Introduction
Undifferentiated pleomorphic sarcoma (UPS), formerly known as malignant fibrous histiocytoma, is one of the most common high-grade soft tissue sarcomas in adults, comprising approximately 5% to 10% of all soft tissue sarcomas.[1] It arises from primitive mesenchymal cells that lack a specific line of differentiation and typically occurs in the deep soft tissues of the extremities, particularly the thigh, followed by the trunk and retroperitoneum. UPS is characterized by marked cellular pleomorphism, atypical mitotic figures, and areas of necrosis on histology. The molecular pathogenesis remains poorly defined, involving highly complex and heterogeneous karyotypes without consistent or targetable genetic alterations, differentiating UPS from other sarcoma subtypes.
The clinical presentation of UPS usually involves a painless, progressively enlarging mass, though symptoms can vary depending on anatomic location and involvement of adjacent structures. Prognosis is determined by several factors, including tumor size, depth, anatomic site, and histologic grade, with high-grade, deep, and large tumors demonstrating an elevated risk of distant metastasis, most commonly to the lungs, followed by lymph nodes, bone, and liver.[2]
Management requires an interprofessional approach and should be centralized at high-volume sarcoma centers to optimize outcomes. The cornerstone of treatment for localized disease is wide surgical resection with negative margins.[1]][3] Radiation therapy is frequently utilized preoperatively or postoperatively to improve local control, particularly for tumors in anatomically constrained sites. Systemic chemotherapy, most often with doxorubicin-based regimens, is considered in select patients with high-risk or metastatic disease, though overall response rates remain modest. Ongoing research into the tumor biology of UPS may pave the way for novel therapeutic approaches, including immunotherapy and molecularly targeted agents.[4][5]
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
The etiology of UPS remains unclear. Most cases are sporadic without identifiable risk factors. UPS may be associated with radiation exposure, with resulting tumors characterized by aggressive clinical behavior. UPS may also arise in the setting of chronic tissue injury, eg, areas of prior trauma, surgical scars, or chronic inflammation, although these associations are not consistently observed. From a molecular standpoint, UPS does not have a characteristic driving mutation.[1][5][4]
Epidemiology
The incidence and prevalence of UPS are challenging to quantify due to historical misclassification (many tumors previously classified as UPS were likely other undifferentiated sarcomas) and the lack of specific tracking in national cancer registries (eg, the SEER database), where UPS is grouped within broader categories of high-grade or unclassified soft tissue sarcomas. Nevertheless, UPS is estimated to comprise approximately 5% to 10% of all adult soft tissue sarcomas, making it one of the most common high-grade sarcoma subtypes in this population. The estimated annual incidence of soft tissue sarcomas in the United States is roughly 13,000 cases, suggesting that approximately 650 to 1,300 of these may be UPS. Global incidence rates are likely similar, as no geographic or racial disparities have been reported.[6]
UPS predominantly occurs in older adults, with peak incidence between the ages of 50 and 70, and is rare in pediatric populations. A slight male predominance has been observed, though sex differences in outcomes have not been established. Improved histopathologic classification and the adoption of advanced molecular diagnostics have led to a decline in UPS diagnoses, as many previously labeled cases have been reclassified into distinct, genetically defined sarcoma subtypes.[1]
Pathophysiology
UPS arises from primitive mesenchymal cells that have undergone genetic alterations leading to dysregulated cellular proliferation, loss of differentiation, and resistance to apoptosis. UPS tumors exhibit complex karyotypes and commonly harbor copy number alterations and mutations in tumor suppressor genes, eg, TP53, RB, and PTEN, along with disruptions in cell cycle regulators like CCNE1 and ATRX, the latter being involved in telomere maintenance and cellular senescence. These molecular changes drive genomic instability and unchecked proliferation. Unlike most sarcomas, however, UPS is not associated with a defining genetic mutation.[4]
Histopathology
Histopathologic evaluation is critical in establishing a definitive diagnosis of UPS. Core needle biopsy is the preferred technique for initial tissue sampling, preferably with image guidance that can help target the most abnormal appearing regions of the tumor. Biopsy sites should be along the eventual site of surgical incision. Fine needle aspiration and open incisional biopsies are seldom indicated.
A thorough postoperative pathology report should include the anatomic location of the tumor, depth of invasion, maximal dimension, histologic grade, presence or absence of necrosis, margin status, vascular invasion, mitotic index, nature and extent of inflammatory infiltrate, and tumor-node-metastasis (TNM) staging according to established guidelines.[5][4]
Gross Histologic Examination
UPS typically presents as a large, poorly circumscribed, infiltrative soft tissue mass on gross examination. The cut surface often appears variegated, with areas of tan-white, gray, or fleshy tissue interspersed with zones of necrosis and hemorrhage. The tumor may demonstrate a lobulated appearance and extend into adjacent structures, including fascia, muscle, or subcutaneous fat. Regions of myxoid change, cystic degeneration, or fibrosis may also be present. Lesions are frequently firm to the touch due to a dense fibrous stroma, but softer areas of necrosis and hemorrhage can create heterogeneity. Tumor margins are usually irregular and indistinct, reflecting their locally aggressive nature and propensity for deep tissue invasion. In some cases, satellite nodules or multifocality may be noted, particularly in recurrent disease.[1]
Microscopic Examination
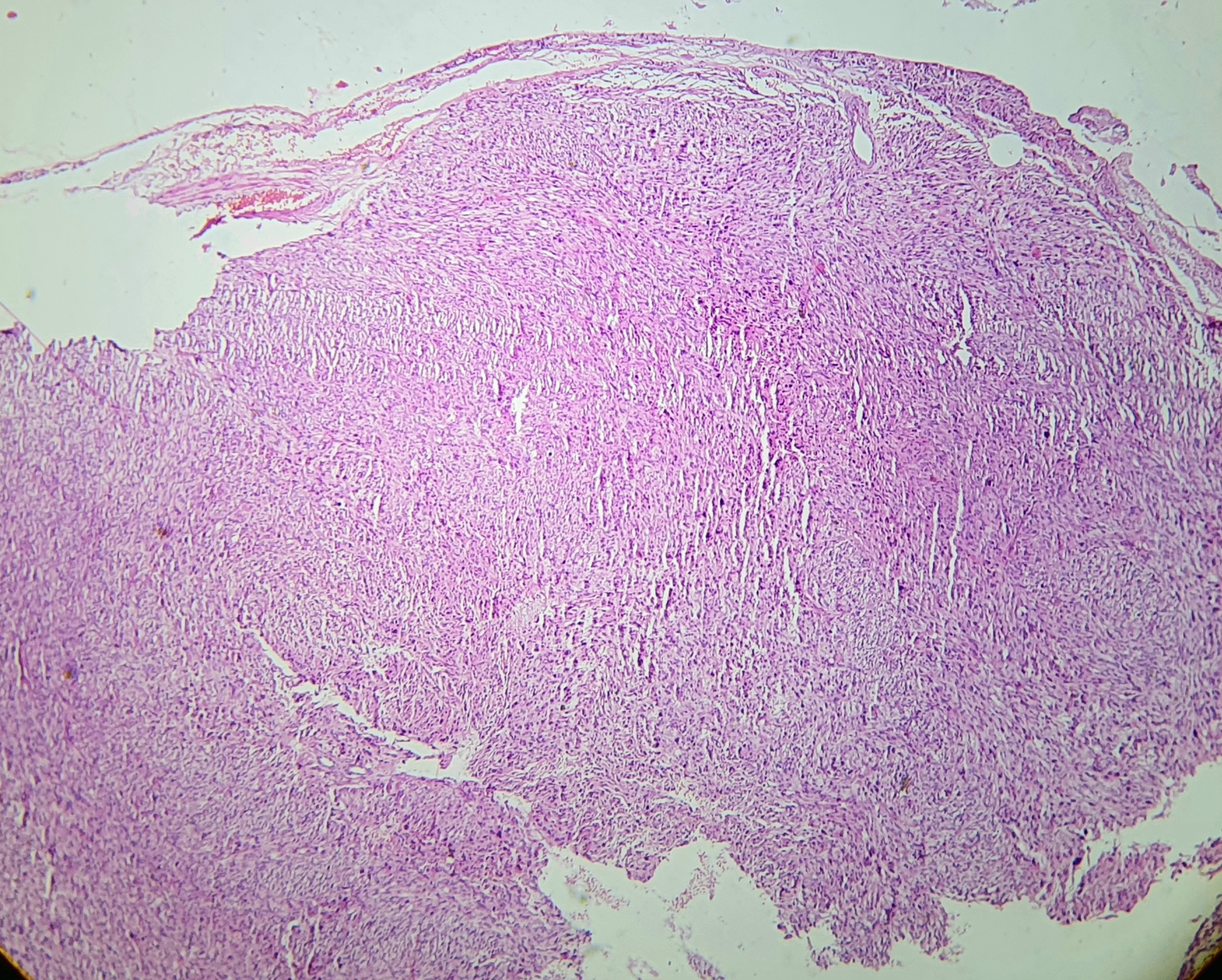
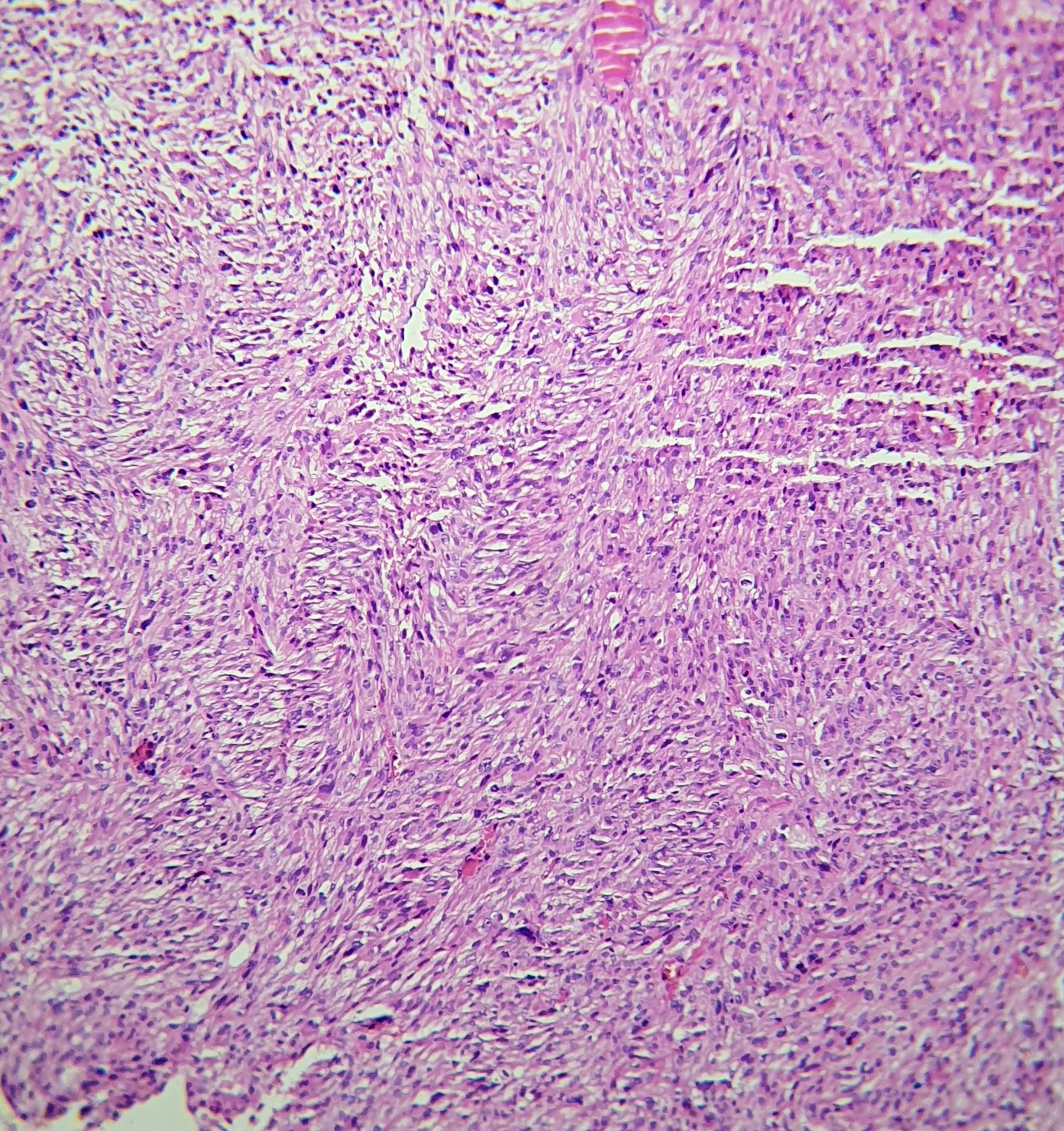
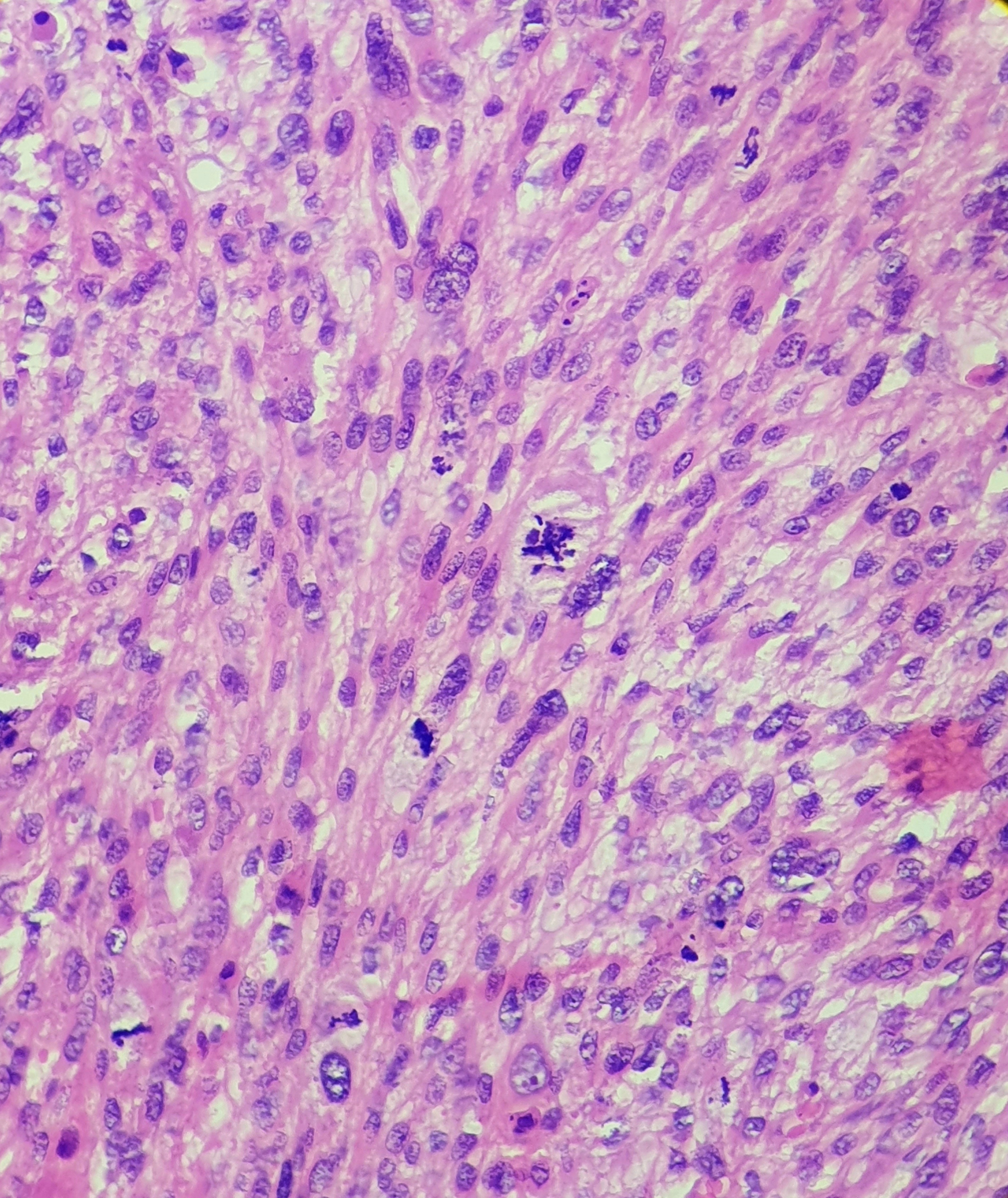
On light microscopy, UPS typically demonstrates markedly pleomorphic spindle cells arranged in storiform, fascicular, or sheet-like patterns within a fibrotic stroma, accompanied by a high mitotic index (see Image. Histologic Examination of Undifferentiated Pleomorphic Sarcoma). Tumor invasion may extend into the deep dermis, subcutis, fascia, and skeletal muscle (see Image. Tumor Invasion of Undifferentiated Pleomorphic Sarcoma). Deep-seated tumors are defined as those involving or penetrating beyond the superficial fascia. While the superficial versus deep designation primarily serves prognostic purposes, deep soft tissue invasion and the absence of solar elastosis are beneficial in distinguishing UPS from atypical fibroxanthoma.
Immunohistochemical Examination
The diagnosis of UPS is ultimately one of exclusion, requiring a comprehensive immunohistochemical (IHC) panel to rule out other morphologically similar entities, including cytokeratins to exclude sarcomatoid carcinoma, S100 and SOX10 for melanoma, smooth muscle actin and desmin for pleomorphic myogenic sarcomas. In cases of intra-abdominal or intrathoracic tumors, MDM2 and CDK4 staining assist in excluding dedifferentiated liposarcoma. Recent findings have shown overlapping expression of LN2, CD10, and ezrin in both UPS and atypical fibroxanthoma, challenging prior assumptions regarding the specificity of LN2 for UPS. In addition, IHC markers, eg, vimentin, p53, and Ki-67 may be expressed in UPS, though they lack diagnostic specificity.[1][4]
History and Physical
Clinical History
The clinical presentation of UPS is often insidious, with patients typically reporting a painless, enlarging soft tissue mass. This tumor most commonly arises in the deep soft tissues of the extremities, particularly the thigh, but can also be found in the trunk or, less commonly, the retroperitoneum. Symptoms may be absent for extended periods, with the lesion often discovered incidentally or once it attains a size sufficient to cause discomfort or functional impairment. Pain or neurological symptoms may occur due to mass effect or compression of adjacent neurovascular structures. Constitutional symptoms are rare unless metastatic disease is present.[5]
Physical Examination
On physical examination, UPS generally presents as a firm, nontender, and immobile mass located deep to the fascia. The overlying skin is usually intact without discoloration or ulceration. Lesions are often large at diagnosis, sometimes exceeding 5 cm in diameter, reflecting their deep location and indolent growth. Involvement of adjacent structures, such as nerves or vessels, may be suspected if associated limb weakness, paresthesia, or diminished pulses are present. Regional lymphadenopathy is uncommon as UPS typically metastasizes hematogenously, most frequently to the lungs. A thorough musculoskeletal and neurologic examination is essential to assess functional status and establish a baseline before intervention.[5]
Evaluation
The evaluation of UPS requires an interprofessional and systematic approach involving imaging, histologic confirmation, and metastatic staging, in accordance with guidelines from the National Comprehensive Cancer Network (NCCN), the European Society for Medical Oncology (ESMO), and other international sarcoma working groups.[5][7]
Laboratory Studies
Laboratory studies are not diagnostic but are often helpful in planning treatment. A complete blood count (CBC), renal and hepatic function panels, and coagulation profile are standard in preoperative evaluation. In rare instances, elevated lactate dehydrogenase (LDH) may suggest aggressive or metastatic disease, although this is not specific to UPS. Additional biomarkers or molecular profiling may be obtained for stratification in patients receiving systemic therapy or enrolled in clinical trials.
Radiographic Evaluation
Magnetic resonance imaging (MRI) of the primary tumor site is the imaging modality of choice for most soft tissue sarcomas. MRI provides detailed information on tumor size, location, compartmental involvement, and relationships with neurovascular bundles and bone. UPS typically demonstrates heterogeneous signal intensity on T2-weighted images and irregular enhancement on postcontrast sequences, often with central necrosis. Diffusion-weighted and subtraction imaging can assist in distinguishing viable tumor from hemorrhagic or necrotic areas. In addition, MRI can help identify the extent of perilesional edema and potential infiltration through fascial planes.[5]
Computed tomography (CT) is utilized for suspected retroperitoneal or truncal lesions. CT scans with contrast are useful initial studies. Moreover, given the relative ease of access to CT and its cheaper and more readily available nature, this modality is often the first imaging study pursued. All patients with high-grade soft tissue sarcomas, including UPS, require staging with a chest CT scan due to the high propensity for pulmonary metastases. This is a class I recommendation in the NCCN guidelines. Whole-body positron emission tomography (PET)/CT may be used selectively, particularly in cases where multifocal disease is suspected or for treatment response monitoring, though not routinely required.[5]
Biopsy
Histologic confirmation via core needle biopsy, performed under imaging guidance, is mandatory before definitive treatment. Biopsy should be performed in a manner that does not compromise future surgical margins. The diagnosis of UPS is one of exclusion, necessitating a comprehensive immunohistochemical workup to rule out other pleomorphic sarcoma subtypes, including dedifferentiated liposarcoma, leiomyosarcoma, malignant peripheral nerve sheath tumor, and solitary fibrous tumor (see Image. Undifferentiated Pleomorphic Sarcoma Biopsy). Immunostaining panels typically include desmin, SMA, S100, CD34, MDM2, CDK4, and nuclear STAT6 markers. Genomic profiling or fluorescence in situ hybridization (FISH) may be required to exclude specific translocation-associated sarcomas.[5][8]
Treatment / Management
Managing UPS involves an interprofessional team, including surgery, radiation, and chemotherapy clinicians. Due to the rarity of this condition and the significant gaps in the current understanding of UPS, enrolling all patients in clinical trials whenever possible should be considered.
Differential Diagnosis
The most common differential diagnoses of UPS are other types of soft tissue sarcomas, which must be distinguished by clinical history, physical examination, and immunohistochemistry markers, including atypical fibroxanthoma, liposarcoma, leiomyosarcoma, angiosarcoma, fibrosarcoma, myxofibrosarcoma, dermatofibrosarcoma protuberans, osteosarcoma, and malignant peripheral nerve sheath tumor.[9][10] Metastases, desmoplastic melanoma, and spindle-cell squamous cell carcinoma may also resemble UPS clinical or histopathological morphology.[11]
Surgical Oncology
Surgical resection is the mainstay for curative intent treatment for UPS. The primary goal is an en-bloc resection with negative microscopic margins (R0 resection), as local recurrence is significantly associated with positive margins. When feasible, wide local excision with a goal of at least 1 cm margins or the inclusion of an anatomic barrier (eg, fascia) is highly recommended.[12] For extremity or trunk-based tumors, limb-sparing surgery should be the standard of care, with amputation reserved for cases where critical neurovascular structures are extensively involved, and reconstruction is not feasible. In the retroperitoneum, complete gross resection, including adjacent organs, may be necessary given the infiltrative nature of high-grade sarcomas in that location. Close coordination with plastic and reconstructive surgery may be required for closure or functional preservation.[5][7]
Radiation Oncology
Adjuvant or neoadjuvant radiation therapy is frequently utilized in the management of UPS, particularly for high-grade tumors or those with close or positive margins. According to randomized data, including the landmark Yang study and subsequent trials, radiation reduces the risk of local recurrence but has not demonstrated a survival benefit.[13] Preoperative radiation (typically 50 Gy) is often preferred for extremity sarcomas, as it allows for a smaller radiation field and better tissue oxygenation.
However, radiation therapy carries a higher risk of postoperative wound complications. Postoperative radiation (generally 60–66 Gy) is indicated when margins are close or positive or when tumors are unexpectedly upstaged. In the retroperitoneum, preoperative radiation is more controversial. Adjuvant or neoadjuvant radiation is typically reserved for selected cases, as shown by the STRASS trial, which did not demonstrate a clear benefit in all comers but suggested a possible role in liposarcoma. For UPS, individualized assessment of recurrence risk and expected morbidity is essential in guiding radiotherapy use.[14][5][7]
Medical Oncology
Systemic therapy plays a role in resectable and metastatic UPS.[5][7]
Adjuvant Chemotherapy
For high-risk localized tumors (generally >5 to 10 cm, deep, and high grade), adjuvant chemotherapy with doxorubicin and ifosfamide may be indicated. The survival benefit remains modest and must be weighed against the significant toxicity.[15]
Neoadjuvant and Perioperative Chemotherapy
Recent meta-analyses and trials, eg, EORTC-STBSG 62931 and ISG-STS 1001, suggest that selected patients, particularly those with poor prognostic features as determined by nomograms (eg, Sarculator), may derive a survival advantage from perioperative chemotherapy.[16][17]
Metastatic Disease
In the metastatic or unresectable setting, doxorubicin remains the first-line agent, alone or in combination with ifosfamide. Other regimens may be used as second-line therapy, such as gemcitabine-docetaxel or gemcitabine-dacarbazine. Targeted therapies, including pazopanib, a multikinase inhibitor, have shown modest efficacy and are approved for pretreated soft tissue sarcomas. Immunotherapy with PD-1 inhibitors, particularly pembrolizumab and nivolumab (alone or in combination with ipilimumab), has demonstrated meaningful response rates in UPS, distinguishing it as one of the more immune-responsive sarcoma subtypes. Clinical trials should be considered for patients with advanced or recurrent disease, especially at high-volume sarcoma centers.[18][19]
Staging
Trunk and extremities UPS is staged according to the TNM and histologic grade (G) (see Table 1. Tumor, Node, Metastasis, and Grade Criteria).[20][21] G is defined by the differentiation, mitotic count, and necrosis extension of the tumor, as stated by the French Federation of Cancer Centers Sarcoma Group (FNCLCC) (see Table 2. Histologic Grade). After TNM and G evaluation, the disease is classified into stages I to IV for therapeutic purposes (see Table 3. Tumor Staging).
Table 1. Tumor, Node, Metastasis, and Grade Criteria
|
T: Primary Tumor |
|
|
Tx |
Primary tumor cannot be assessed |
|
T0 |
No evidence of primary tumor |
|
T1 |
Tumor ≤5 cm in greatest dimension |
|
T2 |
Tumor >10 cm and ≤15 cm |
|
T4 |
Tumor >15 cm in greatest dimension |
|
N: Regional Lymph Nodes |
|
|
N0 |
No regional lymph node metastasis or unknown lymph node status |
|
N1 |
Regional lymph node metástasis |
|
M: Distant Metastasis |
|
|
M0 |
No distant metástasis |
|
M1 |
Distant metástasis |
|
G: Histologic Grade |
|
|
GX |
Grade cannot be assessed |
|
G1 |
Total differentiation, mitotic count, and necrosis score 2 or 3 |
|
G2 |
Total differentiation, mitotic count, and necrosis score 4 or 5 |
|
G3 |
Total differentiation, mitotic count, and necrosis score 6, 7, or 8 |
Table 2. Histologic Grade
|
Histologic Grade |
The sum of differentiation, mitotic activity, and extent of necrosis scores. |
|
|
Tumor Differentiation |
||
|
1 |
Closely resembling normal adult mesenchymal tissue. |
|
|
2 |
Certain histologic typing |
|
|
3 |
Embryonal, synovial, Ewing, primitive neuroectodermal, and undifferentiated sarcoma |
|
|
Mitotic Activity |
||
|
1 |
0-9 mitoses per 10 high-power field |
|
|
2 |
10-19 mitoses per 10 high-power field |
|
|
3 |
≥20 mitoses per 10 high-power field |
|
|
Tumor Necrosis |
||
|
0 |
No necrosis |
|
|
1 |
<50% necrosis |
|
|
2 |
≥50% necrosis |
|
Table 3. Tumor Staging
|
Stage |
T |
N |
M |
G |
|
IA |
T1 |
N0 |
M0 |
G1, GX |
|
IB |
T2 |
N0 |
M0 |
G1, GX |
|
|
T3 |
N0 |
M0 |
G1, GX |
|
|
T4 |
N0 |
M0 |
G1, GX |
|
II |
T1 |
N0 |
M0 |
G2, G3 |
|
IIIA |
T2 |
N0 |
M0 |
G2, G3 |
|
IIIB |
T3 |
N0 |
M0 |
G2, G3 |
|
|
T4 |
N0 |
M0 |
G2, G3 |
|
|
Any T |
N1 |
M0 |
Any G |
|
IV |
Any T |
Any N |
M1 |
Any G |
Staging of head, neck, abdominal, thoracic, and retroperitoneum tumors can be consulted in the American Joint Committee on Cancer Staging (AJCC) Manual Eighth Edition, 2017.[20]
Prognosis
Early disease recognition and an adequate treatment strategy are the most critical interventions to improve overall prognosis. In a current retrospective study of 319 patients from 3 tertiary care centers, recurrences and metastases occurred in 14.1% and 7.8% of the cases, respectively.[22] The recurrence risk was significantly increased with preoperative tumors >5 cm, invasion beyond the subcutaneous fat, and advanced AJCC staging. Besides these, metastasis risk was also significantly increased in 2 to 5 cm tumors and those with lymphatic or vascular invasion. However, an essential limitation of this work is that atypical fibroxanthoma diagnoses were pooled together with UPS. In a UPS-specific report, posttreatment local recurrence (15%) was statistically associated with advanced age and inadequate surgical margins, whilst posttreatment metastatic disease (37.6%) was significantly relevant in tumors ≥5 cm.[23]
The overall survival rates for the 5-year and 10-year periods were 60% and 48%, respectively. Interestingly, in another publication, the 5-year disease-specific survival and recurrence rates were significantly worse in radiation-associated UPS than sporadic UPS, suggesting distinct mutational profiles might exist for the disease.[24]
Follow-up visits are essential to detect local recurrences or metastasis. Physical examination of stage I tumors should be performed at 3- to 6-month intervals for the first 2 years and then annually.[20] Imaging studies are not generally indicated unless prompted by clinical signs and symptoms. Stage II to IV disease must be followed every 2 to 6 months for 2 to 3 years, then every 6 months for 2 years, and annually thereafter. Postoperative MRI with and without contrast or contrast CT are recommended for primary tumor site assessment. Subsequent imaging of the affected and distant areas may be warranted based on the individual risk of recurrence.
Complications
Undifferentiated Pleomorphic Sarcoma Complications
UPS is a high-grade soft tissue malignancy associated with various complications that can arise from the tumor itself, its local and systemic effects, and the treatment modalities used.
Local complications primarily result from the tumor's aggressive and infiltrative behavior. Large or deeply situated tumors can compress or invade adjacent neurovascular structures, leading to pain, sensory deficits, or motor dysfunction. In the extremities, such involvement may result in impaired limb function or even necessitate amputation in cases where critical structures cannot be preserved during resection. In retroperitoneal locations, UPS may compress or infiltrate vital organs, leading to bowel obstruction, hydronephrosis, or vascular compromise.
UPS has a high propensity for hematogenous metastasis, most commonly to the lungs. Pulmonary metastases may be asymptomatic or present with cough, hemoptysis, pleuritic pain, or dyspnea. Less commonly, liver, bone, or brain metastases may occur, contributing to systemic symptoms and organ-specific dysfunction. High-grade tumors are also at risk for early recurrence, particularly if resection margins are inadequate.
Treatment-Related Complications
Treatment-related complications are also significant. Surgical resection, especially when involving large or anatomically complex tumors, can lead to wound healing problems, seroma or hematoma formation, infection, and long-term functional impairment. In the case of preoperative or postoperative radiation therapy, patients may experience fibrosis, joint stiffness, lymphedema, delayed wound healing, and secondary radiation-induced malignancies. Chemotherapy-related toxicity includes myelosuppression, cardiotoxicity (particularly with doxorubicin), nephrotoxicity, and fatigue, all of which may limit treatment intensity or duration.
Finally, psychosocial distress, decreased quality of life, and physical disability are essential but sometimes underrecognized complications, particularly in patients requiring extensive surgeries or facing recurrent or metastatic disease. As such, early engagement of rehabilitation, pain management, psychosocial support, and survivorship care is essential to comprehensive management.[1][5][7]
Deterrence and Patient Education
UPS is a rare and aggressive type of cancer that starts in the body's soft tissues, eg, muscles, fat, or connective tissue. It most often develops in the arms or legs but can also occur in the back, abdomen, or other areas. The cause is usually unknown and is more commonly seen in older adults.
UPS usually presents as a painless lump or swelling that gradually increases in size. Because it may not cause symptoms early on, UPS is often discovered when the tumor becomes large or begins to press on nearby structures. Diagnosis typically involves imaging studies, such as MRI or CT scans, followed by a biopsy to confirm the type of cancer. A pathologist will examine the tissue under a microscope and perform special tests to determine that the tumor does not show signs of a more specific type of soft tissue cancer.
The primary treatment for UPS is surgery to remove the tumor completely. The goal is to take out the cancer with a margin of healthy tissue to lower the risk of it coming back. Radiation therapy may also be used before or after surgery to help reduce the risk of recurrence. In some cases, especially when the cancer has spread or is difficult to remove, chemotherapy may be recommended.
Survival and recovery from this tumor depend on several factors, including the size and location of the tumor, whether it has spread, and how completely it can be removed. Regular follow-up is essential because UPS can return or spread to other parts of the body, most commonly the lungs.
Enhancing Healthcare Team Outcomes
Effective management of UPS demands a highly collaborative, interprofessional approach that centers on patient needs and safety throughout the care continuum. Physicians, particularly oncologists and surgeons, play a central role in diagnosis and treatment planning, relying on advanced imaging and tissue biopsy to guide clinical decisions. Advanced practitioners and nurses support these efforts by conducting thorough assessments, educating patients, and monitoring for treatment-related adverse effects. Pharmacists contribute by managing chemotherapy regimens, mitigating adverse drug reactions, and ensuring safe medication use. Radiologists and pathologists are critical for accurate diagnosis, using MRI and CT imaging, as well as immunohistochemistry, to differentiate UPS from other sarcomas. Open communication among all team members ensures a cohesive interpretation of diagnostic data and timely intervention.
As patients progress through treatment, ethical complexities and care coordination challenges often emerge, especially in cases of recurrent or metastatic disease. To address these effectively, interprofessional teams must prioritize shared decision-making, aligning treatment goals with each patient’s values and preferences. Nurses and social workers facilitate continuity of care, help manage psychosocial distress, and ensure that patients have access to rehabilitation services and survivorship planning. Regular case conferences and team huddles help define roles and improve accountability, minimizing delays and errors. By maintaining clear communication and mutual respect across interprofessional teams, healthcare professionals can significantly enhance patient-centered care, improve clinical outcomes, and foster a culture of safety and performance excellence in the management of UPS.
Media
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
References
Crago AM, Cardona K, Koseła-Paterczyk H, Rutkowski P. Management of Myxofibrosarcoma and Undifferentiated Pleomorphic Sarcoma. Surgical oncology clinics of North America. 2022 Jul:31(3):419-430. doi: 10.1016/j.soc.2022.03.006. Epub [PubMed PMID: 35715142]
Makris EA, Tran TB, Delitto DJ, Lee B, Ethun CG, Grignol V, Harrison Howard J, Bedi M, Clark Gamblin T, Tseng J, Roggin KK, Chouliaras K, Votanopoulos K, Cullinan D, Fields RC, Cardona K, Poultsides G, Kirane A. Natural history of undifferentiated pleomorphic sarcoma: Experience from the US Sarcoma Collaborative. Journal of surgical oncology. 2024 Jun:129(7):1354-1363. doi: 10.1002/jso.27620. Epub 2024 Apr 1 [PubMed PMID: 38562002]
Ferry MJ, Lewis T. Undifferentiated Pleomorphic Sarcoma. The Journal of the American Osteopathic Association. 2020 Aug 1:120(8):543. doi: 10.7556/jaoa.2020.089. Epub [PubMed PMID: 32717090]
Sun H, Liu J, Hu F, Xu M, Leng A, Jiang F, Chen K. Current research and management of undifferentiated pleomorphic sarcoma/myofibrosarcoma. Frontiers in genetics. 2023:14():1109491. doi: 10.3389/fgene.2023.1109491. Epub 2023 Feb 16 [PubMed PMID: 36873946]
von Mehren M, Kane JM, Agulnik M, Bui MM, Carr-Ascher J, Choy E, Connelly M, Dry S, Ganjoo KN, Gonzalez RJ, Holder A, Homsi J, Keedy V, Kelly CM, Kim E, Liebner D, McCarter M, McGarry SV, Mesko NW, Meyer C, Pappo AS, Parkes AM, Petersen IA, Pollack SM, Poppe M, Riedel RF, Schuetze S, Shabason J, Sicklick JK, Spraker MB, Zimel M, Hang LE, Sundar H, Bergman MA. Soft Tissue Sarcoma, Version 2.2022, NCCN Clinical Practice Guidelines in Oncology. Journal of the National Comprehensive Cancer Network : JNCCN. 2022 Jul:20(7):815-833. doi: 10.6004/jnccn.2022.0035. Epub [PubMed PMID: 35830886]
Level 1 (high-level) evidenceMa J, Groisberg R, Shao C, Zhong W. Incidence of Undifferentiated Pleomorphic Sarcoma (UPS) in the United States. Sarcoma. 2024:2024():6735002. doi: 10.1155/2024/6735002. Epub 2024 Oct 29 [PubMed PMID: 39502684]
Gronchi A, Miah AB, Dei Tos AP, Abecassis N, Bajpai J, Bauer S, Biagini R, Bielack S, Blay JY, Bolle S, Bonvalot S, Boukovinas I, Bovee JVMG, Boye K, Brennan B, Brodowicz T, Buonadonna A, De Álava E, Del Muro XG, Dufresne A, Eriksson M, Fagioli F, Fedenko A, Ferraresi V, Ferrari A, Frezza AM, Gasperoni S, Gelderblom H, Gouin F, Grignani G, Haas R, Hassan AB, Hecker-Nolting S, Hindi N, Hohenberger P, Joensuu H, Jones RL, Jungels C, Jutte P, Kager L, Kasper B, Kawai A, Kopeckova K, Krákorová DA, Le Cesne A, Le Grange F, Legius E, Leithner A, Lopez-Pousa A, Martin-Broto J, Merimsky O, Messiou C, Mir O, Montemurro M, Morland B, Morosi C, Palmerini E, Pantaleo MA, Piana R, Piperno-Neumann S, Reichardt P, Rutkowski P, Safwat AA, Sangalli C, Sbaraglia M, Scheipl S, Schöffski P, Sleijfer S, Strauss D, Strauss S, Sundby Hall K, Trama A, Unk M, van de Sande MAJ, van der Graaf WTA, van Houdt WJ, Frebourg T, Casali PG, Stacchiotti S, ESMO Guidelines Committee, EURACAN and GENTURIS. Electronic address: clinicalguidelines@esmo.org. Soft tissue and visceral sarcomas: ESMO-EURACAN-GENTURIS Clinical Practice Guidelines for diagnosis, treatment and follow-up(☆). Annals of oncology : official journal of the European Society for Medical Oncology. 2021 Nov:32(11):1348-1365. doi: 10.1016/j.annonc.2021.07.006. Epub 2021 Jul 22 [PubMed PMID: 34303806]
Level 1 (high-level) evidenceAriizumi T, Kawashima H, Yamagishi T, Oike N, Murayama Y, Umezu H, Endo N, Ogose A. Diagnostic accuracy of fine needle aspiration cytology and core needle biopsy in bone and soft tissue tumor: A comparative study of the image-guided and blindly performed procedure. Annals of diagnostic pathology. 2022 Aug:59():151936. doi: 10.1016/j.anndiagpath.2022.151936. Epub 2022 Mar 18 [PubMed PMID: 35427924]
Level 2 (mid-level) evidenceNascimento AF, Raut CP. Diagnosis and management of pleomorphic sarcomas (so-called "MFH") in adults. Journal of surgical oncology. 2008 Mar 15:97(4):330-9. doi: 10.1002/jso.20972. Epub [PubMed PMID: 18286476]
Hornick JL. Cutaneous soft tissue tumors: how do we make sense of fibrous and "fibrohistiocytic" tumors with confusing names and similar appearances? Modern pathology : an official journal of the United States and Canadian Academy of Pathology, Inc. 2020 Jan:33(Suppl 1):56-65. doi: 10.1038/s41379-019-0388-4. Epub 2019 Oct 25 [PubMed PMID: 31653978]
Henderson MT, Hollmig ST. Malignant fibrous histiocytoma: changing perceptions and management challenges. Journal of the American Academy of Dermatology. 2012 Dec:67(6):1335-41. doi: 10.1016/j.jaad.2012.04.013. Epub 2012 Jun 5 [PubMed PMID: 22677489]
Gronchi A, Lo Vullo S, Colombo C, Collini P, Stacchiotti S, Mariani L, Fiore M, Casali PG. Extremity soft tissue sarcoma in a series of patients treated at a single institution: local control directly impacts survival. Annals of surgery. 2010 Mar:251(3):506-11. doi: 10.1097/SLA.0b013e3181cf87fa. Epub [PubMed PMID: 20130465]
Yang JC, Chang AE, Baker AR, Sindelar WF, Danforth DN, Topalian SL, DeLaney T, Glatstein E, Steinberg SM, Merino MJ, Rosenberg SA. Randomized prospective study of the benefit of adjuvant radiation therapy in the treatment of soft tissue sarcomas of the extremity. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 1998 Jan:16(1):197-203 [PubMed PMID: 9440743]
Level 1 (high-level) evidenceBonvalot S,Gronchi A,Le Péchoux C,Swallow CJ,Strauss D,Meeus P,van Coevorden F,Stoldt S,Stoeckle E,Rutkowski P,Rastrelli M,Raut CP,Hompes D,De Paoli A,Sangalli C,Honoré C,Chung P,Miah A,Blay JY,Fiore M,Stelmes JJ,Dei Tos AP,Baldini EH,Litière S,Marreaud S,Gelderblom H,Haas RL, Preoperative radiotherapy plus surgery versus surgery alone for patients with primary retroperitoneal sarcoma (EORTC-62092: STRASS): a multicentre, open-label, randomised, phase 3 trial. The Lancet. Oncology. 2020 Oct; [PubMed PMID: 32941794]
Level 1 (high-level) evidenceItaliano A, Delva F, Mathoulin-Pelissier S, Le Cesne A, Bonvalot S, Terrier P, Trassard M, Michels JJ, Blay JY, Coindre JM, Bui B. Effect of adjuvant chemotherapy on survival in FNCLCC grade 3 soft tissue sarcomas: a multivariate analysis of the French Sarcoma Group Database. Annals of oncology : official journal of the European Society for Medical Oncology. 2010 Dec:21(12):2436-2441. doi: 10.1093/annonc/mdq238. Epub 2010 May 3 [PubMed PMID: 20439343]
Gronchi A, Palmerini E, Quagliuolo V, Martin Broto J, Lopez Pousa A, Grignani G, Brunello A, Blay JY, Tendero O, Diaz Beveridge R, Ferraresi V, Lugowska I, Merlo DF, Fontana V, Marchesi E, Braglia L, Donati DM, Palassini E, Bianchi G, Marrari A, Morosi C, Stacchiotti S, Bagué S, Coindre JM, Dei Tos AP, Picci P, Bruzzi P, Casali PG. Neoadjuvant Chemotherapy in High-Risk Soft Tissue Sarcomas: Final Results of a Randomized Trial From Italian (ISG), Spanish (GEIS), French (FSG), and Polish (PSG) Sarcoma Groups. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2020 Jul 1:38(19):2178-2186. doi: 10.1200/JCO.19.03289. Epub 2020 May 18 [PubMed PMID: 32421444]
Level 1 (high-level) evidencePasquali S, Pizzamiglio S, Touati N, Litiere S, Marreaud S, Kasper B, Gelderblom H, Stacchiotti S, Judson I, Dei Tos AP, Verderio P, Casali PG, Woll PJ, Gronchi A, EORTC – Soft Tissue and Bone Sarcoma Group. The impact of chemotherapy on survival of patients with extremity and trunk wall soft tissue sarcoma: revisiting the results of the EORTC-STBSG 62931 randomised trial. European journal of cancer (Oxford, England : 1990). 2019 Mar:109():51-60. doi: 10.1016/j.ejca.2018.12.009. Epub 2019 Jan 25 [PubMed PMID: 30690293]
Level 1 (high-level) evidenceJudson I, Verweij J, Gelderblom H, Hartmann JT, Schöffski P, Blay JY, Kerst JM, Sufliarsky J, Whelan J, Hohenberger P, Krarup-Hansen A, Alcindor T, Marreaud S, Litière S, Hermans C, Fisher C, Hogendoorn PC, dei Tos AP, van der Graaf WT, European Organisation and Treatment of Cancer Soft Tissue and Bone Sarcoma Group. Doxorubicin alone versus intensified doxorubicin plus ifosfamide for first-line treatment of advanced or metastatic soft-tissue sarcoma: a randomised controlled phase 3 trial. The Lancet. Oncology. 2014 Apr:15(4):415-23. doi: 10.1016/S1470-2045(14)70063-4. Epub 2014 Mar 5 [PubMed PMID: 24618336]
Level 1 (high-level) evidenceNivolumab with or without ipilimumab treatment for metastatic sarcoma (Alliance A091401): two open-label, non-comparative, randomised, phase 2 trials., D'Angelo SP,Mahoney MR,Van Tine BA,Atkins J,Milhem MM,Jahagirdar BN,Antonescu CR,Horvath E,Tap WD,Schwartz GK,Streicher H,, The Lancet. Oncology, 2018 Mar [PubMed PMID: 29370992]
Level 1 (high-level) evidencevon Mehren M, Kane JM, Bui MM, Choy E, Connelly M, Dry S, Ganjoo KN, George S, Gonzalez RJ, Heslin MJ, Homsi J, Keedy V, Kelly CM, Kim E, Liebner D, McCarter M, McGarry SV, Meyer C, Pappo AS, Parkes AM, Paz IB, Petersen IA, Poppe M, Riedel RF, Rubin B, Schuetze S, Shabason J, Sicklick JK, Spraker MB, Zimel M, Bergman MA, George GV. NCCN Guidelines Insights: Soft Tissue Sarcoma, Version 1.2021. Journal of the National Comprehensive Cancer Network : JNCCN. 2020 Dec 2:18(12):1604-1612. doi: 10.6004/jnccn.2020.0058. Epub 2020 Dec 2 [PubMed PMID: 33285515]
Cates JMM. The AJCC 8th Edition Staging System for Soft Tissue Sarcoma of the Extremities or Trunk: A Cohort Study of the SEER Database. Journal of the National Comprehensive Cancer Network : JNCCN. 2018 Feb:16(2):144-152. doi: 10.6004/jnccn.2017.7042. Epub [PubMed PMID: 29439175]
Winchester D, Lehman J, Tello T, Chimato N, Hocker T, Kim S, Chang J, Markey J, Yom SS, Ryan W, Mully T, Hodge D, Otley C, Arron ST. Undifferentiated pleomorphic sarcoma: Factors predictive of adverse outcomes. Journal of the American Academy of Dermatology. 2018 Nov:79(5):853-859. doi: 10.1016/j.jaad.2018.05.022. Epub 2018 May 19 [PubMed PMID: 29787841]
Vodanovich DA, Spelman T, May D, Slavin J, Choong PFM. Predicting the prognosis of undifferentiated pleomorphic soft tissue sarcoma: a 20-year experience of 266 cases. ANZ journal of surgery. 2019 Sep:89(9):1045-1050. doi: 10.1111/ans.15348. Epub 2019 Jul 30 [PubMed PMID: 31364245]
Level 3 (low-level) evidenceDineen SP, Roland CL, Feig R, May C, Zhou S, Demicco E, Sannaa GA, Ingram D, Wang WL, Ravi V, Guadagnolo A, Lev D, Pollock RE, Hunt K, Cormier J, Lazar A, Feig B, Torres KE. Radiation-Associated Undifferentiated Pleomorphic Sarcoma is Associated with Worse Clinical Outcomes than Sporadic Lesions. Annals of surgical oncology. 2015 Nov:22(12):3913-20. doi: 10.1245/s10434-015-4453-z. Epub 2015 Mar 6 [PubMed PMID: 25743327]
Level 2 (mid-level) evidence