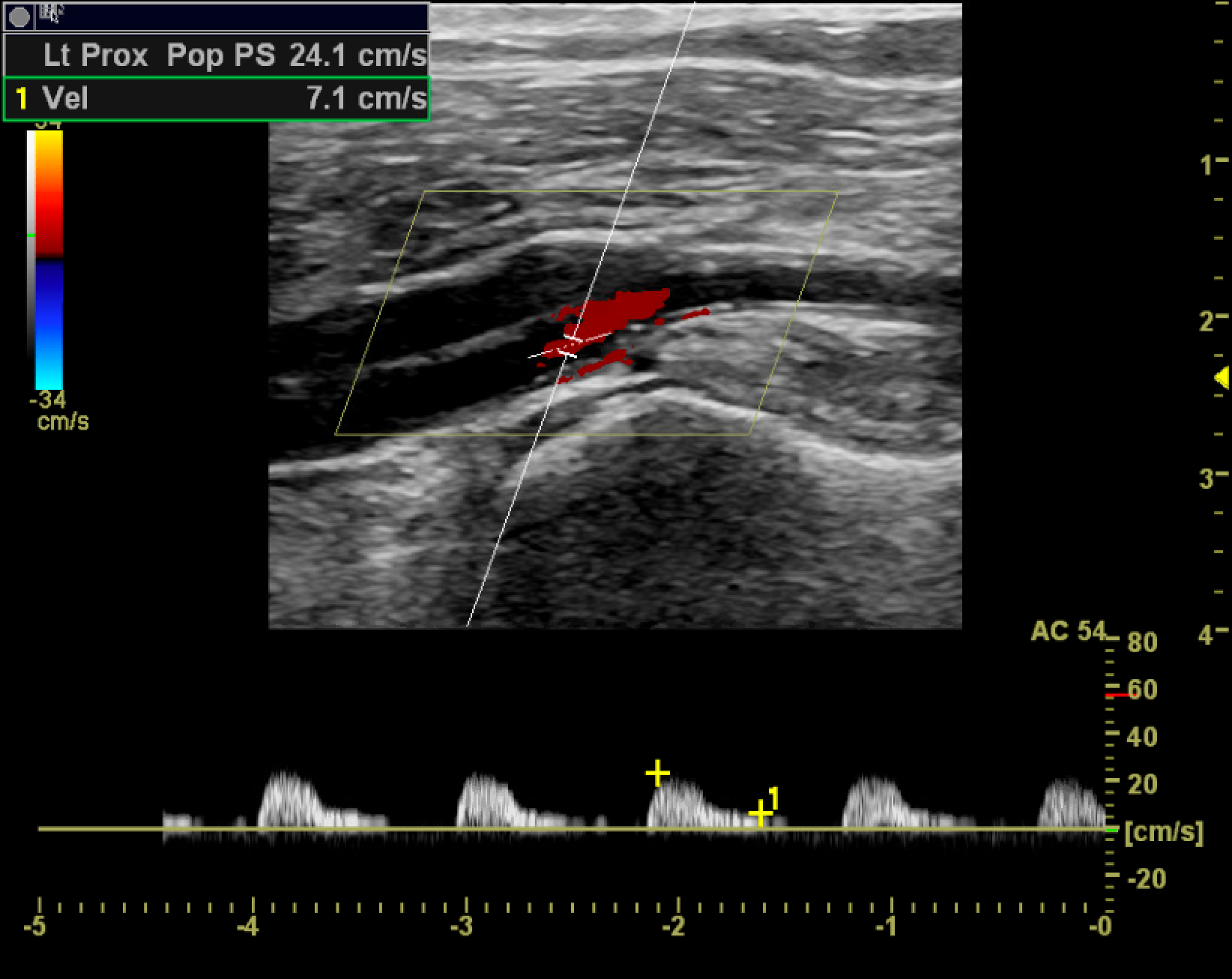
A monophasic waveform exhibits slow, blunted antegrade systolic flow that persists into diastole. Monophasic arterial waveforms are always abnormal and are often observed downstream from stenoses or in collateral vessels that form in response to occlusive disease. At an area of stenosis, maximum peak systolic velocity (PSV) of greater than 200cm/s or PSV ratio at the stenosis compared to a normal segment proximal or distal to the stenosis greater than 2.0 to 2.5 are diagnostic of hemodynamically significant flow reduction (see Image. Hemodynamically Significant Stenosis With Turbulent Color Flow and Spectral Broadening).[6] In the setting of complete occlusion, there may be a pre-occlusive thump in the waveform as the occlusion stops forward blood flow, absence of color flow at the occlusion, and distal reconstitution with low-velocity, monophasic waveforms. As the stenosis progresses to more than 50% of the vessel diameter, altered flow dynamics may result in exertional symptoms. For symptoms to occur during rest, approximately 90% stenosis may be needed.[7]
A common sequela of atherosclerotic disease is the formation of aneurysms. Aneurysms are saccular or fusiform areas of focal arterial enlargement greater than 1.5 times the average normal vessel diameter. They arise secondary to an intrinsic abnormality of the arterial walls as well as from trauma, vasculitis, or infection. A true aneurysm involves all 3 layers of the arterial wall: the intima, media, and adventitia. Complications include compression of adjacent structures, rupture, and thromboembolism, which can result in ischemia. The risk of complications depends on the aneurysm size, with most complications occurring at diameters greater than 2 cm. Aneurysms on sonography appear as focal areas of arterial enlargement, often with mural thrombus and turbulent flow. Peripheral thrombus can give the appearance of a normal luminal caliber.
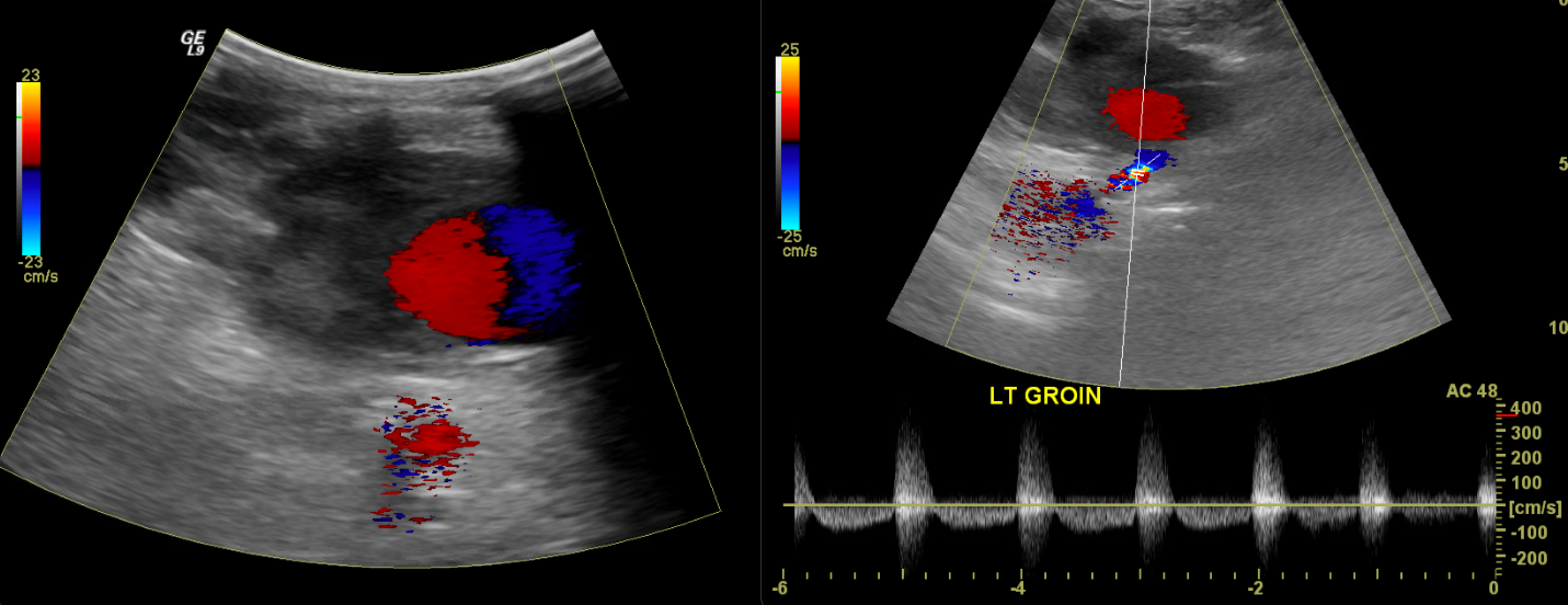
Popliteal artery aneurysms are the most common peripheral aneurysms, accounting for nearly 70%. They are often bilateral and associated with abdominal aortic aneurysms. The second most common peripheral location is the common femoral artery. These should be distinguished from pseudoaneurysms, which are commonly found in the proximal femoral area, primarily resulting from iatrogenic complications related to vascular access. Unlike true aneurysms, a pseudoaneurysm is a disruption in the arterial wall continuity resulting in a contained, pulsatile hematoma. Under ultrasound, pseudoaneurysms appear as defined collections of swirling color flow, often with a communicating neck and "to-and-fro" flow from an adjacent artery.[8] See Image. Pseudoaneurysm. Management includes watchful waiting, ultrasound-guided compression, or direct thrombin injection. In some cases, surgical ligation or endovascular stenting can be performed.
In posttraumatic settings, arteriovenous fistulae (AVF) may develop. AVF are direct communications between an artery and a vein, often with high-velocity turbulent flow between the vessels. See Image. Arteriovenous Fistula. The proximal arterial waveforms assume a low-resistance morphology, and the distal venous waveforms assume high flow, multiphasic arterialized waveforms. The strong pulsatile flow at the site of AVF causes tissue vibration artifacts, where perivascular tissue motion is misregistered as color flow, alerting the sonographer to the presence of an AVF. These are often treated by excluding the AVF with a covered stent, embolizing (occluding) the outflow or draining vein, or surgical ligation.[9]
Indications
Clinical signs and symptoms of the PAD, such as claudication, rest pain, and skin changes, confirming arterial disease suggested by other modalities, monitoring the progression of the disease, monitoring prior surgical interventions (bypass and stent-grafts), monitoring sites of prior percutaneous intervention, surgical mapping, and trauma.[10]
Equipment
A 3.5-5 MHZ low-frequency transducer is used for the aortoiliac portion, while a 5.0-7.5 or higher MHz linear transducer is preferred for the distal external iliac arteries and downstream vessels. A lower-frequency transducer may be required in patients with a larger body habitus.
Personnel
Ultrasound technicians perform the majority of peripheral ultrasound examinations. Any appropriately trained physician is qualified, and a point-of-care ultrasound provided by competent providers can aid in triaging care.
Technique or Treatment
Grayscale ultrasound enables the visualization of the arterial system's morphology and the characterization of plaque. Plaque may be anechoic and indistinguishable from the vessel lumen. In these cases, color Doppler can help delineate the vessel's true lumen. Color Doppler can also help differentiate between arteries and veins based on the direction of blood flow. It helps quickly delineate turbulent flow areas and stenoses by looking for areas of color aliasing.
The complete peripheral arterial duplex exam begins at the abdominal aorta and follows the arterial system vessels to the ankle. The vessels are evaluated in both long- and short-axis views using B-mode and pulsed Doppler. The exam is performed by placing the patient in the supine position. A low-frequency transducer is used to evaluate the aorta from just below the xiphoid process and from just to the left of the umbilicus. The celiac and superior mesenteric arteries serve as useful landmarks. The common and proximal external iliac arteries are then interrogated with the transducer at the angle of the iliac crest. The internal iliac artery takes off from the common iliac artery, differentiating it from the external iliac artery. Graded compression can help displace the overlying bowel, improving visualization.
The distal external iliac arteries and remaining vessels are comparatively superficial, and a higher-frequency transducer is preferred. The distal external iliac arteries are identified from the groin and traced down as the common femoral, proximal portions of the profunda femoris, and superficial femoral arteries. At the adductor canal, the superficial femoral artery courses posteriorly; therefore, positioning the patient prone can help assess the distal superficial femoral artery, popliteal artery, and the origin of the anterior tibial artery (the first branch of the popliteal artery). Finally, the tibial arteries are traced upward from the level of the ankle - posterior to the medial malleolus for the posterior tibial artery, posterior to the lateral malleolus for the peroneal, and anterior to the ankle for the anterior tibial artery.
The areas most prone to the development of atherosclerotic disease are those exposed to higher degrees of shear stress. These include the common iliac artery, superficial femoral artery at the adductor canal, central popliteal artery, and the tibioperoneal trunk. Areas of suspicion are interrogated and graded by comparing the pre-stenotic, stenotic, and poststenotic segments. It usually takes at least a 50% decrease in vessel diameter (corresponding to a 75% decrease in cross-sectional area) to become hemodynamically significant. At 51% to 75% luminal narrowing, the PSV of the stenotic segment is often twice that of the regular proximal segment. The poststenotic segment exhibits a degree of turbulence and may display monophasic waveforms. At a stenosis of 76% to 99%, increased pulsatility becomes apparent in the pre-stenotic segment. The stenotic segment's PSV is often greater than 4 times that of the proximal normal segment and demonstrates monophasic waveforms. There is significant turbulence in the poststenotic segment and a tardus parvus waveform (a prolonged systolic upstroke and a decreased systolic amplitude with a rounded peak) distal to the stenosis.[11][12] See Image. Monophasic Tardus and Parvus Waveforms.
Clinical Significance
PAD is a common condition with profound implications that extend beyond limb loss. It is associated with increased cardiovascular risk and decreased quality of life metrics, including depression.[13] Duplex ultrasonography is easily accessible, uses no ionizing radiation, and is typically less expensive than other cross-sectional imaging modalities. When used in conjunction with other noninvasive physiological tests, ultrasound can effectively and efficiently diagnose and grade disease to help plan management and monitor disease progression or response to therapy.
Enhancing Healthcare Team Outcomes
Diagnosing and treating PAD requires cooperation and communication between healthcare providers. A multidisciplinary team often provides medical and invasive interventional management for the patient. PAD involves interventional radiologists, interventional cardiologists, vascular and plastic surgeons, endocrinologists, and primary care physicians. There are multiple forms of lifestyle, medical, and surgical management available (as well as various guidelines for management). Therefore, the entire healthcare team and the patient must make an informed decision to achieve the best possible patient-centered outcomes.[14]
Media
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
References
Virani SS, Alonso A, Benjamin EJ, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Chang AR, Cheng S, Delling FN, Djousse L, Elkind MSV, Ferguson JF, Fornage M, Khan SS, Kissela BM, Knutson KL, Kwan TW, Lackland DT, Lewis TT, Lichtman JH, Longenecker CT, Loop MS, Lutsey PL, Martin SS, Matsushita K, Moran AE, Mussolino ME, Perak AM, Rosamond WD, Roth GA, Sampson UKA, Satou GM, Schroeder EB, Shah SH, Shay CM, Spartano NL, Stokes A, Tirschwell DL, VanWagner LB, Tsao CW, American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee. Heart Disease and Stroke Statistics-2020 Update: A Report From the American Heart Association. Circulation. 2020 Mar 3:141(9):e139-e596. doi: 10.1161/CIR.0000000000000757. Epub 2020 Jan 29 [PubMed PMID: 31992061]
Mittleider D. Noninvasive Arterial Testing: What and When to Use. Seminars in interventional radiology. 2018 Dec:35(5):384-392. doi: 10.1055/s-0038-1676328. Epub 2019 Feb 5 [PubMed PMID: 30728654]
Rose SC, Nelson TR. Ultrasonographic modalities to assess vascular anatomy and disease. Journal of vascular and interventional radiology : JVIR. 2004 Jan:15(1 Pt 1):25-38 [PubMed PMID: 14709684]
Guilcher A, Lanéelle D, Hoffmann C, Guillaumat J, Constans J, Bressollette L, Le Hello C, Boissier C, Bura-Rivière A, Jaquinandi V, Omarjee L, Lacroix P, Pernod G, Abbadie F, Sevestre MA, Boulon C, Mahé G. Comparison of the Use of Arterial Doppler Waveform Classifications in Clinical Routine to Describe Lower Limb Flow. Journal of clinical medicine. 2021 Jan 26:10(3):. doi: 10.3390/jcm10030464. Epub 2021 Jan 26 [PubMed PMID: 33530374]
Sibley RC 3rd, Reis SP, MacFarlane JJ, Reddick MA, Kalva SP, Sutphin PD. Noninvasive Physiologic Vascular Studies: A Guide to Diagnosing Peripheral Arterial Disease. Radiographics : a review publication of the Radiological Society of North America, Inc. 2017 Jan-Feb:37(1):346-357. doi: 10.1148/rg.2017160044. Epub 2016 Sep 30 [PubMed PMID: 27689831]
Donnelly R, Hinwood D, London NJ. ABC of arterial and venous disease. Non-invasive methods of arterial and venous assessment. BMJ (Clinical research ed.). 2000 Mar 11:320(7236):698-701 [PubMed PMID: 10710584]
Gupta P, Lyons S, Hedgire S. Ultrasound imaging of the arterial system. Cardiovascular diagnosis and therapy. 2019 Aug:9(Suppl 1):S2-S13. doi: 10.21037/cdt.2019.02.05. Epub [PubMed PMID: 31559150]
Dawson J, Fitridge R. Update on aneurysm disease: current insights and controversies: peripheral aneurysms: when to intervene - is rupture really a danger? Progress in cardiovascular diseases. 2013 Jul-Aug:56(1):26-35. doi: 10.1016/j.pcad.2013.05.002. Epub 2013 Jun 21 [PubMed PMID: 23993236]
Madani H, Farrant J, Chhaya N, Anwar I, Marmery H, Platts A, Holloway B. Peripheral limb vascular malformations: an update of appropriate imaging and treatment options of a challenging condition. The British journal of radiology. 2015 Mar:88(1047):20140406. doi: 10.1259/bjr.20140406. Epub 2014 Dec 19 [PubMed PMID: 25525685]
American Institute of Ultrasound in Medicine (AIUM), American College of Radiology (ACR), Society of Radiologists in Ultrasound (SRU). AIUM practice guideline for the performance of peripheral arterial ultrasound examinations using color and spectral doppler imaging. Journal of ultrasound in medicine : official journal of the American Institute of Ultrasound in Medicine. 2014 Jun:33(6):1111-21. doi: 10.7863/ultra.33.6.1111. Epub [PubMed PMID: 24866622]
Level 1 (high-level) evidenceRose SC. Noninvasive vascular laboratory for evaluation of peripheral arterial occlusive disease: Part I--hemodynamic principles and tools of the trade. Journal of vascular and interventional radiology : JVIR. 2000 Oct:11(9):1107-14 [PubMed PMID: 11041465]
Stewart JH, Grubb M. Understanding vascular ultrasonography. Mayo Clinic proceedings. 1992 Dec:67(12):1186-96 [PubMed PMID: 1469931]
Level 3 (low-level) evidenceRuo B, Liu K, Tian L, Tan J, Ferrucci L, Guralnik JM, McDermott MM. Persistent depressive symptoms and functional decline among patients with peripheral arterial disease. Psychosomatic medicine. 2007 Jun:69(5):415-24 [PubMed PMID: 17556643]
Level 2 (mid-level) evidenceDhand S. Multidisciplinary Approach to PAD: Who's on Your Team? Seminars in interventional radiology. 2018 Dec:35(5):378-383. doi: 10.1055/s-0038-1676094. Epub 2019 Feb 5 [PubMed PMID: 30728653]