Definition/Introduction
Nuclear medicine uses radioactive materials and their emitted radiation from the body to diagnose and treat disease. Unstable atoms (radionuclides) are typically administered orally or intravenously and, less commonly, intra-arterially, directly into the CSF spaces, peritoneum, or joint space. These radionuclides are often chelated (labeled or tagged) with other molecules that provide them with their physiologic properties, forming a radiopharmaceutical and allowing the combination to localize preferentially to organs of interest. Specialized cameras are used to record the radiation emission from these unstable atoms, allowing for the localization of pathology and guiding treatment.
In the field of medicine, nuclear physics helps us understand:
- Which atoms and their tagged molecules can be used in diagnostics.
- Which radioactive atoms can be used in therapeutics
- Precautions necessary to protect the general public from radiation
- Precautions necessary to minimize the patient's radiation
- The type of shielding and other protective measures necessary in the handling of certain radioactive products
- The proper disposal of radioactive substances in the nuclear medicine department
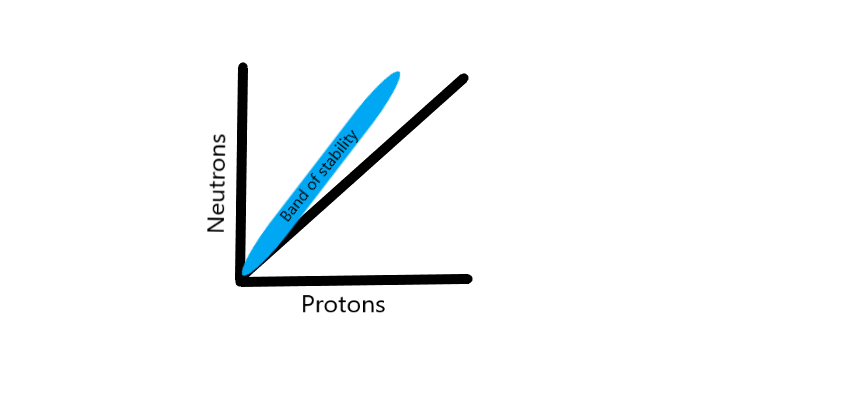
The nucleus of an atom consists of protons and neutrons held together by a strong nuclear force. Atoms are stable when this strong nuclear force can keep its constituent protons and neutrons within the nucleus. When the number of neutrons and protons are plotted on a graph, stable nuclei have been found to fall within a narrow region called the “band of stability” that closely approximates a 1:1 ratio of neutrons to protons in low atomic number atoms (see Graph. Band of Stability). As the atomic weight of an atom increases, this ratio skews towards a higher ratio of neutrons when compared to protons. This band of stability helps us understand the basis of nuclear physics, as atoms located outside of this band undergo reactions (decay) in an attempt to reach this band of stability.
Types of Decay
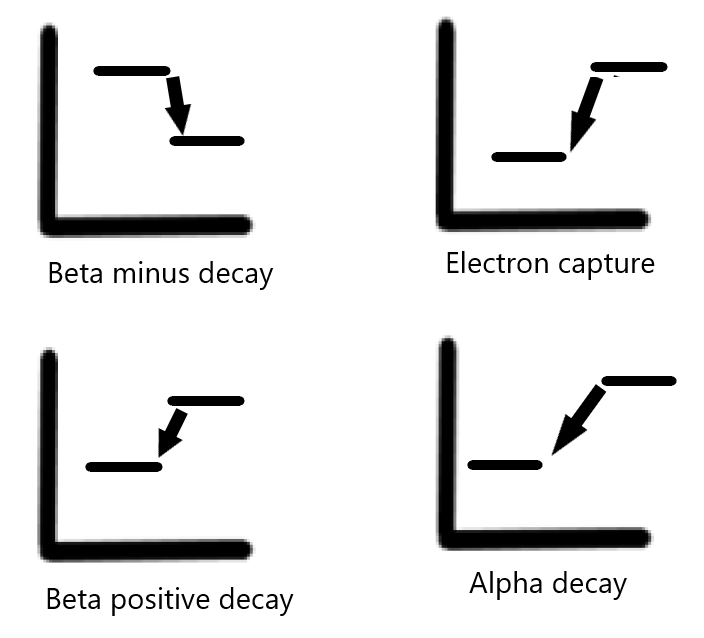
Depending on the imbalance between protons and neutrons, different types of nuclear decay occur (see Graph. Types of Decay When Plotted on a Graph).[1][2] These include:
- Beta positive decay: occurring with an excess of protons.
- Electron capture: occurring with an excess of protons.
- Beta minus decay: occurring with an excess of neutrons.
- Alpha decay: occurring with atoms with high atomic weights that are unstable.
Beta positive decay: Beta positive decay occurs when an atom has an excess of protons (and a low number of neutrons). In this process, a positively charged proton is converted into a neutral neutron. This process results in the emission of a positron and a neutrino, a particle that is electrically neutral and has essentially zero mass. A positron is akin to a positively charged version of an electron. Depending on its energy, the positron traverses a short distance until its energy decreases to 1.02 MeV, at which point it encounters an electron, resulting in an annihilation event. The result of an annihilation event is the emission of two photons, each measuring 511 keV in energy, emitted 180 degrees opposite in direction from each other. This emission of 2 photons is the basis of PET imaging.
Electron capture: Electron capture results from the “capture” of a negatively charged electron by a proton-heavy nucleus, resulting in a neutron. This electron is usually located in the K shell of an atom. The process of electron capture is an isobaric transition, as the atomic mass (ie, the total number of protons and neutrons) remains unchanged. The atomic number, based on the number of protons in the nucleus, decreases since a proton is now transformed into a neutron. The electron capture process is also an isomeric transition, resulting in the emission of photons that can be utilized for imaging purposes.
Beta minus decay: Beta minus decay occurs when an atom contains an excess of neutrons.[1] In this process, a neutron is transformed into a proton, resulting in the release of a beta particle and an antineutrino. A beta particle is akin to an electron. However, it is distinguished from an electron due to its origin in the nucleus. The release of an anti-neutrino and a beta particle balances the change of charge in the process. The atomic number also increases by 1, considering the neutron is now transformed into a proton. This process is an isobaric transition, as the mass of the atom remains unchanged. Beta minus imaging is not useful for imaging but is useful in therapy.
Alpha decay: Alpha decay occurs when the nucleus emits an alpha particle. An alpha particle is essentially a helium-4 atom and consists of 2 protons and 2 neutrons. Alpha particles have a charge of +2 and are emitted by heavy nuclides.
Isomeric transition: After the isobaric transition, excess energy is emitted in the form of isomeric transition, mostly by emitting gamma photons. The energy of these gamma photons is variable and depends on the intermediate and final states of the nucleus undergoing an isomeric transition. These packets of released energy in the form of gamma photons often provide a unique fingerprint for different radionuclides, as each releases a predictable amount of energy to reach its stable state. The intermediate states can be short, or as in the case of “metastable states”, can be long, as is the case with technetium 99-metastable radionuclides used in imaging. Technetium 99-metastable radionuclides release 140 keV photons before reaching their stable ground state.
Production of radionuclides: Atoms with an excess of neutrons and protons can be generated for medical imaging and therapy. Radioactive material not occurring naturally is generated by bombardment and fission, which results in unstable isotopes (a nucleus with an “unfavorable” neutron: proton ratio). The bombardment occurs via the irradiation of a nucleus with either neutrons (in a nuclear reactor) or other charged particles from a cyclotron. Charged particles in a cyclotron used to irradiate neutrons can include alpha particles, deuterons, or protons. The daughter isotopes resulting from bombardment are easily separated in cyclotrons where transmutation occurs (a change in atomic number) and less easily in processes involving neutron bombardment, where atomic numbers are not changed. Neutron bombardment and nuclear fission generally result in nuclei with a neutron excess, and cyclotron-produced isotopes are neutron deficient.
Half-life: The physical half-life of a radionuclide denotes the amount of time it takes for its activity to be reduced to half of its existing activity. The biological half-life of a radionuclide denotes the amount of time it takes for the radionuclide to reduce to half of its concentration within the human body (often through excretion or exhalation). The effective half-life describes the combination of these 2 aspects of a radionuclide.
Issues of Concern
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Issues of Concern
Safety: Plastic and lead are commonly used to shield radiation workers from gamma photons and beta radiation. In the context of beta particle emission, plastic serves as an initial absorber of beta emission shielding. Higher atomic materials such as lead result in radiative interactions, which can increase the radiation exposure dose. However, the use of lead shielding is crucial in protecting against gamma photon emission and X-rays.
Clinical Significance
Various radionuclides are used in clinical imaging and treatment.
Common radionuclides used in imaging through the emission of gamma photons include:
- Technetium-99m: Technetium is used in a wide range of clinical applications when combined with various other compounds.[3] Clinical applications include evaluation for acute cholecystitis, bile leak, neonatal jaundice (Tc-99m mebrofenin), renal function (Tc-99m MAG3), and structure evaluation (Tc-99m DMSA), evaluation for pulmonary embolism (Tc-99m MAA), bone disorders (Tc-99m MDP), evaluation of cardiac function (Tc-99m sestamibi), parathyroid adenoma imaging (also Tc-99m sestamibi), gastrointestinal bleeding (Tc-99m tagged RBCs) and gastrointestinal transit studies (Tc-99m sulfur colloid).
- Iodine-123: Iodine-123 is used in the evaluation of Parkinson disease (I-123 ioflupane), the evaluation of pheochromocytoma, paraganglioma, neuroblastoma (I-123 MIBG, a norepinephrine analog), and in the evaluation of thyroid disease such as Graves disease (I-123).[4]
- Iodine-131: Iodine-131 is mainly used for the therapy of benign and malignant thyroid diseases.
- Indium-111: Indium-111 CSF leak (In-111 DTPA), evaluation of neuroendocrine tumors (In-111 pentreotide), and infection (In-111 labeled WBCs).[5]
- Xenon-133: Xenon-133 is used for evaluating lung ventilation, often in the context of workup for pulmonary embolism.[6]
Commonly radionuclides used in imaging through positron emission include:
- Fluorine-18: Fluorine-18 is most well known for its use in the evaluation of tumors; however, it is also used in the evaluation of cardiac viability, brain metabolism, infection, dementia (in the form of F-18-FDG, a radionuclide glucose analog) and prostate cancer (F-18 fluciclovine, an amino acid analog).[7]
- Nitrogen-13: Nitrogen-13 is used in the evaluation of cardiac perfusion and is preferred for quantifying myocardial blood flow.[8]
- Rubidium-82: Rubidium-82 is used in the evaluation of myocardial perfusion.
Radionuclides used in therapy include:
- Yttrium-90: Yttrium-90 is a beta emitter and is used commonly in the treatment of liver malignancy via selective intra-arterial injection.[9]
- Radium-223: An alpha emitter used for the treatment of bone metastases in patients with prostate cancer that is resistant to other treatments.
- Lutetium-177: Lutetium-177 is a beta emitter used for the treatment of gastrointestinal and pancreatic neuroendocrine tumors that are refractory to other treatments.
- Strontium-89 and Samarium-153: Beta emitters used as a palliative treatment measure for bony metastasis.[10]
Nursing, Allied Health, and Interprofessional Team Interventions
The use of nuclear medicine radiotracers in clinical practice necessitates close coordination among multiple healthcare professionals. The medical physicist plays a crucial role in calculating potential radiation doses to both patients and the public. The nuclear pharmacist is responsible for preparing radiotracers, while the nuclear medicine technologist is involved in screening, preparing, and imaging the patient. The nuclear medicine physician oversees all aspects of nuclear medicine care, including therapy direction and image interpretation. In cases involving inpatient care, nurses and the inpatient team may contribute during admissions related to treatment or complications. Referring providers are essential in identifying appropriate patients and providing initial education on the role of nuclear medicine. Given the radiation doses often involved, especially with therapeutic radiopharmaceuticals, the involvement of this interprofessional team is critical. Clear communication among team members ensures accurate dosing, effective radiation shielding for both workers and the public, and optimized patient outcomes in both diagnostic and therapeutic settings.
Media
(Click Image to Enlarge)
(Click Image to Enlarge)
References
Donya M, Radford M, ElGuindy A, Firmin D, Yacoub MH. Radiation in medicine: Origins, risks and aspirations. Global cardiology science & practice. 2014:2014(4):437-48. doi: 10.5339/gcsp.2014.57. Epub 2014 Dec 31 [PubMed PMID: 25780797]
Groch MW. Radioactive decay. Radiographics : a review publication of the Radiological Society of North America, Inc. 1998 Sep-Oct:18(5):1247-56; quiz 1245-6 [PubMed PMID: 9747617]
Kane SM, Padda IS, Patel P, Davis DD. Technetium-99m. StatPearls. 2025 Jan:(): [PubMed PMID: 32644439]
Kim PD, Tran HD. I-123 Uptake. StatPearls. 2024 Jan:(): [PubMed PMID: 32644740]
Padda IS, Nguyen M. Radioactive Iodine Therapy. StatPearls. 2024 Jan:(): [PubMed PMID: 32491673]
Bajaj T, Cascella M, Borger J. Xenon. StatPearls. 2025 Jan:(): [PubMed PMID: 31082041]
Ashraf MA, Goyal A. Fludeoxyglucose (18F). StatPearls. 2025 Jan:(): [PubMed PMID: 32491585]
Kapoor M, Heston TF, Kasi A. PET Scanning. StatPearls. 2025 Jan:(): [PubMed PMID: 32644515]
Asafo-Agyei KO, Samant H. Hepatocellular Carcinoma. StatPearls. 2024 Jan:(): [PubMed PMID: 32644603]
Munjal A, Gupta N. Radiopharmaceuticals. StatPearls. 2024 Jan:(): [PubMed PMID: 32119327]