Introduction
Infection control refers to the policies and procedures implemented to prevent and minimize the spread of infections in hospitals and other healthcare settings, with the primary purpose of reducing the incidence of infections. Infection control as a formal entity was established in the United States in the early 1950s. By the late 1950s and 1960s, a small number of hospitals began to recognize health care–associated infections and implemented some of these infection control concepts. These early infection control programs primarily focused on the surveillance and identification of risk factors for health care–associated infections.[1] However, most infection control programs were organized and managed by large academic centers rather than public health agencies, resulting in sporadic efficiency and suboptimal outcomes.
The current era in infection control began in the late 20th and early 21st centuries, marked by 3 pivotal events. These events included the Institute of Medicine's 1999 report on errors in health care, the establishment of National Patient Safety Goals by the Joint Commission in 2003, and the 2004 and 2006 publications of significant reductions in bloodstream infection rates through the standardization of the central venous catheter insertion process.[2][3][4][5]
This new era in healthcare epidemiology is characterized by consumer demands for greater transparency and accountability, increased scrutiny and regulation, and expectations for rapid reductions in health care–associated infection rates.[6] The role of infection control is to prevent and reduce the risk of health care–associated infections through comprehensive programs that include surveillance, isolation, outbreak management, environmental hygiene, employee health, education, and infection prevention policies and management.[7]
Function
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Function
The significance and function of infection control are substantial. Nearly 2 million health care-acquired infections occur each year in the United States, resulting in approximately 100,000 deaths annually.[7] Because of the severity of this issue, New York State implemented Chapter 768, which mandates healthcare professionals to complete a 2-hour continuing education training of New York State Department of Education every 4 years, covering Centers for Disease Control and Prevention (CDC) standards of infection control and the possibility of unprofessional conduct due to a lack of compliance with the law. This course meets the New York State Chapter 768 mandatory requirement. Exemptions from the Department of Health are available under certain conditions. A healthcare professional may qualify for an exemption if they have completed equivalent coursework, are not currently practicing in New York, do not provide direct patient care, or if their clinical practice does not necessitate infection control or barrier precautions. In addition, exemptions apply if the professional does not supervise staff who provide direct patient care or reprocess patient care equipment.
State law requires healthcare professionals to be familiar with the following elements of infection control in the healthcare setting:
- Adherence to scientifically accepted principles and practices of infection control, and monitoring the performance of professionals for whom the health professional is responsible.
- Modes and mechanisms of transmission of pathogenic organisms and strategies for control and prevention.
- Use of engineering and work practice controls to reduce the opportunity for patient and worker exposure to potentially infectious material.
- Selection and use of barriers and personal protective equipment (PPE) to prevent patient and healthcare worker contact with potentially infectious substances.
- Creation and maintenance of a safe environment for patient care through the application of infection control practices and principles for cleaning, disinfection, and sterilization.
- Prevention and management of infectious or communicable diseases in healthcare professionals.
- Sepsis awareness and education.
Required Documentation
Clinicians, Physician Assistants, and Specialist Assistants
- Upon completing the course, retain the Certificate of Completion.
- If affiliated with a hospital, submit a copy of the Certificate of Completion to your hospital personnel office or administration. Hospitals are required to verify course completion before credentialing healthcare professionals.
- If not affiliated with a hospital, submit the Certificate of Completion to the Department of Health.
- Retain a copy of the Certificate of Completion for at least 6 years.
Nurses, Dentists, Dental Hygienists, Podiatrists, and Optometrists
- Upon completing the course, retain the Certification of Completion.
- Maintain a copy of the Certificate of Completion in the event you are asked to submit it.
- Attest to completing this requirement to the State Education Department on the initial licensure or registration application and each subsequent registration.
Issues of Concern
Element 1: Adherence to Scientifically Accepted Principles and Practices of Infection Control and Monitoring the Performance of Professionals for whom the Healthcare Professional is Responsible
New York State healthcare providers have legal and professional obligations to follow scientifically accepted principles and practices of infection prevention and control and to ensure that any staff under their supervision also adhere to these practices. In addition to the legal requirements for infection control training, both the New York State Board of Regents, responsible for licensure and regulation of healthcare professionals, and New York State health regulations define unprofessional conduct for healthcare professionals as including failure to use scientifically accepted infection control practices, including:
- Adherence to scientifically accepted standards for handwashing; aseptic technique; use of gloves and other barriers for preventing bi-directional contact with blood and body fluids; thorough cleaning followed by sterilization or disinfection of medical devices; disposal of non-reusable materials and equipment; and cleaning between patients of objects that are visibly contaminated or subject to touch contamination with blood or body fluids.
- Use of scientifically accepted injury prevention techniques or engineering controls to reduce the opportunity for patient and employee exposures.
- Performance monitoring of all personnel, licensed or unlicensed, for whom the licensee is responsible, regarding infection control techniques.[New York State Education Department, Office of the Professions. Rules of the Board of Regents. Part 29][New York State. Department of Health. Section 92-2.1 - Required use of infection control practices]
Healthcare professionals should take the New York State statute and regulations seriously, as the consequences are potentially severe. The statute sets minimum compliance rules and outlines consequences for non-compliance, which may include citations, fines, and professional liability. Employee or patient complaints concerning poor infection control practices may result in an investigation by the Department of Education or the Department of Health. Healthcare professionals who fail to use appropriate infection control techniques, are aware of violations, or are responsible for lax staff compliance and education may be charged with professional misconduct and receive disciplinary action or revocation of their professional licenses.
In addition to state requirements, the Joint Commission identifies infection prevention and control and antibiotic stewardship as integral to quality healthcare delivery. The Commission has established infection control standards for hospitals, long-term care facilities, home care services, and office-based surgery centers.[The Joint Commission. Infection Prevention and Control & Antibiotic Stewardship][8][9] Standards to prevent health care–associated infections are endorsed by the CDC, Joint Commission, Society for Healthcare Epidemiology, Infectious Diseases Society of America, Association for Professionals in Infection Control and Epidemiology, and the American Hospital Association, among other professional organizations.[10][11][12] Furthermore, breaches in infection control standards increase the risks of health care–acquired infections and their associated adverse health events for patients and other healthcare professionals exposed to potentially infectious materials.[13]
Element 2: Modes and Mechanisms of Transmission of Pathogenic Organisms and Strategies for Control and Prevention
The Chain of Infection: Clinicians need to understand the chain of infection to better prevent and treat infections. Pathogens include bacteria, fungi, parasites, viruses, protozoa, and prions. Reservoirs are the natural habitat where pathogens live, grow, and reproduce. Many infectious diseases have human, animal, and environmental reservoirs. Pathogens can leave the reservoir and cause infection when a portal of exit is available, such as through the respiratory tract, gastrointestinal tract, genitourinary tract, blood, secretions, or open wounds. Transmission of a pathogen to a new host occurs through 1 or more of several routes: [14]
- Direct contact transmission occurs when microorganisms are transferred from one person to another without the involvement of a contaminated intermediate.
- Indirect transmission occurs when the transfer occurs through a contaminated intermediate person or object.
- Vehicles are contaminated inanimate objects, such as surfaces, medical equipment, or medications, that may passively carry a pathogen from its reservoir to a new host.
- Vectors are living entities, such as mosquitoes, fleas, or ticks, that may carry a pathogen from a reservoir to a host or from host to host.
- Droplet transmission occurs when respiratory droplets transmit the infection from the respiratory tract to susceptible mucosal surfaces of the recipient, commonly from coughing, sneezing, intubation, or suctioning.
- Airborne transmission occurs when droplet nuclei or small particles containing infectious agents remain in the air over time and distance. The dispersion may occur by air currents over long distances.
Factors Influencing the Outcomes of Exposures: Not all exposures to pathogens result in infection. Pathogens require a portal of entry such as an open wound; vascular access; mucous membrane; or the respiratory, gastrointestinal, or genitourinary tracts. Natural barriers, such as intact skin, intestinal, and respiratory cilia, prevent or reduce portals of entry. Natural host factors, including gastric acid, normal flora, inflammatory responses, and humoral and cell-mediated immunity, inactivate, kill, and remove pathogens from the host.[15] Therefore, most pathogens require a susceptible host—one lacking immunity to the pathogen or insufficient barriers to infection.
Pathogen-specific factors also influence outcomes. Pathogens with higher infectivity cause infections in a higher proportion of exposed people. Highly pathogenic pathogens cause clinical disease in a high proportion of infected people. Virulent pathogens cause severe disease in a high proportion of people with clinical infection. The transmission of infectious diseases requires a minimum number of individuals to cause disease and environmental factors that promote the growth and spread of the pathogen.
Methods to Prevent the Spread of Pathogens: Standard precautions should be applied to all patient care, including hand hygiene, the use of PPE, appropriate patient placement, cleaning and disinfecting of patient care equipment, management of textiles and laundry, safe injection practices, and proper disposal of needles and other sharp objects.
Gloves can also be used as a standard precaution; new gloves must be used for each patient and disposed of after each patient interaction. Other PPE includes facial protection, such as procedure or surgical masks, goggles, and a face shield, and a gown, which should be worn before entering the patient's room.
Hands should be cleaned with either soap and water or an alcohol-based rub in the following situations—before direct patient contact, after contact with patient skin or body fluids, before donning and after removing sterile gloves, before eating, after using a restroom, and anytime the hands are visibly dirty or soiled. Soap and water should be used when hands are visibly dirty, soiled, or contaminated. Hands that are not visibly soiled may be cleaned with either an alcohol-based hand sanitizer or with soap and water.[16] For either method, hands should be rubbed vigorously together for at least 15 seconds, ensuring that all surfaces of the hands and fingers are covered. If your hands feel dry within 15 seconds after applying hand sanitizer, it may indicate that an inadequate amount was applied.
The efficacy of alcohol-based hand sanitizers is affected by the type, concentration, and volume of alcohol used; the duration of skin contact; and whether the hands were wet at the time of application. Contamination of alcohol-based sanitizers is rare; however, liquid soap dispensers can become contaminated when additional soap is added to top off a partially empty dispenser.[16]
Infected patients require early identification, separation, and isolation if necessary, along with the implementation of necessary precautions and treatment. These measures must be enforced to control such outbreaks.
- Contact precautions: Used for patients with known or suspected infections that can be transmitted through contact. Patients under contact precautions should be placed in single rooms or separated from other patients by at least 3 ft, with limited transport and movement. In addition to standard precautions, healthcare providers attending to these patients should wear a gown and gloves, use disposable patient care equipment, and follow thorough cleaning and disinfection protocols. Patients with acute infectious diarrhea, vesicular rash, respiratory tract infection caused by a multidrug-resistant organism, abscess, or draining wound that cannot be covered need to be under contact precautions.[14]
- Droplet precautions: Used for patients with known or suspected infections that can be transmitted by air droplets through coughing, sneezing, or talking. Patients under droplet precautions should be placed in single rooms. If no single room is available, an infectious disease specialist should be consulted. Special air handling or ventilation is not generally necessary for droplet precautions. Healthcare providers attending to these patients should wear standard procedure or surgical masks; respirators are not required. These patients should wear masks during transport and movement. Patients with known or suspected influenza, pertussis, or meningococcal disease should be placed on droplet precautions.[14]
- Airborne precautions: Used for patients with known or suspected infections that are transmissible by the airborne route. These patients are required to be in a single-patient airborne infection isolation room with all the previously mentioned protections. Healthcare providers attending to these patients must wear respirators that are donned before entering their rooms. The most important pathogens that need airborne precautions are tuberculosis, measles, chickenpox, and disseminated herpes zoster.[14]
Multiple of these indications might require more than one precaution to ensure the efficient standard and transmission-based precautions. For example, patients with suspected Clostridium difficile infection require contact and standard precautions, whereas patients with tuberculosis require airborne, contact, and standard precautions.
Healthcare professionals can take several actions to protect themselves and reduce their risk of health care–associated infections. Complete and up-to-date vaccination protects healthcare professionals against several common pathogens. Timely post-exposure prophylaxis can reduce the risk of infection among individuals who have recently been exposed to certain bacteria and viruses. Thorough cleaning, disinfection, and sterilization of patient care equipment; cleaning of the healthcare environment; proper ventilation; waste management; linen and laundry management; and hygienic food services play a crucial role in protecting both patients and staff. The application of engineering and work practice controls in healthcare settings reduces the likelihood of exposure to potentially infectious materials. Each of these elements is discussed further within this course. Staff should be educated and trained on how they can protect patients, their coworkers, and themselves.
Element 3: Use of Engineering and Work Practice Controls to Reduce the Opportunity for Patient and Healthcare Worker Exposure to Potentially Infectious Material
Many common medical and nursing procedures put healthcare professionals and patients at risk of exposure to potentially infectious material. These activities include, but are not limited to:
- Manually disassembling contaminated needles and scalpels
- Improper or delayed sharps disposal
- Recapping contaminated needles
- Performing procedures with poor visualization, such as blind suturing
- Contact with wounds, open skin lesions, or dermatitis
- Irrigation, suctioning, and other procedures that may result in splash or spray of body fluids
- Contact with the eyes, nose, mouth, or other mucous membranes with contaminated hands
- Sharing blood monitoring devices, such as glucometers and lancets
High-risk activities should be avoided when possible. When such activities are medically necessary or unavoidable, healthcare professionals should use engineering and work practice controls to reduce their risks.
Safe Injection Practices: Safe injection practices and procedures protect both patients and healthcare professionals. Unsafe injection practices can and have led to transmission of human HIV, hepatitis B (HBV), hepatitis C (HCV), and pathogens such as viruses, bacteria, fungi, and parasites. According to the World Health Organization, in 2000, 21 million cases of HBV, 2 million cases of HCV, and 260,000 cases of HIV worldwide were due to unsafe injection practices.[17] Iatrogenic transmission of infectious diseases can result in the notification and testing of thousands of exposed patients, referral of healthcare providers for disciplinary action, and malpractice lawsuits by patients and caregivers.[18]
HBV, HCV, HIV, bacteria, and other microorganisms can be present in sufficient quantities to transmit infection in contaminated equipment even in the absence of visible blood or clouding. Therefore, the absence of visible signs of contamination on used medical equipment does not mean that the equipment is free of potentially infectious material.
Healthcare professionals preparing or administering injections should follow aseptic technique:
- Draw up medications in a designated clean area that is physically separate from contaminated materials.
- Use a new, sterile syringe and needle to draw up medications.
- Perform proper hand hygiene before handling medications.
- Disinfect the rubber septum of an open medication vial with alcohol before puncturing it. This step may be bypassed when using a new, unopened vial.
- Never leave a needle inserted into a medication vial or intravenous (IV) bag for multiple uses. The open bore of the needle provides a direct route for contamination of the medication.
- Never use the same syringe to administer medications to more than one patient, even if the needle is changed between patients or if the medication is administered into IV tubing. All components of the IV line from the infusate to the patient's catheter are interconnected and potentially exposed to the patient's blood.
- Never enter a vial with a needle or syringe that has been used on a different patient.
- Never use single-dose vials for more than one patient, nor combine leftover contents from more than one vial.
- Whenever possible, dedicate multidose vials of medications to individual patients.
- Discard medication bottles once they have expired or whenever concerns arise about their sterility.
- Never use medical devices labelled for single-patient use on more than one patient.
Surveillance and Evaluation of Exposure Incidents: The primary aim of healthcare epidemiology and surveillance programs is to assess the rates of infections and identify high-risk groups, settings where exposures occur, and devices and circumstances that increase the risk of exposure. Although all sharp devices pose a risk of injury and disease transmission if not used and disposed of properly, some devices have a higher risk of disease transmission and injury. Hollow-bore needles used for venous access are associated with the highest rates of bloodborne pathogen transmission.[19] Butterfly needles and peripheral venous catheters have higher rates of needlestick and sharps injuries.[20]
Generally, hospitals target surveillance for health care–associated infections in areas with the highest infection rates, including surgery, hematology/oncology, and intensive care units (ICUs). However, surveillance has expanded in recent years to include hospital-wide surveillance.[21] This change has also been facilitated by the widespread adoption of electronic health records in most hospitals in the United States. Most hospitals have developed sophisticated algorithms in their electronic health systems that can streamline surveillance and identify patients at the highest risk for health care–associated infections.
Public health agencies mandate hospitals to report specific infections to strengthen the public health surveillance system.[22] Microorganism outbreaks can be identified through the surveillance system. Once a particular infection's monthly rate crosses the 95% confidence interval threshold, an investigation is warranted for a possible outbreak. Clusters of infections occurring at lower rates can also be reported by healthcare providers or laboratory staff, followed by an investigation to determine whether this cluster constitutes an outbreak.
Engineering Controls: Engineering controls are physical controls that isolate or remove infectious materials from the workplace.
When possible, safety syringes and other devices with sharps injury prevention features should be used.[23] Sharps without built-in safety features are associated with higher rates of injury than those with engineered protections.[24] Mechanisms with continuous or passive protective features are preferred over those that require user activation. Infection control practitioners should assess the safety and usability of new devices before purchasing them. They should consider replacing traditional devices without safety features with safer devices whenever possible. Staff should be trained on the proper use and disposal of safety devices. Needlestick injuries can nonetheless occur with safety-engineered devices; the most commonly reported reasons for such injuries include challenges using the safety features and improper disposal of sharps.[25]
Sharps containers should be closeable, puncture-resistant, leakproof, and labeled or color-coded in accordance with United States Occupational Safety and Health Administration (OSHA) standards.[Occupational Safety and Health Administration. 1910.1030 - Bloodborne pathogens] Patients and caregivers who use injection medications should be educated on the proper disposal of needles and other sharps.
Work Practice Controls: Work practice controls are actions that reduce the likelihood of exposure to infectious materials by modifying the way medical tasks are performed. Hand hygiene is the most significant and effective work practice for preventing the transmission of infections.[26] PPE should be selected appropriately to the risk level and donned and doffed according to guidelines. Blood and bodily fluid spills should be cleaned promptly with initial removal of the biological material, followed by decontamination. Contaminated devices and patient care materials should be decontaminated or disposed of according to guidelines.[27] Please refer to "Elements 2, 4, and 5" in the Issues of Concern section for more information on handwashing, PPE, and cleaning, respectively.
Healthcare professionals should be educated on infection control practices. Staff should be instructed to avoid unnecessary use of needles and other sharp objects, avoid recapping needles unless medically necessary, use a one-handed technique or safety device when recapping cannot be avoided, use designated safe zones for passing sharps, use forceps or other equipment to safely disassemble sharp equipment, and discard used sharps into a puncture-resistant sharps container immediately after use. Education should be reinforced regularly to enhance and promote understanding.
Antimicrobial stewardship programs help control antimicrobial resistance, improve outcomes, and reduce healthcare costs. Antimicrobial stewardship should be programmed to monitor antimicrobial susceptibility profiles, anticipating and assessing any new antimicrobial resistance patterns. These trends should be correlated with the antimicrobial agents used to assess susceptibility patterns.[28] Antimicrobial stewardship programs can be designed as either active or passive initiatives, targeting either pre-prescription or post-prescription periods. An active program includes prescription restrictions and preauthorization in the pre-prescription period, whereas passive initiatives include education, guidelines, and antimicrobial susceptibility reports. An active post-prescription program focuses on providing real-time feedback to clinicians regarding antibiotic usage, dose, bioavailability, and susceptibility, with automatic conversion of intravenous to oral formulations. In contrast, a passive post-prescription program involves integrating electronic medical records to generate alerts for prolonged prescriptions and mismatches between antibiotics and microorganisms.[29]
Element 4: Selection and Use of Barriers and Personal Protective Equipment to Prevent Patient and Healthcare Worker Contact with Potentially Infectious Material
In a world of evolving diseases, accidental industrial or commercial contamination, and foreign and domestic terrorism, healthcare providers must be able to deliver safe and efficient care to patients while wearing PPE and barriers. PPE is specialized clothing or equipment worn to protect the wearer against infectious or other hazardous materials. Barriers are a subset of PPE that protect the wearer's skin and mucous membranes against exposure to potentially infectious materials. Common examples of PPE and barriers include:
- Gloves: Protect the hands.
- Available in sterile, nonsterile, and utility types; care should be taken to select the correct type based on the patient's and wearer's needs.
- Materials include natural rubber latex, nitrile, and vinyl.
- Aprons, gowns, and laboratory coats: Protect skin and clothing.
- May be fluid impervious, fluid resistant, or permeable.
- Masks: Protect the mouth and nose.
- Include surgical and procedure masks that attach with ties or ear loops.
- Respirators: Protect the respiratory tract from airborne infections.
- Include particulate, elastomeric, or powered air-purifying
- Face shields: Protect eyes, face, mouth, and nose.
- Goggles and safety glasses: Protect the eyes.
PPE selection is based on the following factors:
- Anticipated exposure type, such as contact, splash, respiratory droplets, or airborne transmission.
- Anticipated volume of infectious materials.
- Category of isolation precaution required.
- Durability, appropriateness, and fit for the task.
The appropriate level of PPE must be selected based on the specific task or hazard. More restrictive PPE levels confer more health benefits to wearers but also proportionally increase the risk of physiological stress. All PPE use should comply with OSHA standards.[30]
- Level A offers the highest level of protection and is appropriate for situations involving unknown threats. Level A protects operators from liquids, vapors, and gases. This level encompasses a positive-pressure full-face mask connected to a self-contained breathing apparatus (SCBA), a completely encapsulated suit, outer chemical-resistant gloves, inner chemical-resistant gloves, and chemical-resistant boots.
- Level B offers protection from liquids and gases, but not vapors. This level consists of positive-pressure, full-faced SCBA, hooded chemical-resistant clothing, outer chemical-resistant gloves, inner chemical-resistant gloves, chemical-resistant boots, boot covers, and a face shield.
- Level C is most commonly used in healthcare facilities and consists of a full- or half-face mask, air-purifying respirator, chemical hooded resistant clothing, outer and inner chemical-resistant gloves, chemical-resistant boots, boot covers, and a face shield.
- Level D provides the least amount of protection and entails a work uniform, gloves, boots, safety glasses, and a face shield.
PPE should be made available in a variety of sizes consistent with staff needs. Well-fitting PPE is more effective for preventing contamination and is associated with better functional performance.[31][32][33] Respirators require a proper seal around the face and, therefore, require fit testing to ensure a proper fit.[34] In addition, healthcare professionals should conduct brief positive- and negative-pressure fit checks each time a respirator is put on. To conduct a fit check, the wearer should hold both hands in front of the respirator on their face and then quickly inhale and exhale through it. A properly fitting respirator with a good seal collapses on inhalation and has no leakage on exhalation.[35]
Single-use PPE must be discarded after each patient encounter, and all PPE should be replaced immediately if damaged or visibly soiled. Disposable PPE should never be reused, as it can serve as a vector for cross-contamination between patients. Reusable PPE, on the other hand, may be cleaned and reused according to the manufacturer's instructions. Federal, state, or local health agencies may recommend strategies to conserve the supply of PPEs, such as extended use, during supply shortages.
Key Principles:
- Work from clean to dirty.
- Clean: The inside and back of the PPE and any other areas of PPE not in contact with infectious organisms.
- Dirty: The outside front of the PPE and any surfaces in contact with potentially infectious materials or contaminated surfaces.
- Perform hand hygiene.
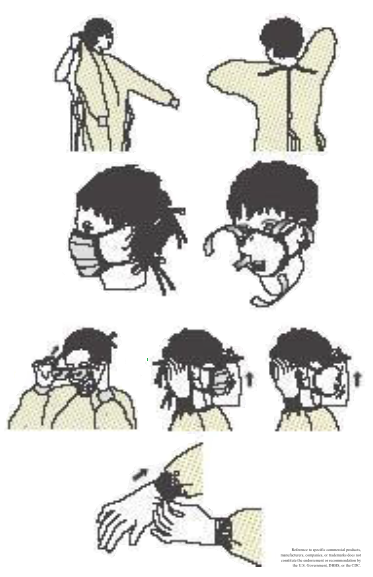
- Don PPE before entering the room in the following order:
- Gown
- Mask or respiratory
- Goggles or a face shield
- Gloves
- Continue the use of PPE during contact.
- Keep your hands away from your face.
- Limit surface touches.
- Change when heavily contaminated.
- When contact is completed, remove PPE at the doorway or immediately outside the room, taking care to avoid self-contamination. Discard PPE in the following order:
- Gloves
- Goggles or a face shield
- Gown
- Mask or respirator
- Perform hand hygiene immediately after removing PPE.
Steps for Donning and Doffing PPE:
- Gloves
- Locate the correct size.
- Insert your hand into the gloves over the isolation gown.
- Avoid touching yourself with contaminated gloves.
- Change gloves after each use.
- Remove at the doorway, before leaving the room, by peeling away from the hand, turning the gloves inside out without touching the contaminated area, and disposing of them properly. Never reuse.
- Aprons and gowns
- Choose the appropriate type and size based on the required level of isolation and protection.
- The opening is located at the back with secure ties at the neck and waist. Two gowns may be worn if one is too small (one in the front and one in the back); wear them front to back.
- Remove front to back, turning the contaminated region outside to the inside. Discard appropriately. Never reuse.
- Masks and respirators
- A mask or respirator should fit snugly over the nose and mouth.
- Place the mask over the nose, mouth, and chin with the nose piece of the bridge of the nose and secure the ties or elastic band.
- To remove, first grasp and break the bottom tie, then the top tie, and remove. Avoid touching the contaminated area, and discard appropriately. Never reuse.
- Goggles and face shields
- Goggles should fit snugly around the eyes; a face shield should cover the forehead and extend below the chin.
- Position over the eyes and face, and secure using earpieces or a headband, adjusting for a snug fit.
- Remove with a clean, ungloved hand by lifting away from the face. Avoid touching the contaminated area, and discard appropriately. Never reuse.
- Respiratory protection
- Select a fit-tested respirator and place it over the nose, mouth, and chin; secure and adjust it on the head; perform a fit-check.
- Remove by first lifting the bottom elastic off the head, followed by the top elastic, and discarding appropriately. Never reuse.
Element 5: Creation and Maintenance of a Safe Environment for Patient Care Through the Application of Infection Control Practices and Principles for Cleaning, Disinfection, and Sterilization
Healthcare professionals should understand the principles of infection control, including cleaning, disinfection, and sterilization. The following definitions provide a foundational understanding of the concepts.[36]
- Contamination: The presence of microorganisms on a surface.
- Cleaning: Scrubbing of visible soil and foreign materials from objects and surfaces using water, soaps, detergents, or enzymatic products.
- Disinfection: A process of using chemicals or wet pasteurization to eliminate pathogenic microorganisms.
- Low-level disinfectants destroy all vegetative bacteria but not bacterial spores and are classified as disinfectants.
- Intermediate-level disinfectants destroy all vegetative bacteria but not bacterial spores. These agents are classified as tuberculocidal.
- High-level disinfectants kill all organisms except bacterial spores and are approved as chemical germicide sterilants.
- Decontamination: A process of using chemicals or physical techniques to destroy, inactivate, or remove bloodborne pathogens on a surface.
- Sterilization: Chemical or physical destruction or removal of all pathogenic microorganisms and their spores from a surface. Effective sterilization techniques are essential, and failure could lead to significant morbidity and mortality. The most commonly employed techniques are as follows:
- Heat method: Heat is used to kill microbes.
- Wet heat/steam: Autoclaves use steam heated to 121 to 134 °C under pressure to kill or deactivate all microbes, bacterial spores, and viruses by hydrolysis and coagulation of cell proteins.
- Dry heat: Bacteria are exposed to high temperatures by flaming, incineration, or a hot air oven. This method is typically used for needles, scalpels, and scissors.
- Filtration: Filtering with a pore size that is too small for microbes to pass through is used to remove bacteria; however, it does not remove viruses or phages.
- Radiation: Exposing packed materials to radiation such as UV, X-rays, and gamma rays for sterilization. UV radiation is safer but has low penetration. X-rays are used for sterilizing large packages and pallet loads of medical devices. Gamma radiation has high penetration and is used to sterilize disposable medical equipment, such as syringes, needles, cannulas, and IV sets, as well as food.
- Chemical method: A reliable method to kill microbes, but it can damage the material to be sterilized.
- Gas: Gases such as ethylene oxide and carbon dioxide penetrate quickly into the material, similar to steam. However, they are expensive and may pose explosion risks.
Reprocessing Procedures: Health professionals should be aware of the OSHA and CDC guidelines, which utilize the Spaulding classification system, depending on the need for disinfection or sterilization, and categorize items as critical, semicritical, or noncritical.[27] Recommendations for cleaning, disinfection, and sterilization vary depending on several factors:
- The presence of lumens, hinges, or crevices that may be sources for internal or external contamination
- The material composition and design of the equipment
- The frequency of contact of the instrument with patient surfaces and operators' hands
- The types and numbers of microorganisms to which they are exposed
Pre-cleaning is the first step of reprocessing. This critical step prepares medical equipment, devices, and instruments for reprocessing by removing visible soil, debris, and liquids from internal and external surfaces. This step should be done as soon as possible after use, and the cleaning solutions should be changed regularly.
The choice of cleaning, disinfection, or sterilization method should be guided by the equipment's intended use and the manufacturer's instructions.
- Noncritical items come in contact with intact skin but not with mucous membranes. Common examples include crutches, a stethoscope, blood pressure cuffs, a toilet, a sink, and bedpans. Noncritical reusable items may be cleaned in the location where they are used rather than being transported to a central processing area. These items can be cleaned manually with detergent or in an automatic washer, or disinfected with a low-level disinfectant, such as sodium hypochlorite, improved hydrogen peroxide, iodophor disinfectants, phenolic disinfectants, quaternary ammonium compounds, or a combination of peracetic acid and hydrogen peroxide.[36] Environmental cleaning can be enhanced with no-touch room decontamination methods, such as hydrogen peroxide vapor or UV light.[37]
- Semicritical items come in contact with mucous membranes and nonintact skin. Common examples include oral thermometers, nasopharyngoscopes, and vaginal specula. Semicritical items require high-level chemical disinfectants that are cleared by the Food and Drug Administration, such as hydrogen peroxide, glutaraldehyde, ortho-phthalaldehyde, or peracetic acid with hydrogen peroxide. Some semicritical medical equipment may have challenges due to potential damage to the instrument. Staff responsible for disinfecting this equipment should be trained and competency tested in safe and proper reprocessing techniques.[36]
- Critical items directly or indirectly enter sterile tissue or the vascular system. Common examples include surgical instruments, duodenoscopes, injection needles, or cardiac catheters. These items must be sterile before coming in contact with tissue and should be cleaned to remove debris, followed by sterilization. Steam sterilization is preferred where possible; however, heat-sensitive materials may be sterilized using ethylene oxide, hydrogen peroxide gas plasma, vaporized hydrogen peroxide, hydrogen peroxide with ozone, or liquid chemical sterilants if other sterilizing methods are not possible.[36]
- Prions are unusually resistant to decontamination and sterilization procedures.[36] Consultation with an infection control specialist is essential for guidance on cleaning and disinfecting when prion disease, such as Creutzfeldt-Jakob disease, is confirmed or suspected.
The manufacturer's guidelines should always be referred to ensure that the selected reprocessing method is compatible with the equipment's materials and the recommended time, temperature, and pressure for reprocessing.
Reprocessing Effectiveness:
The effectiveness of reprocessing medical equipment, devices, and instruments depends on multiple factors:
- Prior cleaning and removal of organic matter and biofilms
- Selection and use of disinfectants and sterilization techniques
- Monitoring the activity and stability of disinfectants, contact time, and usage
- Proper handling, packaging, and storage post-disinfection or sterilization
All items must be cleaned to eliminate organic material before disinfection or sterilization. Disinfectants and steriliants must be applied for sufficient durations to achieve full effectiveness. The recommended contact time for low-level disinfectants is at least 1 minute. In contrast, the exposure time for most high-level disinfectants ranges from 8 to 45 minutes, and the temperature typically varies from 20 to 25 °C. Most liquid chemical sterilants have exposure times ranging from 3 to 12 hours, except for 0.2% peracetic acid, which has an exposure time of 12 minutes at 50 to 56 °C. In addition, these sterilants must be administered at the recommended concentration, pH, and temperature.[36]
Biofilms, when present, can also interfere with the effectiveness of disinfectants. Although biofilms have traditionally been associated with wet or damp surfaces, such as endoscopes and tubing, they have also been reported to occur on dry surfaces. Microbes in biofilms are resistant to many detergents and disinfectants.[38] Sodium hypochlorite and steam treatment are more effective against biofilms; however, some microbes may remain viable even after these techniques are utilized.[39][40]
Therefore, staff responsible for reprocessing should closely monitor reprocessing equipment and materials to ensure the following: [41]
- The efficacy of manual and mechanical cleaning processes
- The quality of water used in cleaning
- The activity and stability of disinfectants and liquid chemical sterilants
- The correct functioning of the equipment involved in reprocessing
- Adequate contact time, temperature, concentration, and pH during disinfection and sterilization, as recommended
- The removal of any recalled or expired chemicals or equipment
- Proper handling and storage of medical equipment after reprocessing
Where possible, staff should use objective measures of reprocessing efficacy, such as rapid cleaning monitors, biological and chemical monitors, and indicator strips or tape.
Potential Sources of Contamination and Cross-contamination: Breaks in infection control practices at any step in reprocessing, storage, or handling can undermine the disinfection or sterility of medical instruments, devices, or equipment. Specific factors that are known to contribute to contamination and cross-contamination include:
- Reuse of single-use or disposable equipment
- Failure to maintain hand hygiene
- Failure to clean surfaces between patients
- Failure to reprocess equipment between patients
- Inadequate cleaning, disinfection, or sterilization
- Contamination of detergent, disinfectant, or rinse solutions
- Improper storage and handling of reprocessed medical equipment
- Failure to monitor reprocessing efficacy
Expectations of Healthcare Professionals: Healthcare professionals working in settings where cleaning and reprocessing of medical equipment is performed separately from patient care areas should understand Standard and Universal Precautions and the core concepts and principles of cleaning, disinfection, and sterilization, and follow recommended safe practices for handling sterile instruments, equipment, and devices in medical settings. These professionals should also verify and follow their local processes for handling and preparing used equipment before submission for reprocessing, such as pre-cleaning or soaking.
Healthcare professionals with primary or supervisory responsibility for reprocessing should know and follow recommended procedures and select and use appropriate reprocessing methods and materials for medical instruments, equipment, and devices, based on their intended use and manufacturer recommendations. Key factors to consider include:
- Antimicrobial efficacy
- Ease of use
- Stability
- Compatibility with items to be reprocessed, including factors such as heat tolerance, moisture sensitivity, corrosiveness, leeching, penetrability, and disintegration
- Potential for patient, occupational, or environmental toxicity
- Odor
- Time constraints and requirements for the selected methods
- Costs
- Monitoring requirements and recommendations
Element 6: Prevention and Management of Infectious or Communicable Diseases in Healthcare Professionals
Healthcare professionals are at particular risk of acquiring institutionally related infections such as HBV, HIV, influenza, measles, mumps, rubella, and varicella. Whenever possible, nonimmune healthcare professionals should not be assigned to care for patients with confirmed or suspected infection with vaccine-preventable diseases.[14] New York State and OSHA have several requirements regarding the vaccination of healthcare professionals, in addition to the CDC vaccine recommendations. These requirements and recommendations are summarized in the table below.
Table 1. Requirements and Recommendations of Vaccinations for Healthcare Professionals
| Pathogen | Requirement | Recommendation | Notes |
| Measles | Healthcare professionals at regulated facilities, including but not limited to hospitals, nursing homes, hospices, home care, and ambulatory surgery centers, must demonstrate immunity unless they have a medical exemption (New York State requirement) | 2 doses, or other evidence of immunity, are recommended for all healthcare professionals |
N.Y. Comp. Codes R. & Regs. tit. 10, §§ 405.3, 415.26, 751.6(d), 763.13, 766.11, 794.3, [42] |
| Mumps | N/A | 2 doses, or other evidence of immunity, are recommended for all healthcare professionals | [42] |
| Rubella | Healthcare professionals at regulated facilities, including but not limited to hospitals, nursing homes, hospices, home care, and ambulatory surgery centers, must demonstrate immunity unless they have a medical exemption (New York State requirement) | At least 1 dose, or other evidence of immunity, is recommended for all healthcare professionals | N.Y. Comp. Codes R. & Regs. tit. 10, §§ 405.3, 415.26, 751.6(d), 763.13, 766.11, 794.3, [42] |
| Hepatitis B | Hospitals and other employers of public employees must offer and make available the complete hepatitis B vaccine series to employees at risk of occupational exposure to blood and other body fluids (OSHA guidelines) | A complete 3-dose series, or other evidence of immunity, is recommended for all healthcare professionals | 29 CFR § 1910.1030, [42] |
| Influenza | Healthcare professionals at regulated facilities, including but not limited to hospitals, nursing homes, hospices, home care, and ambulatory surgery centers, must either provide documentation of influenza vaccination during the current season or wear a surgical or procedure mask at all times while in areas where patients or residents may be present during periods when the Commissioner of Health determines that the influenza season is underway | Annual vaccination with the current season's vaccine is recommended for all healthcare professionals | N.Y. Comp. Codes R. & Regs. tit. 10, § 2.59, [42] |
| Varicella | N/A | 2 doses, or other evidence of immunity, are recommended for all healthcare professionals | [42] |
| Tdap | N/A | 1 dose of Tdap is recommended for any healthcare professional without documentation of previous Tdap vaccination, followed by booster doses of either Tdap or Td every 10 years | [42] [43] |
Abbreviations: Tdap, Tetanus, diphtheria, and acellular pertussis; OSHA, Occupational Safety and Health Administration.
Regulated facilities in New York State must provide health status assessments to new healthcare professionals and conduct them annually (or more frequently if necessary) to determine whether they have a health impairment that could pose a potential risk to patients. This health status assessment must include a screening for tuberculosis exposure, infection, or disease, and any prior tuberculosis testing or treatment. Healthcare professionals should receive baseline interferon-gamma release assays (IGRAs) or tuberculin skin tests (TSTs), and repeat IGRA or TST as indicated by screenings or known exposures to tuberculosis. Annual IGRA or TST screening of asymptomatic low-risk healthcare professionals is no longer required in New York State, although individual facilities may set more restrictive policies. Positive results on IGRA or TST tests should be followed up with chest X-rays and other clinical evaluations, and treatment of latent tuberculosis infection, where applicable.[N.Y. Comp. Codes R. & Regs. tit. 10, §§ 404.12, 405.3, 415.26, 751.6, 763.13, 766.11, 794.3, 1001.11]
All healthcare professionals should promptly report all signs and symptoms of personal illness to their supervisor, including but not limited to fever, cough, rash, vesicles, nausea, vomiting, diarrhea, and draining wounds. These professionals should be evaluated by a licensed medical professional and assessed for post-exposure prophylaxis (if applicable), temporary work restrictions, furlough, or clearance for return to work.[N.Y. Comp. Codes R. & Regs. tit. 10, §§ 405.3, 415.26, 751.6, 763.13, 766.11, 794.3]
Healthcare professionals must be properly trained in avoiding needlestick and sharp injuries, as percutaneous exposure is a common cause of HIV, HBV, and HCV transmission. Education for healthcare professionals on bloodborne pathogens should include information on HBV, HCV, and HIV diseases; HBV vaccine recommendations; hand hygiene; Standard and Universal Precautions; appropriate PPE and barrier precautions; and sharps safety.
If a healthcare worker is exposed to bodily fluids, the affected area should be washed immediately with soap and water. If a mucous membrane is exposed, it should be flushed with water immediately. Healthcare professionals should be educated to report occupational exposures, as treatment is more effective when administered immediately after the exposure.
Healthcare professionals exposed to blood or other potentially infectious material should be promptly evaluated by a licensed medical professional. The evaluation should include a risk assessment and consideration of testing the source-patient if their HIV, HBV, and HCV statuses are unknown. In addition, healthcare professionals have a professional obligation to notify any patients exposed to their blood or other potentially infectious materials. Any exposure should be recorded in the healthcare worker's medical records.
Post-exposure prophylaxis should be administered promptly following current New York State and CDC guidelines. Healthcare facilities should ensure 24/7 access to a designated post-exposure prophylaxis provider available for education and administration of hepatitis B immunoglobulin, HBV vaccine, and HIV antiretroviral agents. These facilities should collaborate with their local or state health department to administer post-exposure prophylaxis for measles, mumps, rubella, pertussis, or varicella to nonimmune individuals exposed to these diseases and to notify the public when necessary.[44]
The United States Public Health Services no longer recommends different HIV post-exposure prophylaxis regimens depending on the severity of exposure. Instead, all individuals with occupational exposures to HIV should receive a regimen consisting of at least 3 antiretroviral agents, such as a dual nucleoside reverse transcriptase inhibitor and an integrase strand transfer inhibitor combined with either a protease inhibitor (boosted with ritonavir) or a non-nucleoside reverse transcriptase inhibitor.[Centers for Disease Control and Prevention. Updated U.S. Public Health Service guidelines for the management of occupational exposures to HIV and recommendations for postexposure prophylaxis]
Treatment for healthcare personnel potentially exposed to HBV depends on the HBV vaccination and serologic status of the exposed individual, as well as the HBV status of the source patient.[45]
Table 2. Post-Exposure Treatment of Healthcare Personnel Based on Hepatitis B Vaccination and Serologic Status
| Healthcare Personnel Status | Post-exposure testing results for the source patient (HBsAg) | Post-exposure testing results for healthcare personnel (anti-HBs) | HBIG post-exposure prophylaxis | HBV vaccination post-exposure prophylaxis | Post-vaccination serologic testing |
| Documented responder after complete HBV vaccine series | No action needed | No action needed | No action needed | No action needed | No action needed |
| Documented nonresponder after 2 complete HBV vaccine series | Positive or unknown | No action needed | 2 doses of HBIG separated by 1 month | No action needed | No action needed |
| Negative | No action needed | No action needed | No action needed | No action needed | |
| Response unknown after 1 complete HBV vaccine series | Positive or unknown | <10 mIU/mL | 1 dose of HBIG | Initiate revaccination | Yes |
| ≥10 mIU/mL | No action needed | No action needed | No action needed | ||
| Negative | <10 mIU/mL | No action needed | Initiate revaccination | Yes | |
| ≥10 mIU/mL | No action needed | No action needed | No action needed | ||
| Unvaccinated or incompletely vaccinated | Positive or unknown | No action needed | 1 dose of HBIG | Complete HBV vaccine series | Yes |
| Negative | No action needed | No action needed | Complete HBV vaccine series | Yes |
Abbreviations: HBV, hepatitis B; HBIG, hepatitis B immunoglobulin.
Any healthcare personnel infected with HIV, HBV, HCV, or any other bloodborne pathogen should be evaluated by healthcare personnel for their risk of occupational transmission. The evaluation should include an assessment of their professional practice and assigned duties, techniques within their scope of practice that may pose a risk of transmission to patients, their compliance with infection control standards, the presence of open or draining wounds or weeping dermatitis, and their overall physical and cognitive health.
Element 7: Sepsis Awareness and Education
Sepsis is a life-threatening medical emergency that arises when the body's dysregulated response to an infection causes injury to its tissues and organs. Due to its significant morbidity and mortality and increased incidence in institutional settings, all healthcare professionals should be familiar with the signs, symptoms, and treatment of sepsis. Sepsis is most commonly triggered by bacterial infections, but it can also be triggered by viral, fungal, and other microbial infections. Sepsis and its sequelae represent a continuum of clinical and pathophysiologic severity, resulting in progressive physiologic failure of several interdependent organ systems.[46]
Septic shock is a subset of sepsis in which underlying circulation and cellular abnormalities are severe enough to increase the risk of mortality. Sepsis progresses to septic shock and multi-organ failure due to a worsening circulatory insufficiency characterized by hypovolemia, myocardial depression, increased metabolic demands, and vasoregulatory perfusion abnormalities. The typical hemodynamic pattern in septic shock consists of low cardiac filling pressures or low central venous pressures, and low systemic vascular resistance. With low preload and afterload, cardiac output must increase to compensate, typically with increased heart rate.[46] Please see StatPearls' companion resources, "Bacterial Sepsis" and "Septic Shock," for further information.
Sepsis is associated with high morbidity and mortality and has recently been identified as affecting nearly 1.7 million adults in the United States every year. Before 2000, mortality was noted to be as high as 50% in severe sepsis and patients with septic shock.[47][48] Despite advancements in technology and treatment, the mortality rate remains between 15% and 25% nationwide, with 1 in 3 patients who die in the hospital succumbing to sepsis, accounting for $23.7 billion in health expenditures in 2013.[49][50][51]
Nearly 9 in 10 cases of sepsis had community onset, but over 70% had a recent healthcare encounter or had chronic conditions that are typically associated with frequent healthcare encounters, suggesting that a high proportion of community-acquired sepsis may have been acquired in healthcare settings.[49][51] The most common infections leading to sepsis are pneumonia, urinary tract infections, gastrointestinal infections, and skin or soft tissue infections.[49] The comorbidities most commonly occurring in patients with sepsis are diabetes mellitus, chronic pulmonary disease, renal disease, congestive heart failure, and cancer.[51] Infants, very young children, and individuals older than 65 are the age groups at highest risk of sepsis.[52][53][52] Tracheostomy, mechanical ventilation, anticoagulant use, transfusion of red blood cells, and indwelling urinary or central venous catheters are all risk factors for ICU-acquired sepsis.[54]
In 2014, New York State launched its Sepsis Care Improvement Initiative to increase the early detection and timely treatment of suspected sepsis and septic shock, and to improve sepsis outcomes. Rory's Regulations, initially adopted in 2013 and revised in 2018, require New York State hospitals to adopt, implement, and periodically update protocols for early recognition and treatment of sepsis and septic shock. These protocols must contain, at a minimum:
- A process for screening and early recognition of patients with sepsis and septic shock.
- A process to rapidly identify and document individuals appropriate for treatment of sepsis and septic shock, including explicit criteria defining certain patients who should be excluded from the protocols, such as patients who are receiving palliative care.
- Guidelines for hemodynamic support, including monitoring, therapeutic endpoints, and time frame goals
- For infants and children, guidelines for fluid resuscitation with explicit time frames for vascular access and fluid delivery consistent with current, evidence-based guidelines for severe sepsis and septic shock with defined therapeutic goals for children.
- A procedure for identifying the source of infection and delivering early antibiotics with specific time frame goals.[N.Y. Comp. Codes R. & Regs. tit. 10, §§ 405.2 and 405.4]
The New York State Department of Health estimates that the Sepsis Care Improvement Initiative saved more than 16,000 lives between 2015 and 2019.[New York State Department of Health. NYS Sepsis Care Improvement Initiative and NYS Regulations]
Diagnosis and Treatment: Early recognition and treatment of sepsis are critical for improving clinical outcomes.[47] Signs and symptoms of sepsis and septic shock vary widely depending on the type of infection and host factors, and may range from subtle manifestations to florid shock. However, common symptoms and signs of sepsis include:
- Altered mental status
- Fever
- Hypotension
- Tachycardia
- Sweaty or clammy skin
- Shortness of breath
- Severe pain or discomfort
However, older adults, patients with diabetes mellitus, and individuals taking beta blockers may have atypical symptoms, including suppression of tachycardia despite dropping blood pressure.[55] Infants and newborns with sepsis can also have atypical clinical characteristics, including apnea, bradycardia, periodic breathing, listlessness, poor feeding, unstable body temperatures, and grey skin.[56][57][58] Therefore, healthcare providers should maintain a high index of suspicion for sepsis among patients with known or suspected infections, even when they present atypically.
Multiple clinical scoring systems have been developed to better predict which adults are at risk of sepsis and may experience worse outcomes. However, there is a wide variation in the sensitivity and specificity of these tools, and no gold standard exists for diagnosing sepsis in adults.[59] Elevated serum lactate levels are associated with a higher risk of mortality in patients with suspected sepsis. Some studies have suggested an association between point-of-care lactate testing of patients presenting with infections and lower mortality from sepsis; however, results have been inconsistent.[60] Lactate testing alone is neither sensitive nor specific enough to either confirm or rule out a diagnosis of sepsis, but it may be used as an adjunct to other diagnostic tests.[59]
In contrast, the Society of Critical Care Medicine recommends that healthcare providers use the Phoenix Sepsis Score to assess infants and children for sepsis. This tool assesses respiratory, cardiovascular, coagulation, and neurologic function. Children with a Phoenix Sepsis Score of 2 or higher meet the criteria for sepsis, whereas those with a score of at least 2, including at least 1 point on the cardiovascular scale, meet the criteria for septic shock.[52]
Healthcare providers should initiate fluid resuscitation with at least 30 mL/kg of intravenous crystalloid fluid as soon as possible after recognition of shock or sepsis shock, even if shock is only suspected.[59] A retrospective cohort study of adults treated for severe sepsis or septic shock in an emergency department found that failure to receive 30 mL/kg of crystalloid fluid within 3 hours of presentation was associated with higher mortality rates and longer lengths of ICU stay.[61] Continued fluid administration after the initial bolus should be guided by a careful assessment of organ perfusion and intravascular volume to balance resuscitation needs with the risks of fluid accumulation.[59] Please see StatPearls' companion resource, "Bacterial Sepsis," for further information.
Clinicians attending to people with sepsis should assess their patients for potential foci of infection and implement rapid source control, such as removal of potentially contaminated devices, abscess drainage, or debridement of necrotic tissue.[59] Timely source control of patients with sepsis is associated with reduced mortality.[62][63] Some experts recommend source control within 6 to 12 hours of presentation or onset; however, research findings on the optimal timing of source control have not been consistent. However, prompt identification and source control should be prioritized.[59]
Healthcare providers treating patients with suspected sepsis or septic shock should obtain a full panel of microbiological cultures to identify the causative agent and antimicrobial sensitivity. In addition, they should consider noninfectious causes and regularly re-evaluate and consider alternative diagnoses throughout the treatment course, particularly if the patient's clinical course is atypical for sepsis. More than 1 in 3 patients initially diagnosed with sepsis are ultimately diagnosed with noninfectious causes.[59]
Intravenous broad-spectrum antimicrobial therapy should be started as soon as possible after diagnosing severe sepsis or septic shock, ideally within 1 hour of recognition. Delays in initiating antibiotic therapy have been associated with increased mortality. Patients with suspected sepsis but not shock should be rapidly evaluated for both infectious and noninfectious causes, and broad-spectrum antibiotics should be initiated, ideally within 3 hours of presentation, if concern for infection persists after investigation. Once a causative pathogen has been detected and its sensitivities assessed, the treating healthcare provider should de-escalate antimicrobial treatment by discontinuing any antimicrobials that are no longer necessary or switching from broad-spectrum to narrower-spectrum antimicrobials where appropriate.[59]
Patient Education and Prevention: Patients and their caregivers should be educated on measures to reduce the risk of sepsis, including:
- Hand hygiene
- Wound care
- Vaccination against Neisseria meningitidis, Streptococcus pneumoniae, and Haemophilus influenzae type B
- Seeking emergent medical care if they or their children have infections that worsen despite treatment, high fevers that do not respond to antipyretics, altered mental status, or difficulty breathing.
Clinical Significance
Health care–acquired infections are a significant cause of morbidity and mortality, costing billions of dollars annually. The most common health care–associated infections include urinary tract infections, surgical site infections, pneumonia, and bloodstream infections. The following table describes the most common healthcare-acquired pathogens, according to the CDC.[CDC. Healthcare-Associated Infections (HAIs)] Health professionals should be aware of each disease and how to avoid its spread.
Table 3. Common Pathogens Associated with Health care–Associated Infections
| Pathogen | Reservoir | Sites of Infection | Risk Factors | Transmission | Risk Reduction | Treatment | Other Considerations |
| Acinetobacter [64] | Soil and water | Blood, lungs, urinary tract, and wounds | Immunocompromise, indwelling catheters, open wounds, ventilator use, and prolonged hospital stay | Contact with contaminated surfaces or equipment and person-to-person contact, particularly through contaminated hands | Hand hygiene, disinfection of medical devices, and rigorous environmental cleaning | Antibiotic treatment should be selected after culture and sensitivity reports are obtained. Often carbapenem-resistant and usually multidrug-resistant. | Rarely occur outside of healthcare settings, but can cause thousands of infections in hospitalized patients and hundreds of deaths |
| Burkholderia cepacia [65] | Soil and water | Blood, lungs, and urinary tract | Immunocompromise, chronic lung disease, and especially cystic fibrosis | Contact with contaminated medicines, contaminated medical devices, and person-to-person contact | Hand hygiene, particularly before and after contact with wounds or medical devices, and rigorous cleaning of healthcare settings | Treatment decisions should be made on a case-by-case basis. May be resistant to most antibiotics. | Burkholderia cepacia complex poses a minimal medical risk to healthy individuals |
| Clostridium sordellii [66][67] | Soil | Joints, blood, peritoneum, endocardium, lungs, and muscle tissue | Women are at the highest risk of infection following the end of pregnancy | Currently unknown | Antibiotic prophylaxis before medical abortion is the only known preventive measure | Supportive measures such as IV fluids. Typically is susceptible to beta-lactam, clindamycin, chloramphenicol, and tetracycline, but resistant to aminoglycosides and sulfonamides. | May cause severe toxic shock syndrome |
| Carbapenem-resistant Enterobacteriaceae [68] | Human and animal gastrointestinal systems | Blood, lungs, urinary tract, wounds, and meninges | Urinary catheters, urinary retention, mechanical ventilation, intravenous catheters, prolonged antibiotic use, immunocompromise, high Charlson's comorbidity index, frequent hospitalization, and ICU admission | Person-to-person contact and contaminated medical equipment | Private rooms and dedicated equipment for patients with infection and colonization, contact precautions, hand hygiene, and avoidance of long-term urinary catheters | Treatment decisions should be made on a case-by-case basis after review of sensitivity reports. Often multidrug resistant. Individuals with colonization rarely need treatment. | Frequently fatal |
| ESBL-producing Enterobacteriaceae [68][69] | Human gastrointestinal tract, skin, food, water, and soil | Urinary tract and blood | Hospital or long-term care stay, travel to tropical and subtropical regions, including Asia and Southeast Asia | Person-to-person contact, contaminated surfaces, and contaminated food or water | Frequent hand hygiene and safe food and water practices while traveling abroad | Hospitalization with IV carbapenem antibiotics | ESBLs break down and destroy penicillins and cephalosporins, rendering these antibiotics ineffective |
| Gram-negative bacteria [70] | Widely dispersed in nature | Gastrointestinal system, nervous system, urinary tract, blood, wounds, and surgical sites | Immunocompromise, antimicrobial overuse, and hospital or long-term care stay | Person-to-person contact or exposure to contaminated surfaces or equipment | Hand hygiene | Treatment decisions should be made on a case-by-case basis after review of sensitivity reports. Can be multidrug resistant. | Significant worldwide public health concern due to high rates of antibiotic resistance, morbidity, and mortality |
| Klebsiella [71] | Human gastrointestinal tract | Blood, lungs, meninges, surgical sites, and wounds | Ventilator use, intravenous catheters, and prolonged antibiotic use | Person-to-person contact, contaminated water or soil, and contaminated medical equipment | Hand hygiene and contact precautions | Treatment decisions should be made on a case-by-case basis after review of sensitivity reports. Can be multidrug resistant. | Rarely causes infection in healthy individuals |
| Staphylococcus aureus [72][73][72] | Human nose and skin | Blood, endocardium, bone, lungs, and skin | Chronic medical conditions, IV drug use, immunocompromise, ICU stay, and inserted medical devices | Person-to-person contact and exposure to contaminated surfaces or equipment | Hand hygiene and hospital decontamination measures | Depends largely on the type of infection and the presence or absence of drug-resistant strains, but penicillin is the drug of choice for sensitive isolates | Carried by approximately 30% of individuals |
| MRSA [74] | Human nose and skin | Blood, endocardium, bone, lungs, and skin | Prolonged hospitalization, ICU admission, long-term care stay, recent antibiotic use, open wounds, hemodialysis, central venous access, long-term indwelling urinary catheter placement, and IV drug use | Skin-to-skin contact and exposure to contaminated surfaces or equipment | Hand hygiene, contact precautions, and private rooms for individuals with infection or colonization | Depends on the type of disease, local Staphylococcus aureus resistance patterns, availability of the drug, adverse effect profile, and individual patient profile, but vancomycin is the drug of choice | Common cause of both hospital- and community-acquired infections with significant morbidity, mortality, length of stay, and cost burden |
| Nontuberculous mycobacteria [75] | Soil, dust, and water | Lungs, soft tissue, lymph nodes, blood, surgical sites, and medical devices | Chronic lung disease and immunocompromise | Exposure to contaminated water or equipment | Water management, hand hygiene, and contact precautions | Typically requires a combination of 2 to 3 antimicrobial agents for 6-12 months | Also referred to as atypical mycobacteria, MOTT, or environmental mycobacteria |
| Pseudomonas aeruginosa [76] | Soil and water | Blood, lungs, and wounds | Hospitalization, ventilator use, indwelling catheters, open wounds, and burn victims | Person-to-person contact, exposure to contaminated surfaces or equipment, and exposure to contaminated water | Hand hygiene, daily room cleaning, and water management | Guided by culture and sensitivity analysis. Treatment options are limited for multidrug-resistant strains. | A frequent cause of morbidity and mortality in the hospital |
Abbreviations: IV, intravenous; ESBL, extended-spectrum beta-lactamase; MRSA, methicillin-resistant Staphylococcus aureus; MOTT, mycobacteria other than tuberculosis.
Enhancing Healthcare Team Outcomes
All healthcare professionals share the responsibility of preventing infection and maintaining an aseptic environment when possible. Over 100 years of data show improved patient outcomes, regardless of the disease process, when healthcare professionals collaborate as an interprofessional team.[77]
The prevention and management of infectious diseases involve nearly every aspect of health care. With the implementation of bundled care, communication between clinicians, nursing staff, respiratory therapists, and pharmacists becomes increasingly essential, as these interventions must be implemented swiftly and accurately. Delayed identification of infectious diseases increases morbidity and mortality, emphasising the importance of prevention. Nursing is on the front lines of this issue, as they routinely have the highest level of contact with patients and have access to all aspects of the facility. Their observations and recommendations should be taken seriously by all members of the interprofessional healthcare team.
Multiple curricula and recommendations have been proposed for addressing interprofessional team development in the prevention and treatment of infectious diseases.[78] Developing a successful strategy for preventing, managing, and treating infectious diseases requires an interprofessional team approach, leading to improved outcomes.
Media
(Click Image to Enlarge)
(Click Image to Enlarge)
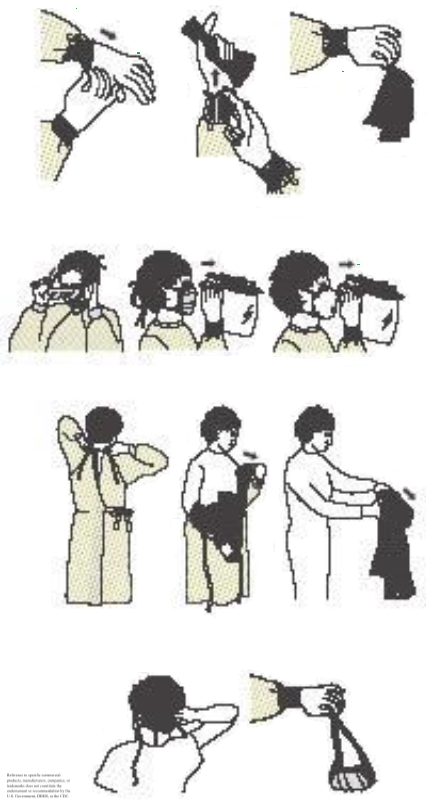
Proper Removal of Personal Protective Equipment. The schematic demonstrates the step-by-step process of removing personal protective equipment (PPE), which includes removing gloves, gown, eye protection, mask, and performing hand hygiene. Each step should be performed slowly and carefully, avoiding unnecessary contact with contaminated surfaces.
References
Forder AA. A brief history of infection control - past and present. South African medical journal = Suid-Afrikaanse tydskrif vir geneeskunde. 2007 Nov:97(11 Pt 3):1161-4 [PubMed PMID: 18250929]
Melker RJ. The Institute of Medicine report on medical errors. The New England journal of medicine. 2000 Aug 31:343(9):664-5 [PubMed PMID: 10979813]
Level 3 (low-level) evidence. JCAHO approves National Patient Safety Goals for 2003. Joint Commission perspectives. Joint Commission on Accreditation of Healthcare Organizations. 2002 Sep:22(9):1-3 [PubMed PMID: 12233144]
Level 3 (low-level) evidenceBerenholtz SM, Pronovost PJ, Lipsett PA, Hobson D, Earsing K, Farley JE, Milanovich S, Garrett-Mayer E, Winters BD, Rubin HR, Dorman T, Perl TM. Eliminating catheter-related bloodstream infections in the intensive care unit. Critical care medicine. 2004 Oct:32(10):2014-20 [PubMed PMID: 15483409]
Level 2 (mid-level) evidencePronovost P, Needham D, Berenholtz S, Sinopoli D, Chu H, Cosgrove S, Sexton B, Hyzy R, Welsh R, Roth G, Bander J, Kepros J, Goeschel C. An intervention to decrease catheter-related bloodstream infections in the ICU. The New England journal of medicine. 2006 Dec 28:355(26):2725-32 [PubMed PMID: 17192537]
Level 2 (mid-level) evidenceEdmond M, Eickhoff TC. Who is steering the ship? External influences on infection control programs. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2008 Jun 1:46(11):1746-50. doi: 10.1086/587987. Epub [PubMed PMID: 18419420]
Habboush Y, Yarrarapu SNS, Guzman N. Infection Control. StatPearls. 2025 Jan:(): [PubMed PMID: 30085559]
Pritchard V. Joint Commission standards for long-term care infection control: putting together the process element. Joint Commission on Accreditation of Healthcare Organizations. American journal of infection control. 1999 Feb:27(1):27-34 [PubMed PMID: 9949375]
Popovich ML. The Joint Commission's home care standards for infection control. Home care provider. 1999 Feb:4(1):40-1 [PubMed PMID: 10222937]
Klompas M, Branson R, Cawcutt K, Crist M, Eichenwald EC, Greene LR, Lee G, Maragakis LL, Powell K, Priebe GP, Speck K, Yokoe DS, Berenholtz SM. Strategies to prevent ventilator-associated pneumonia, ventilator-associated events, and nonventilator hospital-acquired pneumonia in acute-care hospitals: 2022 Update. Infection control and hospital epidemiology. 2022 Jun:43(6):687-713. doi: 10.1017/ice.2022.88. Epub 2022 May 20 [PubMed PMID: 35589091]
Calderwood MS, Anderson DJ, Bratzler DW, Dellinger EP, Garcia-Houchins S, Maragakis LL, Nyquist AC, Perkins KM, Preas MA, Saiman L, Schaffzin JK, Schweizer M, Yokoe DS, Kaye KS. Strategies to prevent surgical site infections in acute-care hospitals: 2022 Update. Infection control and hospital epidemiology. 2023 May:44(5):695-720. doi: 10.1017/ice.2023.67. Epub 2023 May 4 [PubMed PMID: 37137483]
Patel PK, Advani SD, Kofman AD, Lo E, Maragakis LL, Pegues DA, Pettis AM, Saint S, Trautner B, Yokoe DS, Meddings J. Strategies to prevent catheter-associated urinary tract infections in acute-care hospitals: 2022 Update. Infection control and hospital epidemiology. 2023 Aug:44(8):1209-1231. doi: 10.1017/ice.2023.137. Epub 2023 Aug 25 [PubMed PMID: 37620117]
Manchikanti L, Falco FJ, Benyamin RM, Caraway DL, Helm Ii S, Wargo BW, Hansen H, Parr AT, Singh V, Hirsch JA. Assessment of infection control practices for interventional techniques: a best evidence synthesis of safe injection practices and use of single-dose medication vials. Pain physician. 2012 Sep-Oct:15(5):E573-614 [PubMed PMID: 22996856]
Siegel JD, Rhinehart E, Jackson M, Chiarello L, Health Care Infection Control Practices Advisory Committee. 2007 Guideline for Isolation Precautions: Preventing Transmission of Infectious Agents in Health Care Settings. American journal of infection control. 2007 Dec:35(10 Suppl 2):S65-164 [PubMed PMID: 18068815]
Level 1 (high-level) evidenceYatim KM, Lakkis FG. A brief journey through the immune system. Clinical journal of the American Society of Nephrology : CJASN. 2015 Jul 7:10(7):1274-81. doi: 10.2215/CJN.10031014. Epub 2015 Apr 6 [PubMed PMID: 25845377]
Boyce JM, Pittet D, Healthcare Infection Control Practices Advisory Committee, HICPAC/SHEA/APIC/IDSA Hand Hygiene Task Force. Guideline for Hand Hygiene in Health-Care Settings. Recommendations of the Healthcare Infection Control Practices Advisory Committee and the HICPAC/SHEA/APIC/IDSA Hand Hygiene Task Force. Society for Healthcare Epidemiology of America/Association for Professionals in Infection Control/Infectious Diseases Society of America. MMWR. Recommendations and reports : Morbidity and mortality weekly report. Recommendations and reports. 2002 Oct 25:51(RR-16):1-45, quiz CE1-4 [PubMed PMID: 12418624]
Level 1 (high-level) evidence. WHO Best Practices for Injections and Related Procedures Toolkit. 2010 Mar:(): [PubMed PMID: 23741781]
Schaefer MK, Perkins KM, Perz JF. Patient Notification Events Due to Syringe Reuse and Mishandling of Injectable Medications by Health Care Personnel-United States, 2012-2018: Summary and Recommended Actions for Prevention and Response. Mayo Clinic proceedings. 2020 Feb:95(2):243-254. doi: 10.1016/j.mayocp.2019.08.024. Epub 2019 Dec 26 [PubMed PMID: 31883694]
Tomkins SE, Elford J, Nichols T, Aston J, Cliffe SJ, Roy K, Grime P, Ncube FM. Occupational transmission of hepatitis C in healthcare workers and factors associated with seroconversion: UK surveillance data. Journal of viral hepatitis. 2012 Mar:19(3):199-204. doi: 10.1111/j.1365-2893.2011.01543.x. Epub 2011 Oct 19 [PubMed PMID: 22329374]
Ottino MC, Argentero A, Argentero PA, Garzaro G, Zotti CM. Needlestick prevention devices: data from hospital surveillance in Piedmont, Italy-comprehensive analysis on needlestick injuries between healthcare workers after the introduction of safety devices. BMJ open. 2019 Nov 19:9(11):e030576. doi: 10.1136/bmjopen-2019-030576. Epub 2019 Nov 19 [PubMed PMID: 31748292]
Assanasen S, Edmond M, Bearman G. Impact of 2 different levels of performance feedback on compliance with infection control process measures in 2 intensive care units. American journal of infection control. 2008 Aug:36(6):407-13. doi: 10.1016/j.ajic.2007.08.008. Epub [PubMed PMID: 18675146]
Dudeck MA, Horan TC, Peterson KD, Allen-Bridson K, Morrell G, Pollock DA, Edwards JR. National Healthcare Safety Network (NHSN) Report, data summary for 2010, device-associated module. American journal of infection control. 2011 Dec:39(10):798-816. doi: 10.1016/j.ajic.2011.10.001. Epub [PubMed PMID: 22133532]
. WHO Guideline on the Use of Safety-Engineered Syringes for Intramuscular, Intradermal and Subcutaneous Injections in Health Care Settings. 2016:(): [PubMed PMID: 27748094]
Kasteler SD, Reid M, Lee PC, Sparer-Fine E, Laramie AK. Sharps Injuries Among Medical Trainees and Attending Physicians. Academic medicine : journal of the Association of American Medical Colleges. 2023 Jul 1:98(7):805-812. doi: 10.1097/ACM.0000000000005187. Epub 2023 Jun 23 [PubMed PMID: 36812071]
Schuurmans J, Lutgens SP, Groen L, Schneeberger PM. Do safety engineered devices reduce needlestick injuries? The Journal of hospital infection. 2018 Sep:100(1):99-104. doi: 10.1016/j.jhin.2018.04.026. Epub 2018 May 5 [PubMed PMID: 29738783]
Lotfinejad N, Peters A, Tartari E, Fankhauser-Rodriguez C, Pires D, Pittet D. Hand hygiene in health care: 20 years of ongoing advances and perspectives. The Lancet. Infectious diseases. 2021 Aug:21(8):e209-e221. doi: 10.1016/S1473-3099(21)00383-2. Epub [PubMed PMID: 34331890]
Level 3 (low-level) evidenceSehulster L, Chinn RY, CDC, HICPAC. Guidelines for environmental infection control in health-care facilities. Recommendations of CDC and the Healthcare Infection Control Practices Advisory Committee (HICPAC). MMWR. Recommendations and reports : Morbidity and mortality weekly report. Recommendations and reports. 2003 Jun 6:52(RR-10):1-42 [PubMed PMID: 12836624]
Level 3 (low-level) evidencePolk RE, Hohmann SF, Medvedev S, Ibrahim O. Benchmarking risk-adjusted adult antibacterial drug use in 70 US academic medical center hospitals. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2011 Dec:53(11):1100-10. doi: 10.1093/cid/cir672. Epub 2011 Oct 13 [PubMed PMID: 21998281]
MacDougall C, Polk RE. Variability in rates of use of antibacterials among 130 US hospitals and risk-adjustment models for interhospital comparison. Infection control and hospital epidemiology. 2008 Mar:29(3):203-11. doi: 10.1086/528810. Epub [PubMed PMID: 18257689]
Level 2 (mid-level) evidenceHolland MG, Cawthon D. Personal protective equipment and decontamination of adults and children. Emergency medicine clinics of North America. 2015 Feb:33(1):51-68. doi: 10.1016/j.emc.2014.09.006. Epub 2014 Nov 15 [PubMed PMID: 25455662]
Verbeek JH, Rajamaki B, Ijaz S, Sauni R, Toomey E, Blackwood B, Tikka C, Ruotsalainen JH, Kilinc Balci FS. Personal protective equipment for preventing highly infectious diseases due to exposure to contaminated body fluids in healthcare staff. The Cochrane database of systematic reviews. 2020 May 15:5(5):CD011621. doi: 10.1002/14651858.CD011621.pub5. Epub 2020 May 15 [PubMed PMID: 32412096]
Level 1 (high-level) evidenceDrabek T, Boucek CD, Buffington CW. Wearing the wrong size latex surgical gloves impairs manual dexterity. Journal of occupational and environmental hygiene. 2010 Mar:7(3):152-5. doi: 10.1080/15459620903481660. Epub [PubMed PMID: 20017056]
Brisbine BR, Radcliffe CR, Jones MLH, Stirling L, Coltman CE. Does the fit of personal protective equipment affect functional performance? A systematic review across occupational domains. PloS one. 2022:17(11):e0278174. doi: 10.1371/journal.pone.0278174. Epub 2022 Nov 30 [PubMed PMID: 36449531]
Level 1 (high-level) evidenceHackett L, Zhang MM, Casey M, Miller J, Smith J, Low C, Aldridge E, Owen PJ, Buntine P. N-95/P2 respirator compliance with fit testing recommendations and respirator satisfaction amongst hospital staff. Infection, disease & health. 2024 Aug:29(3):144-151. doi: 10.1016/j.idh.2024.04.001. Epub 2024 May 3 [PubMed PMID: 38702235]
Level 3 (low-level) evidenceRegli A, Sommerfield A, von Ungern-Sternberg BS. The role of fit testing N95/FFP2/FFP3 masks: a narrative review. Anaesthesia. 2021 Jan:76(1):91-100. doi: 10.1111/anae.15261. Epub 2020 Sep 15 [PubMed PMID: 32932556]
Level 3 (low-level) evidenceRutala WA, Boyce JM, Weber DJ. Disinfection, sterilization and antisepsis: An overview. American journal of infection control. 2023 Nov:51(11S):A3-A12. doi: 10.1016/j.ajic.2023.01.001. Epub [PubMed PMID: 37890951]
Level 3 (low-level) evidenceHavill NL. Best practices in disinfection of noncritical surfaces in the health care setting: creating a bundle for success. American journal of infection control. 2013 May:41(5 Suppl):S26-30. doi: 10.1016/j.ajic.2012.10.028. Epub [PubMed PMID: 23622744]
Otter JA, Vickery K, Walker JT, deLancey Pulcini E, Stoodley P, Goldenberg SD, Salkeld JA, Chewins J, Yezli S, Edgeworth JD. Surface-attached cells, biofilms and biocide susceptibility: implications for hospital cleaning and disinfection. The Journal of hospital infection. 2015 Jan:89(1):16-27. doi: 10.1016/j.jhin.2014.09.008. Epub 2014 Oct 2 [PubMed PMID: 25447198]
Almatroudi A, Gosbell IB, Hu H, Jensen SO, Espedido BA, Tahir S, Glasbey TO, Legge P, Whiteley G, Deva A, Vickery K. Staphylococcus aureus dry-surface biofilms are not killed by sodium hypochlorite: implications for infection control. The Journal of hospital infection. 2016 Jul:93(3):263-70. doi: 10.1016/j.jhin.2016.03.020. Epub 2016 Apr 12 [PubMed PMID: 27140421]
Song L, Wu J, Xi C. Biofilms on environmental surfaces: evaluation of the disinfection efficacy of a novel steam vapor system. American journal of infection control. 2012 Dec:40(10):926-30. doi: 10.1016/j.ajic.2011.11.013. Epub 2012 Mar 12 [PubMed PMID: 22418602]
Alfa MJ. Monitoring and improving the effectiveness of cleaning medical and surgical devices. American journal of infection control. 2013 May:41(5 Suppl):S56-9. doi: 10.1016/j.ajic.2012.12.006. Epub [PubMed PMID: 23622750]
Advisory Committee on Immunization Practices, Centers for Disease Control and Prevention (CDC). Immunization of health-care personnel: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR. Recommendations and reports : Morbidity and mortality weekly report. Recommendations and reports. 2011 Nov 25:60(RR-7):1-45 [PubMed PMID: 22108587]
Havers FP, Moro PL, Hunter P, Hariri S, Bernstein H. Use of Tetanus Toxoid, Reduced Diphtheria Toxoid, and Acellular Pertussis Vaccines: Updated Recommendations of the Advisory Committee on Immunization Practices - United States, 2019. MMWR. Morbidity and mortality weekly report. 2020 Jan 24:69(3):77-83. doi: 10.15585/mmwr.mm6903a5. Epub 2020 Jan 24 [PubMed PMID: 31971933]
Territo H, Vaqar S, Justiz Vaillant AA. HIV Prophylaxis (Archived). StatPearls. 2025 Jan:(): [PubMed PMID: 30521273]
Schillie S, Vellozzi C, Reingold A, Harris A, Haber P, Ward JW, Nelson NP. Prevention of Hepatitis B Virus Infection in the United States: Recommendations of the Advisory Committee on Immunization Practices. MMWR. Recommendations and reports : Morbidity and mortality weekly report. Recommendations and reports. 2018 Jan 12:67(1):1-31. doi: 10.15585/mmwr.rr6701a1. Epub 2018 Jan 12 [PubMed PMID: 29939980]
Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, Bellomo R, Bernard GR, Chiche JD, Coopersmith CM, Hotchkiss RS, Levy MM, Marshall JC, Martin GS, Opal SM, Rubenfeld GD, van der Poll T, Vincent JL, Angus DC. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA. 2016 Feb 23:315(8):801-10. doi: 10.1001/jama.2016.0287. Epub [PubMed PMID: 26903338]
Level 3 (low-level) evidenceRivers E, Nguyen B, Havstad S, Ressler J, Muzzin A, Knoblich B, Peterson E, Tomlanovich M, Early Goal-Directed Therapy Collaborative Group. Early goal-directed therapy in the treatment of severe sepsis and septic shock. The New England journal of medicine. 2001 Nov 8:345(19):1368-77 [PubMed PMID: 11794169]
Level 1 (high-level) evidenceFriedman G, Silva E, Vincent JL. Has the mortality of septic shock changed with time. Critical care medicine. 1998 Dec:26(12):2078-86 [PubMed PMID: 9875924]
Level 1 (high-level) evidenceNovosad SA, Sapiano MR, Grigg C, Lake J, Robyn M, Dumyati G, Felsen C, Blog D, Dufort E, Zansky S, Wiedeman K, Avery L, Dantes RB, Jernigan JA, Magill SS, Fiore A, Epstein L. Vital Signs: Epidemiology of Sepsis: Prevalence of Health Care Factors and Opportunities for Prevention. MMWR. Morbidity and mortality weekly report. 2016 Aug 26:65(33):864-9. doi: 10.15585/mmwr.mm6533e1. Epub 2016 Aug 26 [PubMed PMID: 27559759]
Dantes RB, Epstein L. Combatting Sepsis: A Public Health Perspective. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2018 Sep 28:67(8):1300-1302. doi: 10.1093/cid/ciy342. Epub [PubMed PMID: 29846544]
Level 3 (low-level) evidenceRhee C, Dantes R, Epstein L, Murphy DJ, Seymour CW, Iwashyna TJ, Kadri SS, Angus DC, Danner RL, Fiore AE, Jernigan JA, Martin GS, Septimus E, Warren DK, Karcz A, Chan C, Menchaca JT, Wang R, Gruber S, Klompas M, CDC Prevention Epicenter Program. Incidence and Trends of Sepsis in US Hospitals Using Clinical vs Claims Data, 2009-2014. JAMA. 2017 Oct 3:318(13):1241-1249. doi: 10.1001/jama.2017.13836. Epub [PubMed PMID: 28903154]
Schlapbach LJ, Watson RS, Sorce LR, Argent AC, Menon K, Hall MW, Akech S, Albers DJ, Alpern ER, Balamuth F, Bembea M, Biban P, Carrol ED, Chiotos K, Chisti MJ, DeWitt PE, Evans I, Flauzino de Oliveira C, Horvat CM, Inwald D, Ishimine P, Jaramillo-Bustamante JC, Levin M, Lodha R, Martin B, Nadel S, Nakagawa S, Peters MJ, Randolph AG, Ranjit S, Rebull MN, Russell S, Scott HF, de Souza DC, Tissieres P, Weiss SL, Wiens MO, Wynn JL, Kissoon N, Zimmerman JJ, Sanchez-Pinto LN, Bennett TD, Society of Critical Care Medicine Pediatric Sepsis Definition Task Force. International Consensus Criteria for Pediatric Sepsis and Septic Shock. JAMA. 2024 Feb 27:331(8):665-674. doi: 10.1001/jama.2024.0179. Epub [PubMed PMID: 38245889]
Level 3 (low-level) evidenceMartin GS, Mannino DM, Moss M. The effect of age on the development and outcome of adult sepsis. Critical care medicine. 2006 Jan:34(1):15-21 [PubMed PMID: 16374151]
He Y, Xu J, Shang X, Fang X, Gao C, Sun D, Yao L, Zhou T, Pan S, Zou X, Shu H, Yang X, Shang Y. Clinical characteristics and risk factors associated with ICU-acquired infections in sepsis: A retrospective cohort study. Frontiers in cellular and infection microbiology. 2022:12():962470. doi: 10.3389/fcimb.2022.962470. Epub 2022 Jul 28 [PubMed PMID: 35967847]
Level 2 (mid-level) evidenceGirard TD, Opal SM, Ely EW. Insights into severe sepsis in older patients: from epidemiology to evidence-based management. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2005 Mar 1:40(5):719-27 [PubMed PMID: 15714419]
Stoll BJ, Hansen NI, Sánchez PJ, Faix RG, Poindexter BB, Van Meurs KP, Bizzarro MJ, Goldberg RN, Frantz ID 3rd, Hale EC, Shankaran S, Kennedy K, Carlo WA, Watterberg KL, Bell EF, Walsh MC, Schibler K, Laptook AR, Shane AL, Schrag SJ, Das A, Higgins RD, Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network. Early onset neonatal sepsis: the burden of group B Streptococcal and E. coli disease continues. Pediatrics. 2011 May:127(5):817-26. doi: 10.1542/peds.2010-2217. Epub 2011 Apr 25 [PubMed PMID: 21518717]
Level 2 (mid-level) evidenceKausch SL, Lake DE, Di Fiore JM, Weese-Mayer DE, Claure N, Ambalavanan N, Vesoulis ZA, Fairchild KD, Dennery PA, Hibbs AM, Martin RJ, Indic P, Travers CP, Bancalari E, Hamvas A, Kemp JS, Carroll JL, Moorman JR, Sullivan BA, Prematurity-Related Ventilatory Control (Pre-Vent) Investigators. Apnea, Intermittent Hypoxemia, and Bradycardia Events Predict Late-Onset Sepsis in Infants Born Extremely Preterm. The Journal of pediatrics. 2024 Aug:271():114042. doi: 10.1016/j.jpeds.2024.114042. Epub 2024 Apr 2 [PubMed PMID: 38570031]
Bekhof J, Reitsma JB, Kok JH, Van Straaten IH. Clinical signs to identify late-onset sepsis in preterm infants. European journal of pediatrics. 2013 Apr:172(4):501-8. doi: 10.1007/s00431-012-1910-6. Epub 2012 Dec 28 [PubMed PMID: 23271492]
Evans L, Rhodes A, Alhazzani W, Antonelli M, Coopersmith CM, French C, Machado FR, Mcintyre L, Ostermann M, Prescott HC, Schorr C, Simpson S, Wiersinga WJ, Alshamsi F, Angus DC, Arabi Y, Azevedo L, Beale R, Beilman G, Belley-Cote E, Burry L, Cecconi M, Centofanti J, Coz Yataco A, De Waele J, Dellinger RP, Doi K, Du B, Estenssoro E, Ferrer R, Gomersall C, Hodgson C, Møller MH, Iwashyna T, Jacob S, Kleinpell R, Klompas M, Koh Y, Kumar A, Kwizera A, Lobo S, Masur H, McGloughlin S, Mehta S, Mehta Y, Mer M, Nunnally M, Oczkowski S, Osborn T, Papathanassoglou E, Perner A, Puskarich M, Roberts J, Schweickert W, Seckel M, Sevransky J, Sprung CL, Welte T, Zimmerman J, Levy M. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock 2021. Intensive care medicine. 2021 Nov:47(11):1181-1247. doi: 10.1007/s00134-021-06506-y. Epub 2021 Oct 2 [PubMed PMID: 34599691]
Morris E, McCartney D, Lasserson D, Van den Bruel A, Fisher R, Hayward G. Point-of-care lactate testing for sepsis at presentation to health care: a systematic review of patient outcomes. The British journal of general practice : the journal of the Royal College of General Practitioners. 2017 Dec:67(665):e859-e870. doi: 10.3399/bjgp17X693665. Epub 2017 Nov 20 [PubMed PMID: 29158243]
Level 1 (high-level) evidenceKuttab HI, Lykins JD, Hughes MD, Wroblewski K, Keast EP, Kukoyi O, Kopec JA, Hall S, Ward MA. Evaluation and Predictors of Fluid Resuscitation in Patients With Severe Sepsis and Septic Shock. Critical care medicine. 2019 Nov:47(11):1582-1590. doi: 10.1097/CCM.0000000000003960. Epub [PubMed PMID: 31393324]
Kim H, Chung SP, Choi SH, Kang GH, Shin TG, Kim K, Park YS, Han KS, Choi HS, Suh GJ, Kim WY, Lim TH, Ko BS, Korean Shock Society (KoSS) Investigators. Impact of timing to source control in patients with septic shock: A prospective multi-center observational study. Journal of critical care. 2019 Oct:53():176-182. doi: 10.1016/j.jcrc.2019.06.012. Epub 2019 Jun 17 [PubMed PMID: 31247517]
Level 2 (mid-level) evidenceMartínez ML, Ferrer R, Torrents E, Guillamat-Prats R, Gomà G, Suárez D, Álvarez-Rocha L, Pozo Laderas JC, Martín-Loeches I, Levy MM, Artigas A, Edusepsis Study Group. Impact of Source Control in Patients With Severe Sepsis and Septic Shock. Critical care medicine. 2017 Jan:45(1):11-19 [PubMed PMID: 27611975]
Brady MF, Jamal Z, Pervin N. Acinetobacter. StatPearls. 2025 Jan:(): [PubMed PMID: 28613535]
Tavares M, Kozak M, Balola A, Sá-Correia I. Burkholderia cepacia Complex Bacteria: a Feared Contamination Risk in Water-Based Pharmaceutical Products. Clinical microbiology reviews. 2020 Jun 17:33(3):. doi: 10.1128/CMR.00139-19. Epub 2020 Apr 15 [PubMed PMID: 32295766]
Aronoff DM. Clostridium novyi, sordellii, and tetani: mechanisms of disease. Anaerobe. 2013 Dec:24():98-101. doi: 10.1016/j.anaerobe.2013.08.009. Epub 2013 Sep 12 [PubMed PMID: 24036420]
Vidor C, Awad M, Lyras D. Antibiotic resistance, virulence factors and genetics of Clostridium sordellii. Research in microbiology. 2015 May:166(4):368-74. doi: 10.1016/j.resmic.2014.09.003. Epub 2014 Oct 5 [PubMed PMID: 25290059]
Ramirez D, Giron M. Enterobacter Infections. StatPearls. 2025 Jan:(): [PubMed PMID: 32644722]
Woerther PL, Andremont A, Kantele A. Travel-acquired ESBL-producing Enterobacteriaceae: impact of colonization at individual and community level. Journal of travel medicine. 2017 Apr 1:24(suppl_1):S29-S34. doi: 10.1093/jtm/taw101. Epub [PubMed PMID: 28520999]
Oliveira J, Reygaert WC. Gram-Negative Bacteria. StatPearls. 2025 Jan:(): [PubMed PMID: 30855801]
Hu Y, Anes J, Devineau S, Fanning S. Klebsiella pneumoniae: Prevalence, Reservoirs, Antimicrobial Resistance, Pathogenicity, and Infection: A Hitherto Unrecognized Zoonotic Bacterium. Foodborne pathogens and disease. 2021 Feb:18(2):63-84. doi: 10.1089/fpd.2020.2847. Epub 2020 Oct 30 [PubMed PMID: 33124929]
Wertheim HF, Melles DC, Vos MC, van Leeuwen W, van Belkum A, Verbrugh HA, Nouwen JL. The role of nasal carriage in Staphylococcus aureus infections. The Lancet. Infectious diseases. 2005 Dec:5(12):751-62 [PubMed PMID: 16310147]
Ondusko DS, Nolt D. Staphylococcus aureus. Pediatrics in review. 2018 Jun:39(6):287-298. doi: 10.1542/pir.2017-0224. Epub [PubMed PMID: 29858291]
Siddiqui AH, Koirala J. Methicillin-Resistant Staphylococcus aureus. StatPearls. 2025 Jan:(): [PubMed PMID: 29489200]
Bethencourt Mirabal A, Nguyen AD, Ferrer G. Lung Nontuberculous Mycobacterial Infections. StatPearls. 2025 Jan:(): [PubMed PMID: 31869064]
Wilson MG, Pandey S. Pseudomonas aeruginosa. StatPearls. 2025 Jan:(): [PubMed PMID: 32491763]
Frenk J, Chen L, Bhutta ZA, Cohen J, Crisp N, Evans T, Fineberg H, Garcia P, Ke Y, Kelley P, Kistnasamy B, Meleis A, Naylor D, Pablos-Mendez A, Reddy S, Scrimshaw S, Sepulveda J, Serwadda D, Zurayk H. Health professionals for a new century: transforming education to strengthen health systems in an interdependent world. Lancet (London, England). 2010 Dec 4:376(9756):1923-58. doi: 10.1016/S0140-6736(10)61854-5. Epub 2010 Nov 26 [PubMed PMID: 21112623]
Chung HO, Medina D, Fox-Robichaud A. Interprofessional sepsis education module: a pilot study. CJEM. 2016 Mar:18(2):143-5. doi: 10.1017/cem.2015.42. Epub 2015 Jun 11 [PubMed PMID: 26063312]
Level 3 (low-level) evidence