Introduction
Micropenis is an objective diagnosis characterized by a stretched penile length that is more than 2.5 SD below the mean compared to age-matched normal values. The penis appears otherwise normal in appearance, function, and structure.[1][2] The diagnostic criteria vary depending on age, geographic region, and ethnicity.[2][3][4][5][6]
In cases of micropenis, the scrotum, testicles, median raphe, foreskin, and the position of the urethral meatus are typically normal.[7] The condition is usually diagnosed at birth or during early childhood.[1]
As a general guide, the stretched penile lengths that generally suggest a micropenis are listed below.
- Newborns or babies: Less than 1.9 cm.[2][4]
- Prepubertal boys: 3.8 cm or less.[2][8]
- Adults: Less than 7.5 cm.[9][10][11][12]
More detailed information on age-specific stretched penile lengths can be found in the History and Physical section below.
Given the significant psychological distress and anxiety associated with the condition, timely and accurate diagnosis of micropenis is essential. Once the diagnosis is confirmed, treatment should be initiated promptly and coordinated by a multidisciplinary healthcare team.
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
The human penis originates from the genital tubercle. By 5 to 6 weeks of gestation, this structure appears as a bulge or elevation of the perineum, known as the genital swellings, located on either side of the cloacal membrane.[4][13][14] These swellings comprise the following 3 germ layers:
- Ectoderm, which forms the penile skin and prepuce
- Mesoderm, which develops into the corpora cavernosa
- Endoderm, which gives rise to the penile urethra [4][14]
In humans, chromosomal sex is determined at fertilization as either female (XX) or male (XY). Early in fetal development, the embryo possesses both mesonephric (Wolffian) ducts, which can develop into male reproductive structures, and paramesonephric (Müllerian) ducts, which can develop into female reproductive structures. The presence or absence of the SRY gene, located on the Y chromosome, determines the regression or development of these embryonic structures during organogenesis. The SRY gene stimulates gonadal differentiation into testicular tissue in males. Please see StatPearls' companion resources, "Embryology, Mullerian-Inhibiting Factor" and "Embryology, Wolffian Ducts," for more information.
At this early stage of development, the testes secrete 3 essential hormones that are crucial for the sexual differentiation process in males, as listed below.
- Anti-Müllerian hormone (AMH): AMH is secreted by the Sertoli cells in the testes and prevents the development of female reproductive organs by promoting the regression of the Müllerian (paramesonephric) ducts in males. Please see StatPearls' companion resources, "Embryology, Mullerian-Inhibiting Factor" and "Embryology, Mullerian Ducts (Paramesonephric Ducts)," for more information.
- Dihydrotestosterone (DHT): DHT is responsible for the development of male sexual characteristics, including scrotal maturation, penile growth, and testicular enlargement. Please see StatPearls' companion resource, "Biochemistry, Dihydrotestosterone," for more information.
- Testosterone: This hormone stimulates the development of the mesonephric ducts into the internal male reproductive structures, including the seminal vesicles, vas deferens, and epididymis. Please see StatPearls' companion resource, "Physiology, Testosterone," for more information.
Under the influence of these three hormones, the male reproductive and genitourinary systems begin to develop during the late first trimester, between 8 and 12 weeks of gestation. Please see StatPearls' companion resources, "Embryology, Mullerian-Inhibiting Factor," "Embryology, Wolffian Ducts," and "Embryology, Urethral Folds," for more information.
Maternal human chorionic gonadotropin (hCG) stimulates Leydig cells to produce testosterone, which is then metabolized in utero into the more potent DHT. High levels of fetal androgens during the second trimester further accelerate penile growth and anatomical development.[15] Micropenis typically results from reduced testosterone production in utero during the third trimester (usually beginning around 12 weeks), assuming normal development up to that time.[8] Please see StatPearls' companion resources, "Biochemistry, Dihydrotestosterone" and "Embryology, Sexual Development," for more information.
In the postnatal period, further development of male sexual characteristics is regulated by hormones controlled by the hypothalamic-pituitary axis, including gonadotropin-releasing hormone (GnRH), follicle-stimulating hormone (FSH), and luteinizing hormone (LH).[8] The highest levels of postnatal androgens occur during the so-called "mini-puberty" period, which begins after the first month of life and typically lasts until around 6 months of age.[16][17][18][19] Disruptions at any point in this hormonal cascade may lead to undervirilization, genital abnormalities, or micropenis.
Androgen deficiency before 12 weeks of gestation typically causes genital ambiguity, whereas deficiency occurring after 12 weeks results in isolated micropenis.[8] Micropenis usually arises from insufficient fetal testosterone production after the 12th week of gestation and/or during the postnatal “mini-puberty” period within the first 3 months of life.[2][8] The most common cause of reduced fetal and neonatal testosterone is hypothalamic or pituitary dysfunction leading to hypogonadotropic hypogonadism, followed by primary testicular failure, which presents as hypergonadotropic hypogonadism.[2][8][4][20]
Micropenis may occur with any of the following conditions:
- 5-Alpha (5α)-reductase deficiency. (Please see StatPearls' companion resource, "5-Alpha-Reductase Deficiency," for more information.)
- 17-Beta hydroxysteroid dehydrogenase (17β-HSD) deficiency.[16]
- Addison disease. (Please see StatPearls' companion resource, "Addison Disease," for more information.)
- Androgen insensitivity due to receptor defects and/or resistance. (Please see StatPearls' companion resource, "Androgen Insensitivity Syndrome," for more information.)
- Anorchia (absence of testes).
- Congenital hypogonadotropic hypogonadism. (Please see StatPearls' companion resource, "Hypopituitarism," for more information.)
- Down syndrome (Please see StatPearls' companion resource, "Down Syndrome," for more information.)
- Fetal exposure to pesticides and other toxic chemicals.[21]
- Gonadal dysgenesis. (Please see Statpearls' companion resource, "Ambiguous Genitalia and Disorders of Sexual Differentiation," for more information.)
- Growth hormone deficiency.[22]
- Hypopituitarism, which is characterized by hypoglycemia, hypogonadotropic hypogonadism, and growth disturbances. (Please see StatPearls' companion resource, "Hypopituitarism," for more information.)
- Kallmann syndrome, which is characterized by hypogonadotropic hypogonadism, osteoporosis, hearing impairment, and anosmia. (Please see StatPearls' companion resource, "Kallmann Syndrome," for more information.)
- Kleefstra syndrome.[23]
- Klinefelter syndrome (47,XXY). This is associated with small testicles, infertility, gynecomastia, poor coordination, and reading difficulties.[24] (Please see StatPearls' companion resource, "Klinefelter Syndrome," for more information.)
- Lawrence-Moon-Biedl syndrome.[25][26]
- H-receptor defects.[16]
- Noonan syndrome, which is characterized by hypertelorism, short neck, low-set ears, skeletal malformation, bleeding disorders, and pulmonary valve stenosis. (Please see StatPearls' companion resource, "Noonan Syndrome," for more information.)
- Primary testicular failure.
- Prader-Willi syndrome, which is characterized by hypotonia, obesity, intellectual disabilities, undescended testes, micropenis, small hands and feet (Please see StatPearls' companion resource, "Prader-Willi Syndrome," for more information.)
- Rare forms of congenital adrenal hyperplasia (deficiency in steroidogenic acute regulatory protein [STAR], 3-beta hydroxysteroid dehydrogenase [3β-HSD], and 17α-hydroxylase). (Please see StatPearls' companion resources, "17-Hydroxylase Deficiency" and "Congenital Adrenal Hyperplasia," for more information.)
- Robinow syndrome, which is a rare inherited disorder primarily affecting the skeleton, is characterized by short arms, legs, and fingers, genital anomalies, and facial distortion.[27]
- Silver-Russell syndrome (also known as Russell-Silver syndrome), which is a rare genetic disorder characterized by dwarfism resulting from intrauterine growth restriction. This is characterized by poor postnatal growth, a relatively large head, triangular facial features, a prominent forehead, and body asymmetry. Intelligence is typically unaffected.[28][29][30]
- Trisomies of chromosomes 8, 13, 18, and 21. (Please see StatPearls' companion resources, "Trisomy 13" and "Edwards Syndrome," for more information.)
- Vanishing testes syndrome.[2][8][31][32]
In some cases, the etiology remains unclear even after performing an extensive evaluation.
Epidemiology
The incidence of micropenis in North America is approximately 1.5 per 10,000 male newborns.[33] Globally, the average incidence is estimated at 0.6% of all males, or about 1 in every 300 male births.[34] However, reported rates vary widely due to differences in diagnostic criteria across countries and study populations.[6][34]
Research indicates statistically significant differences in mean penile length among newborns of different ethnic backgrounds—Black newborns (3.2–3.5 cm), Caucasians (2.6 cm), East Indians (2.5 cm), and Chinese (2.3 cm).[3][6][35][36] Other studies have proposed population-specific penile length standards for Brazilian, Indian, Japanese, and Turkish infants and children.[5][8][37][38][39][40]
A 2024 systematic review and meta-analysis confirmed that mean stretched penile length and flaccid circumference vary significantly across geographical regions. The longest mean stretched penile lengths were observed in individuals from North and South America (9.86 cm), followed by those from Europe (9.71 cm), the Eastern Mediterranean (9.3 cm), Africa (9.22 cm), Southeast Asia (8.21 cm), and the Western Pacific region (8 cm).[5] These findings indicate that the stretched penile length threshold for diagnosing micropenis varies substantially by geographical region.[5]
A recent systematic review and meta-analysis found that the average global penile length has gradually increased over time, with a 24% rise observed over the past 29 years.[41]
History and Physical
The clinical diagnosis of micropenis is typically made following a thorough history and physical examination. Once diagnosed, additional testing is warranted to identify any associated abnormalities and determine the underlying etiology. A detailed maternal history is the first step in evaluating micropenis. Factors such as consanguinity and a family history of ambiguous genitalia increase the likelihood of inherited conditions. In addition, it is also important to inquire about maternal medication use, as exposure to anti-androgens (eg, flutamide, testolactone, enzalutamide, and spironolactone) during pregnancy can disrupt in-utero virilization.
Physical examination is the most critical component in evaluating micropenis. Signs of undervirilization associated with low blood pressure and tachycardia may suggest adrenal insufficiency related to rare forms of congenital adrenal hyperplasia (CAH). Accurate measurement of stretched penile length is essential during the physical examination. The penile shaft should be fully stretched, and the length measured dorsally from the pubic symphysis—after compressing the suprapubic fat pad—to the tip of the glans, with the foreskin retracted as much as possible. Taking the average of multiple measurements helps improve accuracy.
Stretched Penile Length
Various techniques have been described for measuring stretched penile length in neonates and infants.[42] These include the use of rigid rulers, flexible measuring tapes, vernier calipers, cotton swabs, wooden spatulas, tongue depressors, modified syringes with suction, and ultrasonography.[42] Proper retraction of the foreskin is essential to accurately identify the distal landmark—the tip of the glans.[42] If adequate foreskin retraction is not possible, the measurement will be less accurate. For instance, in infants with phimosis or redundant foreskin that limits glans exposure, the final measurement may only serve as an approximate estimate.[42]
- Variations in the degree of stretching applied can create inaccuracies in the measurements.[42]
- When using a ruler or a flexible tape, parallax errors may occur.[42]
- Most rulers and measuring tapes have the zero mark a few millimeters from the actual edge, which must be considered during measurement.[42]
Having an assistant greatly aids in obtaining an accurate measurement of stretched penile length using tools such as a flexible tape measure, ruler, spatula, tongue depressor, cotton swab, or the syringe and suction technique.[42][43][44][45][46][47]
A rigid ruler is the most commonly used and simplest method for measuring a stretched penile length.[42] The ruler allows for depression of the prepubic fat pad and alignment along the stretched penis. An assistant can use a second ruler placed perpendicularly to gently press against the glans, helping to accurately identify the distal extension.[42] If the penis has curvature, such as from chordee, a flexible tape measure may be preferred, although it typically requires assistance for accurate measurement.[42][48][49] Vernier calipers are not specifically designed for this anatomical application and must be used with caution to avoid injury.[42][50]
The penis should be stretched until increased resistance is felt to ensure consistency in measurement.[42][43][44][51] Accurately measuring stretched penile length in a crying or squirming newborn can be difficult, and the assistance of a second person is often essential when using any of the standard techniques.[42][43][44][45][46][47] The commonly used methods for measuring stretched penile length in neonates are discussed below.
Rigid Marker Technique
- While holding and stretching an infant's penis with one hand, the examiner places a tongue depressor at the base of the pubic symphysis.[42]
- The examiner then grasps the tongue depressor with the same hand along with the stretched penis.
- A marker is used to indicate the distal tip of the penis on the tongue depressor.
- The marked distance on the tongue depressor is subsequently measured.
- Having an assistant perform the marking can simplify the process.
- Alternatively, a cotton swab or wooden spatula may be used in place of the tongue depressor.
Syringe and Suction Technique
- A 10 mL disposable syringe is used by first removing the piston and cutting off the entire luer lock end, leaving only the cylinder.
- The piston is then reinserted into the cut end of the cylinder, and the flanged, uncut end is placed over the penis.
- The modified syringe is firmly pressed onto the pubic area.
- By gently withdrawing the piston, a mild suction is created, which draws the penis into the syringe barrel while excluding scrotal tissue and prepubic fat.
- The clinician or an assistant can then mark the length of the penis on the syringe cylinder with a marker for later measurement.
- The syringe and suction method has been suggested to be technically simple, more reproducible, and less operator-dependent than other techniques for measuring stretched penile length.[42][52]
Ultrasonography
- Ultrasonography can be used to accurately and reliably measure stretched penile length.[42][53]
- Ultrasound is generally considered more accurate than manual measurement methods.[42]
- This technique offers reproducibility, eliminates variability from manual traction, and is not affected by factors such as obesity, phimosis, body habitus, redundant foreskin, or excessive prepubic fat.[12][42][53][54]
- Ultrasound may be the optimal measurement modality for patients with a buried penis, extreme obesity, excessive foreskin, severe phimosis, or a webbed scrotum.[42]
- Prenatal measurement of fetal penile length is also possible using ultrasonography.[55]
A stretched penile length measuring less than 2.5 SD below the mean for the corresponding age and geographic region confirms the diagnosis of micropenis.[2] After age 5, penile growth is typically minimal until testosterone levels increase at puberty. Please see StatPearls' companion resource, "Physiology, Puberty," for more information.
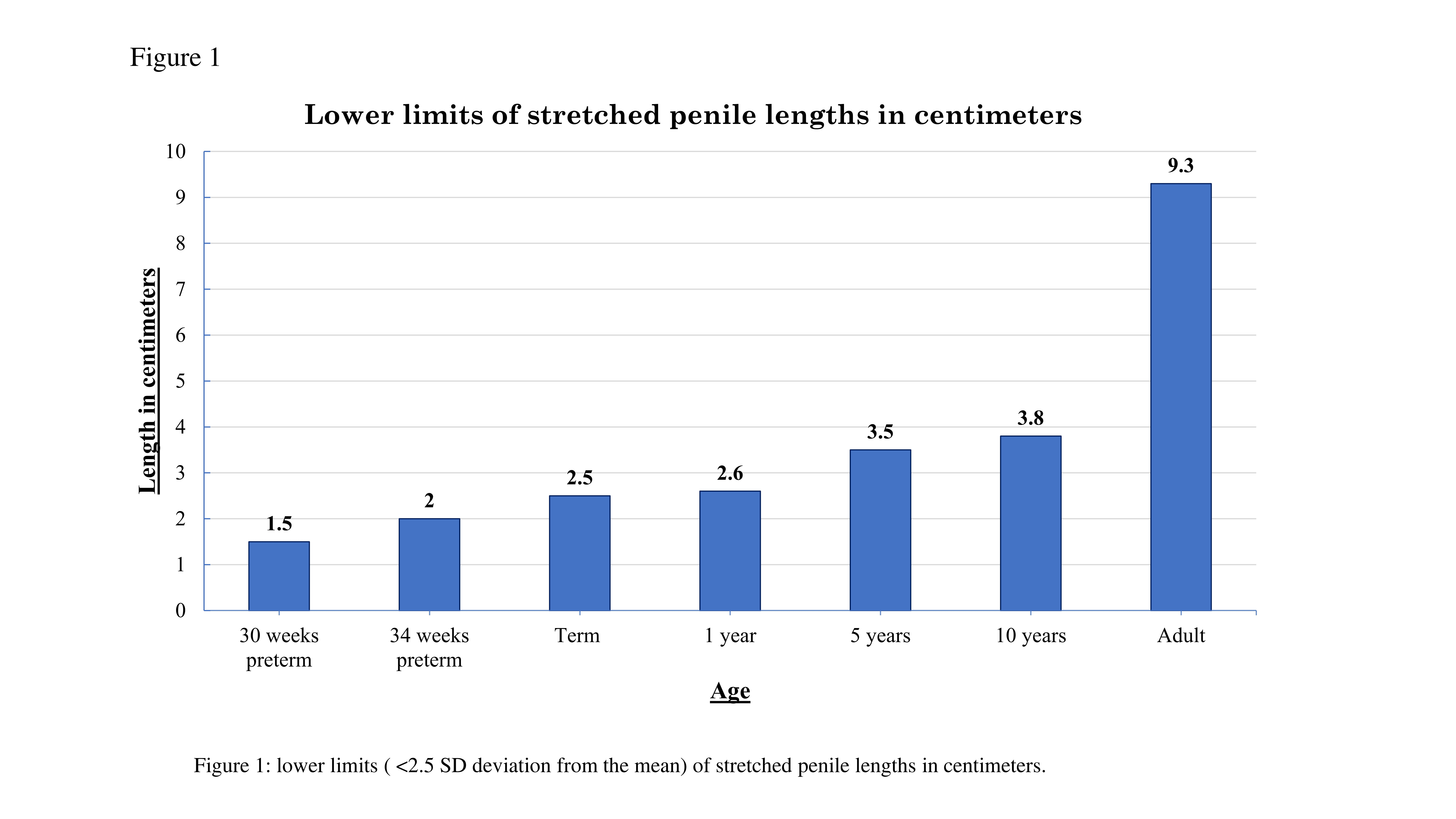
Suggested lower normal limits of stretched penile lengths (in centimeters) according to age are listed below (see Image 1. Age-Specific Lower Limits [<2.5 SD Below the Mean] of Stretched Penile Lengths [in centimeters]).[2][9][10][11][12][39][56][57]
- Preterm infants born at 30 weeks of gestation: 1.5 cm
- Preterm infants born at 34 weeks of gestation: <2 cm
- Term infants: 2.5 cm
- Individuals aged 1: 2.6 cm
- Individuals aged 5: 3.5 cm
- Individuals aged 10: 3.8 cm
- Adults: 7.5 cm
As average penile size varies significantly across geographic regions, an international consensus recommends using a stretched penile length of less than 2 cm at birth and less than 4 cm after age 5 as a reasonable global standard for defining micropenis.[8]
Careful and comprehensive palpation of the gonads is a crucial step in evaluating micropenis. Key aspects to assess and document include the appearance and maturity of the scrotal sac, the size and location of both testes, the presence and position of the urethral meatus, any curvature of the penile shaft, and any associated dysmorphic features. Additional areas to examine include facial anomalies, skin hyperpigmentation, hypertension, unusual height, gynecomastia, and abnormalities in hearing or smell.[8]
The majority of prepubertal boys brought in by their parents for evaluation of possible micropenis are obese, with significant suprapubic fat pads and a buried but normally sized penis.
A diagnosis of micropenis can be reasonably made based on stretched penile length relative to age, as follows:
- In full-term neonates, a stretched penile length of less than 1.9 cm indicates congenital micropenis.[2][4]
- At the age of 5, a stretched penile length of less than 4 cm is considered a micropenis.[2][8]
- In adults, a stretched penile length of less than 7.5 cm (2.95 inches) confirms the diagnosis.[9][10][11][12]
Evaluation
Once a physical examination confirms the presence of micropenis, further evaluation is essential to identify the underlying etiology and guide the treatment plan. A standardized diagnostic approach typically includes the components mentioned below.[2][8]
- Baseline hormonal panel: The levels of DHT, growth hormone, LH, FSH, testosterone, and androstenedione (a precursor sex hormone) should be assessed.[2][7][8]
- Evaluation of congenital adrenal dysfunctions: The levels of progesterone, 17-OH progesterone, androstenedione, and 17-hydroxypregnenolone should be assessed to rule out rare types of congenital adrenal hyperplasia associated with male undervirilization.[2][8]
- Serum glucose assessment: Hypoglycemia may indicate hypopituitarism. If detected, serum glucose, electrolytes, insulin, thyroid-stimulating hormone (TSH), thyroxine (T4), and cortisol levels should be evaluated for potential cortisol deficiency. Please see StatPearls' companion resource, "Addison Disease," for more information.[58][59]
- Counterregulatory hormone assessment: Cortisol and human growth hormone have essential roles in maintaining serum glucose levels. Deficiency in one or both may contribute to hypoglycemia. Please see StatPearls' companion resources, "Neonatal Hypoglycemia" and "Hypoglycemia," for more information.
- hCG stimulation test: This is used to evaluate gonadal function by measuring serum testosterone levels before and after hCG administration.[2][8][16][60][61]
- hCG is typically administered intramuscularly (IM) at a dosage of 1000 IU daily for 3 days or 1500 IU every other day for 2 weeks.
- A post-stimulation testosterone level below 300 ng/dL suggests gonadal dysgenesis.
- If LH and FSH levels are concurrently elevated and testosterone levels remain low, this may indicate testicular absence or primary testicular failure.
- Defects in testosterone synthesis can be assessed by measuring testosterone precursors, such as 17-hydroxyprogesterone (17-OH progesterone), dehydroepiandrosterone, and androstenedione, before and after administration of hCG.
- Inhibin B and AMH levels may be useful in selected cases to determine the presence of functional testicular tissue.[62] Low levels of AMH combined with normal inhibin B suggest persistent Müllerian duct syndrome.[62]
- Karyotype analysis is recommended to confirm a 46,XY genotype.[8]
- Pelvic ultrasound is used to evaluate the gonads and internal genital structures. The presence of female internal genitalia may indicate a virilized phenotypic female (46,XX genotype), which is often associated with 21-hydroxylase deficiency. Please see Statpearls' companion resources, "Ambiguous Genitalia and Disorders of Sexual Differentiation," "21-Hydroxylase Deficiency," and "Congenital Adrenal Hyperplasia," for more information.
- Pituitary gland hypofunction (hypopituitarism) should be suspected if there is evidence of hormonal dysfunction or hypoglycemia (Please see StatPearls' companion resource, "Hypopituitarism," for more information.)[63][64]
If both LH and testosterone levels are elevated, the diagnosis can be confirmed by examining DHT levels and the testosterone-to-DHT ratio.[34]
- A low DHT level with an elevated testosterone-to-DHT ratio suggests 5α-reductase deficiency, as the enzyme is responsible for converting testosterone into DHT.[34][65] Please see StatPearls' companion resource, "5-Alpha-Reductase Deficiency," for more information.
- A high DHT level with a low testosterone-to-DHT ratio points to partial androgen insensitivity syndrome. Please see StatPearls' companion resource, "Androgen Insensitivity Syndrome," for more information.[34][66]
Normal serum levels of inhibin B and AMH (both produced by active Sertoli cells) indicate the presence of functional testicular tissue.[16][62] Low AMH levels combined with normal inhibin B levels suggest persistent Müllerian duct syndrome.[16][67]
Findings of associated hypospadias, cryptorchidism, or incomplete scrotal fusion suggest a disorder of sexual development. Please see Statpearls' companion resource, "Ambiguous Genitalia and Disorders of Sexual Differentiation," for more information.
Congenital Hypopituitarism
Congenital hypopituitarism is rare, with an estimated incidence of 1 in 4000 to 1 in 10,000 births.[68] The condition usually presents with nonspecific findings, which makes early diagnosis challenging.[58][59] Hypoglycemia is often the first sign of hypopituitarism in newborns with micropenis.[58][59] Other common signs include prolonged neonatal jaundice, poor weight gain, abnormal electrolytes (notably, hyponatremia without hyperkalemia), hemodynamic instability, body temperature dysregulation, nystagmus, strabismus, short stature, optic nerve atrophy, cleft lip or palate, and septo-optic dysplasia.[63][64][68][69][70] Additional findings may include recurrent urinary tract infections, apnea, lethargy, seizures, polyuria, increased body fat, and midline central nervous system malformations.[59] Please see StatPearls' companion resource, "Hypopituitarism," for more information.
A diagnostic workup for neonatal hypopituitarism should include assessments of adrenocorticotropic hormone (ACTH), TSH, T4, LH, FSH, prolactin, cortisol, and growth hormone.[64] A magnetic resonance imaging (MRI) of the brain should be performed to evaluate the pituitary gland if hypopituitarism is suspected.[59][63]
- In cases of hypoglycemia, cortisol deficiency should be promptly investigated and quickly treated if diagnosed.[58][59]
- Birth weight is generally normal, as fetal growth is not usually affected.[58][59]
- Congenital hypopituitarism may result from developmental disorders of the pituitary gland, birth trauma, asphyxia, or genetic mutations, although the precise etiology of the disorder is not well understood.[58][64]
- Acquired forms of hypopituitarism are quite rare in neonates.
- Congenital hypopituitarism may involve isolated or multiple hormonal deficiencies.[71]
- Neonates exhibiting nystagmus should undergo an evaluation of pituitary function.[59][72]
- Genetic testing should also be considered to help determine the underlying cause.[58]
Congenital Growth Hormone Deficiency
Congenital growth hormone deficiency is the most common isolated pituitary disorder in childhood, with an estimated incidence of 1 in 3500 births in the United States.[58][59][73] Growth hormone stimulation testing is contraindicated in infants aged 1 or younger.[58]
Key clinical features of congenital growth hormone deficiency include hypoglycemia and micropenis.[58][59] Interestingly, fetal growth and birth weight are typically normal despite the deficiency.
Treatment / Management
Most children with micropenis achieve normal penile size and length by adulthood without treatment.[1][74] Monitoring the overall growth rate may be more significant than measuring and following the stretched penile length.[1] Body mass index (BMI) is the most significant predictor of eventual penile growth by adulthood, whereas baseline stretched penile length is surprisingly a less reliable indicator.[1][75]
Clinical trials evaluating medical and surgical interventions for micropenis have yielded variable results.[1] Treatment approach typically depends on the primary etiology, age at presentation, degree of penile atrophy, and desired outcome. A reasonable plan involves initiating medical therapy first, followed by surgical intervention if medical treatment fails to achieve the desired results. As many patients experience normalization of penis size after puberty, careful shared decision-making and thorough discussion of options are essential.[1][74]
The goals of micropenis management include:
- Ability to achieve normal sexual function.
- Preserve sperm count and fertility.
- Minimize social embarrassment related to micropenis.
- Ensure normal urinary function in the standing position.
Medical Treatment
The standard medical treatment of congenital micropenis would generally include:
- Testosterone is available as an IM injection or a topical gel for children with micropenis. Testosterone is administered either as an IM injection or a topical gel in children with micropenis. A commonly used, effective, traditional dosing regimen involves administering testosterone IM at a dosage of 25 mg every 3 weeks for a total duration of 3 months.[76][77] (B2)
- The use of 5% testosterone topical cream in children and infants aged 8 or younger has shown promising outcomes.[82]
- In a study, 45 out of 50 patients (90%) demonstrated a statistically significant increase in both penile length and serum testosterone levels after 30 days of treatment with the topical hormone cream.[82]
- Children aged 12 and older may be treated with IM hCG at a dose of 1500 to 2000 IU administered weekly for 6 weeks. Please refer to the "Mini-puberty" section below for further information.[78]
- DHT 2.5% gel, a potent metabolite of testosterone, has demonstrated efficacy primarily in case reports and small series, particularly in infants with 5α-reductase deficiency.[79][83][84]
- One protocol involved the topical application of DHT to the periscrotal region 3 times a day for 5 weeks.[2]
- In selected newborns, DHT therapy has been associated with an increase in stretched penile length of at least 120%.[84]
- Topical DHT may be considered in cases where standard testosterone therapy fails.[2][84]
- Caregivers, particularly mothers, should exercise caution when administering topical therapy by wearing gloves to prevent inadvertent skin absorption.
(B2)
- Boys with isolated growth hormone deficiency may benefit from hormonal supplements, which can promote penile growth along with other physiological effects.[20][85]
Human recombinant FSH and LH therapy may be considered in children with hypogonadotropic hypogonadism, where it has been shown to promote significant penile growth.[2][8][80][86][87](B3)
A favorable response would be at least a 3.5 cm increase or a doubling of the stretched penile length after the initial course of testosterone therapy.[2][60][77][88] A course of testosterone therapy can be repeated if the initial response is inadequate.[2][16][77] If there is still no response, topical DHT therapy should be considered.[2][84] Please see StatPearls' companion resource, "Kallmann Syndrome," for more information.
Patients with hypogonadotropic hypogonadism will experience a progressive decrease in penile androgen responsiveness, which naturally becomes more pronounced during early adulthood.[2][89] As the concentration of penile androgen receptors increases with early testosterone treatment, androgen therapy should optimally be initiated before the early adulthood period.[2][89]
Supplemental testosterone therapy is relatively ineffective for treating micropenis in adults or children after puberty.[81] However, initiating testosterone therapy at the onset of puberty may provide some benefit.[77] As most testosterone formulations are designed for adult male hypogonadism, clinicians should carefully monitor androgen levels in boys undergoing therapy to avoid supratherapeutic dosing.[90]
Many boys with micropenis caused by gonadotropin deficiency will not undergo normal puberty. In these cases, testosterone should be administered to induce puberty, with gradual dose increases to reach full adult replacement levels and closely mimic natural pubertal progression.[91] To preserve fertility, treatment with hCG and recombinant FSH is recommended to stimulate endogenous testosterone production and support spermatogenesis.[92][93][94][95] Ideally, this therapy should be managed under the supervision of a specialist in male fertility and reproductive medicine.(A1)
Mini-puberty
Mini-puberty is a normal, temporary phase of increased gonadotropin production that briefly reaches adult levels, beginning within the first few months of life.[17][18] During a "mini-puberty" phase, gonadotropin levels typically peak around 1 month after birth and persist until approximately 6 months of age.[19] This mini-puberty phase is absent in patients with congenital hypogonadotropic hypogonadism—a rare genetic disorder affecting approximately 1 in 4000 to 1 in 15,000 male births annually.[17][18] (B3)
Congenital hypogonadotropic hypogonadism is characterized by a deficiency in hypothalamic GnRH production during both fetal and postnatal development.[17][18][96] Kallmann syndrome accounts for about 50% of all cases of male congenital hypogonadotropic hypogonadism.[96] Patients with this condition do not experience a normal mini-puberty, which further contributes to their penile size disorder. Diagnosis is established by measuring serum gonadotropin and testosterone levels within the first 2 months after birth.[18][97] (B3)
Evidence suggests that gonadotropin replacement therapy to mimic the natural "mini-puberty" period can help normalize penile size.[17][18][98][99][100][101] This approach may also reduce the incidence of cryptorchidism and enhance future male fertility.[18][98] Clinically, patients with congenital hypogonadotropic hypogonadism may present with both micropenis and cryptorchidism, although the condition is usually not diagnosed until puberty.[17][102](A1)
Treatment of congenital hypogonadotropic hypogonadism includes the administration of recombinant LH and FSH during infancy.[8][87] The recommended regimen is 21.3 IU of FSH and 20 to 40 IU of LH twice weekly for about 7 months.[8] Alternatively, a continuous infusion may also be used, as both protocols have yielded promising results.[8][18][80][86][87][103][104](B3)
Experimental studies have shown that reducing lysyl oxidase activity using anti-lysyl oxidase, combined with passive penile stretching via an external vacuum erection device (VED), can promote penile lengthening through tunica albuginea remodeling in a rat model.[105][106] This effect was more pronounced in prepubertal rats compared to adults.[107] Anti-lysyl oxidase therapy, with or without VED treatment, did not impact erectile function or alter the microscopic structure of the corpus cavernosum.[107] A maximum penile length increase of nearly 20% was observed.[107] The addition of hCG had no significant effect on penile growth.[106](B2)
Combining testosterone therapy with growth hormone supplementation produced normal-sized penises in an animal model of micropenis—an outcome neither therapy achieved individually.[108]
Surgical Treatment
Penile reconstruction may be considered when medical treatment fails to produce adequate results. However, most patients with micropenis achieve a normal penis size by adulthood without surgical intervention.[1][74] Although surgery can provide initially satisfactory outcomes, many treated patients later report dissatisfaction with penile appearance.[1][74] Therefore, surgical intervention for micropenis in children is rarely indicated, given the typically favorable outcomes without surgery, potential procedural risks, and inconsistent patient satisfaction.[1][8][74]
The suspensory support of the penis is provided by 4 pelvic ligaments—the arcuate, dense vertical, fundiform, and suspensory ligaments.[109] Of these, the fundiform and suspensory ligaments can be surgically released without compromising the stability of an erect penis.[109] This procedure typically increases the average flaccid stretched penile length from 7.4 cm to 10.7 cm without affecting erectile function or stability.[109][110] However, penile lengthening alone is generally insufficient to achieve a penis suitable for sexual intercourse, and phalloplasty is often required in adults to achieve satisfactory results.[111]
Complete penile reconstruction (phalloplasty)
Phalloplasty with urethroplasty can be successfully performed in adults with micropenis who have realistic expectations, with generally favorable outcomes reported.[81][112][113][114][115][116][117][118][119][120] The addition of a penile prosthesis can restore erectile function in the reconstructed penis.[121][122][123][124][125] Overall patient satisfaction following phalloplasty ranges from approximately 77% to 95%.[126][127][128][129][130][131](A1)
According to the American Urological Association and the Urology Care Foundation, phalloplasty is a surgical procedure to reconstruct the penis. This procedure is generally appropriate for adult men with severe micropenis when the condition causes significant distress, dysfunction, or negatively affects quality of life. Phalloplasty is particularly recommended for individuals with a stretched penile length of less than 7.5 cm (2.95 inches).[9]
Various surgical techniques for total phalloplasty, such as radial artery–based forearm skin flaps, can restore normal penile length, achieve favorable cosmetic outcomes, and enable acceptable sexual function when combined with a penile prosthesis.[112][113][114][115][116][117][132] Additional flap options include the sensate osteocutaneous fibular, free (septocutaneous) fibular, free scapular, suprapubic abdominal wall, pedicled anterolateral thigh, musculocutaneous latissimus dorsi, and vertical rectus abdominis flaps.[133][134] Among these, the radial forearm flap remains the most commonly used technique, followed by the anterolateral thigh flap.[133][134](A1)
Urethral reconstruction (urethroplasty)
Urethroplasty can be performed using various techniques, including the integrated (tube-in-tube) method, superficial circumflex iliac artery perforator flap, buccal mucosa grafts, radial forearm flap, or the "Big Ben" 2-stage approach for urethral lengthening.[120][128][135][136] However, phalloplasty and urethroplasty procedures are associated with a high rate of complications—such as scarring, penile insensitivity, fistula formation, urethral strictures, and patient dissatisfaction—often necessitating surgical repairs and modifications.[125][128][137][138][139][140] The primary goal of urethral reconstruction is to enable the patient to void while standing following phalloplasty.(B3)
Optimal results in adult patients with severe micropenis are achieved when expectations are realistic. Many men face significant psychological challenges that can interfere with sexual activity, including poor self-image and the effects of previous traumatic sexual experiences.[34][119][137] Psychological support is recommended and often essential for addressing these issues.[34][118][137] (B2)
An overall lack of systematic studies exists that compares the different treatment options for micropenis in terms of overall outcomes, patient satisfaction, genital appearance, quality of life, sexual function and enjoyment, and psychosocial development. Most existing studies include diverse sexually dysmorphic diagnoses, have small sample sizes, and lack long-term follow-up.[4]
Gender Assignment in Cases of Micropenis
Most prepubertal males with micropenis and bilaterally descended functional testes respond well to testosterone supplementation therapy.[4][34][76][78][132][141]
Gender reassignment to female with genitoplasty surgery is usually not recommended for isolated micropenis.[132][142][143] However, it may be considered in exceptional circumstances, such as in individuals with incomplete androgen insensitivity syndrome. Even in these cases, the decision remains a matter of controversy.[132][142][143]
- The consensus among most experts and professional societies is that 46,XY male infants with isolated micropenis and functional testes should be raised as males, as they typically respond to testosterone therapy. An exception may be made in carefully selected cases involving incomplete androgen insensitivity syndrome.[132][142][143][144][145][146][147][148][149][150] (B3)
- Complete androgen insensitivity syndrome results in phenotypic females. The consensus is that individuals with this condition should be raised as females despite their 46,XY genotype.[146][150] The condition is typically diagnosed during evaluation for female infertility.[148] Please see StatPearls' companion resource, "Androgen Insensitivity Syndrome," for more information.
- Children with 5α-reductase deficiency and micropenis are likely to experience spontaneous penile lengthening at puberty and are therefore raised as males.[146] The enzyme 5α-reductase converts testosterone into the more potent DHT, which is necessary for the early development of the male penis.[146] Please see StatPearls' companion resources, "5-Alpha Reductase Deficiency" and "Biochemistry, Dihydrotestosterone," for more information.
Differential Diagnosis
Accurate diagnosis of micropenis requires careful distinction from other conditions that cause the appearance of a small penis for appropriate management and the prevention of unnecessary interventions.
- The most important differential diagnosis is pseudo-micropenis—a condition in which the penis appears small due to the unusual prominence of the surrounding peripubic tissue or the presence of a web that attaches the penis to the underlying scrotal skin.[2]
- A meticulous and comprehensive physical examination is essential to rule out pseudo-micropenis and avoid unnecessary invasive diagnostic evaluation with significant psychosocial stress and anxiety for the patient and his family.
- A buried penis can be mistaken for a micropenis.
- Chordee of the penis and hypospadias are surgical conditions that cause abnormal curvature of the penile shaft, which can lead to underestimation of penile length.
- Overly flaccid penile skin or foreskin, excessive fatty tissue, buried penis, and postoperative scarring from prior penile surgery can all interfere with obtaining an accurate measurement of stretched penile length.[2]
Prognosis
Micropenis carries a generally good prognosis, especially if detected and treated early.
Complications
Micropenis and phalloplasty surgery can be associated with various complications, including:
- Dissatisfaction with penile size, girth, function, or appearance in adulthood (which is often related to a lack of social and psychological support, poor surgical outcomes, or unrealistic preoperative expectations)
- Erectile dysfunction
- Flap necrosis
- Hematoma formation
- High rate of reoperation
- Infertility (often due to low sperm count or underlying hormonal disorders)
- Loss of the phalloplasty flap due to tissue necrosis (reported in <7% of cases) [127]
- Penile edema
- Poor self-image
- Postoperative scarring
- Psychogenic sexual dysfunction
- Unrecognized underlying pathologies
- Unsatisfactory cosmetic appearance
- Urethral fistulas (up to 50%) and strictures (up to 38%) [127]
- Various psychological issues
- Wound infection and dehiscence [127][151][152]
Deterrence and Patient Education
Early recognition of micropenis enables timely initiation of treatment, which can significantly improve the prognosis.[34] Notably, it is important to reassure families that the condition often resolves by adulthood, and if surgery is needed, it can be performed more safely and effectively at that time.
Young boys with micropenis may experience significant embarrassment and are at risk of bullying by peers. Therefore, healthcare providers should offer psychological support, counseling, and appropriate interventions to these individuals to help address emotional and social challenges.
Pearls and Other Issues
Key facts to keep in mind regarding micropenis include:
- Circumcision is not recommended in children with micropenis.[8]
- Elevated serum testosterone may indicate either androgen insensitivity syndrome (partial or incomplete) or 5-alpha reductase deficiency. The testosterone-to-DHT ratio can help differentiate between the two conditions.[8]
- The normal testosterone-to-DHT ratio is 2-3:1, but it can increase to as high as 26:1 following biochemical stimulation (hCG).
- If the stimulated testosterone-to-DHT ratio is 27 or more, it suggests 5α-reductase deficiency. See the companion StatPearls' resource, "Biochemistry, Dihydrotestosterone" and "5-Alpha-Reductase Deficiency," for more information.
- A stimulated testosterone-to-DHT ratio of 26 or lower is more consistent with partial androgen insensitivity syndrome. Please see StatPearls' companion resource, "Androgen Insensitivity Syndrome," for more information.[150]
- The normal testosterone-to-DHT ratio is 2-3:1, but it can increase to as high as 26:1 following biochemical stimulation (hCG).
- Elevated 17-OH progesterone levels suggest virilizing congenital adrenal hyperplasia.[8]
- An androstenedione-to-testosterone ratio greater than 20 indicate 17β-HSD type-3 deficiency.[8]
- Elevated deoxycorticosterone levels are associated with 17α-hydroxylase deficiency.[153]
- If hypogonadotropic hypogonadism is diagnosed, an MRI of the brain is suggested.[8]
- In prepubertal males with normal testes, testosterone treatment for micropenis is likely to be successful.
- Low levels of FSH, LH, and testosterone suggest the possibility of other hormonal abnormalities in the anterior pituitary. Evaluation should include growth hormone, insulin-like growth factor 1, cortisol, TSH, and free T4 levels, along with a brain MRI.[8]
- Testosterone therapy will be relatively ineffective in adult men and post-pubertal males.[81]
- The simultaneous presentation of micropenis and cryptorchidism suggests a diagnosis of congenital hypogonadotropic hypogonadism.[8][154]
- Topical DHT may be effective when topical testosterone therapy fails, particularly in patients with 5α-reductase deficiency.[2][79][84][155] Seethe companion StatPearls' resource, "5-Alpha-Reductase Deficiency," for more information.
Enhancing Healthcare Team Outcomes
Micropenis is a clinical finding that may indicate an underlying systemic hormonal abnormality or a genetic condition. As it can be a manifestation of various conditions, a comprehensive, multidisciplinary approach is essential for accurate diagnosis and effective management. The interdisciplinary healthcare team often includes adult and pediatric endocrinologists, urologists, plastic surgeons, and psychologists to address the medical, surgical, and psychosocial aspects of the condition.
Ancillary services, including those provided by geneticists, psychologists, social workers, and pharmacists, are essential for supporting families and maintaining the mental well-being of both patients and their families. As most cases of micropenis are identified during the early neonatal period, nursing assessments are often the first step in recognizing the condition and initiating further evaluation.
Although standardized evaluation and management guidelines have been proposed, the wide variability in presentation and underlying causes makes a comprehensive history and physical examination essential before initiating diagnostic testing. Medical and surgical treatments for micropenis generally yield satisfactory clinical outcomes; however, social and psychological dissatisfaction may persist, highlighting the need for ongoing psychosocial support.
Media
(Click Image to Enlarge)
References
Amirkashani D, Abdollahi Sarvi M, Masoumi M. Long-term outcomes of untreated micropenis: growth patterns and predictive factors. Frontiers in pediatrics. 2025:13():1501259. doi: 10.3389/fped.2025.1501259. Epub 2025 Apr 30 [PubMed PMID: 40370978]
Hatipoğlu N, Kurtoğlu S. Micropenis: etiology, diagnosis and treatment approaches. Journal of clinical research in pediatric endocrinology. 2013:5(4):217-23. doi: 10.4274/Jcrpe.1135. Epub [PubMed PMID: 24379029]
Cheng PK, Chanoine JP. Should the definition of micropenis vary according to ethnicity? Hormone research. 2001:55(6):278-81 [PubMed PMID: 11805431]
Stancampiano MR, Suzuki K, O'Toole S, Russo G, Yamada G, Faisal Ahmed S. Congenital Micropenis: Etiology And Management. Journal of the Endocrine Society. 2022 Feb 1:6(2):bvab172. doi: 10.1210/jendso/bvab172. Epub 2021 Nov 15 [PubMed PMID: 35036822]
Mostafaei H, Mori K, Katayama S, Quhal F, Pradere B, Yanagisawa T, Laukhtina E, König F, Motlagh RS, Rajwa P, Salehi-Pourmehr H, Hajebrahimi S, Shariat SF. A Systematic Review and Meta-Analysis of Penis Length and Circumference According to WHO Regions: Who has the Biggest One? Urology research & practice. 2025 Mar 7:50(5):291-301. doi: 10.5152/tud.2025.24038. Epub [PubMed PMID: 40248849]
Level 1 (high-level) evidenceLópez-Soto Á, Bueno-González M, Urbano-Reyes M, Garví-Morcillo J, Meseguer-González JL, Martínez-Uriarte J, García-Izquierdo O, Donate-Legaz JM, Leante-Castellanos JL, Martínez-Cendán JP. Stretched penile length at birth: a systematic review. Journal of pediatric endocrinology & metabolism : JPEM. 2021 Oct 26:34(10):1211-1223. doi: 10.1515/jpem-2021-0189. Epub 2021 Jul 29 [PubMed PMID: 34323056]
Level 1 (high-level) evidenceTsang S. When size matters: a clinical review of pathological micropenis. Journal of pediatric health care : official publication of National Association of Pediatric Nurse Associates & Practitioners. 2010 Jul-Aug:24(4):231-40. doi: 10.1016/j.pedhc.2009.05.001. Epub 2009 Jul 23 [PubMed PMID: 20620849]
Khadilkar V, Mondkar SA. Micropenis. Indian journal of pediatrics. 2023 Jun:90(6):598-604. doi: 10.1007/s12098-023-04540-w. Epub 2023 Apr 20 [PubMed PMID: 37079255]
Kayes O, Shabbir M, Ralph D, Minhas S. Therapeutic strategies for patients with micropenis or penile dysmorphic disorder. Nature reviews. Urology. 2012 Sep:9(9):499-507. doi: 10.1038/nrurol.2012.150. Epub 2012 Aug 14 [PubMed PMID: 22890302]
Ghanem H, Glina S, Assalian P, Buvat J. Position paper: Management of men complaining of a small penis despite an actually normal size. The journal of sexual medicine. 2013 Jan:10(1):294-303. doi: 10.1111/j.1743-6109.2012.02725.x. Epub 2012 Apr 18 [PubMed PMID: 22512935]
Veale D, Miles S, Bramley S, Muir G, Hodsoll J. Am I normal? A systematic review and construction of nomograms for flaccid and erect penis length and circumference in up to 15,521 men. BJU international. 2015 Jun:115(6):978-86. doi: 10.1111/bju.13010. Epub 2015 Mar 2 [PubMed PMID: 25487360]
Level 1 (high-level) evidenceWessells H,Lue TF,McAninch JW, Penile length in the flaccid and erect states: guidelines for penile augmentation. The Journal of urology. 1996 Sep; [PubMed PMID: 8709382]
Cohn MJ. Development of the external genitalia: conserved and divergent mechanisms of appendage patterning. Developmental dynamics : an official publication of the American Association of Anatomists. 2011 May:240(5):1108-15. doi: 10.1002/dvdy.22631. Epub 2011 Apr 4 [PubMed PMID: 21465625]
Baskin L, Shen J, Sinclair A, Cao M, Liu X, Liu G, Isaacson D, Overland M, Li Y, Cunha GR. Development of the human penis and clitoris. Differentiation; research in biological diversity. 2018 Sep-Oct:103():74-85. doi: 10.1016/j.diff.2018.08.001. Epub 2018 Aug 23 [PubMed PMID: 30249413]
Johnson P, Maxwell D. Fetal penile length. Ultrasound in obstetrics & gynecology : the official journal of the International Society of Ultrasound in Obstetrics and Gynecology. 2000 Apr:15(4):308-10 [PubMed PMID: 10895450]
Grumbach MM. A window of opportunity: the diagnosis of gonadotropin deficiency in the male infant. The Journal of clinical endocrinology and metabolism. 2005 May:90(5):3122-7 [PubMed PMID: 15728198]
Rohayem J, Alexander EC, Heger S, Nordenström A, Howard SR. Mini-Puberty, Physiological and Disordered: Consequences, and Potential for Therapeutic Replacement. Endocrine reviews. 2024 Jul 12:45(4):460-492. doi: 10.1210/endrev/bnae003. Epub [PubMed PMID: 38436980]
Swee DS, Quinton R. Congenital Hypogonadotrophic Hypogonadism: Minipuberty and the Case for Neonatal Diagnosis. Frontiers in endocrinology. 2019:10():97. doi: 10.3389/fendo.2019.00097. Epub 2019 Feb 21 [PubMed PMID: 30846970]
Level 3 (low-level) evidencede Zegher F, Devlieger H, Veldhuis JD. Pulsatile and sexually dimorphic secretion of luteinizing hormone in the human infant on the day of birth. Pediatric research. 1992 Nov:32(5):605-7 [PubMed PMID: 1480465]
Menon PS, Khatwa UA. The child with micropenis. Indian journal of pediatrics. 2000 Jun:67(6):455-60 [PubMed PMID: 10932967]
Gaspari L, Paris F, Jandel C, Kalfa N, Orsini M, Daurès JP, Sultan C. Prenatal environmental risk factors for genital malformations in a population of 1442 French male newborns: a nested case-control study. Human reproduction (Oxford, England). 2011 Nov:26(11):3155-62. doi: 10.1093/humrep/der283. Epub 2011 Aug 25 [PubMed PMID: 21868402]
Level 2 (mid-level) evidenceStagi S, Tufano M, Chiti N, Cerutti M, Li Pomi A, Aversa T, Wasniewska M. Management of Neonatal Isolated and Combined Growth Hormone Deficiency: Current Status. International journal of molecular sciences. 2023 Jun 14:24(12):. doi: 10.3390/ijms241210114. Epub 2023 Jun 14 [PubMed PMID: 37373261]
Lee R, Lee MS, Moon JE. A Korean male with Kleefstra syndrome presented with micropenis. Annals of pediatric endocrinology & metabolism. 2023 Dec:28(4):308-311. doi: 10.6065/apem.2244174.087. Epub 2023 Dec 31 [PubMed PMID: 38173384]
Shrestha A, Parajuli B, Pandit A. Case Report of 49,XXXXY Syndrome: A Rare Variation of Klinefelter Syndrome With Seizure Disorder and ASD. Clinical case reports. 2025 Mar:13(3):e70257. doi: 10.1002/ccr3.70257. Epub 2025 Feb 26 [PubMed PMID: 40018421]
Level 3 (low-level) evidenceKumar A, Husain A Sr, Saleem A, Khawaja UA, Virani S. Laurence-Moon-Bardet-Biedl Syndrome: A Rare Case With a Literature Review. Cureus. 2020 Nov 5:12(11):e11355. doi: 10.7759/cureus.11355. Epub 2020 Nov 5 [PubMed PMID: 33304690]
Level 3 (low-level) evidenceAbdulla AB, Niloy AA, Shah TA, Biswas SK, Imran AK, Murshed KM, Ahmed M. Laurence Moon Bardet Biedl Syndrome. Mymensingh medical journal : MMJ. 2009 Jan:18(1 Suppl):S124-128 [PubMed PMID: 19377420]
Goitia-Cárdenas M, Azotla-Vilchis CO, Miranda-Lora AL. Robinow syndrome and its response to growth hormone treatment: a case report and review of the literature. Boletin medico del Hospital Infantil de Mexico. 2023 Jul 12:80(Supl 1):040-046. doi: 10.24875/BMHIM.22000101. Epub 2023 Jul 12 [PubMed PMID: 36917807]
Level 3 (low-level) evidenceAdam MP, Feldman J, Mirzaa GM, Pagon RA, Wallace SE, Amemiya A, Saal HM, Harbison MD, Netchine I. Silver-Russell Syndrome. GeneReviews(®). 1993:(): [PubMed PMID: 20301499]
Galeva S, Diglio G, Stoilov B, Uchikova E, Pop L. Prenatal Detection of Silver-Russell Syndrome: A First Trimester Suspicion and Diagnostic Approach. Medicina (Kaunas, Lithuania). 2025 Jan 16:61(1):. doi: 10.3390/medicina61010145. Epub 2025 Jan 16 [PubMed PMID: 39859127]
Giabicani E, Perrière A, Netchine I. Silver-Russell Syndrome in 2025: Is It Still a Distinct Diagnostic Entity? The Journal of clinical endocrinology and metabolism. 2025 Jan 2:():. pii: dgae902. doi: 10.1210/clinem/dgae902. Epub 2025 Jan 2 [PubMed PMID: 39745816]
Dağdeviren Çakır A, Baş F, Akın O, Şıklar Z, Özcabı B, Berberoğlu M, Kardelen AD, Bayramoğlu E, Poyrazoğlu Ş, Aydın M, Törel Ergür A, Gökşen D, Bolu S, Aycan Z, Tüysüz B, Ercan O, Evliyaoğlu O. Clinical Characteristics and Growth Hormone Treatment in Patients with Prader-Willi Syndrome. Journal of clinical research in pediatric endocrinology. 2021 Aug 23:13(3):308-319. doi: 10.4274/jcrpe.galenos.2021.2020.0228. Epub 2021 Feb 10 [PubMed PMID: 33565750]
Wei X, Li S, He Y. NR5A1-related 46,XY partial gonadal dysgenesis: A case report and literature review. Medicine. 2023 Dec 29:102(52):e36725. doi: 10.1097/MD.0000000000036725. Epub [PubMed PMID: 38206718]
Level 3 (low-level) evidenceNelson CP, Park JM, Wan J, Bloom DA, Dunn RL, Wei JT. The increasing incidence of congenital penile anomalies in the United States. The Journal of urology. 2005 Oct:174(4 Pt 2):1573-6 [PubMed PMID: 16148654]
Al-Beltagi M, Saeed NK, Bediwy AS, Shaikh MA, Elbeltagi R. Microphallus early management in infancy saves adulthood sensual life: A comprehensive review. World journal of clinical pediatrics. 2024 Jun 9:13(2):89224. doi: 10.5409/wjcp.v13.i2.89224. Epub 2024 Jun 9 [PubMed PMID: 38947989]
Chikani UN, Chinawa JM, Ikefuna AN, Ibekwe MU. Stretched penile length of healthy term neonates: normative values among Igbo babies in southeastern Nigeria. Journal of tropical pediatrics. 2015 Feb:61(1):69-73. doi: 10.1093/tropej/fmu064. Epub 2014 Dec 1 [PubMed PMID: 25466913]
Kareem AJ, Elusiyan JBE, Kareem AO. Stretched penile length and total serum testosterone in term male neonates. The Pan African medical journal. 2020:37():61. doi: 10.11604/pamj.2020.37.61.21123. Epub 2020 Sep 15 [PubMed PMID: 33244324]
Gabrich PN, Vasconcelos JS, Damião R, Silva EA. Penile anthropometry in Brazilian children and adolescents. Jornal de pediatria. 2007 Sep-Oct:83(5):441-6. doi: 10.2223/JPED.1671. Epub 2007 Aug 3 [PubMed PMID: 17676248]
Level 2 (mid-level) evidenceMatsuo N, Ishii T, Takayama JI, Miwa M, Hasegawa T. Reference standard of penile size and prevalence of buried penis in Japanese newborn male infants. Endocrine journal. 2014:61(9):849-53 [PubMed PMID: 24931740]
Cinaz P, Yeşilkaya E, Onganlar YH, Boyraz M, Bideci A, Camurdan O, Karaoğlu AB. Penile anthropometry of normal prepubertal boys in Turkey. Acta paediatrica (Oslo, Norway : 1992). 2012 Jan:101(1):e33-6. doi: 10.1111/j.1651-2227.2011.02386.x. Epub 2011 Oct 4 [PubMed PMID: 21682766]
Level 2 (mid-level) evidenceNanda PM, Yadav J, Dayal D, Kumar R, Kumar P, Kumar J, Kaur H, Sikka P. Estimation of Reference Values for External Genitalia Parameters in North Indian Preterm and Term Male Newborns. Indian journal of pediatrics. 2024 Jun:91(6):556-563. doi: 10.1007/s12098-023-04703-9. Epub 2023 Jun 30 [PubMed PMID: 37389773]
Belladelli F, Del Giudice F, Glover F, Mulloy E, Muncey W, Basran S, Fallara G, Pozzi E, Montorsi F, Salonia A, Eisenberg ML. Worldwide Temporal Trends in Penile Length: A Systematic Review and Meta-Analysis. The world journal of men's health. 2023 Oct:41(4):848-860. doi: 10.5534/wjmh.220203. Epub 2023 Feb 15 [PubMed PMID: 36792094]
Level 1 (high-level) evidenceGoel P, Choudhury P, Saroya KK, Jain V, Dhua AK, Yadav DK, Anand S, Agarwala S, Sharma K, Agrawal V, Saha S, Singh H, Sharma N, Singh VP. Advancing Precision in Penile Length Measurement: Evidence-based Synthesis of Stretched Penile Length INdicator Technique (SPLINT). Journal of Indian Association of Pediatric Surgeons. 2024 Sep-Oct:29(5):492-504. doi: 10.4103/jiaps.jiaps_11_24. Epub 2024 Sep 9 [PubMed PMID: 39479430]
Badawy M, Fawaz LA, Abd El Baky H, Elkhashab A, Hussein AA, Mira MF. Reference values for penile and clitoral lengths of healthy term Egyptian newborn infants. Journal of paediatrics and child health. 2022 Jan:58(1):157-162. doi: 10.1111/jpc.15686. Epub 2021 Aug 9 [PubMed PMID: 34369621]
Fawaz LMA, Mira M, Ibrahim SY, Maarouf YS, Badawi NE. Stretched penile length and anogenital distance in Egyptian boys aged one month to five years. Journal of pediatric urology. 2021 Feb:17(1):110.e1-110.e7. doi: 10.1016/j.jpurol.2020.11.007. Epub 2020 Nov 6 [PubMed PMID: 33221178]
Asafo-Agyei SB, Ameyaw E, Chanoine JP, Nguah SB. Normative penile anthropometry in term newborns in Kumasi, Ghana: a cross-sectional prospective study. International journal of pediatric endocrinology. 2017:2017():2. doi: 10.1186/s13633-017-0042-1. Epub 2017 Jan 26 [PubMed PMID: 28149308]
Level 2 (mid-level) evidenceAl-Herbish AS. Standard penile size for normal full term newborns in the Saudi population. Saudi medical journal. 2002 Mar:23(3):314-6 [PubMed PMID: 11938424]
Lian WB, Lee WR, Ho LY. Penile length of newborns in Singapore. Journal of pediatric endocrinology & metabolism : JPEM. 2000 Jan:13(1):55-62 [PubMed PMID: 10689638]
Engel JD, Sutherland DE, Williams SB, Wagner KR. Changes in penile length after robot-assisted laparoscopic radical prostatectomy. Journal of endourology. 2011 Jan:25(1):65-9. doi: 10.1089/end.2010.0382. Epub 2010 Nov 29 [PubMed PMID: 21114411]
Smith AM, Jolley D, Hocking J, Benton K, Gerofi J. Does penis size influence condom slippage and breakage? International journal of STD & AIDS. 1998 Aug:9(8):444-7 [PubMed PMID: 9702591]
Bhat A, Upadhyay R, Bhat M, Sabharwal K, Singla M, Kumar V. Penile anthropometry in North Indian children. Indian journal of urology : IJU : journal of the Urological Society of India. 2015 Apr-Jun:31(2):106-10. doi: 10.4103/0970-1591.152917. Epub [PubMed PMID: 25878409]
Vasudevan G, Manivarmane, Bhat BV, Bhatia BD, Kumar S. Genital standards for south Indian male newborns. Indian journal of pediatrics. 1995 Sep-Oct:62(5):593-6 [PubMed PMID: 10829928]
Ozbey H, Temiz A, Salman T. A simple method for measuring penile length in newborns and infants. BJU international. 1999 Dec:84(9):1093-4 [PubMed PMID: 10571642]
Khairil OA, Zulfiqar A, Thambidorai CR, Nizam JM, Ahmad JT, Jamil MA. Ultrasound measurement of corporeal (penile) length in newborns. The Medical journal of Malaysia. 2005 Oct:60(4):469-74 [PubMed PMID: 16570709]
Smith DP, Rickman C, Jerkins GR. Ultrasound evaluation of normal penile (corporeal) length in children. The Journal of urology. 1995 Aug:154(2 Pt 2):822-4 [PubMed PMID: 7609188]
Soto ÁL, González JLM, López-Perez R, Fernández ML, Martínez-Uriarte J, Izquierdo OG. Sonographic measure techniques of fetal penile length. Obstetrics & gynecology science. 2020 Sep:63(5):555-564. doi: 10.5468/ogs.20087. Epub 2020 Aug 19 [PubMed PMID: 32810976]
Feldman KW, Smith DW. Fetal phallic growth and penile standards for newborn male infants. The Journal of pediatrics. 1975 Mar:86(3):395-8 [PubMed PMID: 1113226]
Lee PA, Houk CP, Ahmed SF, Hughes IA, International Consensus Conference on Intersex organized by the Lawson Wilkins Pediatric Endocrine Society and the European Society for Paediatric Endocrinology. Consensus statement on management of intersex disorders. International Consensus Conference on Intersex. Pediatrics. 2006 Aug:118(2):e488-500 [PubMed PMID: 16882788]
Level 3 (low-level) evidenceBosch I Ara L, Katugampola H, Dattani MT. Congenital Hypopituitarism During the Neonatal Period: Epidemiology, Pathogenesis, Therapeutic Options, and Outcome. Frontiers in pediatrics. 2020:8():600962. doi: 10.3389/fped.2020.600962. Epub 2021 Feb 2 [PubMed PMID: 33634051]
Bautista G. Overview of Congenital Hypopituitarism for the Neonatologist. NeoReviews. 2022 May 1:23(5):e300-e310. doi: 10.1542/neo.23-5-e300. Epub [PubMed PMID: 35490188]
Level 3 (low-level) evidenceSultan C, Paris F, Jeandel C, Lumbroso S, Galifer RB. Ambiguous genitalia in the newborn. Seminars in reproductive medicine. 2002 Aug:20(3):181-8 [PubMed PMID: 12428198]
Gueguen T, Martinerie L, Castets S, Menut V, Villanueva C, Lambert AS, Perge K, Bouhours-Nouet N, Levaillant L, Avril T, Simon D, de Kerdanet M, Lahlou N, Baron S, Reynaud R, Nicolino M, Bouvattier C, Coutant R. Inhibin B and AMH for Diagnosis of Hypogonadotropic Hypogonadism in Boys Under One Year of Age: A Case-Control Study. The Journal of clinical endocrinology and metabolism. 2025 Apr 7:():. pii: dgaf219. doi: 10.1210/clinem/dgaf219. Epub 2025 Apr 7 [PubMed PMID: 40193582]
Level 2 (mid-level) evidenceAdan L, Couto-Silva AC, Trivin C, Metz C, Brauner R. Congenital gonadotropin deficiency in boys: management during childhood. Journal of pediatric endocrinology & metabolism : JPEM. 2004 Feb:17(2):149-55 [PubMed PMID: 15055348]
Hamilton J, Blaser S, Daneman D. MR imaging in idiopathic growth hormone deficiency. AJNR. American journal of neuroradiology. 1998 Oct:19(9):1609-15 [PubMed PMID: 9802480]
Kurtoğlu S, Özdemir A, Hatipoğlu N. Neonatal Hypopituitarism: Approaches to Diagnosis and Treatment. Journal of clinical research in pediatric endocrinology. 2019 Feb 20:11(1):4-12. doi: 10.4274/jcrpe.galenos.2018.2018.0036. Epub 2018 May 9 [PubMed PMID: 29739730]
McBride JA, Carson CC, Coward RM. Diagnosis and management of testosterone deficiency. Asian journal of andrology. 2015 Mar-Apr:17(2):177-86. doi: 10.4103/1008-682X.143317. Epub [PubMed PMID: 25532575]
Androgen insensitivity syndrome: a review., Batista RL,Costa EMF,Rodrigues AS,Gomes NL,Faria JA Jr,Nishi MY,Arnhold IJP,Domenice S,Mendonca BB,, Archives of endocrinology and metabolism, 2018 Mar-Apr [PubMed PMID: 29768628]
Cima LN, Grosu I, Draghici IM, Enculescu AC, Chirita-Emandi A, Andreescu N, Puiu M, Barbu CG, Fica S. Persistent Müllerian Duct Syndrome with Supernumerary Testicles Due to a Novel Homozygous Variant in the AMHR2 Gene and Literature Review. Diagnostics (Basel, Switzerland). 2024 Nov 21:14(23):. doi: 10.3390/diagnostics14232621. Epub 2024 Nov 21 [PubMed PMID: 39682529]
Davis SW, Castinetti F, Carvalho LR, Ellsworth BS, Potok MA, Lyons RH, Brinkmeier ML, Raetzman LT, Carninci P, Mortensen AH, Hayashizaki Y, Arnhold IJ, Mendonça BB, Brue T, Camper SA. Molecular mechanisms of pituitary organogenesis: In search of novel regulatory genes. Molecular and cellular endocrinology. 2010 Jul 8:323(1):4-19. doi: 10.1016/j.mce.2009.12.012. Epub 2009 Dec 16 [PubMed PMID: 20025935]
Mehta A, Hindmarsh PC, Mehta H, Turton JP, Russell-Eggitt I, Taylor D, Chong WK, Dattani MT. Congenital hypopituitarism: clinical, molecular and neuroradiological correlates. Clinical endocrinology. 2009 Sep:71(3):376-82. doi: 10.1111/j.1365-2265.2009.03572.x. Epub 2009 Mar 6 [PubMed PMID: 19320653]
Lammoglia JJ, Eyzaguirre F, Unanue N, Román R, Codner E, Cassorla F, Mericq V. [Congenital hypopituitarism: report of 23 cases]. Revista medica de Chile. 2008 Aug:136(8):996-1006 [PubMed PMID: 18949183]
Level 3 (low-level) evidenceLadd JM, Pyle-Eilola AL, Mamilly L, Chaudhari M, Henry RK. Clinical Presentation of Congenital Hypopituitarism: Lessons From a Large Academic Centre. Clinical endocrinology. 2025 Jun:102(6):649-655. doi: 10.1111/cen.15213. Epub 2025 Feb 4 [PubMed PMID: 39905798]
Bertsch M, Floyd M, Kehoe T, Pfeifer W, Drack AV. The clinical evaluation of infantile nystagmus: What to do first and why. Ophthalmic genetics. 2017 Jan-Feb:38(1):22-33. doi: 10.1080/13816810.2016.1266667. Epub [PubMed PMID: 28177849]
Parks JS. Congenital Hypopituitarism. Clinics in perinatology. 2018 Mar:45(1):75-91. doi: 10.1016/j.clp.2017.11.001. Epub [PubMed PMID: 29406008]
Han JH, Lee JP, Lee JS, Song SH, Kim KS. Fate of the micropenis and constitutional small penis: do they grow to normalcy in puberty? Journal of pediatric urology. 2019 Oct:15(5):526.e1-526.e6. doi: 10.1016/j.jpurol.2019.07.009. Epub 2019 Jul 23 [PubMed PMID: 31447312]
Mancini M, Pecori Giraldi F, Andreassi A, Mantellassi G, Salvioni M, Berra CC, Manfrini R, Banderali G, Folli F. Obesity Is Strongly Associated With Low Testosterone and Reduced Penis Growth During Development. The Journal of clinical endocrinology and metabolism. 2021 Oct 21:106(11):3151-3159. doi: 10.1210/clinem/dgab535. Epub [PubMed PMID: 34283215]
Guthrie RD, Smith DW, Graham CB. Testosterone treatment for micropenis during early childhood. The Journal of pediatrics. 1973 Aug:83(2):247-52 [PubMed PMID: 4717581]
Bin-Abbas B, Conte FA, Grumbach MM, Kaplan SL. Congenital hypogonadotropic hypogonadism and micropenis: effect of testosterone treatment on adult penile size why sex reversal is not indicated. The Journal of pediatrics. 1999 May:134(5):579-83 [PubMed PMID: 10228293]
Nerli RB, Guntaka AK, Patne PB, Hiremath MB. Penile growth in response to hormone treatment in children with micropenis. Indian journal of urology : IJU : journal of the Urological Society of India. 2013 Oct:29(4):288-91. doi: 10.4103/0970-1591.120107. Epub [PubMed PMID: 24235789]
Becker D, Wain LM, Chong YH, Gosai SJ, Henderson NK, Milburn J, Stott V, Wheeler BJ. Topical dihydrotestosterone to treat micropenis secondary to partial androgen insensitivity syndrome (PAIS) before, during, and after puberty - a case series. Journal of pediatric endocrinology & metabolism : JPEM. 2016 Feb:29(2):173-7. doi: 10.1515/jpem-2015-0175. Epub [PubMed PMID: 26352087]
Level 2 (mid-level) evidenceStoupa A, Samara-Boustani D, Flechtner I, Pinto G, Jourdon I, González-Briceño L, Bidet M, Laborde K, Chevenne D, Millischer AE, Lottmann H, Blanc T, Aigrain Y, Polak M, Beltrand J. Efficacy and Safety of Continuous Subcutaneous Infusion of Recombinant Human Gonadotropins for Congenital Micropenis during Early Infancy . Hormone research in paediatrics. 2017:87(2):103-110. doi: 10.1159/000454861. Epub 2017 Jan 12 [PubMed PMID: 28081535]
Falcone M, Bettocchi C, Carvalho J, Ricou M, Boeri L, Capogrosso P, Cocci A, Corona G, Gül M, Hatzichristodoulou G, Jones TH, Kadioğlu A, Kalkanli A, Martinez-Salamanca JI, Milenkovic U, Morgado LA, Russo GI, Serefoğlu EC, Tharakan T, Verze P, Minhas S, Salonia A. European Association of Urology Guidelines on Penile Size Abnormalities and Dysmorphophobia: Summary of the 2023 Guidelines. European urology focus. 2024 May:10(3):432-441. doi: 10.1016/j.euf.2023.08.012. Epub 2023 Sep 13 [PubMed PMID: 37709592]
Arisaka O, Hoshi M, Kanazawa S, Nakajima D, Numata M, Nishikura K, Oyama M, Nitta A, Kuribayashi T, Kano K, Nakayama Y, Yamashiro Y. Systemic effects of transdermal testosterone for the treatment of microphallus in children. Pediatrics international : official journal of the Japan Pediatric Society. 2001 Apr:43(2):134-6 [PubMed PMID: 11285063]
Ong YC, Wong HB, Adaikan G, Yong EL. Directed pharmacological therapy of ambiguous genitalia due to an androgen receptor gene mutation. Lancet (London, England). 1999 Oct 23:354(9188):1444-5 [PubMed PMID: 10543676]
Level 3 (low-level) evidenceBertelloni S, Scaramuzzo RT, Parrini D, Baldinotti F, Tumini S, Ghirri P. Early diagnosis of 5alpha-reductase deficiency in newborns. Sexual development : genetics, molecular biology, evolution, endocrinology, embryology, and pathology of sex determination and differentiation. 2007:1(3):147-51. doi: 10.1159/000102103. Epub [PubMed PMID: 18391525]
Levy JB, Husmann DA. Micropenis secondary to growth hormone deficiency: does treatment with growth hormone alone result in adequate penile growth? The Journal of urology. 1996 Jul:156(1):214-6 [PubMed PMID: 8648808]
Bougnères P, François M, Pantalone L, Rodrigue D, Bouvattier C, Demesteere E, Roger D, Lahlou N. Effects of an early postnatal treatment of hypogonadotropic hypogonadism with a continuous subcutaneous infusion of recombinant follicle-stimulating hormone and luteinizing hormone. The Journal of clinical endocrinology and metabolism. 2008 Jun:93(6):2202-5. doi: 10.1210/jc.2008-0121. Epub 2008 Apr 1 [PubMed PMID: 18381569]
Level 3 (low-level) evidenceSwee DS, Quinton R. Managing congenital hypogonadotrophic hypogonadism: a contemporary approach directed at optimizing fertility and long-term outcomes in males. Therapeutic advances in endocrinology and metabolism. 2019:10():2042018819826889. doi: 10.1177/2042018819826889. Epub 2019 Feb 10 [PubMed PMID: 30800268]
Level 3 (low-level) evidenceMain KM, Schmidt IM, Skakkebaek NE. A possible role for reproductive hormones in newborn boys: progressive hypogonadism without the postnatal testosterone peak. The Journal of clinical endocrinology and metabolism. 2000 Dec:85(12):4905-7 [PubMed PMID: 11134160]
Borsellino A, Spagnoli A, Vallasciani S, Martini L, Ferro F. Surgical approach to concealed penis: technical refinements and outcome. Urology. 2007 Jun:69(6):1195-8 [PubMed PMID: 17572214]
Suarez A MC, Israeli JM, Kresch E, Telis L, Nassau DE. Testosterone therapy in children and adolescents: to whom, how, when? International journal of impotence research. 2022 Nov:34(7):652-662. doi: 10.1038/s41443-021-00525-5. Epub 2022 Jan 7 [PubMed PMID: 34997199]
Nordenström A, Ahmed SF, van den Akker E, Blair J, Bonomi M, Brachet C, Broersen LHA, Claahsen-van der Grinten HL, Dessens AB, Gawlik A, Gravholt CH, Juul A, Krausz C, Raivio T, Smyth A, Touraine P, Vitali D, Dekkers OM. Pubertal induction and transition to adult sex hormone replacement in patients with congenital pituitary or gonadal reproductive hormone deficiency: an Endo-ERN clinical practice guideline. European journal of endocrinology. 2022 Apr 21:186(6):G9-G49. doi: 10.1530/EJE-22-0073. Epub 2022 Apr 21 [PubMed PMID: 35353710]
Level 1 (high-level) evidenceLee JA, Ramasamy R. Indications for the use of human chorionic gonadotropic hormone for the management of infertility in hypogonadal men. Translational andrology and urology. 2018 Jul:7(Suppl 3):S348-S352. doi: 10.21037/tau.2018.04.11. Epub [PubMed PMID: 30159241]
Ramasamy R, Armstrong JM, Lipshultz LI. Preserving fertility in the hypogonadal patient: an update. Asian journal of andrology. 2015 Mar-Apr:17(2):197-200. doi: 10.4103/1008-682X.142772. Epub [PubMed PMID: 25337850]
Kim D, Lee S, Cho YH, Kang MJ, Ku CR, Chi H, Ahn J, Lee K, Han J, Chi S, Song MY, Cha SH, Lee EJ. Long-acting recombinant human follicle-stimulating hormone (SAFA-FSH) enhances spermatogenesis. Frontiers in endocrinology. 2023:14():1132172. doi: 10.3389/fendo.2023.1132172. Epub 2023 Feb 23 [PubMed PMID: 36909328]
Zacharin M, Sabin MA, Nair VV, Dabadghao P. Addition of recombinant follicle-stimulating hormone to human chorionic gonadotropin treatment in adolescents and young adults with hypogonadotropic hypogonadism promotes normal testicular growth and may promote early spermatogenesis. Fertility and sterility. 2012 Oct:98(4):836-42. doi: 10.1016/j.fertnstert.2012.06.022. Epub 2012 Jul 3 [PubMed PMID: 22763096]
Boehm U, Bouloux PM, Dattani MT, de Roux N, Dodé C, Dunkel L, Dwyer AA, Giacobini P, Hardelin JP, Juul A, Maghnie M, Pitteloud N, Prevot V, Raivio T, Tena-Sempere M, Quinton R, Young J. Expert consensus document: European Consensus Statement on congenital hypogonadotropic hypogonadism--pathogenesis, diagnosis and treatment. Nature reviews. Endocrinology. 2015 Sep:11(9):547-64. doi: 10.1038/nrendo.2015.112. Epub 2015 Jul 21 [PubMed PMID: 26194704]
Level 3 (low-level) evidenceStuckey BGA, Nolan JD, Hurley DM, Martin GB. Congenital hypogonadotrophic hypogonadism, induction of minipuberty, and future fertility. Endocrinology, diabetes & metabolism case reports. 2023 Jul 17:2023(3):. pii: 23-0038. doi: 10.1530/EDM-23-0038. Epub 2023 Jul 17 [PubMed PMID: 37458575]
Level 3 (low-level) evidenceRhys-Evans S, Howard SR. Combined gonadotropin therapy to replace mini-puberty in male infants with congenital hypogonadotropic hypogonadism. Annals of the New York Academy of Sciences. 2024 Jul:1537(1):32-40. doi: 10.1111/nyas.15177. Epub 2024 Jun 25 [PubMed PMID: 38924109]
Rhys-Evans S, d'Aniello F, Alexander EC, Dinah IF, Heger S, Nordenstrom A, Rohayem J, Howard SR. Gonadotropin Therapy for Mini-Puberty Induction in Male Infants With Hypogonadotropic Hypogonadism. The Journal of clinical endocrinology and metabolism. 2025 Mar 17:110(4):e921-e931. doi: 10.1210/clinem/dgae874. Epub [PubMed PMID: 39673783]
Alexander EC, Faruqi D, Farquhar R, Unadkat A, Ng Yin K, Hoskyns R, Varughese R, Howard SR. Gonadotropins for pubertal induction in males with hypogonadotropic hypogonadism: systematic review and meta-analysis. European journal of endocrinology. 2024 Jan 3:190(1):S1-S11. doi: 10.1093/ejendo/lvad166. Epub [PubMed PMID: 38128110]
Level 1 (high-level) evidenceMesas-Aróstegui MA, Hita-Contreras F, López-Siguero JP. A Therapeutic Proposal for Mini-Puberty in Male Infants with Hypogonadotropic Hypogonadism: A Retrospective Case Series. Journal of clinical medicine. 2024 Nov 20:13(22):. doi: 10.3390/jcm13226983. Epub 2024 Nov 20 [PubMed PMID: 39598127]
Level 2 (mid-level) evidenceSwee DS, Quinton R. Current concepts surrounding neonatal hormone therapy for boys with congenital hypogonadotropic hypogonadism. Expert review of endocrinology & metabolism. 2022 Jan:17(1):47-61. doi: 10.1080/17446651.2022.2023008. Epub 2022 Jan 7 [PubMed PMID: 34994276]
Main KM, Schmidt IM, Toppari J, Skakkebaek NE. Early postnatal treatment of hypogonadotropic hypogonadism with recombinant human FSH and LH. European journal of endocrinology. 2002 Jan:146(1):75-9 [PubMed PMID: 11751071]
Kohva E, Huopio H, Hietamäki J, Hero M, Miettinen PJ, Raivio T. Treatment of gonadotropin deficiency during the first year of life: long-term observation and outcome in five boys. Human reproduction (Oxford, England). 2019 May 1:34(5):863-871. doi: 10.1093/humrep/dez040. Epub [PubMed PMID: 31067328]
Li T, Fu FD, Wu CJ, Qin F, Wang R, Yuan JH. Anti-lysyl oxidase combined with a vacuum device induces penile lengthening by remodeling the tunica albuginea. Asian journal of andrology. 2020 Sep-Oct:22(5):485-492. doi: 10.4103/aja.aja_120_19. Epub [PubMed PMID: 31736474]
Level 2 (mid-level) evidenceLi T, Tian Y, Zhong Q, Chen P, Zhang J, Du G, Li L, Jiang Y, Jiang K. HCG supplement did not accelerate tunica albuginea remodeling to facilitate penile growth. Scientific reports. 2023 Oct 2:13(1):16519. doi: 10.1038/s41598-023-38888-y. Epub 2023 Oct 2 [PubMed PMID: 37783699]
Li T, Fu F, Wu C, Qin F, Yuan J. Characteristics of penile growth in pubertal rats and a non-invasive method to lengthen the penis. Andrology. 2020 Nov:8(6):1884-1894. doi: 10.1111/andr.12845. Epub 2020 Jul 12 [PubMed PMID: 32578359]
Level 2 (mid-level) evidenceOh JK, Im YJ, Park K, Paick JS. Effects of combined growth hormone and testosterone treatments in a rat model of micropenis. Endocrine connections. 2018 Nov:7(11):1150-1157. doi: 10.1530/EC-18-0200. Epub [PubMed PMID: 30352406]
Danino MA, Benkahdra M, El Khatib A, Yafi N, Trouilloud P, Danino RP, Laurent R. Anatomical Study of the Penile Suspensory System: A Surgical Application to Micropenis. Plastic and reconstructive surgery. Global open. 2023 Jan:11(1):e4728. doi: 10.1097/GOX.0000000000004728. Epub 2023 Jan 10 [PubMed PMID: 36699228]
Campbell J, Gillis J. A review of penile elongation surgery. Translational andrology and urology. 2017 Feb:6(1):69-78. doi: 10.21037/tau.2016.11.19. Epub [PubMed PMID: 28217452]
Callens N, Hoebeke P. Phalloplasty: A panacea for 46,XY disorder of sex development conditions with penile deficiency? Endocrine development. 2014:27():222-33. doi: 10.1159/000363666. Epub 2014 Sep 9 [PubMed PMID: 25247659]
Yao A, Ingargiola MJ, Lopez CD, Sanati-Mehrizy P, Burish NM, Jablonka EM, Taub PJ. Total penile reconstruction: A systematic review. Journal of plastic, reconstructive & aesthetic surgery : JPRAS. 2018 Jun:71(6):788-806. doi: 10.1016/j.bjps.2018.02.002. Epub 2018 Feb 14 [PubMed PMID: 29622476]
Level 1 (high-level) evidenceDe Fontaine S, Loréa P, Wespes E, Schulman C, Goldschmidt D. Complete phalloplasty using the free radial forearm flap for correcting micropenis associated with vesical exstrophy. The Journal of urology. 2001 Aug:166(2):597-9 [PubMed PMID: 11458075]
Callens N, De Cuypere G, T'Sjoen G, Monstrey S, Lumen N, Van Laecke E, Hoebeke P, Cools M. Sexual quality of life after total phalloplasty in men with penile deficiency: an exploratory study. World journal of urology. 2015 Jan:33(1):137-43. doi: 10.1007/s00345-014-1283-8. Epub 2014 Mar 29 [PubMed PMID: 24682594]
Level 2 (mid-level) evidenceSarıkaya S, Ralph DJ. Mystery and realities of phalloplasty: a systematic review. Turkish journal of urology. 2017 Sep:43(3):229-236. doi: 10.5152/tud.2017.14554. Epub 2017 Aug 3 [PubMed PMID: 28861290]
Level 1 (high-level) evidenceGaraffa G, Antonini G, Gentile V, Ralph DJ. Phalloplasty for the genetic male. Translational andrology and urology. 2012 Jun:1(2):103-8. doi: 10.3978/j.issn.2223-4683.2012.05.05. Epub [PubMed PMID: 26816694]
Kristinsson S, Johnson M, Ralph D. Review of penile reconstructive techniques. International journal of impotence research. 2021 Apr:33(3):243-250. doi: 10.1038/s41443-020-0246-4. Epub 2020 Mar 9 [PubMed PMID: 32152468]
Callens N, De Cuypere G, Van Hoecke E, T'sjoen G, Monstrey S, Cools M, Hoebeke P. Sexual quality of life after hormonal and surgical treatment, including phalloplasty, in men with micropenis: a review. The journal of sexual medicine. 2013 Dec:10(12):2890-903. doi: 10.1111/jsm.12298. Epub 2013 Aug 23 [PubMed PMID: 23981815]
Level 2 (mid-level) evidenceEscoffier A, Morel-Journel N, Terrier M, Paganelli L, Boucher F, Ruffion A, Carnicelli D, Neuville P. Functional and surgical outcomes after phalloplasty in cis men. World journal of urology. 2022 Nov:40(11):2635-2640. doi: 10.1007/s00345-022-04141-w. Epub 2022 Sep 16 [PubMed PMID: 36112209]
D'Arpa S, Claes K, Lumen N, Oieni S, Hoebeke P, Monstrey S. Urethral Reconstruction in Anterolateral Thigh Flap Phalloplasty: A 93-Case Experience. Plastic and reconstructive surgery. 2019 Feb:143(2):382e-392e. doi: 10.1097/PRS.0000000000005278. Epub [PubMed PMID: 30688908]
Level 3 (low-level) evidenceLo Re M, Alonso Isa M, Garcia Rojo E, Manfredi C, Fraile Poblador A, Pezzoli M, Cocci A, Sessa F, Minervini A, Romero-Otero J. Advancements in penile lengthening techniques concurrent with penile prosthesis placement: a narrative review. Asian journal of andrology. 2025 Apr 18:():. doi: 10.4103/aja202512. Epub 2025 Apr 18 [PubMed PMID: 40247692]
Level 3 (low-level) evidenceMarchand S, Morel-Journel N, Carnicelli D, Boucher F, Ruffion A, Neuville P. Surgical Outcomes of the ZSI475 FtM Inflatable Erectile Prosthesis Implantation After Phalloplasty. Urology. 2025 Apr:198():217-221. doi: 10.1016/j.urology.2025.01.002. Epub 2025 Jan 9 [PubMed PMID: 39798615]
Shah BB, Kent M, Valenzuela R. Advanced Penile Length Restoration Techniques to Optimize Penile Prosthesis Placement Outcomes. Sexual medicine reviews. 2021 Oct:9(4):641-649. doi: 10.1016/j.sxmr.2020.05.007. Epub 2020 Jul 9 [PubMed PMID: 32653404]
Chung E, Moon DG, Hui J, Chang HC, Hakim L, Nagao K, Tan R, Mak SK, Tantiwongse K, Lin H, Mai DBT, Nguyen Q, Tan HM, Sato Y, Jiann BP, Park K, Xin ZC, Park HJ. Clinical recommendations on penile reconstructive and prosthetic surgery: a consensus statement from the Asia-Pacific Society of Sexual Medicine. Sexual medicine. 2023 Apr:11(2):qfad003. doi: 10.1093/sexmed/qfad003. Epub 2023 Apr 11 [PubMed PMID: 37056790]
Level 3 (low-level) evidenceMonstrey S, Hoebeke P, Selvaggi G, Ceulemans P, Van Landuyt K, Blondeel P, Hamdi M, Roche N, Weyers S, De Cuypere G. Penile reconstruction: is the radial forearm flap really the standard technique? Plastic and reconstructive surgery. 2009 Aug:124(2):510-518. doi: 10.1097/PRS.0b013e3181aeeb06. Epub [PubMed PMID: 19644267]
Alba B, Nolan IT, Weinstein B, O'Neill E, Fritsch A, Jacobs KM, Schechter L. Gender-Affirming Phalloplasty: A Comprehensive Review. Journal of clinical medicine. 2024 Oct 8:13(19):. doi: 10.3390/jcm13195972. Epub 2024 Oct 8 [PubMed PMID: 39408031]
Falagario UG, Piramide F, Pang KH, Durukan E, Tzelves L, Ricapito A, Baekelandt L, Checcucci E, Carrion DM, Bettocchi C, Esperto F. Techniques for Penile Augmentation Surgery: A Systematic Review of Surgical Outcomes, Complications, and Quality of Life. Medicina (Kaunas, Lithuania). 2024 May 2:60(5):. doi: 10.3390/medicina60050758. Epub 2024 May 2 [PubMed PMID: 38792941]
Level 1 (high-level) evidenceBerli JU, Ferrin PC, Buuck C, Cylinder I, Putnam C, Dy GW, Peters BR, Llado-Farrulla M, Sajadi KP, Annen A. Long-term Urologic Outcomes Using the Big Ben Method for Phalloplasty. Plastic and reconstructive surgery. 2025 May 7:():. doi: 10.1097/PRS.0000000000012010. Epub 2025 May 7 [PubMed PMID: 40331576]
Heston AL, Esmonde NO, Dugi DD 3rd, Berli JU. Phalloplasty: techniques and outcomes. Translational andrology and urology. 2019 Jun:8(3):254-265. doi: 10.21037/tau.2019.05.05. Epub [PubMed PMID: 31380232]
Falcone M, Timpano M, Oderda M, Cocci A, Morelli G, Preto M, Polito C, Giorgio IR, Gideon B, Gontero P. Suprapubic pedicled phalloplasty in transgender men: a multicentric retrospective cohort analysis. International journal of impotence research. 2020 Dec:33(8):808-814. doi: 10.1038/s41443-020-0238-4. Epub 2020 Feb 7 [PubMed PMID: 32034312]
Level 2 (mid-level) evidenceAscha M, Massie JP, Morrison SD, Crane CN, Chen ML. Outcomes of Single Stage Phalloplasty by Pedicled Anterolateral Thigh Flap versus Radial Forearm Free Flap in Gender Confirming Surgery. The Journal of urology. 2018 Jan:199(1):206-214. doi: 10.1016/j.juro.2017.07.084. Epub 2017 Jul 29 [PubMed PMID: 28765066]
Khorashad BS, Gardner M, Lee PA, Kogan BA, Sandberg DE. Recommendations for 46,XY Disorders/Differences of Sex Development Across Two Decades: Insights from North American Pediatric Endocrinologists and Urologists. Archives of sexual behavior. 2024 Aug:53(8):2939-2956. doi: 10.1007/s10508-024-02942-1. Epub 2024 Jul 22 [PubMed PMID: 39039338]
Babaei A, Safarinejad MR, Farrokhi F, Iran-Pour E. Penile reconstruction: evaluation of the most accepted techniques. Urology journal. 2010 Jun 10:7(2):71-8 [PubMed PMID: 20535691]
Dabernig J, Chan LK, Schaff J. Phalloplasty with free (septocutaneous) fibular flap sine fibula. The Journal of urology. 2006 Nov:176(5):2085-8 [PubMed PMID: 17070265]
Xu KY, Watt AJ. The Pedicled Anterolateral Thigh Phalloplasty. Clinics in plastic surgery. 2018 Jul:45(3):399-406. doi: 10.1016/j.cps.2018.03.011. Epub 2018 May 1 [PubMed PMID: 29908629]
Peters BR, Sajadi KP, Berli JU. Big Ben Method Phalloplasty: Step by Step. Plastic and reconstructive surgery. Global open. 2023 Jul:11(7):e5126. doi: 10.1097/GOX.0000000000005126. Epub 2023 Jul 17 [PubMed PMID: 37465284]
Falcone M, Blecher G, Anfosso M, Christopher AN, Ralph DJ. Total Phallic Reconstruction in the Genetic Male. European urology. 2021 May:79(5):684-691. doi: 10.1016/j.eururo.2020.07.025. Epub 2020 Aug 14 [PubMed PMID: 32800729]
Leriche A, Timsit MO, Morel-Journel N, Bouillot A, Dembele D, Ruffion A. Long-term outcome of forearm flee-flap phalloplasty in the treatment of transsexualism. BJU international. 2008 May:101(10):1297-300. doi: 10.1111/j.1464-410X.2007.07362.x. Epub 2008 Jan 8 [PubMed PMID: 18190640]
Paganelli L, Morel-Journel N, Carnicelli D, Ruffion A, Boucher F, Maucort-Boulch D, Paparel P, Terrier M, Neuville P. Determining the outcomes of urethral construction in phalloplasty. BJU international. 2023 Mar:131(3):357-366. doi: 10.1111/bju.15915. Epub 2022 Oct 26 [PubMed PMID: 36221955]
Elliott SP. Trauma and Genital and Urethral Reconstruction. The Journal of urology. 2025 Jun 3:():101097JU0000000000004610. doi: 10.1097/JU.0000000000004610. Epub 2025 Jun 3 [PubMed PMID: 40460289]
Cimador M, Catalano P, Ortolano R, Giuffrè M. The inconspicuous penis in children. Nature reviews. Urology. 2015 Apr:12(4):205-15. doi: 10.1038/nrurol.2015.49. Epub 2015 Apr 7 [PubMed PMID: 25850928]
Calikoglu AS. Should boys with micropenis be reared as girls? The Journal of pediatrics. 1999 May:134(5):537-8 [PubMed PMID: 10228285]
Raveenthiran V. Neonatal Sex Assignment in Disorders of Sex Development: A Philosophical Introspection. Journal of neonatal surgery. 2017 Jul-Sep:6(3):58. doi: 10.21699/jns.v6i3.604. Epub 2017 Aug 10 [PubMed PMID: 28920018]
Lee PA, Mazur T, Houk CP, Blizzard RM. Growth Hormone Deficiency Causing Micropenis: Lessons Learned From a Well-Adjusted Adult. Pediatrics. 2018 Jul:142(1):. pii: e20174168. doi: 10.1542/peds.2017-4168. Epub [PubMed PMID: 29959177]
Wisniewski AB, Migeon CJ. Long-term perspectives for 46,XY patients affected by complete androgen insensitivity syndrome or congenital micropenis. Seminars in reproductive medicine. 2002 Aug:20(3):297-304 [PubMed PMID: 12428209]
Level 3 (low-level) evidenceRaveenthiran V. Controversies of Sex Re-assignment in Genetic Males with Congenital Inadequacy of the Penis. Indian journal of pediatrics. 2017 Sep:84(9):700-708. doi: 10.1007/s12098-017-2412-3. Epub 2017 Jul 8 [PubMed PMID: 28687949]
Wisniewski AB, Migeon CJ, Gearhart JP, Rock JA, Berkovitz GD, Plotnick LP, Meyer-Bahlburg HF, Money J. Congenital micropenis: long-term medical, surgical and psychosexual follow-up of individuals raised male or female. Hormone research. 2001:56(1-2):3-11 [PubMed PMID: 11815721]
Chen MJ, Vu BM, Axelrad M, Dietrich JE, Gargollo P, Gunn S, Macias CG, McCullough LB, Roth DR, Sutton VR, Karaviti LP. Androgen Insensitivity Syndrome: Management Considerations from Infancy to Adulthood. Pediatric endocrinology reviews : PER. 2015 Jun:12(4):373-87 [PubMed PMID: 26182482]
Delli Paoli E, Di Chiano S, Paoli D, Lenzi A, Lombardo F, Pallotti F. Androgen insensitivity syndrome: a review. Journal of endocrinological investigation. 2023 Nov:46(11):2237-2245. doi: 10.1007/s40618-023-02127-y. Epub 2023 Jun 10 [PubMed PMID: 37300628]
Mohan S, Kapoor G, Raman DK. Partial androgen insensitivity syndrome: a diagnostic and therapeutic dilemma. Medical journal, Armed Forces India. 2011 Oct:67(4):382-4. doi: 10.1016/S0377-1237(11)60093-2. Epub 2011 Oct 22 [PubMed PMID: 27365856]
Aliani F, Haghshenas Z, Vosough Dizaj A, Arabipoor A, Vesali S, Ashrafi M. Birth prevalence of genital anomalies among males conceived by intracytoplasmic sperm injection cycles: A cross-sectional study. International journal of reproductive biomedicine. 2023 Jan:21(1):53-60. doi: 10.18502/ijrm.v21i1.12666. Epub 2023 Feb 8 [PubMed PMID: 36875504]
Level 2 (mid-level) evidenceEl Kholy M, Hamza RT, Saleh M, Elsedfy H. Penile length and genital anomalies in Egyptian male newborns: epidemiology and influence of endocrine disruptors. Journal of pediatric endocrinology & metabolism : JPEM. 2013:26(5-6):509-13. doi: 10.1515/jpem-2012-0350. Epub [PubMed PMID: 23509209]
Tian Y, Hou L, Xiang S, Tian X, Xu J. Congenital adrenal hyperplasia disorder due to 17 α-hydroxylase deficiency: a case report. Gynecological endocrinology : the official journal of the International Society of Gynecological Endocrinology. 2023 Aug 18:39(1):2250001. doi: 10.1080/09513590.2023.2250001. Epub [PubMed PMID: 37683689]
Level 2 (mid-level) evidenceAl Sayed Y, Howard SR. Panel testing for the molecular genetic diagnosis of congenital hypogonadotropic hypogonadism - a clinical perspective. European journal of human genetics : EJHG. 2023 Apr:31(4):387-394. doi: 10.1038/s41431-022-01261-0. Epub 2022 Dec 15 [PubMed PMID: 36517585]
Level 3 (low-level) evidenceKaya C, Bektic J, Radmayr C, Schwentner C, Bartsch G, Oswald J. The efficacy of dihydrotestosterone transdermal gel before primary hypospadias surgery: a prospective, controlled, randomized study. The Journal of urology. 2008 Feb:179(2):684-8 [PubMed PMID: 18082206]
Level 1 (high-level) evidence