Introduction
Saliva serves multiple functions, including initiating digestion through enzymatic action, providing a chemical buffer that prevents dental caries and aids enamel remineralization, and lubricating the oral cavity and pharynx, which is essential for speech, swallowing, and taste sensation.[1][2] The 3 paired major salivary glands are the parotid, submandibular, and sublingual glands. While the parotids are the largest, the submandibular glands produce the majority of baseline saliva, with the parotids contributing more during mastication.[3] Minor salivary glands, including the recently identified tubarial glands in the nasopharynx, are distributed throughout the upper aerodigestive tract mucosa.[4]
As exocrine glands, each salivary gland secretes a unique mix of serous (proteinaceous/enzymatic) and mucinous (lubricating) components, influencing the types of pathologies that arise. The submandibular gland produces a mixed serous and mucinous secretion combining enzymatic and lubricating functions. The parotid gland predominantly secretes enzyme-rich serous fluid. The sublingual gland mainly produces mucinous, lubricating saliva.[5]
Salivary flow impairment, arising from factors such as dehydration, obstruction, and aging, can thicken saliva and promote stone formation. Salivary calculus composition varies by gland. Submandibular stones typically contain 70% to 80% inorganic material and 20% to 30% organic matter, with the latter including proteins (5%), lipids (1%), and carbohydrates.[6][7] Reduced salivary flow also increases infection risk, frequently presenting in urgent and emergency care. Recurrent painful salivary stones, especially when complicated by bacterial sialadenitis, strongly warrant submandibular gland excision, which will be explored further in this activity.
Anatomy and Physiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Anatomy and Physiology
Submandibular Gland Anatomy
The paired submandibular glands lie within the submandibular triangle. This space is bounded superiorly by the inferior border of the mandible, posteroinferiorly by the posterior belly of the digastric, and anteroinferiorly by the anterior belly of the digastric (see Image. Superficial Neck Anatomy). The mylohyoid forms the deep margin of the triangle, while the superficial boundary is the platysma. The gland curves around the posterior border of the mylohyoid, so that the deep lobe projects into the floor of the mouth and lies adjacent to the hyoglossus muscle medially (see Image. Lateral View of the Salivary Glands).
The Wharton duct, the excretory duct of the submandibular gland, originates from the deep lobe and courses along the floor of the mouth to open at the sublingual papilla, just lateral to the lingual frenulum, where the sublingual gland also drains.[8] Since the duct arises from the superior portion of the gland, myoepithelial contraction must propel saliva upward, a factor thought to predispose the submandibular gland to sialolith formation. The duct measures up to 5 cm in length and 0.5 to 1.5 mm in diameter.[9] This structure crosses over the lingual nerve as the nerve passes from lateral to medial and runs medial to the ipsilateral sublingual gland (see Image. Intersection of Lingual Nerve and Submandibular Duct).
The investing layer of the deep cervical fascia envelops the superficial lobe of the submandibular gland. The facial artery and vein run along this fascial layer, with the marginal mandibular branch of the facial nerve lying superficial to the vein. This nerve supplies the mentalis and depressor anguli oris.[10] This anatomic relationship is often used intraoperatively during the Hayes Martin maneuver. In this technique, the facial vein is divided inferior to the gland and reflected superiorly with the gland’s fascia to protect the marginal mandibular branch of the facial nerve. The maneuver allows safe exposure of the superficial gland during excision or cervical lymph node dissection.
However, division of the facial vein eliminates its availability for future free tissue transfer in head and neck reconstruction. For this reason, many head and neck surgeons preserve the facial vessels whenever feasible, particularly when submandibular gland excision accompanies oncologic cervical lymphadenectomy.
The submandibular gland receives its primary vascular supply from the facial artery, via the sublingual and submental branches, with venous drainage through the facial vein. The lingual artery and sublingual vein provide additional contribution. The facial vein runs across the superficial surface of the gland, crossing the posterior belly of the digastric muscle before draining into the common facial vein and then the internal jugular vein. The facial artery originates from the external carotid artery and follows a tortuous course along the deep aspect of the gland (see Image. Veins, Arteries, and Nerves of the Face and Scalp)
On the medial surface of the submandibular gland, the submandibular ganglion connects to the lingual nerve and provides parasympathetic innervation. The ganglion must be separated from the gland during resection to preserve lingual nerve function (see Image. Maxillary and Mandibular Nerve Distribution). The hypoglossal nerve courses deep and inferior to the lingual nerve and may be encountered during excision, requiring careful preservation (see Image. Muscles, Arteries, and Nerves of the Neck).
Submandibular Gland Physiology
Salivary secretion from the submandibular gland is under autonomic control. Parasympathetic innervation arises from the superior salivatory nucleus of the pons. Preganglionic fibers travel with the facial nerve via the chorda tympani, join the lingual branch of the mandibular nerve (cranial nerve V3, CN V3), and synapse at the submandibular ganglion. Postganglionic fibers then act directly on the gland to stimulate secretion and vasodilation. Sympathetic innervation originates from the superior cervical ganglion. Postganglionic axons form a plexus within the carotid sheath, follow the facial artery and its submental branches, and induce vasoconstriction that reduces salivary output.
The submandibular gland produces a mixed serous and mucinous secretion, with water comprising 98% to 99% of the total volume. Other components include:
- Glycoproteins that confer viscosity
- Enzymes, such as amylase and lysozyme
- Immunoglobulin A
- Epithelial and white blood cells
- Lipids, including cholesterol, fatty acids, triglycerides, glycolipids, and phospholipids
- Electrolytes, such as sodium, potassium, chloride, bicarbonate, calcium, magnesium, and fluoride
Calculi may incorporate these elements along with precipitated hydroxyapatite, frequently accompanied by whitlockite, octacalcium phosphate, and brushite.[11]
Submandibular Gland Pathology
Acute pathologies include obstructive processes of the salivary ductal system, which may result from calculi, stricture, or infection. Unilateral submandibular infections are typically bacterial, with Staphylococcus aureus, Streptococcus pyogenes, Haemophilus influenzae, and viridans streptococci most commonly isolated.[12] These acute bacterial infections often arise secondary to obstruction or conditions that cause salivary stasis, including dehydration, chronic systemic disease, medication use, xerostomia, or prior radiation therapy to the oral cavity. Acute bilateral submandibular infections are more often viral, most commonly due to the mumps virus, coxsackievirus, parainfluenza virus, or human immunodeficiency virus.
Chronic conditions affecting the submandibular glands include neoplasia, autoimmune disease, infiltrative disorders such as amyloidosis or granulomatous disease, and idiopathic enlargement. Tumors of the submandibular glands are distributed approximately 75% benign and 25% malignant, with pleomorphic adenoma the most common benign tumor and adenoid cystic carcinoma the most frequent malignant tumor.[13] Other malignancies that occur with relative frequency include mucoepidermoid carcinoma and adenocarcinoma.[14]
Indications
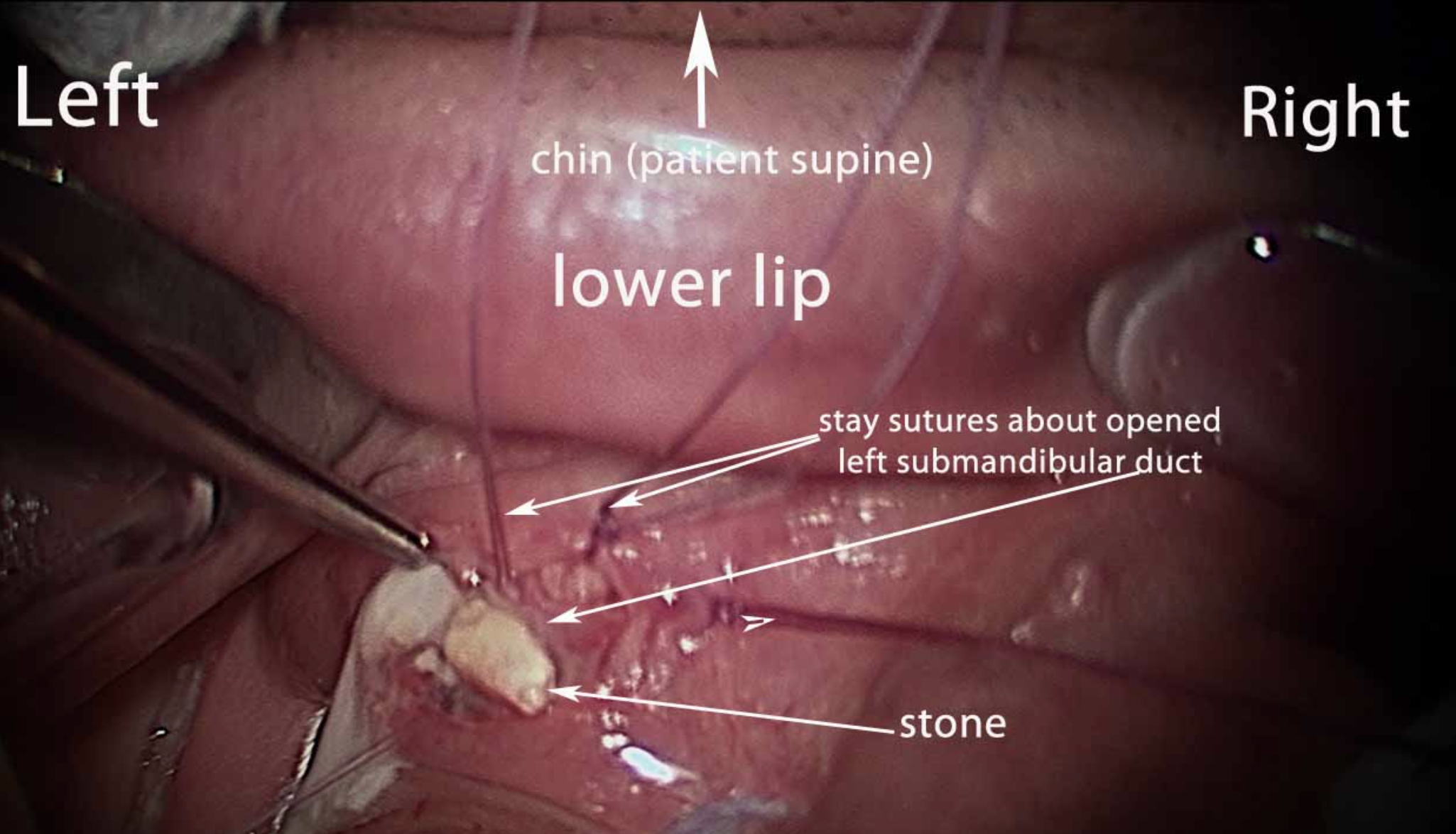
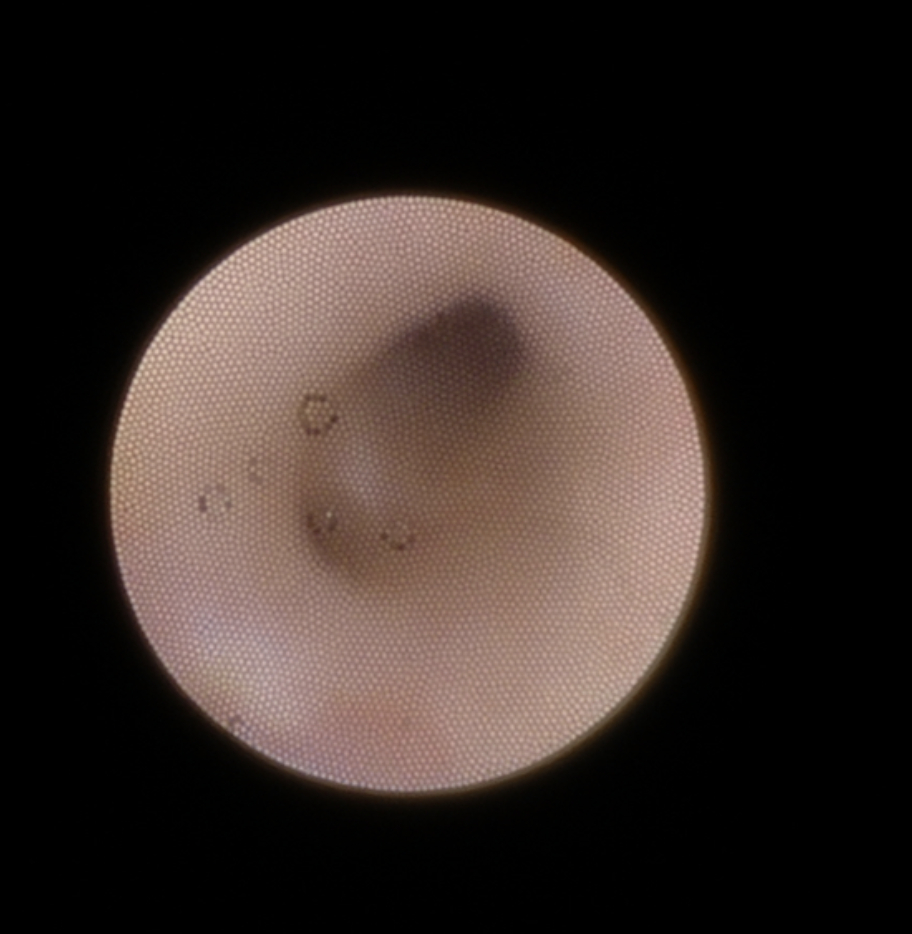
The most common indication for submandibular gland excision is salivary stones with secondary ductal obstruction and sialadenitis. Transoral stone removal may be successful in cases of an isolated calculus within the submandibular duct (see Image. Submandibular Gland Calculi). Sialendoscopy is a widely used minimally invasive treatment that can be both diagnostic and therapeutic, particularly when stones are smaller than 7 mm in diameter (see Image. Endoscopy View from Sialendoscopy).[15] However, most patients with salivary calculi experience recurrent obstruction and sialadenitis that do not resolve with minimally invasive measures alone, often necessitating submandibular gland excision.
Less commonly, excision is performed for refractory sialorrhea, such as in cerebral palsy and congenital syndromes, when botulinum toxin injection therapy fails.[16] When appropriate, this procedure is generally bilateral and frequently combined with parotid duct ligation. Partial resection of the submandibular gland may also be undertaken for cosmetic purposes, typically as part of cervicofacial rhytidectomy. During jawline and upper neck recontouring, with suspension of the underlying fascia to rejuvenate the region, age-related submandibular gland ptosis can create unsightly fullness and obscure the ideal distinction between the lateral mandibular margin and upper neck.[17]
Neoplastic growth is another important indication for submandibular gland excision. A thorough preoperative assessment is essential, as a malignancy cannot be managed with submandibular gland excision alone. Cancer cases may require a formal neck dissection, depending on tumor pathology and staging.
Evaluation of submandibular gland disease begins with a detailed clinical history, including the timing, onset, and progression of symptoms, laterality, and any features suggesting systemic autoimmune, inflammatory, or viral processes. A history of external beam radiation or radioactive iodine therapy should also be elicited, as salivary tissue is particularly radiosensitive. Radiation exposure typically results in xerostomia with thickened secretions. Physical examination focuses on the oral cavity, with bimanual palpation of the floor of the mouth to detect calculi, and thorough inspection of the neck for masses, lesions, or lymphadenopathy. Assessment of facial nerve function and symmetry, with particular attention to movement at the corners of the mouth, is also essential.
Imaging is generally obtained before surgical excision. Ultrasound or contrast-enhanced computed tomography of the neck can characterize neoplastic processes and identify the presence, size, and location of calculi.[18] Cytological evaluation should precede surgical excision when a neoplasm is suspected. Fine needle aspiration or core needle biopsy, often performed under ultrasound guidance, are the primary methods.[19] Core needle biopsy yields a larger sample and reduces nondiagnostic results, but this procedure is associated with higher complication rates, such as bleeding.[20]
Contraindications
Submandibular gland excision has no absolute contraindications, but several relative contraindications and considerations should be recognized before surgery. The presence of a malignant neoplasm often requires more than simple gland excision, as management may necessitate additional lymph node dissection, radiation therapy, or chemotherapy. Acute infection in or around the gland complicates dissection and removal, making preoperative treatment with antibiotics advisable before attempting surgery.
Preoperative medical optimization is essential since excision is usually performed under general anesthesia and frequently offered to older patients with recurrent calculi. Individuals who are poor candidates for general anesthesia may be better treated with conservative approaches. Conditions like trismus that prevent sufficient mouth opening for visualization and effective access contraindicate an intraoral approach to gland removal.
Equipment
Standard equipment for submandibular gland excision includes the following:
- #15 scalpel blade with #3 Bard-Parker handle
- Forceps: DeBakey, Adson
- Clamps: Babcock, Allis, Crile
- Scissors: Metzenbaum, suture scissors
- McCabe dissector
- Retractors: Army-Navy, Greene, Senn/Ragnell, nerve hook
- Electrocautery: monopolar and jeweler's bipolar
- Kittner sponges
- Needle drivers: Halsey, Hagar, or similar types
- Sutures for vessel ligation and skin closure
- Hemostatic agent
Optional equipment may include the following:
- Tissue sealing/hemostatic device (eg, harmonic scalpel)
- Facial nerve monitor
- Sialendoscope (0.8-1.6 mm) with camera, duct dilators, microdrill, and stone retrieval basket
All equipment should be inspected, assembled, and confirmed to be in working order before the procedure.
Personnel
Submandibular gland excision is performed in the operating room under general anesthesia and requires the presence of a circulating nurse, an anesthesia provider, and a surgical scrub technician. A 1st assistant is typically valuable if available. Once the gland is removed, a pathologist evaluates the specimen, and if malignancy is identified, the involvement of medical, surgical, and radiation oncologists may be necessary. A radiologist may also contribute by assessing the extent of disease on imaging.
Preparation
The patient is positioned supine on the operating table with a shoulder roll to achieve gentle neck extension, and the head is rotated away from the operative side. Many surgeons rotate the table 90° toward the operative side to provide adequate working space for the surgical team. The operative field is prepped and draped widely, extending from the nasolabial fold to the clavicles.
The endotracheal tube is secured to the contralateral side, and the ipsilateral oral commissure is left visible to monitor for twitching that may signal manipulation of the marginal mandibular or cervical branches of the facial nerve. Long-acting paralytic agents should be avoided to permit facial and tongue twitching, which is particularly important when electrical nerve monitoring is used. Prophylactic antibiotics are generally unnecessary in clean head and neck procedures, but should be available if infection or abscess is encountered.[21]
Technique or Treatment
A 2- to 3-cm transverse neck incision is planned along the inferior aspect of the submandibular gland, approximately 1 to 2 cm below the inferior margin of the mandible. Local anesthesia is infiltrated along the incision line, taking care to avoid injection along the courses of the marginal mandibular and cervical branches of the facial nerve (see Image. Superficial Nerves of the Head and Neck).
The marginal mandibular branch exits the inferior aspect of the parotid gland, passes over the submandibular gland, and crosses the margin of the mandible at the gonial notch to enter the face and supply target muscles in the perioral region.[22] The cervical branch lies in the upper neck, traveling from the parotid gland to the undersurface of the platysma, and is reliably encountered 1 cm below the midpoint between the mastoid process and the mentum.[23] Blocking these nerves with local anesthetic blunts the twitching response to their intraoperative manipulation, eliminating an early warning against iatrogenic injury.
An existing skin crease is used to conceal the incision when available. Excision of a submandibular malignancy may require a wider and often lower incision to complete the neck dissection. Placement of the incision at least 1 cm inferior to the mandible provides broader access and a superior cosmetic result. After the initial incision, a subplatysmal skin flap may be raised posteriorly. Some surgeons avoid raising a superior skin flap to reduce trauma to the marginal mandibular or cervical branches of the facial nerve. Others elevate the superior flap in a supraplatysmal plane and subsequently split the platysma fibers vertically to identify the marginal mandibular nerve over the gland.
Dissection in proximity to nerves must be performed with care to avoid traction or thermal injury. Many surgeons routinely identify the marginal mandibular or cervical branch of the facial nerve as it crosses the submandibular gland. After identification of the facial artery and vein, these vessels may be dissected away from the gland to preserve vascularity, or they may be ligated with the vein reflected superiorly to protect the marginal mandibular nerve, as previously described. The tortuous course of the facial artery can complicate preservation, as the vessel enters and exits the gland while giving off several intraglandular branches.
Proceeding in a subfascial plane, the submandibular gland is separated from the inferior border of the mandible, and dissection is continued to the mylohyoid muscle (see Image. Submandibular Gland Resection). Working in a subfascial plane protects facial nerve branches, but this plane may be difficult to establish in the presence of inflammation.
The anterior aspect of the superficial lobe of the submandibular gland is first mobilized away from the anterior belly of the digastric muscle. The lobe is then separated from the underlying mylohyoid muscle by ligating any perforating vessels. Division of the motor nerve to the mylohyoid must be avoided.
The posterior belly of the digastric muscle is identified, and the posterior aspect of the submandibular gland is mobilized away from the muscle. The facial vein may be ligated or retracted during this step. Anterior retraction of the posterior border of the mylohyoid muscle exposes the deep lobe of the submandibular gland and the lingual nerve. Preservation of the lingual nerve is critical to avoid dysgeusia and anesthesia of the ipsilateral oral tongue. The nerve is separated from the gland by dividing the rootlets connecting the submandibular ganglion to the gland.
The Wharton duct and its accompanying vein are then identified, with the vein ligated. Deeper dissection allows identification and protection of the hypoglossal nerve, which confirms the identity of the Wharton duct. The duct is ligated as close as possible to the sublingual papilla to prevent retention of calculi after resection.
The remaining attachments of the submandibular gland are released bluntly. Exposure may be improved by applying intraoral pressure with a finger against the gland. The integrity of the marginal mandibular nerve must be confirmed as dissection is completed and the gland is delivered. Meticulous hemostasis is obtained, and a suction drain is typically placed before layered closure is performed.
The transcervical procedure described above is the most commonly used approach for submandibular gland removal. Alternative approaches have been described, including intraoral, intraoral endoscopy-assisted, and robot-assisted procedures. These approaches avoid a neck scar and may employ a variety of incisions, such as intraoral, postauricular, and hairline incisions.[24][25]
The intraoral approach is a viable option for patients who wish to avoid a neck scar or who have strictures or stones located proximally along the submandibular duct. This approach can be challenging, as the lingual and hypoglossal nerves, as well as the submandibular duct, can vary in their locations. When an intraoral approach is undertaken, the endotracheal tube should be placed via the nose and prophylactic antibiotics administered for 24 hours to prevent infection due to wound contamination with oral flora. Temporary lingual nerve paresis is common after intraoral submandibular gland excision, occurring in up to 80% of cases. Hypoglossal nerve injury is infrequent. Marginal mandibular branch injury is nearly unheard of, as this nerve lies opposite the direction of the surgical approach.
The intraoral approach begins with identification of the sublingual papilla, followed by a mucosal incision extending to the retromolar floor of the mouth. The sublingual gland is usually removed through this incision to improve access and exposure of the submandibular gland. The lingual nerve is identified and separated from the gland. The Wharton duct and its accompanying vein are ligated after the hypoglossal nerve is identified and preserved. The posterior border of the mylohyoid muscle is retracted anteriorly to expose the superficial lobe of the gland, which is then dissected from the facial vessels and facial nerve branches in a subfascial plane. Transcervical pressure on the gland facilitates delivery into the oral cavity. Closure is performed with resorbable sutures, and a drain may or may not be left in place.[26]
Complications
Particular attention is paid to the marginal mandibular nerve throughout the procedure to avoid injury. In one series, temporary neuropraxia with full recovery of function after submandibular gland excision occurred in 15.6% of cases. Permanent lower lip weakness was reported in 2.2% of cases. Wound healing complications are comparable to other neck surgeries, ranging from 7% to 22%.[27] Hypoglossal or lingual nerve injury has been observed in fewer than 2% of cases.[28]
Xerostomia is rare after unilateral submandibular gland excision, although the effect on overall salivary gland function remains under investigation.[29] A hematoma may develop in the immediate postoperative period, as the facial artery and vein can bleed significantly and exert compressive effects on the airway. Some patients may also find the scar unsightly, particularly when surgery is performed in the setting of acute infection.
Clinical Significance
With an annual incidence of approximately 60 cases per 100,000 individuals, salivary gland pathologies are frequently encountered by primary and emergency care providers, as well as otolaryngologists and head-and-neck surgeons.[30] Up to 1% of the population may develop salivary calculi, which may or may not be symptomatic. Acute suppurative sialadenitis, although comparatively rare (0.02% of hospital admissions), carries a mortality rate of up to 40%.[31][32] Recognition of these conditions is therefore critical for most clinicians.
Enhancing Healthcare Team Outcomes
Understanding the principles of diagnosis and conservative management of sialadenitis and sialolithiasis, such as hydration, massage, sialogogues (lemon slices or sour candy), warm compresses, and antibiotics, is important for many clinicians and nurses, given the frequency of these conditions. Similarly, familiarity with the indications for surgery or specialist referral helps expedite and facilitate care for affected patients.[33]
Nursing, Allied Health, and Interprofessional Team Monitoring
Awareness of hematoma and nerve injury risk is essential for nurses caring for patients in the postoperative period. Active bleeding from the facial artery requires timely surgical management to reduce the risk of significant blood loss or airway compromise. Early recognition of lower lip weakness may prompt consideration of surgical exploration and facial nerve repair, or the use of conservative strategies such as oral nimodipine.[34]
Media
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
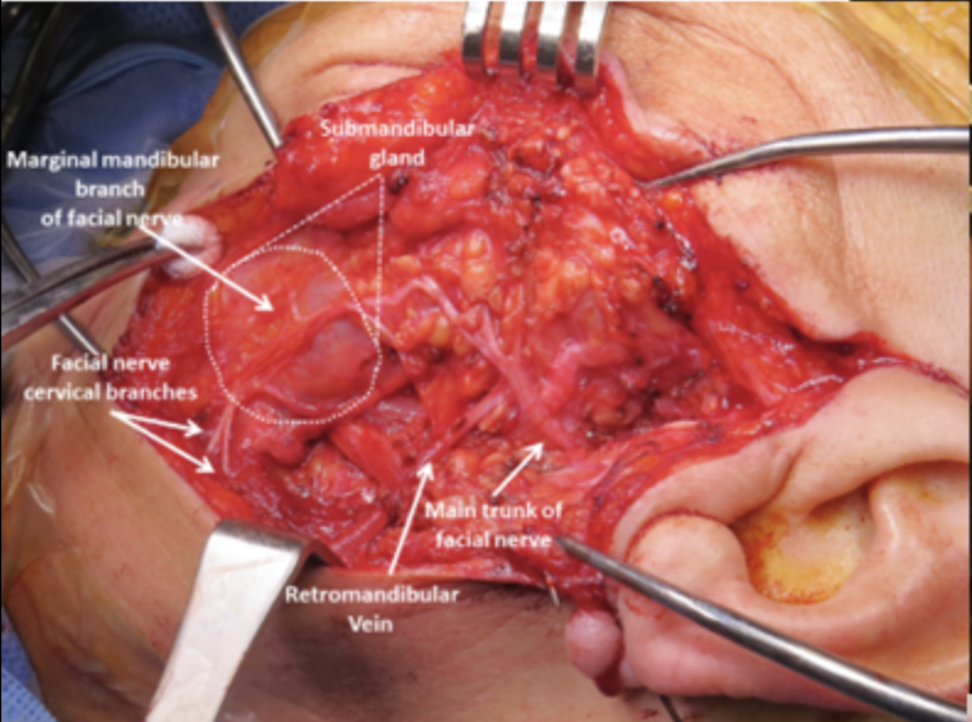
Submandibular Gland Resection. Intraoperative view showing the submandibular gland with identification of the marginal mandibular branch and cervical branches of the facial nerve. The main trunk of the facial nerve and the retromandibular vein are also visible.
Iowa Head and Neck Protocols, Carver College of Medicine, University of Iowa
(Click Image to Enlarge)
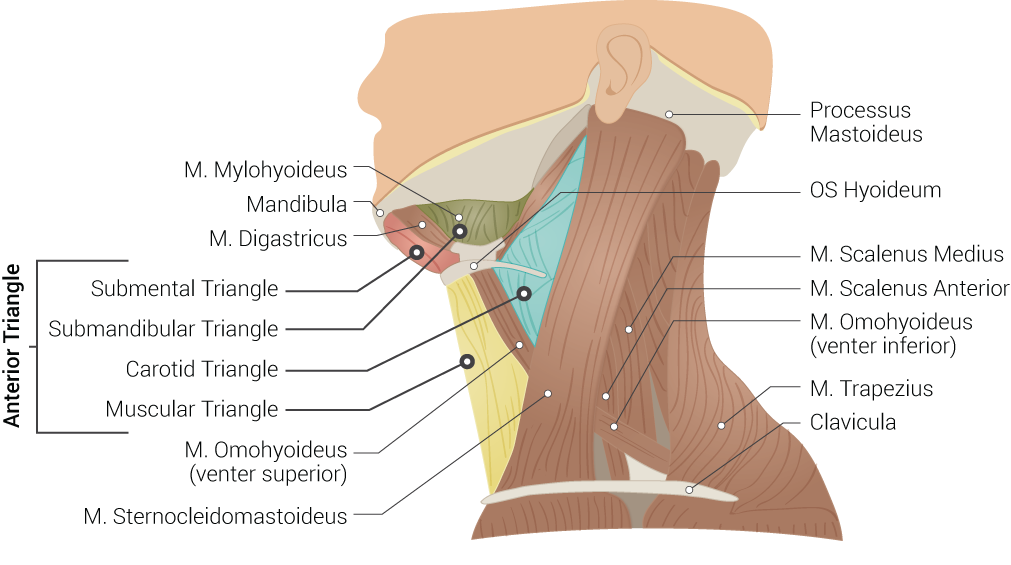
Superficial Neck Anatomy. This left lateral-view illustration shows the anterior and posterior triangles. The anterior triangle is further divided into the submental, submandibular, carotid, and muscular triangles. The muscles in this illustration include the mylohyoideus, digastricus, omohyoideus (venter superior and inferior), sternocleidomastoideus, scalenus medius and anterior, and trapezius. Bony structures include the mandible, mastoid process, left hyoid, and clavicle.
Contributed by B Palmer
(Click Image to Enlarge)
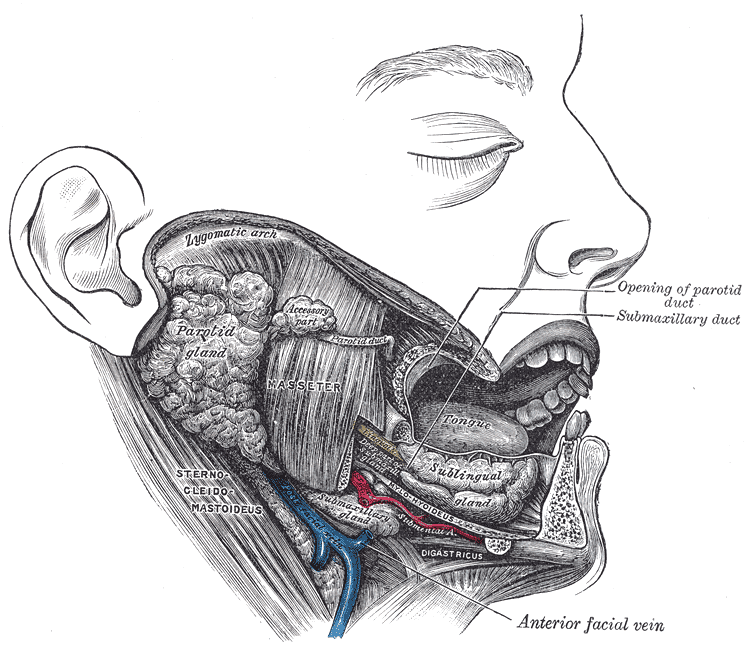
Lateral View of the Salivary Glands. The image shows the parotid, submandibular, and sublingual glands in relation to surrounding structures. The Stensen duct of the parotid gland and the submandibular duct are visible, opening into the oral cavity. Neighboring muscles, vessels, and bony landmarks provide spatial orientation.
Henry Van Dyke Carter, Public Domain, via Wikimedia Commons
(Click Image to Enlarge)
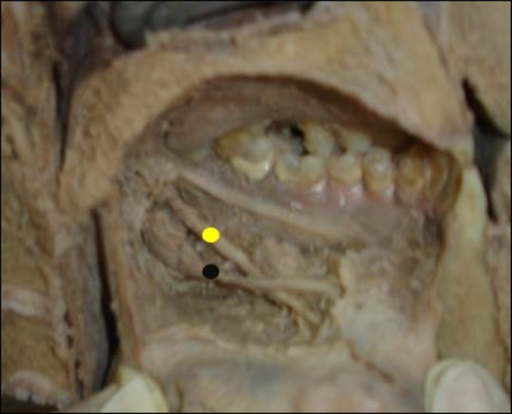
Intersection of Lingual Nerve and Submandibular Duct. The dissection shows the left lingual nerve (yellow) crossing inferior to the submandibular duct (black) in the floor of the oral cavity. The relationship is clinically important during surgical procedures involving the submandibular gland and duct.
With permission: Mendes MB, de Carvalho Leite Leal Nunes CM, de Almeida Lopes MC. Anatomical relationship of lingual nerve to the region of mandibular third molar. J Oral Maxillofac Res. 2014;4(4):e2.
(Click Image to Enlarge)
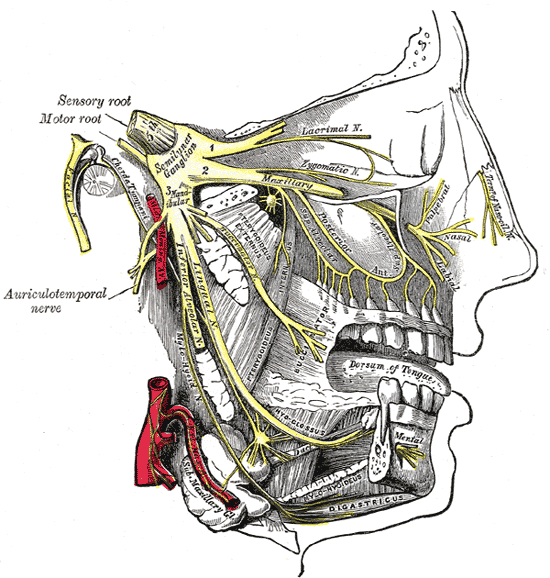
Maxillary and Mandibular Nerve Distribution. This image illustrates the trigeminal nerve and its 3 divisions, originating from the semilunar ganglion. The ophthalmic division gives rise to the lacrimal nerve and nasal branches of the nasociliary nerve. The maxillary division branches into the zygomatic nerves and terminates as the infraorbital nerve, with the associated pterygopalatine (sphenopalatine) ganglion. The mandibular division gives rise to the auriculotemporal, lingual, and inferior alveolar nerves, with the latter continuing as the mental nerve. The otic ganglion near the medial pterygoid and the submandibular ganglion hanging from the lingual nerve are also shown. The image additionally identifies the nerve to the mylohyoid and the digastric muscles.
By Henry Vandyke Carter - Henry Gray (1918) Anatomy of the Human Body (See "Book" section below)Bartleby.com: Gray's Anatomy, Plate 778, Public Domain, https://commons.wikimedia.org/w/index.php?curid=526568
(Click Image to Enlarge)
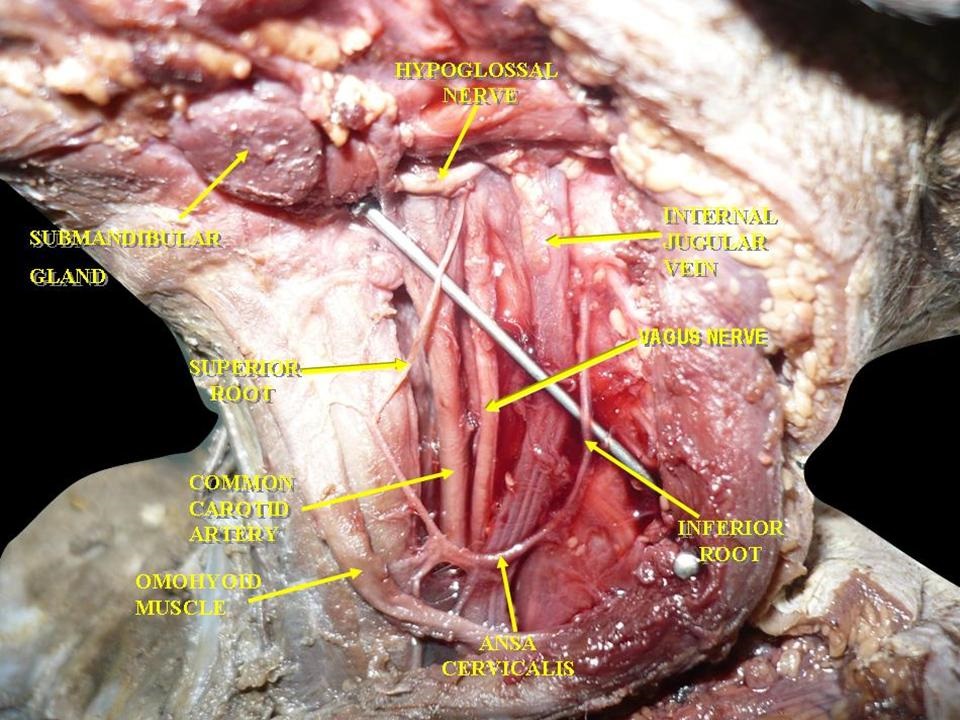
Muscles, Arteries, and Nerves of the Neck. The image shows a dissection of the cervical region. Visible structures include the hypoglossal nerve, vagus nerve, and ansa cervicalis. The submandibular gland, the omohyoid muscle, and the common carotid artery are also identified. The internal jugular vein lies adjacent to the carotid artery. Superior and inferior roots of the ansa cervicalis are clearly demonstrated.
By Anatomist90 - Own work, CC BY-SA 3.0, https://commons.wikimedia.org/w/index.php?curid=25036202
(Click Image to Enlarge)
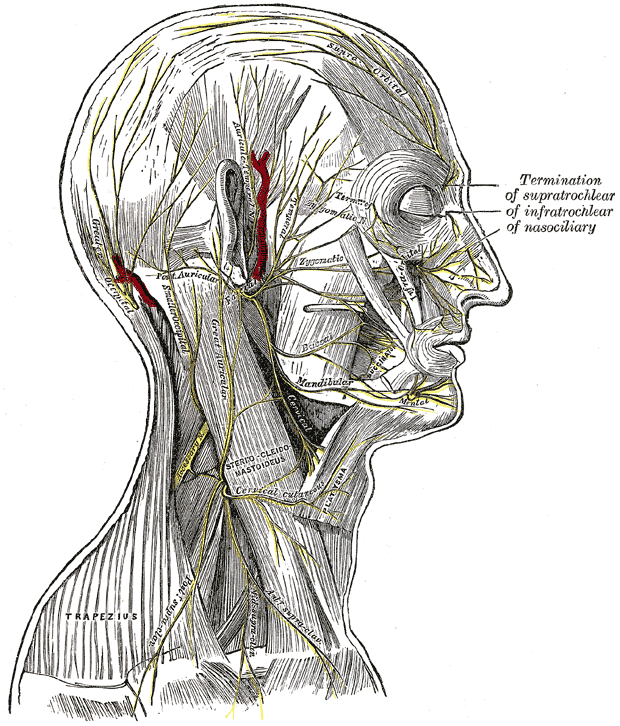
Superficial Nerves of the Head and Neck. The image illustrates the nerves of the scalp, face, and side of the neck. Branches of the facial and auriculotemporal nerves, as well as other cranial nerve divisions, are shown. Terminations of the supratrochlear, infratrochlear, and nasociliary nerves are also labeled. Muscular attachments, vascular structures, and cutaneous distributions are clearly depicted.
Henry Vandyke Carter, Public Domain, via Wikimedia Commons
(Click Image to Enlarge)
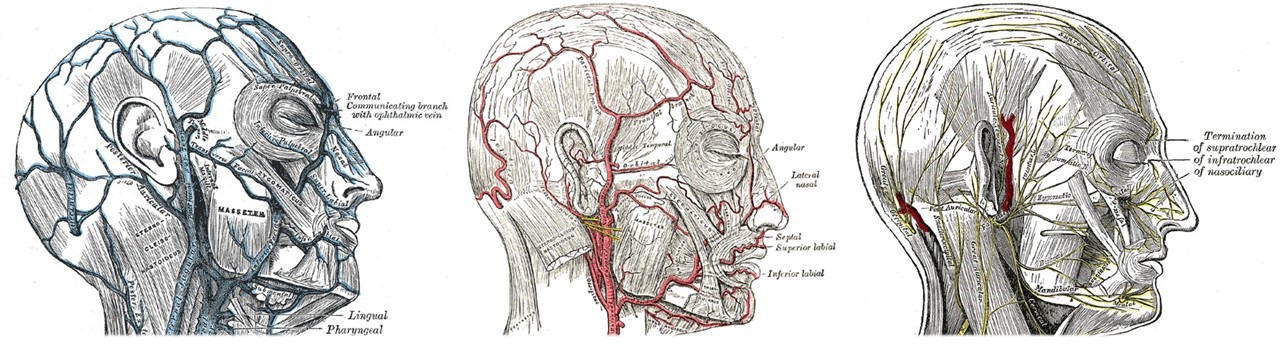
Veins, Arteries, and Nerves of the Face and Scalp. The left image shows venous drainage, including the frontal vein, the communicating branch with the ophthalmic vein, and the angular, masseteric, lingual, and pharyngeal veins. The center image depicts the arterial supply, highlighting the facial artery and its branches, the angular, lateral nasal, septal, superior labial, and inferior labial arteries. The right image shows motor and sensory innervation, including the supratrochlear, infratrochlear, nasociliary, auriculotemporal, zygomaticotemporal, zygomaticofacial, infraorbital, and mental nerves. Facial nerve branches are labeled, including the mandibular, cervical, temporal, zygomatic, buccal, and marginal mandibular branches. Clinically, the venous connection between the angular and ophthalmic veins provides a pathway for infection from the face to the cavernous sinus.
Contributed by Henry Vandyke Carter, Public Domain, via Wikimedia Commons
References
Ogle OE. Excision of Sublingual Gland. Oral and maxillofacial surgery clinics of North America. 2021 May:33(2):161-168. doi: 10.1016/j.coms.2020.12.001. Epub 2021 Jan 29 [PubMed PMID: 33526317]
Chibly AM, Aure MH, Patel VN, Hoffman MP. Salivary gland function, development, and regeneration. Physiological reviews. 2022 Jul 1:102(3):1495-1552. doi: 10.1152/physrev.00015.2021. Epub 2022 Mar 28 [PubMed PMID: 35343828]
Hernandez S, Busso C, Walvekar RR. Parotitis and Sialendoscopy of the Parotid Gland. Otolaryngologic clinics of North America. 2016 Apr:49(2):381-93. doi: 10.1016/j.otc.2015.12.003. Epub 2016 Feb 22 [PubMed PMID: 26912292]
Valstar MH, de Bakker BS, Steenbakkers RJHM, de Jong KH, Smit LA, Klein Nulent TJW, van Es RJJ, Hofland I, de Keizer B, Jasperse B, Balm AJM, van der Schaaf A, Langendijk JA, Smeele LE, Vogel WV. The tubarial salivary glands: A potential new organ at risk for radiotherapy. Radiotherapy and oncology : journal of the European Society for Therapeutic Radiology and Oncology. 2021 Jan:154():292-298. doi: 10.1016/j.radonc.2020.09.034. Epub 2020 Sep 23 [PubMed PMID: 32976871]
Holmberg KV, Hoffman MP. Anatomy, biogenesis and regeneration of salivary glands. Monographs in oral science. 2014:24():1-13. doi: 10.1159/000358776. Epub 2014 May 23 [PubMed PMID: 24862590]
HARRILL JA, KING JS Jr, BOYCE WH. Structure and composition of salivary calculi. The Laryngoscope. 1959 May:69(5):481-92 [PubMed PMID: 13655716]
Osuoji CI, Rowles SL. Studies on the organic composition of dental calculus and related calculi. Calcified tissue research. 1974:16(3):193-200 [PubMed PMID: 4447897]
Garrett JR. Recent advances in physiology of salivary glands. British medical bulletin. 1975 May:31(2):152-5 [PubMed PMID: 1100168]
Level 3 (low-level) evidenceArmstrong MA, Turturro MA. Salivary gland emergencies. Emergency medicine clinics of North America. 2013 May:31(2):481-99. doi: 10.1016/j.emc.2013.01.004. Epub [PubMed PMID: 23601484]
Kaufman-Goldberg T, Flynn JP, Banks CA, Varvares MA, Hadlock TA. Lower Facial Nerve Nomenclature Clarification: Cervical Branch Controls Smile-Associated Lower Lip Depression and Dental Display. Otolaryngology--head and neck surgery : official journal of American Academy of Otolaryngology-Head and Neck Surgery. 2023 Oct:169(4):837-842. doi: 10.1002/ohn.337. Epub 2023 Apr 6 [PubMed PMID: 37021911]
Kraaij S, Karagozoglu KH, Forouzanfar T, Veerman EC, Brand HS. Salivary stones: symptoms, aetiology, biochemical composition and treatment. British dental journal. 2014 Dec 5:217(11):E23. doi: 10.1038/sj.bdj.2014.1054. Epub [PubMed PMID: 25476659]
Porcheri C, Mitsiadis TA. Physiology, Pathology and Regeneration of Salivary Glands. Cells. 2019 Aug 26:8(9):. doi: 10.3390/cells8090976. Epub 2019 Aug 26 [PubMed PMID: 31455013]
Mizrachi A, Bachar G, Unger Y, Hilly O, Fliss DM, Shpitzer T. Submandibular salivary gland tumors: Clinical course and outcome of a 20-year multicenter study. Ear, nose, & throat journal. 2017 Mar:96(3):E17-E20 [PubMed PMID: 28346650]
Level 2 (mid-level) evidenceCarlson ER. Diagnosis and management of salivary lesions of the neck. Atlas of the oral and maxillofacial surgery clinics of North America. 2015 Mar:23(1):49-61. doi: 10.1016/j.cxom.2014.10.005. Epub 2015 Jan 9 [PubMed PMID: 25707565]
Filipov I, Cristache CM, Chirila L, Sandulescu M, Nimigean V. Diagnostic and Interventional Sialendoscopy: A Four-Year Retrospective Study of 89 Patients. Journal of clinical medicine. 2025 Jun 3:14(11):. doi: 10.3390/jcm14113938. Epub 2025 Jun 3 [PubMed PMID: 40507699]
Level 2 (mid-level) evidenceJost WH, Friedman A, Michel O, Oehlwein C, Slawek J, Bogucki A, Ochudlo S, Banach M, Pagan F, Flatau-Baqué B, Csikós J, Cairney CJ, Blitzer A. SIAXI: Placebo-controlled, randomized, double-blind study of incobotulinumtoxinA for sialorrhea. Neurology. 2019 Apr 23:92(17):e1982-e1991. doi: 10.1212/WNL.0000000000007368. Epub 2019 Mar 27 [PubMed PMID: 30918101]
Level 1 (high-level) evidenceMendelson BC, Tutino R. Submandibular Gland Reduction in Aesthetic Surgery of the Neck: Review of 112 Consecutive Cases. Plastic and reconstructive surgery. 2015 Sep:136(3):463-471. doi: 10.1097/PRS.0000000000001526. Epub [PubMed PMID: 25989302]
Level 3 (low-level) evidenceThomas WW, Douglas JE, Rassekh CH. Accuracy of Ultrasonography and Computed Tomography in the Evaluation of Patients Undergoing Sialendoscopy for Sialolithiasis. Otolaryngology--head and neck surgery : official journal of American Academy of Otolaryngology-Head and Neck Surgery. 2017 May:156(5):834-839. doi: 10.1177/0194599817696308. Epub 2017 Mar 14 [PubMed PMID: 28457224]
Żurek M, Fus Ł, Niemczyk K, Rzepakowska A. Salivary gland pathologies: evolution in classification and association with unique genetic alterations. European archives of oto-rhino-laryngology : official journal of the European Federation of Oto-Rhino-Laryngological Societies (EUFOS) : affiliated with the German Society for Oto-Rhino-Laryngology - Head and Neck Surgery. 2023 Nov:280(11):4739-4750. doi: 10.1007/s00405-023-08110-w. Epub 2023 Jul 13 [PubMed PMID: 37439929]
Cho J, Kim J, Lee JS, Chee CG, Kim Y, Choi SI. Comparison of core needle biopsy and fine-needle aspiration in diagnosis of ma lignant salivary gland neoplasm: Systematic review and meta-analysis. Head & neck. 2020 Oct:42(10):3041-3050. doi: 10.1002/hed.26377. Epub 2020 Jul 16 [PubMed PMID: 32671867]
Level 1 (high-level) evidenceChiesa-Estomba CM, Lechien JR, Fakhry N, Melkane A, Calvo-Henriquez C, de Siati D, Gonzalez-Garcia JA, Fagan JJ, Ayad T. Systematic review of international guidelines for perioperative antibiotic prophylaxis in Head & Neck Surgery. A YO-IFOS Head & Neck Study Group Position Paper. Head & neck. 2019 Sep:41(9):3434-3456. doi: 10.1002/hed.25856. Epub 2019 Jul 8 [PubMed PMID: 31282061]
Level 1 (high-level) evidenceBatra AP, Mahajan A, Gupta K. Marginal mandibular branch of the facial nerve: An anatomical study. Indian journal of plastic surgery : official publication of the Association of Plastic Surgeons of India. 2010 Jan:43(1):60-4. doi: 10.4103/0970-0358.63968. Epub [PubMed PMID: 20924452]
Chowdhry S, Yoder EM, Cooperman RD, Yoder VR, Wilhelmi BJ. Locating the cervical motor branch of the facial nerve: anatomy and clinical application. Plastic and reconstructive surgery. 2010 Sep:126(3):875-879. doi: 10.1097/PRS.0b013e3181e3b374. Epub [PubMed PMID: 20463628]
Cammaroto G, Vicini C, Montevecchi F, Bonsembiante A, Meccariello G, Bresciani L, Pelucchi S, Capaccio P. Submandibular gland excision: From external surgery to robotic intraoral and extraoral approaches. Oral diseases. 2020 Jul:26(5):853-857. doi: 10.1111/odi.13340. Epub 2020 Apr 20 [PubMed PMID: 32246560]
de Brito Neves CP, Lira RB, Chulam TC, Kowalski LP. Retroauricular endoscope-assisted versus conventional submandibular gland excision for benign and malignant tumors. Surgical endoscopy. 2020 Jan:34(1):39-46. doi: 10.1007/s00464-019-07173-3. Epub 2019 Dec 6 [PubMed PMID: 31811452]
Singh PP, Goyal M. Our Experience with Intraoral Submandibular Gland Excision. Indian journal of otolaryngology and head and neck surgery : official publication of the Association of Otolaryngologists of India. 2020 Sep:72(3):297-301. doi: 10.1007/s12070-019-01784-x. Epub 2020 Jan 3 [PubMed PMID: 32728538]
Chaukar DA, Deshmukh AD, Majeed T, Chaturvedi P, Pai P, D'cruz AK. Factors affecting wound complications in head and neck surgery: A prospective study. Indian journal of medical and paediatric oncology : official journal of Indian Society of Medical & Paediatric Oncology. 2013 Oct:34(4):247-51. doi: 10.4103/0971-5851.125236. Epub [PubMed PMID: 24604952]
Erbek SS, Köycü A, Topal Ö, Erbek HS, Özlüoğlu LN. Submandibular Gland Surgery: Our Clinical Experience. Turkish archives of otorhinolaryngology. 2016 Mar:54(1):16-20. doi: 10.5152/tao.2016.1467. Epub 2016 Mar 1 [PubMed PMID: 29392010]
Jaguar GC, Lima EN, Kowalski LP, Pellizon AC, Carvalho AL, Alves FA. Impact of submandibular gland excision on salivary gland function in head and neck cancer patients. Oral oncology. 2010 May:46(5):349-54. doi: 10.1016/j.oraloncology.2009.11.018. Epub 2010 Mar 15 [PubMed PMID: 20227906]
Żurek M, Rzepakowska A, Jasak K, Niemczyk K. The Epidemiology of Salivary Glands Pathologies in Adult Population over 10 Years in Poland-Cohort Study. International journal of environmental research and public health. 2021 Dec 24:19(1):. doi: 10.3390/ijerph19010179. Epub 2021 Dec 24 [PubMed PMID: 35010439]
Brown JE. Interventional sialography and minimally invasive techniques in benign salivary gland obstruction. Seminars in ultrasound, CT, and MR. 2006 Dec:27(6):465-75 [PubMed PMID: 17233229]
Kim MJ, Milliren A, Gerold DJ Jr. Salivary Gland Disorders: Rapid Evidence Review. American family physician. 2024 Jun:109(6):550-559 [PubMed PMID: 38905553]
Wolf G, Langer C, Wittekindt C. [Sialolithiasis: Current Diagnostics and Therapy]. Laryngo- rhino- otologie. 2019 Nov:98(11):815-823. doi: 10.1055/a-0896-9572. Epub 2019 Nov 18 [PubMed PMID: 31739357]
Mistry RK, Hohman MH, Al-Sayed AA. Facial Nerve Trauma. StatPearls. 2025 Jan:(): [PubMed PMID: 31971735]